Abstract
Background:
In the era of high active antiretroviral therapy (HAART), with increasing survival of HIV patients, cardiovascular risk has emerged as a leading health issue.
Aims:
This study aims to predict the 10-year cardiovascular disease risk in HIV patients using the Framingham risk score and its modification for HIV-infected patients on HAART, that is, the data on adverse effect of antiretroviral drugs (DAD) prediction equation.
Setting and Design:
This is a case control study.
Materials and Methods:
The present study included 320 subjects (220 HIV infected cases and 100 age, sex and body mass index matched HIV noninfected healthy controls) and was conducted in a tertiary care centre in western Rajasthan. All the patients were subjected to a detailed clinical history, complete physical examination and evaluation of laboratory parameters. We calculated Framingham risk score and DAD prediction equation for these patients and compared these scores between patients on HAART with lipodystrophy, those without lipodystrophy, HAART naive and healthy controls.
Statistical Analysis:
Unpaired t-test was used and statistical analysis was performed using SPSS version 20.
Results:
In our study, 46.67% patients on HAART developed lipodystrophy, of which 17.72% had moderate-to-high risk of cardiovascular risk according to Framingham risk score, which is significantly higher than in patients without lipodystrophy and controls (3.3% and 6%, respectively). Similar high risk was also seen with DAD score. The various risk factors also showed a positive correlation with duration of HAART.
Conclusion:
Our study emphasizes the need for early recognition of cardiovascular risk in HIV-infected patients on HAART, especially in those with lipodystrophy and advocates effective use of risk calculators in these patients.
Keywords: Acquired Immunodeficiency Syndrome, cardiovascular disease, data on adverse effect of antiretroviral drugs risk equation, Framingham risk score, HIV infections, lipodystrophy
INTRODUCTION
As longevity is increasing in HIV-infected individuals, after introduction of highly active antiretroviral therapy (HAART), the risk of cardiovascular disease is emerging as a leading health issue.[1] Multiple potential risk factors contribute to cardiovascular disease in HIV-infected individuals which include HIV infection itself (due to its proatherogenic lipid profile: low high-density lipoprotein [HDL] and elevated low-density lipoprotein [LDL] particles and triglyceride [TG] levels),[2] metabolic syndrome associated with HIV, lipodystrophy syndrome, antiretroviral agents, and duration of intake of these drugs, CD4 counts, viral load, weight, age, and gender.[3]
Furthermore, there is still need to identify and evaluate HIV-specific cardiovascular disease risk factors and to refine existing cardiovascular disease prediction equation and to develop new HIV-specific cardiovascular disease prediction calculators for adults, adolescents, and children.[4] Hence, we conducted a study to predict the 10-year cardiovascular disease risk in HIV patients using the Framingham risk score, and its modification for HIV-infected patients on HAART, that is, the data on adverse effect of antiretroviral drugs (DAD) prediction equation, by measuring the fat redistribution in HIV-infected patients in age group of 18–44 years.[5]
To emphasize the importance of the above statement, we would also compare the cardiovascular disease risk calculated using the Framingham risk score with the results of DAD prediction equation, so that the future research priorities can be identified.
MATERIALS AND METHODS
The present study was multicentric, case–control study conducted in a tertiary care center in Western Rajasthan in 1-year duration. The study population comprised 320 participants, 170 HIV patients on HAART for >6 months and 50 HAART naïve patients (case) and 100 healthy individuals aged between 18 and 44 years who were age, sex, and body mass index (BMI) matched with the study group (control).
Ethical approval was taken from medical college ethics committee. Participants were briefed about the nature of study and written consent was obtained from them. HIV patients with the evidence of clinical sign of active AIDS in the 3 months before study or on HAART for <6 months or those who had changed drugs in ≤6 weeks were excluded from the study. Patients with diabetes, coronary artery disease, hypertension, dyslipidemia or those exposed to drugs such as statins, testosterone, and estrogen or substance abuser, except alcohol and cigarette, and pregnant females were also excluded from the study. Same exclusion criteria were applied for selecting the control group.
Study participants were then subjected to questionnaire which included medical history, health behavior questionnaire, assessment of medication use, with complete physical examination and laboratory data collection. BMI was calculated as weight (kg) divided by height squared (m2) and individuals were defined as underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), and obese (≥30). Lipodystrophy was defined on basis of body fat distribution which was scored as absent (0), present (1), or severe (2) with regard to increased neck fat (anterior or posterior), predominantly dorsocervical fat pad or buffalo hump, increased trunk or chest fat (lipohypertrophy), facial fat loss, and decreased arm and leg fat (lipoatrophy).[6] Blood pressure (BP) was measured using an automated sphygmomanometer, with the patient in the sitting position before the blood test. Same methods were used to estimate the value in both cases and controls to maximize comparability between the groups.
HAART was defined by the Department of Health and Human Services guidelines as follows: (1) ≥2 nucleoside reverse-transcriptase inhibitors (NRTIs) in combination with at least 1 protease inhibitor (PI) or 1 nonnucleoside reverse-transcriptase inhibitor; (2) 1 NRTI in combination with at least 1 protease inhibitor and at least 1 nonnucleoside reverse-transcriptase inhibitor; (3) a regimen containing ritonavir and saquinavir in combination with 1 NRTI and no non-NRTIs; or (4) an abacavir or tenofovir containing regimen of 3 NRTIs in the absence of both protease inhibitors and non-NRTIs, except for the 3-NRTI regimens consisting of abacavir, tenofovir and lamivudine or didanosine, tenofovir, and lamivudine.[7]
The blood sample was collected in the morning after at least 8 h of fasting. Individual were subjected to baseline investigations including hemogram, fasting and postprandial blood sugar, fasting total lipid profile including total cholesterol (TC), HDL, TG estimated by enzymatic methods in a Cobas Mira automatic analyzer and glucose estimated by oxidase method and CD4 counts was also done in HIV-infected patients.
Fasting plasma glucose ≥126 mg/dL (fasting is defined as no calorie intake for 8 h) or 2 h plasma glucose ≥200 mg/dL on oral glucose tolerance test taken for the diagnosis of diabetes mellitus.[8] Elevated levels for cholesterol fractions and triglycerides were defined by ATP lll guideline as TG ≥200 mg/dL, HDL ≤40 mg/dL, and LDL ≥160 mg/dL.[9]
A total of 220 patients of HIV were examined during the study period. Of these, 79 patients on HAART had lipodystrophy (Group A), 91 patients who were on HAART did not develop lipodystrophy (Group B). A total of 50 HIV patients were HAART naive (Group C), and 100 controls were taken (Group D).
Framingham risk score which is based on age, gender, TC, HDL cholesterol, smoking, and BP levels was calculated in study groups and controls.[10] DAD score is used to determine the prevalence cardiovascular disease risk among HIV-infected persons and to investigate any association between such risk factors, stage of HIV disease, and use of antiretroviral therapies (ARTs).[11] On the basis of both these scores, participants were categorized as mild, moderate, and severe cardiovascular disease risk.
Baseline demographic data in the 4 groups were descriptively summarized. Continuous variables were expressed as mean ± standard deviation and mean ± standard error. Categorical variables were presented as percentages. Unpaired t-test was used for calculating statistical significances of the results. Pearson correlation coefficient (r) in linear regression model was used to evaluate the relationship between increasing duration of HAART and various risk factors [Figure 1]. Significance was defined as P < 0.05 for all statistical tests, which were performed using SPSS version 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp).
Figure 1.
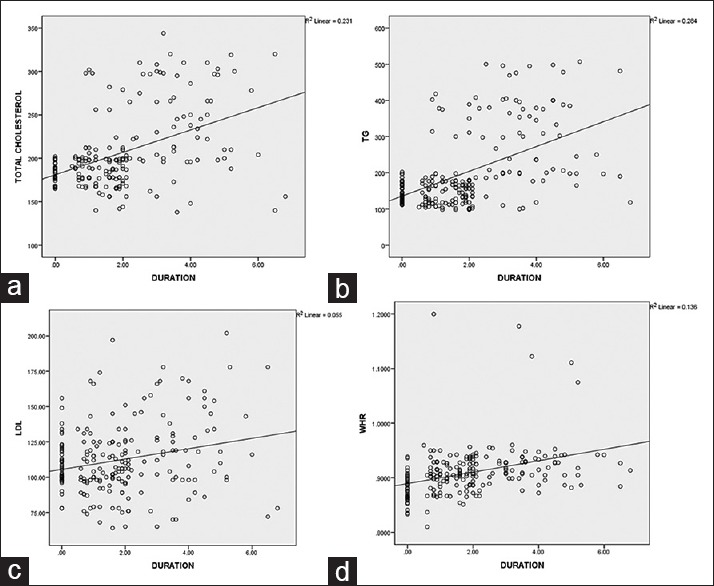
Positive correlation between duration of high active antiretroviral therapy and the lipid abnormalities (a) Pearson corelation = 0.481 (b) Pearson corelation = 0.533 (c) Pearson corelation = 0.234 (d) Pearson corelation = 0.369
RESULTS
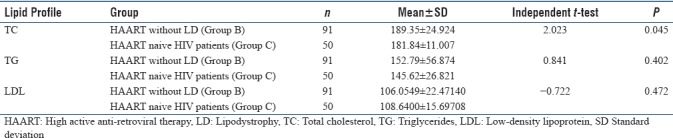
In the present study, we included 220 HIV-infected patients out of which 170 patients were on HAART and 50 patients were HAART naive. We also took 100 age- and BMI-matched healthy controls. Lipodystrophy was found in 79 (46.47%) patients out of 170 patients who were on HAART. In our study, none of the HAART naive patients developed lipodystrophy. The mean value of TC (236 ± 52.88), TG (282.19 ± 119.68) and LDL (121.44 ± 29.86) was significantly (P < 0.005) higher in Group A (HAART with LD) as compared to Group B (HAART without LD), Group C (HAART naive) and control group, while not showing significant difference between Group B and Group C. Significant results are summarized in tables and subjected to independent t-test, and P value is calculated which is highly significant [Tables 1–5].
Table 1.
Comparative analysis of various groups

Table 5.
Comparison between HIV patients on high active anti-retroviral therapy with lipodystrophy and healthy controls

Table 2.
Comparison between HIV patients on high active anti-retroviral therapy with and without lipodystrophy
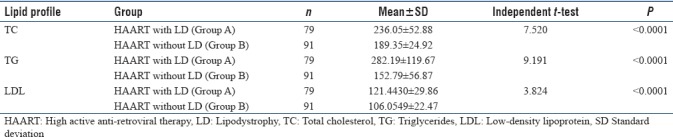
Table 3.
Comparison between HIV patients on high active anti-retroviral therapy with lipodystrophy and high active anti-retroviral therapy naïve
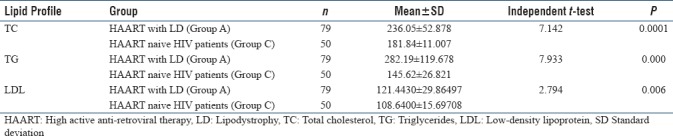
Table 4.
Comparison between HIV patients on high active anti-retroviral therapy without lipodystrophy and high active anti-retroviral therapy naïve
Framingham risk score which is based on age, gender, TC, HDL cholesterol, smoking, and BP levels was calculated in study groups and controls. In our study, 14 (17.72%) patients in Group A had moderate-to-high cardiovascular disease risk by Framingham risk score as compared to only 3 (3.3%) in Group B, 2 (4%) in Group C, and 3 (3%) in control group. This observation shows odds ratio of 6.318 and statistically significant (P < 0.005) cardiovascular disease risk in patients on HAART with lipodystrophy (Group A) as compared to patients on HAART without lipodystrophy (Group B), and when compared to controls (Group D) odds ratio is 6.96 and P value is 0.0031, while the risk of cardiovascular disease between Group B and Group C was not statically significant [Table 6].
Table 6.
Cardiovascular risk assessment in HIV patients and control by Framingham risk score

DAD score is used to determine the cardiovascular disease risk among HIV-infected persons and to investigate any association between such risk factors, stage of HIV disease, and use of ARTs. This score calculated in HIV patients on HAART shows, 42 (53.20%) patients of Group A had moderate-to-high cardiovascular disease risk, while 21 (23.10%) out of 91 patients of Group B had moderate-to-high cardiovascular disease risk. It indicates significant positive correlation between lipodystrophy and cardiovascular risk in HIV patients on HAART, odds ratio is 3.78 and P value 0.0001 [Table 7].
Table 7.
Cardiovascular risk assessment in HIV patients on high active anti-retroviral therapy by data on adverse effect of antiretroviral drugs prediction equation

DISCUSSION
In our study, 220 patients of HIV and 100 age- and BMI-matched healthy controls from a tertiary care center in Western Rajasthan were enrolled in 1-year duration. Of these 220 patients, 170 were on HAART, out of which 157 (92.4%) patients were on 2NRTI+ 1NNRTI regimen and 13 (7.6%) were on 2NRTI+ PI, that is, 100% were exposed to NRTI (stavudine, lamivudine, and zidovudine), 92.4% to NNRTI (Efavirenz, Nevarepine) and 7.6% to PI (ritonavir-boosted lopinavir and atazanavir). In DAD[4,5] study, 13% were treatment naïve, 36% had received NNRTI which was less in comparison to the present study and 87% had received NRTI and 72% had received PI which is almost 10 times that of our study. This suggests relatively lesser use of PI-based regimes in this part of India.
In the present study, the mean duration of exposure to various categories of HAART was 3.68 years and the average duration of exposure to HAART in patients with lipodystrophy is >3 years. Our findings are similar to two studies, one by Hadigan et al.,[12] in which the mean duration of HAART was 3 years, and the CREATE-1[13] study which also had similar duration of exposure to HAART and development of lipodystrophy.
Out of 79 patients who developed lipodystrophy, 72.2% had lipohypertrophy and 27.8% had lipoatrophy. The present study had this higher prevalence of lipohypertrophy as also seen in a similar study by Safrin and Grunfeld[14] and Tien and Grunfeld.[15]
In the present study, lipid profiles of patients with lipodystrophy were significantly altered in comparison to that of patients without lipodystrophy. In patients with lipodystrophy TC (236.05 ± 52.88), TG (282.19 ± 119.68), LDL (121.44 ± 29.86) in mg/dl and TC: HDL (8.01 ± 2.54) were significantly higher in comparison to the patients without lipodystrophy, TC (189.35 ± 24.92), TG (152.79 ± 56.87), LDL (106.05 ± 22.47) in mg/dl, and TC: HDL (5.22 ± 1.25), (P < 0.0001) and controls TC (178.15 ± 21.31), TG (150.47 ± 28.77), LDL (96.51 ± 13.54) in mg/dl, and TC: HDL (4.79 ± 1.02) (P < 0.0001). The findings of the study were similar to those seen in the data collection on adverse events of anti-HIV drugs (DAD) study,[5] which showed elevated TC (>6.2 mmol/L: 239 mg/dL) among NNRTI + PI (OR, 5.48) with lipodystrophy compared to the prevalence among ART-naive participants and cases without lipodystrophy.
In the present study, on predicting the cardiovascular disease risk using Framingham risk score it was found that, in patients with lipodystrophy 65 (82.3%) out of 79 patients had minimum (<15%) risk of cardiovascular disease per decade and 14 (17.72%) patients had moderate (≥15-<25%) -to-high (≥25%) risk of cardiovascular disease per decade as compared to patients without lipodystrophy having 88 (96.7%) patients with minimal risk and 3 (3.3%) patients with moderate-to-high risk out of a total of 91 patients. In the control group, only 6 (6%) out of 100 cases had moderate-to-high risk of cardiovascular disease per decade as calculated by Framingham risk score. The finding in the present study were similar to those of Hadigan et al.[12] and CREATE-1 study[13] who too predicted a higher risk among patients with lipodystrophy and a lower risk among patients without lipodystrophy.
When cardiovascular disease risk was predicted using DAD equation, it revealed that, the patients with lipodystrophy had minimum risk (<1%) in 37 (46.8%) and moderate (≥1–<5%)-to-high (≥5%) risk of cardiovascular disease per 5 years in 42 (53.2%) out of a total number of 79 patients. Whereas, in the patients without lipodystrophy, 70 (76.9%) patients had minimal and 21 (23.1%) had moderate risk of cardiovascular disease per 5 years out of a total number of 91 patients. The findings were similar to CREATE-1 study which too found increased and better predicted cardiovascular disease risk by DAD score among cases of HIV on HAART who develop lipodystrophy.[13]
In the present study, smokers were 45 (26.5%) among HIV patients on HAART and 11 (22%) in patients not on HAART. The present study shows smoking is increasingly prevalent amongst HIV patients as was seen in the DAD study.[5]
CONCLUSION
Our study suggests that the presence of lipodystrophy is associated with adverse lipid profile as well as adverse cardiovascular profile as assessed by Framingham and DAD score. Thus patients of HIV on HAART should be screened early for cardiovascular risk and appropriate measures should be taken to prevent progression of this risk into overt disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Arioglu E, Duncan-Morin J, Sebring N, Rother KI, Gottlieb N, Lieberman J, et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133:263–74. doi: 10.7326/0003-4819-133-4-200008150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Caron M, Auclair M, Vigouroux C, Glorian M, Forest C, Capeau J, et al. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001;50:1378–88. doi: 10.2337/diabetes.50.6.1378. [DOI] [PubMed] [Google Scholar]
- 3.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–12. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Friis-Møller N, Thiébaut R, Reiss P, El-Sadr W, Weber R, D'ArminioMonforte A, et al. Predicting the Risk of Coronary Heart Disease (CHD) in HIV-infected Patients: The D:A:D CHD Risk Equation. Paper Presented at: 14th Conference on Retroviruses and Opportunistic Infections; 27 February, 2007; Los Angeles, Calif. [Google Scholar]
- 5.Friis-Møller N, Weber R, Reiss P, Thiébaut R, Kirk O, d'Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients - Association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ, Jr, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–98. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [Last accessed on 2013 Oct 25]. Available from: https://www.aidsinfo.nih.gov/guidelines .
- 8.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2012. [Last accessed on 2013 Oct 25]. Available from: http://www.care.diabetesjournals.org/content/35/Supplement_1/S11 .
- 9.Third Report of the Expert Panel on National Cholesterol Education Program (NCEP) on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or ATP III) Final Report of National Cholesterol Education Program, National Heart, Lung, Blood Institute, National Institute of Health. NIH Publication No. 02-5215 September, 2002 [Google Scholar]
- 10. [Last accessed on 2014 Oct 25]. Available from: https://www.cvdriskchecksecure.com/framinghamriskscore.aspx .
- 11. [Last accessed on 2014 Oct 25]. Available from: http://www.hivpv.org/home/tools/tabid/91/ExamView/mid/500/eid/0/.../Default.aspx .
- 12.Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S, et al. Metformin in the treatment of HIV lipodystrophy syndrome: A randomized controlled trial. JAMA. 2000;284:472–7. doi: 10.1001/jama.284.4.472. [DOI] [PubMed] [Google Scholar]
- 13.Wierzbicki AS, Reynolds TM. Vascular risk screening: Possible or too much, too soon? Int J Clin Pract. 2009;63:989–96. doi: 10.1111/j.1742-1241.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 14.Safrin S, Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS. 1999;13:2493–505. doi: 10.1097/00002030-199912240-00002. [DOI] [PubMed] [Google Scholar]
- 15.Tien PC, Grunfeld C. What is HIV-associated lipodystrophy? Defining fat distribution changes in HIV infection. Curr Opin Infect Dis. 2004;17:27–32. doi: 10.1097/00001432-200402000-00005. [DOI] [PubMed] [Google Scholar]


