Abstract
Background
This project is an effort to understand how orders for IV immunoglobulin (IVIg) are documented and prescribed by physicians, and subsequently, how they are reviewed by insurance companies for the treatment of immune neuropathies.
Methods
A panel of neuromuscular specialists reviewed case records from 248 IVIg-naive patients whose in-home IVIg infusion treatment was submitted to insurance for authorization. After reviewing a case record, 1 panelist was asked to make a diagnosis and to answer several questions about the treatment. A second panelist reviewed the original record and follow-up records that were obtained for reauthorization of additional treatments and was asked to determine whether the patient had responded to the treatment.
Results
Our specialists believed that only 32.2% of 248 patients had an immune neuropathy and were appropriate candidates for IVIg therapy, whereas 46.4% had neuropathies that were not immune mediated. Only 15.3% of cases met electrodiagnostic criteria for a demyelinating neuropathy. Our specialists believed that 36.7% of 128 cases with follow-up records had responded to therapy. In cases in which the initial reviewer had predicted that there would be a response to IVIg, the second reviewer found that 54% had responded. This is compared with a 27% response rate when the first reviewer predicted that there would be no response (p = 0.019).
Conclusions
Our expert review finds that the diagnosis of immune neuropathies made by providers, and subsequently approved for IVIg therapy by payers, is incorrect in a large percentage of cases. If payers include an expert in their review process, it would improve patient selection, appropriate use, and continuation of treatment with this expensive therapeutic agent.
Allen and Lewis1 recently reported that 47% of patients referred to a tertiary care neuromuscular practice with a diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) failed to meet diagnostic criteria for the disease, raising questions about overdiagnosis in the area of immune neuropathies. Their study used research criteria as the gold standard, which broke the diagnostic process into a “present or not present” dichotomy. The reality, however, is that the review process used by insurance carriers may not always adhere to published standards. Although these reviewers have become the de facto referees for determining when IV immunoglobulin (IVIg) treatment is permitted, there are no systematic studies on how treatment decisions are made or on their accuracy in predicting outcomes.
In this project, we asked clinical experts to review cases and assess pretreatment diagnoses, appropriateness of therapy, and the subsequent responses using records submitted to insurance companies for treatment of CIDP and multifocal motor neuropathy (MMN). This study, called “Immunological and Neurological Study of Immune Globulin Habits, Treatments and Standards” (INSIGHTS), aimed at better understanding how IVIg is prescribed and reviewed across the spectrum of clinical practices and how this relates to outcomes.
Methods
Study cohort
The study was part of a quality assurance/quality improvement (QA/QI) project conducted by NuFACTOR, Inc. (“NuFACTOR”), which provides home-based IVIg infusions. NuFACTOR's program evaluated treatment outcomes associated with disease-specific prescribing regimens in patients who received home-based IVIg treatment provided by NuFACTOR. We conducted a retrospective review of the medical records of 248 patients who received home-based IVIg treatment between October 2011 and November 2016. Patients were referred by 154 distinct physicians in 26 states. Each case had been diagnosed with an immune-mediated peripheral neuropathy by the treating physician, was approved for IVIg therapy by an insurance company, and was naive to previous immunoglobulin therapy. The records included the exact written notes, laboratory data, imaging reports, and electrodiagnostic studies that were supplied to the insurance company, with the exception that all patients' protected health information and information about treating physicians, including names, geography, and type of practice, were redacted before the information was entered into the study. To the best of our knowledge, the insurers did not obtain additional documentation directly from prescribers.
Each redacted record was provided to 1 randomly selected initial expert reviewer (IER), chosen from the panel of 8 practicing neuromuscular clinicians (T.D.L., J.S.K., R.B., D.S.S., T.M., G.I.W., L.K., and M.M.D.). After reviewing the record, the IER answered 4 questions listed in table 1. The IER also completed questions regarding the nerve conduction studies, EMG, laboratory values, and clinical phenotypes (pattern of weakness, reflexes, and sensory loss). From the original 248 cases, we obtained 1 or 2 sets of follow-up records from 145 cases. These records were submitted when the treating clinician ordered additional IVIg or when insurance reauthorization was required. Because there was no standard protocol for the treating physicians, follow-up appointment times, doses and intervals of IVIg, and documentation varied between cases.
Table 1.
Questions asked of expert reviewers
A second expert reviewer (SER), blinded to the IER's opinions, evaluated the original note and the follow-up records from these 145 cases under the same redacted protocol. The SER was asked whether, in his/her opinion, the records indicated an “objective improvement” related to IVIg therapy. We intentionally did not provide a specific definition of “objective improvement” to mirror the real-world insurance utilization review process. The SER had the option to deem records as “unreviewable” if records lacked information to form an opinion. This was the situation in 17 of these 145 cases, leaving 52% of the original cohort for formal response analysis.
Among the 128 cases with reviewable follow-up records, patients were classified as “responders” when the SER believed that the records indicated an objective response in either the first, second, or both follow-up records. Specifically, in cases with only 1 follow-up record, “responder” was defined as an objective response in that record and “nonresponder” as no objective response in the single follow-up record. The case was excluded if the SER deemed the record as “unreviewable.” When there were 2 sets of follow-up records, “nonresponder” meant the SER found “no response” in both records or “no response” in 1 record and deemed the other “unreviewable.” The case was excluded if both follow-up records were “unreviewable.”
Data analysis
Data were entered into a Research Electronic Data Capture database housed at the University of Kansas Medical Center. Descriptive statistics were used to characterize the study population (n = 248) on demographic and other diagnostic characteristics at baseline. Statistical analysis was completed by G.J.B. Outcome analyses were based on the 128 participants in whom a response could be evaluated. The importance of the association between baseline clinical and electrophysiologic characteristics and objective responses were evaluated with χ2 tests. In addition, logistic regressions were used to estimate odds ratios and associated 95% confidence intervals for each predictor. Statistical significance was determined based on α = 0.05.
Standard protocol approvals, registrations, and patient consents
This study was conducted as part of a QA/QI study, “NuFACTOR IVIg Treatment Outcomes Assessment and Clinical Guidelines Study,” by NuFACTOR as part of its health care operations. Before the initiation of the study, an independent institutional review board (IRB), the Copernicus Group IRB, determined that the study was a QA/QI study for which IRB oversight was not required. To cover broader analysis of the data beyond NuFACTOR's QA/QI analyses, the IRB at the University of Kansas Medical Center approved the study and issued a waiver of informed consent for the retrospective analysis of previously collected data.
Data availability
All study data are published within this article.
Results
Assessments by expert reviewers
IER and diagnosis of immune neuropathy
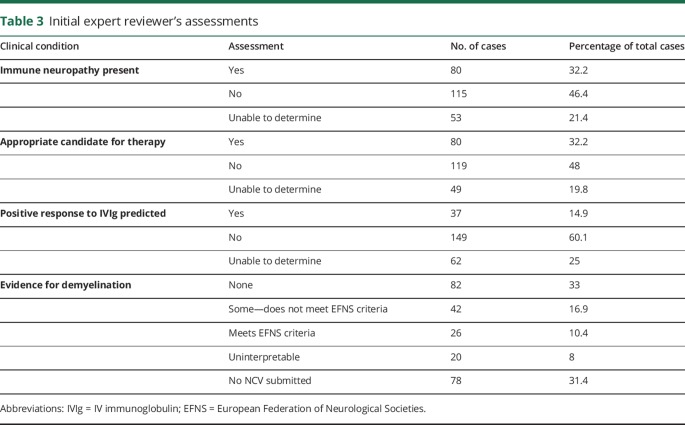
Only 32.3% of overall cases were believed to have an immune-mediated neuropathy; in 46.3% of cases, the documentation supported a non–immune-mediated neuropathy, and in 21.4% of cases, there was insufficient information to make a determination. Table 2 summarizes the diagnoses proposed by the IERs. Responses to questions about the diagnosis, appropriateness of therapy, prediction of a response, and degree of demyelination for each of the original 248 cases are summarized in table 3. Electrodiagnostic studies were not submitted for review in 70 cases.
Table 2.
Neuropathy diagnosis as determined by the initial expert reviewers
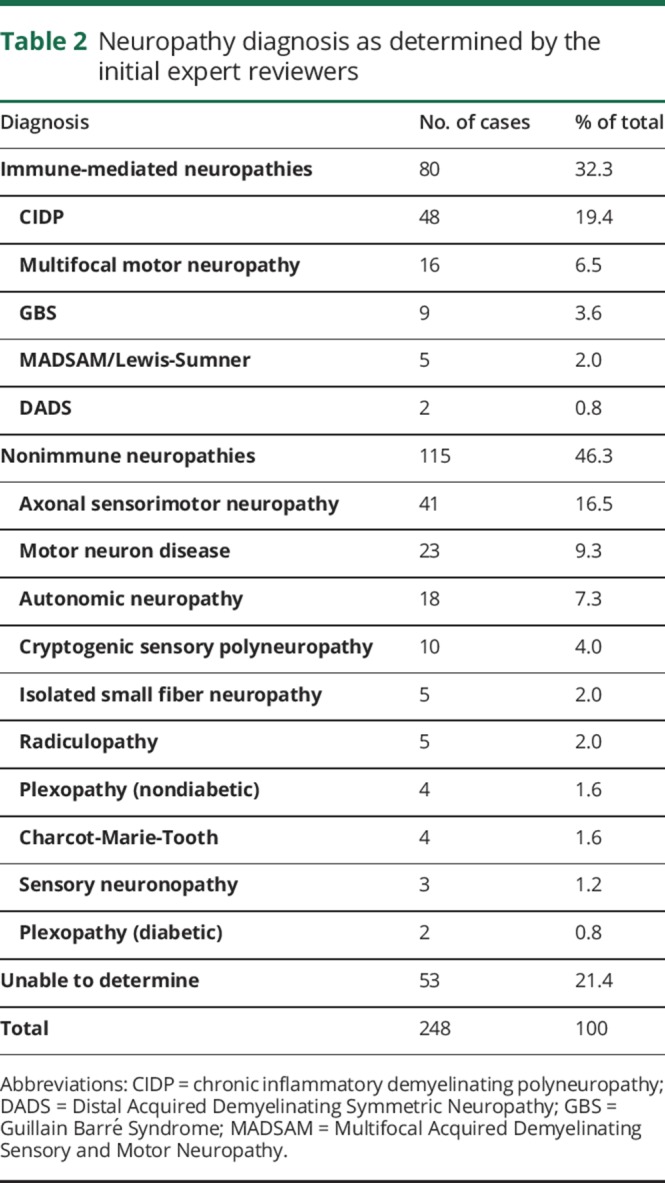
Table 3.
Initial expert reviewer's assessments
SER and response to IVIg
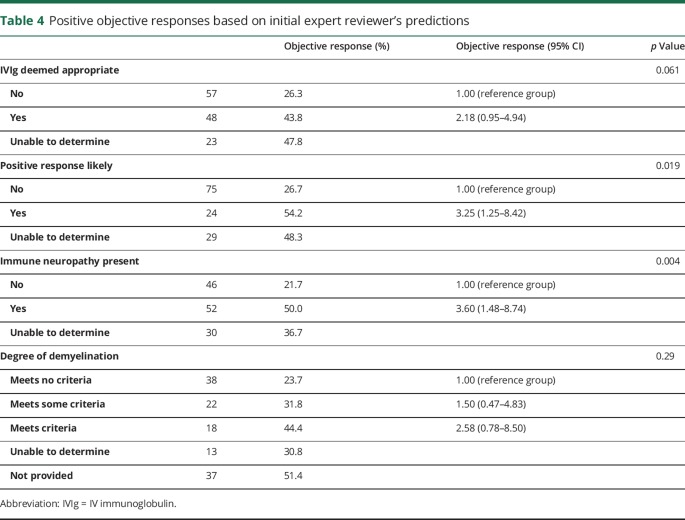
The overall “objective response” rate in the 128 reviewable cases was 36.7% (47/128). There was no difference in the mean loading doses of IVIg between responders (1.91 ± 0.28 g/kg) and nonresponders (1.87 ± 0.46 g/kg) (p = 0.58). The interquartile range for follow-up was 86–234 days for the overall cohort. The responses based on the original predictions are summarized in table 4 for these 128 patients.
Table 4.
Positive objective responses based on initial expert reviewer's predictions
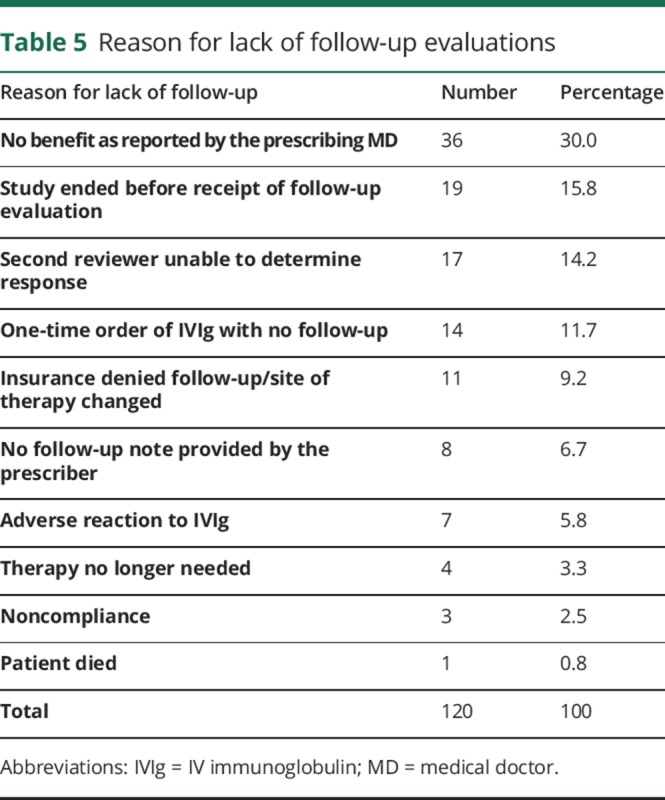
We also collected the various reasons for not having follow-up records in 120 patients who underwent initial review and were not included in the SER evaluation (table 5). The IERs predicted that this cohort was generally more inappropriate for IVIg therapy and less likely to respond to treatment than the 128 cases with follow-up records, but these differences were not significant. In 35 of the 120 cases, the treating physician specifically communicated to NuFACTOR that they did not reorder IVIg because the patient had not responded.
Table 5.
Reason for lack of follow-up evaluations
Discussion
There are now more than 20 sets of diagnostic criteria for CIDP and MMN that range from highly specific, where the goal is to select ideal patients for research, to most sensitive, which capture more cases but risk overdiagnosis.2–5 These multiple efforts reflect a neuropathy field characterized by the absence of a gold standard test for diagnosis and diseases with overlapping findings that require a demanding clinical logic to account for phenotypic, laboratory, and electrodiagnostic features. Complicating the situation, bedside decision makers must calculate the downside of missing a diagnosis in a potentially treatable case that might not fulfill diagnostic criteria.6–8 It should not be surprising that clinicians with differing experiences and values, and who may have diverse decision-making capabilities, can approach the same problem differently.
As a specialty pharmacy, NuFACTOR has been a first-hand observer of the use of IVIg in neuropathy. In addition, many of our experts (J.S.K., T.D.L., R.B., and D.S.S.) have consulted for insurance payers as reviewers and arbiters or have published diagnostic criteria. A decade after food and drug administration (FDA) approvals of treatments for these conditions,9–12 our team wondered if the pendulum may have swung toward excessive use. We designed this QA/QI study to learn about clinical practice decisions by mimicking the review processes used by insurance companies. Because of the multiplicity of published criteria that reflect the diagnostic difficulty, we made the assumption that these would be difficult to use alone in making review decisions. Thus, we asked our expert reviewers to rely on real-world records provided to insurers to make individual assessments about diagnoses and expected treatment responses. As the study progressed, it became clear that “ideal” data do not exist to facilitate the required interactions between patients, physicians, infusion companies, and payers.
Using our methods, diagnostic accuracy appears to be even more pessimistic than the report by Allen and Lewis,1 in which 53% of cases diagnosed with CIDP met electrodiagnostic criteria. In contrast to the earlier work, we included all forms of demyelinating neuropathies, rather than CIDP alone, and focused only on patients naive to IVIg therapy. We found just 15.3% of the cases in which electrodiagnostic studies were provided, all of which were approved by insurance companies, clearly fulfilled electrodiagnostic criteria for a demyelinating neuropathy. In approximately 12% of the provided cases, electrodiagnostic studies were incomplete or could not be interpreted, and no studies were provided in nearly one-third of cases.
In the judgment of our expert reviewers, only 42% of the reviewable cases and 32% of overall cases clearly met diagnostic criteria consistent with an immune neuropathy, whereas nonimmune neuropathies accounted for more than half of reviewable cases. Notably, these values may be an overly optimistic estimation of real-world practice because our cohort solely included patients already approved for IVIg therapy. The a priori exclusion of insurer-denied cases would have had the effect of increasing our estimate if these cases were the clearest examples of conditions other than immune neuropathy.
The overall 36.7% treatment response rate may represent another overestimate. This value would have been lower had we included cases in which the treating physician explained that IVIg had been discontinued specifically because there was no response. Other key biases may also have affected our response rate. IVIg had been reordered, by definition, in all cases the SER examined. We can presume that the treating physicians reordered IVIg because they believed it worked, but anchoring to the original diagnosis might have affected both how they perceived and documented the outcome. This, in turn, could influence the interpretation by our SER. The effect of anchoring could be magnified if a treating physician relied on subjective responses or failed to carefully document examinations. Patient experience may add to this because placebo responses can be as high as 20%.3 Biases may have contributed to the surprisingly high 21.7% “objective” response rate in cases in which our first reviewers diagnosed types of neuropathy that are known not to be immune responsive.
Our panel deemed the records “unreviewable” in more than 20% of cases. This problem typically reflected incomplete, poorly written, or difficult-to-follow notes. Follow-up assessments also often lacked key observations to allow for clear conclusions. The SERs had no way to contact the treating physicians, as could be done in controlled studies, because insurance companies rarely use iterative approaches to track missing information. As a result, we adopted the term “AUNTs” (Analytically Uninterpretable Note Taking) to designate this real-world phenomenon of how experts perceive clinical records.13
Even given these limitations, predictions about who would and would not respond to IVIg treatment by our IERs, paired with the interpretation of the SERs, were significantly better than the overall SER-determined base response estimates. This provides evidence that input from neuromuscular specialists, who understand the complex reasoning processes involved in differential diagnosis of neuropathies, could improve on the current review process.
Our findings also suggest that prescribing some IVIg for patients who do not have immune-mediated neuropathy is unavoidable. This likely reflects realities related to diagnostic uncertainty. For example, IERs believed that twice as many patients were appropriate candidates for a trial of IVIg (32%) as were likely to respond (15%). These low numbers continue to suggest that only few cases are sufficiently documented not only to clearly warrant treatment but also to demonstrate how reviewers may at times be open to therapy, despite skepticism about the eventual benefit. We have previously used the term “UNCLEs” (UNcertain CLinical Entities) to describe a differential diagnosis that cannot reasonably be narrowed to exclude a treatable condition.13 Two of the more common examples in our study were cases likely to have primary muscular atrophy, in which MMN remained on the differential diagnosis14,15 or diabetic neuropathies with features that could not be fully distinguished from CIDP.6 Weak indications for treatment despite a known diagnosis is another factor. For example, Distal Acquired Demyelinating Symmetric Neuropathy with an Immunoglobulin M paraprotein, which is known to respond poorly to IVIg,16 and mild Guillain Barré Syndrome, where progression has ceased before treatment was ordered, were considered reasonable for a trial of IVIg by some of our IERs. It may be important to capture these specific clinical entities, to make providers aware of uncertainty, and, therefore, open to discontinuation of therapy when treatment seems ineffective.
Overall, our findings, consistent with earlier reports suggesting that IVIg is overused in immune neuropathy, call for a change from the current approach. Potential areas for improvement include documentation of key observations, inclusion of expertise in review processes, and capturing data to highlight true and perceived uncertainty as it pertains to diagnosis and treatment responses. Given the cost of IVIg, it is worth ensuring that clinicians submit concise information at the onset and at designated intervals during therapy, as has been proven possible in the IGOS study in acute neuropathies.17 Uncertainty makes it harder to control costs and may foster distrust in communications around treatment decisions. All parties desire to use expensive or scarce resources appropriately and to improve care. Providers might be more enthusiastic about supplying requested documentation and more accepting of decisions to deny insurance coverage for IVIg therapy if they appreciate that decisions were well reasoned, data driven and backed by expert review. Similarly, insurers might be more willing to moderate time-consuming utilization review practices if physicians demonstrate a willingness to participate in a well-validated process to maximize the value of therapy.
Footnotes
Editorial, page 373
Author contributions
T.D. Levine and J.S. Katz developed the study design and organization, served as study codirector during the course of the study, and reviewed data and developed the manuscript. R. Barohn developed the study design and organization and reviewed data and developed the manuscript. L.J. Vaughan ran the day-to-day operations of collecting patient information and acquiring reviews from the expert panel and helped with data analysis and manuscript preparation. M.M. Dimachkie, D.S. Saperstein, T. Mozaffar, and G.I. Wolfe were expert reviewers who analyzed cases during the study and assisted in manuscript preparation. M.S. Mayo was the PI for the Data Coordinating Center for this research project and lead biostatistician. He and his team aided in the design of the study, created and managed the study data capture system, and performed data curation and preliminary and final analyses along with periodic and final study reports to the sponsor. G.J. Badger served to analyze the data at the end of the study and provided statistical support in the preparation of the manuscript. L. Katzin was one of the expert reviewers who analyzed cases during the study. E. Ritt worked on an organizational level during the course of the study and assisted in manuscript preparation. M. Greer, J. DiStefano, and P.M. Schmidt led on an organizational level during the course of the study and assisted in manuscript preparation.
Study funding
This work was funded by NuFACTOR, Inc.
Disclosure
T.D. Levine has received funding for travel and/or speaker honoraria from Grifols, NuFACTOR, Inc., Shire, CSL Behring, Mallinckrodt, and Alnylam; serves as a consultant for NuFACTOR, Inc., Grifols, and Mallinckrodt; serves on speakers' bureaus for Alexion, Grifols, Shire, and CSL Behring; receives research support from Alnylam, Mallinckrodt, CSL Behring, and Grifols; and received financial support from NuFACTOR, Inc. during the course of this study. J.S. Katz has received funding for travel and/or speaker honoraria from Grifols, CSL, Alexion, Mitsubishi Tanabe Pharma America, and Genentech; serves as a consultant for Grifols, NuFACTOR, Inc., Genentech, Mitsubishi Tanabe Pharma America, Denali, CSL Behring, and Shire; serves on the speakers' bureaus for CSL, Alexion, Grifols, and Mitsubishi Tanabe Pharma America; receives research support from the ALS Association and the Muscular Dystrophy Association; and received financial support from NuFACTOR, Inc. during the course of this study. R. Barohn has received funding for travel and/or speaker honoraria from NuFACTOR, Inc., Plan 365 Inc., and Option Care; serves as Associate Editor for Journal of Clinical Neuromuscular Disease; has served as a consultant for Grifols, Shire, and CSL Behring; serves on the speakers' bureaus for NuFACTOR, Inc. and Plan 365 Inc.; receives research support from Novartis, Sanofi Genzyme, BioMarin, Ionis, Teva, Cytocenetics, Eli Lilly, NIH (NCATS and NINDS), FDA OPD, and PCORI; and received financial support from NuFACTOR, Inc. during the course of this study. L.J. Vaughan is Chief Operating Officer for NuFACTOR, Inc. M.M. Dimachkie is a consultant or on the speakers' bureau for Alnylam, Audentes, Baxalta, BioMarin, Catalyst, CSL Behring, Genzyme, Mallinckrodt, Novartis, NuFACTOR, Inc., Octapharma, and Terumo; serves on the editorial board of the Journal of Clinical Neuromuscular Disease; and has received research support from Amicus, Alnylam, Alexion, BioMarin, Bristol-Myers Squibb, Catalyst, CSL Behring, FDA/OPD, GSK, Grifols, MDA, NIH, Novartis, Genzyme, Octapharma, UCB Biopharma, Viromed, and TMA; and received financial support from NuFACTOR, Inc. during the course of this study. D.S. Saperstein serves on a medical advisory board for CSL Behring and received honoraria for teaching courses from Shire and Grifols; serves as a consultant for Grifols, Shire, and CSL Behring; has received research support from CSL Behring; and received financial support from NuFACTOR, Inc. during the course of this study. T. Mozaffar serves on medical and scientific advisory boards for the Myositis Association, Amicus, BioMarin, Genzyme, Idera Pharmaceuticals, and Ultragenyx; has received funding for travel from Genzyme Corporation, BioMarin, and Amicus; serves as a consultant for Amicus, BioMarin, Genzyme, OptionCare, Sarepta, and Ultragenyx; serves on speakers' bureaus for Genzyme Corporation and Grifols; receives research support from Alnylam, Alexion, Amicus, BioMarin, CSL Behring, Genzyme, Ionis, GSK, Grifols, Octapharma, Novartis, Ultragenyx, and NIH; and received financial support from NuFACTOR, Inc. during the course of this study. G.I. Wolfe serves on scientific advisory boards for Grifols and Shire; serves on a speakers' bureau for and has received speaker honoraria from Grifols; serves as a consultant for Grifols, Shire, and CSL Behring; receives research support from CSL Behring and PCORI; and received financial support from NuFACTOR, Inc. during the course of this study. M.S. Mayo serves on scientific advisory boards for Amgen, Bellerophon, and NIH; has served as a consultant for Weil in medico-legal cases; receives research support from the NIH and PCORI; and received financial support from NuFACTOR, Inc. during the course of this study. G.J. Badger has received compensation from NuFACTOR, Inc. for providing statistical support. L. Katzin is Staff Physician for Grand Rounds, LLC and has received financial support from NuFACTOR, Inc. during the course of this study. E. Ritt is Medical Science Liaison for NuFACTOR, Inc. M. Greer serves on scientific advisory boards for Octapharma and Grifols and is Senior Vice President of Sales for NuFACTOR, Inc. J. DiStefano is Director of Clinical Programs for NuFACTOR, Inc. P.M. Schmidt is Chief Executive Officer of NuFACTOR, Inc./FFF Enterprises, Inc. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology 2015;85;498–504. [DOI] [PubMed] [Google Scholar]
- 2.Koski CL, Baumgarten M, Magder LS, et al. . Derivation and validation of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy. J Neurol Sci 2009;277:1–8. [DOI] [PubMed] [Google Scholar]
- 3.Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle Nerve 2004;24:311–324. [DOI] [PubMed] [Google Scholar]
- 4.Van den Bergh PY, Hadden RD, Bouche P, et al. . European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society: first revision. Eur J Neurol 2010;17:356–363. [DOI] [PubMed] [Google Scholar]
- 5.AAN Task Force. Research criteria for the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Neurology 1991;41:617–618. [PubMed] [Google Scholar]
- 6.Chan YC, Allen DC, Fialho D, Mills KR, Hughes RA. Predicting response to treatment in chronic inflammatory demyelinating polyradiculopathy. J Neurol Neurosurg Psychiatry 2006;77:114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajabally YA, Nicolas G, Piéret F, Bouche P, Van den Bergh PY. Validity of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy: a Multicenter European study. J Neurol Neurosurg Psychiatry 2009;80:1364–1368. [DOI] [PubMed] [Google Scholar]
- 8.Saner HW, Latov N. Research criteria for defining patients with CIDP. Neurology 2003;60(8 suppl):S8–S15. [DOI] [PubMed] [Google Scholar]
- 9.Hughes RA, Donofrio P, Bril V, et al. . Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 2008;7:136–144. [DOI] [PubMed] [Google Scholar]
- 10.Hahn AF, Beydoun SR, Lawson V; IVIg in MMN Study Team. A controlled trial of intravenous immunoglobulin in multifocal motor neuropathy. J Peripher Nerv Syst 2013;18:321–330. [DOI] [PubMed] [Google Scholar]
- 11.Leger JM, Bleeker JL, Sommer C, et al. . Efficacy and safety of Privigen in patients with chronic inflammatory demyelinating polyneuropathy: results of a single arm, prospective open label phase III study. J Peripher Nerv Syst 2013;18:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaik I, Bril V, Geloven N, et al. . Subcutaneous gammaglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy. Lancet. Available at: 10.1016/S1474-4422(17)30378-2. Accessed November 6, 2017. [DOI] [Google Scholar]
- 13.Katz JS, Levine TD, Dimachkie M, Barohn R. Improving review processes for IVIg therapy: getting to know our AUNTS and UNCLES. 2017. Presented at PNS Meeting; Sitges, Spain; July 8, 2017. [Google Scholar]
- 14.Simon NG, Ayer G, Lomen-Hoerth C. Is IVIg warranted in progressive lower motor neuron syndromes without conduction block. Neurology 2013;81:2116–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz JS, Barohn RJ, Kojan S, et al. . Axonal multifocal motor neuropathy without conduction block or other features of demyelination. Neurology 2002;58:615–620. [DOI] [PubMed] [Google Scholar]
- 16.Katz JS, Saperstein DS, Gronseth G, Amato AA, Barohn RJ. Distal acquired demyelinating symmetric neuropathy. Neurology 2000;54:615–620. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs BC, van den Berg B, Verboon C, et al. ; IGOS Consortium. International Guillain-Barré Syndrome Outcome Study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain-Barré Syndrome. J Peripher Nerv Syst 2017;22:68–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are published within this article.