PRACTICAL IMPLICATIONS
MRI with inclusion of T2-weighted fluid-attenuated inversion recovery (FLAIR) and susceptibility-weighted imaging sequences may be helpful in identifying delayed posthypoxic leukoencephalopathy and critical illness microbleeds in patients at risk.
A 51-year-old man initially presented to an outside institution with 2 days of emesis, altered mental status, visual hallucinations, and agitation. His medical history was notable for 5 years of cognitive decline (forgetfulness, disorientation; incompletely worked up), anxiety, depression, and heavy alcohol use. No illicit drug use was reported. Initial examination at an outside institution was not well-documented but reported as nonfocal.
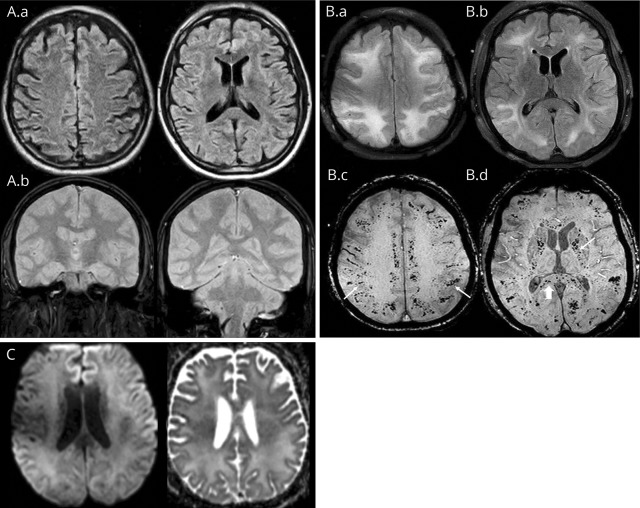
Initial MRI brain was unremarkable (figure, A), lumbar puncture was traumatic, and CSF studies were negative for infection or encephalitis; serum cultures were negative. The patient's outside hospital course was complicated by aspiration pneumonia and acute respiratory distress syndrome (ARDS) requiring intubation, antibiotics, and subsequent tracheostomy. Serum electrolytes and osmoles were normal. There were no hypertensive episodes and extracorporeal membrane oxygenation was not required. Mental status was improving 18 days into admission (mouthing words, following commands) when he developed generalized tonic-clonic seizures. Antiepileptic therapy was initiated. MRI brain was repeated, demonstrating confluent symmetric T2/FLAIR hyperintensities in the white matter of both cerebral hemispheres and extensive microhemorrhages involving the subcortical white matter, corpus callosum, internal capsules, brainstem, and cerebellar peduncles (figure, B, MRI on transfer to our institution); no restricted diffusion or abnormal enhancement was noted (figure, C).
Figure. Imaging.
(A) Axial fluid-attenuated inversion recovery (FLAIR) (A.a) and coronal gradient recalled echo (GRE) (A.b) at presentation to the outside institution show no white matter signal abnormality or hemorrhage. (B) MRI performed 3 weeks after the initial presentation. Axial FLAIR images (B.a, B.b) show extensive confluent hyperintensity in the periventricular, deep, and subcortical white matter. Axial susceptibility-weighted imaging (B.c, B.d) shows numerous microhemorrhages in the subcortical white matter (arrows, B.c), internal capsules (arrow, B.d) and corpus callosum (arrowhead, B.d). (C) MRI performed 3 weeks after the initial presentation. Axial diffusion-weighted imaging and B.a, B.d, and B.c show no restricted diffusion in the white matter. Not pictured is the second MRI FLAIR and GRE obtained at the outside institution prior to transfer, which revealed a similar distribution of leukoencephalopathy and microhemorrhages as the repeat images obtained at our institution and presented in (B).
On presentation at our institution, the patient was not following commands, had intermittent agitation but no focal motor weakness. Continuous EEG only revealed diffuse generalized slowing.
Repeat MRI was consistent with delayed posthypoxic leukoencephalopathy (DPHL) and critical illness–associated microbleeds (CIAM) in the setting of ARDS (figure, B), with no imaging correlate for posterior reversible encephalopathy syndrome. The patient's course at our institution was uncomplicated.
On discharge, the patient was awake, mouthing words, and intermittently following commands, had no sensorimotor deficits, and was discharged to a long-term acute care facility. Six months postdischarge, the patient is walking, talking, and eating independently but continues to have short-term memory deficits.
Discussion
DPHL and CIAM are both independently rare phenomena. CIAM has been described in patients presenting after acute respiratory failure, defined by a pattern of diffuse microbleeds within the juxtacortical white matter and corpus callosum, sparing the deep and periventricular white matter, similar to cases of high-altitude exposure.1,2 The location of microbleeds differs from cerebral amyloid angiopathy (CAA) (cortical) and chronic hypertensive arteriopathy (deep gray matter).1 The pathophysiology of CIAM is not well-understood, but may be related to hypoxia-induced effects on the blood–brain barrier causing extravasation of erythrocytes2 or secondary to disseminated intravascular coagulation.1
Severe hypoxia most commonly causes acute hypoxic–ischemic injury with damage to the gray matter structures, which are more vulnerable to diffuse ischemic injury. Rare cases of DPHL (commonly described in carbon monoxide poisoning) have been described with mild to moderate episodes of hypoxia. These patients show improvement in neurologic examination after the initial insult, followed by delayed second neurologic deterioration 1–4 weeks later, manifesting clinically with change in executive functioning, akinetic mutism, psychomotor retardation, or parkinsonism.3 Our patient's neuropsychiatric examination, though limited by tracheostomy, revealed evidence of changes in executive functioning and agitation.
MRI after secondary decompensation typically reveals extensive symmetric white matter T2/FLAIR hyperintensities with sparing of the deep gray matter, often with restricted diffusion. This tends to be reversible, with patients returning to neurologic baseline 6–12 months after the initial event.3,4 The pathophysiology, while not well-understood, may be due to demyelination, typically occurring 10–14 days after hypoxic insult. Clinical symptoms begin with the necrosis of myelin sheaths.3 DPHL has been described in patients with abnormally low levels of arylsulfatase A, which hydrolyzes key components of myelin.5 Arylsulfatase A was not measured in our patient.
While DPHL is typically thought to present with restricted diffusion in the subcortical white matter, the majority of case reports did not include diffusion-weighted imaging sequences. In one case series, patients had less restricted diffusion compared to T2-FLAIR changes.4 Our patient fits the clinical picture of DPHL, with other etiologies of leukoencephalopathy unlikely, as a lack of restricted diffusion does not rule out DPHL.
We describe a rare case of concomitant CIAM and extensive leukoencephalopathy. Our case was limited in that much of the diagnostic workup was completed at the outside institution. Review of the literature did not reveal pathophysiology that links these diagnoses. We suspect these are 2 distinct processes occurring simultaneously, rather than a new syndrome. While DPHL portends a good neurologic prognosis, the literature on CIAM remains sparse. Studies on microbleeds of CAA and hypertensive arteriopathy indicate a relationship between cerebral microbleeds and cognitive impairment and disability.6 It is unclear whether these studies translate to critical illness microbleeds.7 Further investigation of the relationship between CIAM and DPHL, along with long-term outcomes, is warranted.
Author contributions
Hannah Breit: study concept and design, drafting and revision of manuscript for intellectual content, analysis and interpretation of data. Sayona John: study concept and design, critical revision of manuscript for intellectual content, study supervision. Miral Jhaveri: analysis and interpretation of data, revision of manuscript for intellectual content.
Study funding
No targeted funding reported.
Disclosure
H. Breit reports no disclosures. M. Jhaveri receives publishing royalties from Elsevier. S. John serves as a consultant for Gift of Hope, Illinois Organ Procurement Agency. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Fanou EM, Coutinho M, Shannon P, et al. . Critical illness-associated cerebral microbleeds. Stroke 2017;48:1085–1087. [DOI] [PubMed] [Google Scholar]
- 2.Riech S, Kallenberg K, Moerer O, et al. . The pattern of brain microhemorrhages after severe lung failure resembles the one seen in high-altitude cerebral edema. Crit Care Med 2015;43:386–389. [DOI] [PubMed] [Google Scholar]
- 3.Betts AM, Ritter JL, Kubal WS. Reversible delayed posthypoxic leukoencephalopathy after drug overdose: MRI findings in a collection of patients. Emerg Radiol 2012;19:165–173. [DOI] [PubMed] [Google Scholar]
- 4.Zamora CA, Nauen D, Hynecek R, et al. . Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain Behav 2015;5:e00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottfried JA, Mayer SA, Shungu DC, Chang Y, Duyn JH. Delayed posthypoxic demyelination association with arylsulfatase A deficiency and lactic acidosis on proton MR spectroscopy. Neurology 1997;49:1400–1404. [DOI] [PubMed] [Google Scholar]
- 6.Salazar R, Dubow J. Delayed posthypoxic leukoencephalopathy following a morphine overdose. J Clin Neurosci 2012;19:1060–1062. [DOI] [PubMed] [Google Scholar]
- 7.Werring DJ, Frazer D, Coward L, et al. . Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain J Neurol 2004;127:2265–2275. [DOI] [PubMed] [Google Scholar]