Abstract
Background
Several studies have examined the relationship between body mass index (BMI) and survival from amyotrophic lateral sclerosis (ALS). Many indicate that low BMI at diagnosis or during follow-up may be associated with accelerated progression and shortened survival. This study systematically evaluated the relationship between BMI and survival in patients with ALS.
Methods
The PubMed database was searched to identify all available studies reporting time-to-event data. Eight studies with 6,098 patients fulfilled the eligibility criteria. BMI was considered a continuous and ordered variable. Interstudy heterogeneity was assessed by the Cochran Q test and quantified by the I2 metric. Fixed- or random-effects odds ratios summarized pooled effects after taking interstudy variability into account. Significance was set at p < 0.05.
Results
The ALS survival hazard ratio (HR) decreased approximately by 3% (95% confidence interval [CI]: 2%–5%) for each additional BMI unit when BMI was considered a continuous variable. When BMI was considered a categorical variable, the HRs for “normal” BMI vs “overweight” BMI and “obese” BMI were estimated to be as high as 0.91 (95% CI: 0.79–1.04) and 0.78 (95% CI: 0.60–1.01), respectively. The HR for the comparison of the “normal” BMI vs “underweight” BMI was estimated to be as high as 1.94 (95% CI: 1.42–2.65).
Conclusions
BMI is significantly and inversely associated with ALS survival.
Amyotrophic lateral sclerosis (ALS) represents a heterogeneous group of neurodegenerative disorders.1 It is the third most common neurodegenerative disease and the most frequent form of motor neuron disease, with its onset in adulthood.1 ALS is characterized by the progressive loss of motor neurons and a rapidly progressive paralysis.2,3 It is a serious health problem because respiratory failure usually leads to death within 2–3 years after symptom onset.4,5 Although the cause of ALS is unknown, genetic and environmental factors are likely to confer susceptibility to the risk of developing ALS.6,7
Body mass index (BMI) is a measure of tissue mass expressed in units of kg/m2. Body weight is associated with a number of neurologic disorders, with excess body weight leading to an increased risk of MS, a neuroinflammatory disease of unknown etiology,8–10 and ischemic stroke.11–13 BMI may also be implicated in degenerative processes because decreases in BMI have been observed before the onset of Alzheimer disease, with weight remaining stable or increasing after diagnosis.14 Furthermore, patients with Parkinson disease (PD) have a significantly lower BMI than healthy controls,15 and Hoehn and Yahr stage 3 PD patients present lower BMIs than those with stage 2 disease.15
A number of exogenous factors are associated with the risk of developing ALS, including low BMI.16,17 Moreover, large prospective cohort studies have demonstrated that overweight and obese individuals are at significantly lower risk of developing ALS compared with those with normal premorbid BMIs.18,19 A recent systematic review suggested that a lower BMI is among the risk factors associated with ALS.20
Several studies (including the systematic review mentioned earlier) have examined the relationship between BMI and survival in patients with ALS, and they indicate that a low BMI at diagnosis or during follow-up may lead to faster progression and shorter survival.20–25 We conducted a meta-analysis of studies examining BMI and survival in patients with ALS.
Methods
Literature search
The PubMed database from its inception until November 3, 2017, was systematically searched for studies using the terms “amyotrophic lateral sclerosis,” “body mass index,” “survival,” and “hazards.” The complete search algorithm is available in appendix e-1 (links.lww.com/CPJ/A48). Animal experiments, unpublished data, and congress presentations/abstracts were excluded. Titles and abstracts were reviewed to determine relevance. Studies lacking time-to-event data were excluded. Only articles written in English were included. The final literature search was performed on November 3, 2017. Finally, the bibliographies of the resulting full texts were searched for other relevant citations.
Data extraction
The mean age, male-to-female ratio of the study sample, and the resulting hazard ratios (HRs) were extracted. Because the literature considers BMI as both a continuous and ordered variable, the HRs for each type of BMI description were considered separately (tables 1 and 2). Finally, we registered if the BMI was recorded before the diagnosis or at the initial visit, during the course of the disease, or at the late stages of progression.
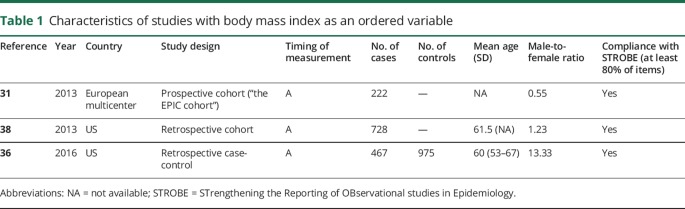
Table 1.
Characteristics of studies with body mass index as an ordered variable
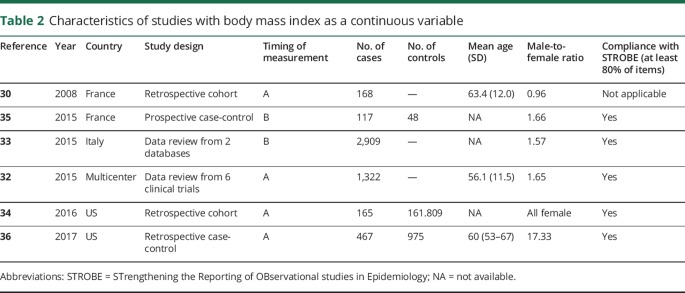
Table 2.
Characteristics of studies with body mass index as a continuous variable
Quality appraisal
Two reviewers (E.D. and V.S.) appraised the articles according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.26 Reporting clarity was considered as “high” if the study authors reported at least 80% of the STROBE checklist items (18 of 22 items) and as “low” otherwise. In cases of disagreement, the 2 authors reached a consensus after consulting a third coauthor (A.G.B.). PRISMA statement for reporting reviews and meta-analyses was applied.27
Statistical analysis
Interstudy heterogeneity was measured and quantified by the I2 metric (“low,” “moderate,” “high,” and “very high” corresponding to values of up to 25%, 50%, 75%, and 100%, respectively). Baujat plots were used to visualize contributions to overall heterogeneity. Fixed- or random-effects odds ratios summarized pooled effects after taking interstudy variability into account. Forest plots were used to visualize effect sizes and their 95% confidence intervals (CIs) for each study and the pooled effects. Subgroup analysis was performed to explain interstudy heterogeneity. The outcomes were stratified in terms of the timing of BMI measurement. The leave-one-out method was used to pinpoint the most influential study. Funnel plots were used to visually assess publication bias, verified by the Egger linear regression test. Significance was set at p < 0.05 for all analyses. All statistical analyses were performed using R statistical environment (“meta” package).28,29
Results
Literature results
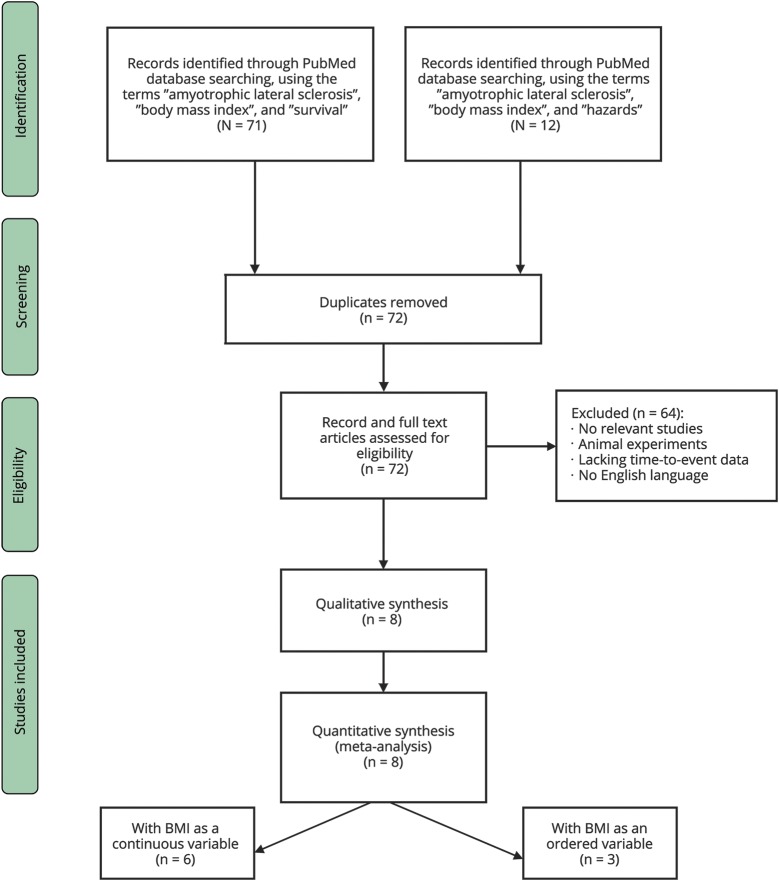
A flowchart of the selection of eligible studies is presented in figure 1. The search of the PubMed database yielded 71 articles using the terms “amyotrophic lateral sclerosis,” “body mass index,” and “survival” and 12 using the terms “amyotrophic lateral sclerosis,” “body mass index,” and “hazards.” After the removal of duplicates, 72 studies (published between October 1995 and October 2017) were available for further review. Two independent reviewers screened records, full-text articles, titles, and abstracts. Sixty-four studies were removed (as not relevant, animal experiments, lacking time-to-event data, or no English language). Eight potentially eligible studies (5 with BMI as a continuous variable, 2 with BMI as an ordered variable, and 1 with BMI both as a continuous and ordered variable), published between 2008 and 2017, were retained and finally included in the quantitative meta-analysis.
Figure 1. Prisma flowchart of the current meta-analysis.
Characteristics of eligible studies
Eight studies involving 6,098 patients fulfilled the eligibility criteria and formed the basis of the current meta-analysis. Desport et al.30 originally studied the effect of BMI on the survival of patients with ALS. Because of the rarity of ALS, most studies were retrospective analyses of data gathered in clinical trials or registries.31–33 The majority of evidence derived from developed countries with increased health care awareness such as Europe and the United States.30,33–38 Most patients were in the sixth decade of life, and there was an obvious variability in the male-to-female ratio among the gathered studies, while the majority of the studies were compliant with the STROBE statement (tables 1 and 2).
BMI as a continuous variable
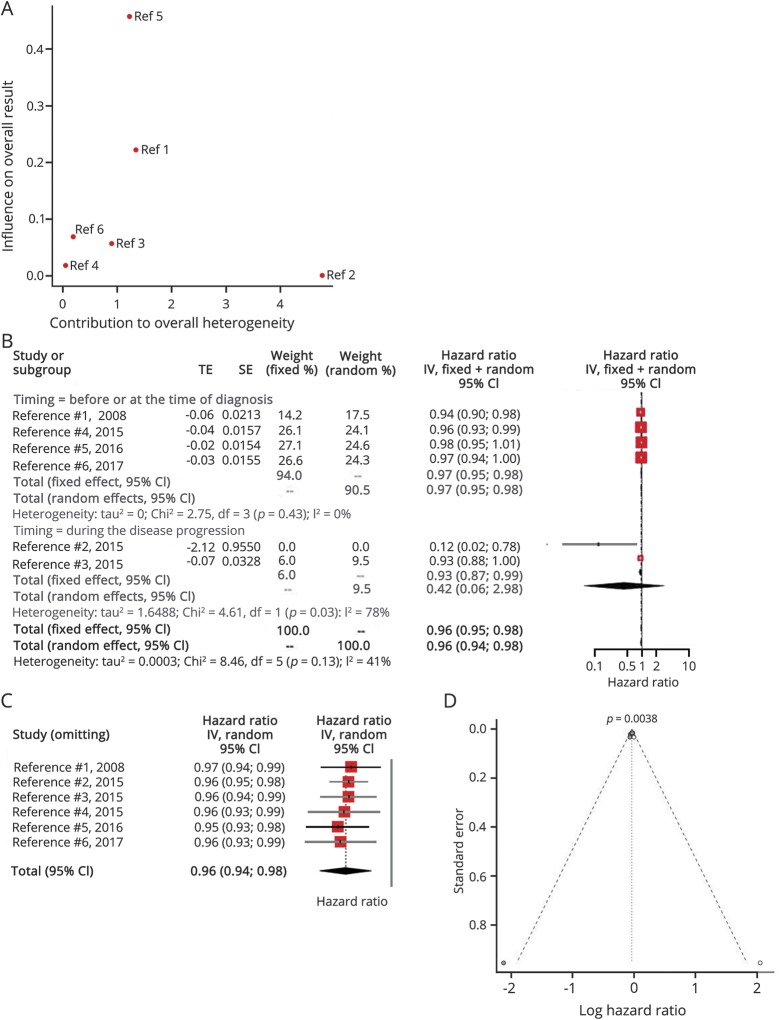
Six articles examined the effect of BMI as a continuous variable on ALS survival (table 2). There was no significant overall interstudy heterogeneity (I2 = 41%, p = 0.13), with most variability attributed to the studies of Eaglehouse et al.34 and Henriques et al.35 (figure 2A). The HR decreased by 3% (95% CI: 2%–5%) for each additional BMI unit when the BMI was measured before or at the time of diagnosis (figure 2B). On the other hand, there was significant statistical heterogeneity (I2 = 78%, p = 0.03) in studies measuring the BMI during the disease progression. No study was based on BMI measurements at late disease stages. All studies equally influenced the overall result (figure 2C). The relevant literature was not free of publication bias (p < 0.05) (figure 2D).
Figure 2. Body mass index (BMI) as a continuous variable.
(A) Baujat plot depicting the influence of each study on the overall result according to the contribution to the overall heterogeneity. Henriques et al. had the major contribution to the overall heterogeneity, whereas the study of Eaglehouse had the major influence on the overall result. BMI is regarded as a continuous variable. (1 = Desport et al.30; 2 = Henriques et al.35; 3 = Lunetta et al.33; 4 = Paganoni et al.32; 5, Eaglehouse et al.34; 6 = Mariosa et al.36) (B) Hazard ratio forest plot of the role of BMI in the amyotrophic lateral sclerosis survival. The fixed- and random-effects estimates along with their 95% confidence intervals are also visualized. BMI is regarded as a continuous variable. (C) Influence analysis according to the leave-one-out method. (D) The funnel plot is indicative of a high risk of publication bias.
BMI as an ordered variable
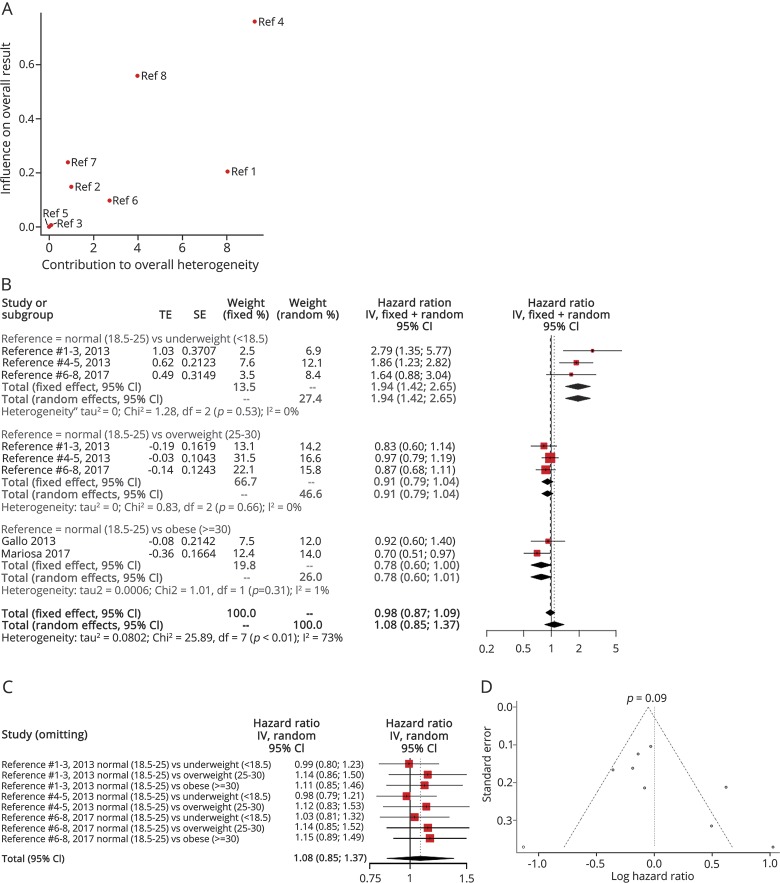
Three articles examined the effect of BMI as a categorical variable on ALS survival (table 1). The “normal” BMI was the reference level in all studies, while the overall heterogeneity was moderate (I2 = 73%, p < 0.01), with the study by Traxinger et al.38 contributing largely to the overall heterogeneity (figure 3A). The heterogeneity was eliminated after stratifying the results according to the individual comparisons. Thus, the HR for the comparison of the “normal” subgroup with the “overweight” subgroup was estimated to be as high as 0.91 (95% CI: 0.79–1.04). Similarly, the HR for the comparison of the “normal” subgroup with the “obese” subgroup was estimated to be as high as 0.78 (95% CI: 0.60–1.01). However, the HR for the comparison of the “normal” subgroup with the “underweight” subgroup was estimated to be as high as 1.94 (95% CI: 1.42–2.65) (figure 3B). The leave-one-out analysis indicated that the most influential study was that of Mariosa et al.36 (figure 3C). The trim-and-fill funnel plot showed that there was no important publication bias, as verified by the Egger linear regression test (p = 0.09) (figure 3D). The measurement of BMI in all studies took place before the diagnosis or at the initial assessment.
Figure 3. Body mass index (BMI) as a categorical variable.
(A) Baujat plot depicting the influence of each study on the overall result according to the contribution to the overall heterogeneity. Traxinger et al. had the major contribution to the overall heterogeneity and the major influence on the overall result. (1 = “Normal vs Underweight”/Gallo et al.31; 2 = “Normal vs Overweight”/Gallo et al.31; 3 = “Normal vs Obese”/Gallo et al.31; 4 = “Normal vs Underweight”/Traxinger et al.38; 5 = “Normal vs Overweight”/Traxinger et al.38; 6 = “Normal vs Underweight”/Mariosa et al.36; 7 = “Normal vs Overweight”/Mariosa et al.36; 8 = “Normal vs Obese”/Mariosa et al.36) (B) Hazard ratio forest plot of the role of BMI in amyotrophic lateral sclerosis (ALS) survival. The fixed- and random-effects estimates along with their 95% confidence intervals (CIs) are also visualized. BMI is regarded as a continuous variable. (C) Influence analysis according to the leave-one-out method. (D) The funnel plot is indicative of a high risk of publication bias.
Discussion
The current meta-analysis concentrated on data from a large number of participants (n = 6,098) to investigate the effect of BMI on the survival of patients with ALS. We report a significant influence of BMI (as measures before the disease diagnosis or progression) on the survival from ALS. More precisely, an increased BMI at diagnosis should be considered among the protective factors in terms of overall survival in patients with ALS, whereas underweight patients are at an increased risk of ALS and death from the disease (HR decreases by 3% per unit of BMI). That was especially true for underweight patients who seemed to have almost twice the risk of death in comparison to normal weight patients (HRs for the “normal” vs “underweight” subgroups were estimated to be as high as 1.94).
A wide spectrum of genetic and exogenous factors has been associated with the risk of developing ALS, including exposure to heavy metals and organic chemicals, participation in sports, occupation, and physical trauma.16 BMI is among the exogenous factors associated with the risk of developing ALS.16,17 Lower presymptomatic BMI and obesity rates have been documented in individuals who go on to develop ALS39,40 consistent with studies indicating an association with BMI before ALS diagnosis.41–43 Consequently, BMI may act as a red flag for ALS in the prediagnostic period.
ALS survival has been associated with age at disease onset, sex, clinical phenotype, respiratory failure at an early stage, treatment with riluzole, and weight loss.44,45 Survival in ALS is also influenced by the functional status and its slope of decline.46 Desport et al.30 were the first to examine the effect of BMI on the survival of patients with ALS. It was reported that a 5% weight loss at the time of diagnosis increased the risk of death in those patients by as much as 30%, whereas each BMI unit loss could increase the risk of death by 20%.25 A relationship was also reported between mortality and BMI, with the longest survival observed in the patients with BMI between 30 and 35 kg/m2. Higher or lower BMI values were correlated with higher mortality.37 Findings from Atassi et al. also supported a relationship between BMI and survival, with overweight (BMI ≥ 25 kg/m2) and obese patients with ALS (BMI ≥ 30 kg/m2) having a 35% and 54% reduced risk of dying, respectively, compared with patients with BMI <25 kg/m2.47 Consequently, BMI seems to influence both the development and the survival of patients with ALS.44,45,48
The precise pathophysiologic processes by which BMI may influence ALS development and survival are only partially understood. A reduction in BMI has consistently been reported in patients with ALS during the course of the disease and has been attributed to hypermetabolism or reduced calorie intake secondary to loss of appetite, dysphagia, or hand muscle weakness48–50; nutritional supplements (e.g., acetylcarnitine) may protect against these symptoms.51,52 There is also some evidence to suggest that the entire human body and cellular metabolism may even contribute to ALS development.34 Moreover, endogenous retroviruses (HERVs)—HERV-K in principal—have been causally associated with ALS,53 and their activity in mammals inversely correlates with body size.54
This meta-analysis has some limitations. First, because of the rarity of ALS, most data are from retrospective analyses of clinical trials or registries. Also, the majority of studies were conducted in very few developed countries with increased health care awareness. Therefore, the influence of ethnic diversity on BMI and ALS cannot be assessed. Only a few potential confounding factors that may influence weight, BMI, and ALS were included. Finally, our study carries all the inherent limitations of using BMI as a measure of obesity.55
In conclusion, this meta-analysis reveals a possible influence for initial BMI on the survival of patients with ALS. Further large-scale collaborative studies, including low- and middle-income countries, would be helpful to elucidate the net effect of BMI on ALS and neurodegeneration. Our findings have important implications for the diagnosis, classification, prognosis, and management of ALS. Monitoring weight and BMI would also help physicians personalize the nutritional needs of patients with ALS.
Acknowledgment
A.-F.A.M. is supported by an educational scholarship from the Alexander S. Onassis Public Benefit Foundation who played no role in the design of the study, collection and/or interpretation of data, or writing of the manuscript.
Author contributions
E. Dardiotis: original idea, study design, investigation, interpretation of results, methodology, project administration, supervision, and writing—original draft preparation and review and editing. V. Siokas: investigation, validation, visualization, resources, methodology, and writing—original draft preparation and review and editing. M. Sokratous: investigation and writing—original draft preparation and review and editing. Z. Tsouris: methodology and writing—original draft preparation. A.-M. Aloizou, D. Florou, M. Dastamani, and A.-F.A. Mentis: writing—review and editing. A.G. Brotis: statistical analysis, software, investigation, interpretation of results, and writing—review and editing.
Study funding
No targeted funding reported.
Disclosure
E. Dardiotis, V. Siokas, M. Sokratous, Z. Tsouris, A.-M. Aloizou, D. Florou, and M. Dastamani report no disclosures. A.-F.A. Mentis serves as Junior Editor for ESR Journal and receives research support from the Alexander S. Onassis Public Benefit Foundation. A.G. Brotis reports no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 2014;17:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel SH, Zhao W, Beers DR, Henkel JS. The microglial-motoneuron dialogue in ALS. Acta Myol 2011;30:4–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Mitropoulos K, Merkouri Papadima E, Xiromerisiou G, et al. Genomic variants in the FTO gene are associated with sporadic amyotrophic lateral sclerosis in Greek patients. Hum Genomics 2017;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med 2001;344:1688–1700. [DOI] [PubMed] [Google Scholar]
- 5.Nicolas A, Kenna KP, Renton AE, et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron 2018;97:1268–1283.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarei S, Carr K, Reiley L, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int 2015;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dardiotis E, Siokas V, Sokratous M, et al. Genetic polymorphisms in amyotrophic lateral sclerosis: evidence for implication in detoxification pathways of environmental toxicants. Environ Int 2018;116:122–135. [DOI] [PubMed] [Google Scholar]
- 8.Mentis AA, Dardiotis E, Grigoriadis N, Petinaki E, Hadjigeorgiou GM. Viruses and endogenous retroviruses in multiple sclerosis: from correlation to causation. Acta Neurol Scand 2017;136:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mentis AA, Dardiotis E, Grigoriadis N, Petinaki E, Hadjigeorgiou GM. Viruses and multiple sclerosis: from mechanisms and pathways to translational research opportunities. Mol Neurobiol 2017;54:3911–3923. [DOI] [PubMed] [Google Scholar]
- 10.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol 2015;15:545–558. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Yue XJ, Li HH, et al. Overweight and obesity in young adulthood and the risk of stroke: a meta-analysis. J Stroke Cerebrovasc Dis 2016;25:2995–3004. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Zhang TT, Yu J, et al. Excess body weight during childhood and adolescence is associated with the risk of multiple sclerosis: a meta-analysis. Neuroepidemiology 2016;47:103–108. [DOI] [PubMed] [Google Scholar]
- 13.Strazzullo P, D'Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke 2010;41:e418–e426. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Scarmeas N, Cosentino S, et al. Change in body mass index before and after Alzheimer's disease onset. Curr Alzheimer Res 2014;11:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Marck MA, Dicke HC, Uc EY, et al. Body mass index in Parkinson's disease: a meta-analysis. Parkinsonism Relat Disord 2012;18:263–267. [DOI] [PubMed] [Google Scholar]
- 16.Chio A, Mora G, Moglia C, et al. Secular trends of amyotrophic lateral sclerosis: the Piemonte and Valle d'Aosta register. JAMA Neurol 2017;74:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingre C, Roos PM, Piehl F, Kamel F, Fang F. Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol 2015;7:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longinetti E, Mariosa D, Larsson H, et al. Physical and cognitive fitness in young adulthood and risk of amyotrophic lateral sclerosis at an early age. Eur J Neurol 2017;24:137–142. [DOI] [PubMed] [Google Scholar]
- 19.O'Reilly EJ, Wang H, Weisskopf MG, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang MD, Little J, Gomes J, Cashman NR, Krewski D. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology 2017;61:101–130. [DOI] [PubMed] [Google Scholar]
- 21.Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology 1999;53:1059–1063. [DOI] [PubMed] [Google Scholar]
- 22.Limousin N, Blasco H, Corcia P, et al. Malnutrition at the time of diagnosis is associated with a shorter disease duration in ALS. J Neurol Sci 2010;297:36–39. [DOI] [PubMed] [Google Scholar]
- 23.Jawaid A, Murthy SB, Wilson AM, et al. A decrease in body mass index is associated with faster progression of motor symptoms and shorter survival in ALS. Amyotroph Lateral Scler 2010;11:542–548. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T, Nagaoka U, Nakayama Y, et al. Reduction rate of body mass index predicts prognosis for survival in amyotrophic lateral sclerosis: a multicenter study in Japan. Amyotroph Lateral Scler 2012;13:363–366. [DOI] [PubMed] [Google Scholar]
- 25.Marin B, Desport JC, Kajeu P, et al. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 2011;82:628–634. [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. ISBN 3-900051-07-0. Available at: http://www.R-project.org. [Google Scholar]
- 29.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. 1st ed Cham, Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 30.Desport JC, Marin B, Funalot B, Preux PM, Couratier P. Phase angle is a prognostic factor for survival in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2008;9:273–278. [DOI] [PubMed] [Google Scholar]
- 31.Gallo V, Wark PA, Jenab M, et al. Prediagnostic body fat and risk of death from amyotrophic lateral sclerosis: the EPIC cohort. Neurology 2013;80:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paganoni S, Hyman T, Shui A, et al. Pre-morbid type 2 diabetes mellitus is not a prognostic factor in amyotrophic lateral sclerosis. Muscle Nerve 2015;52:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lunetta C, Lizio A, Melazzini MG, Maestri E, Sansone VA. Amyotrophic Lateral Sclerosis Survival Score (ALS-SS): a simple scoring system for early prediction of patient survival. Amyotroph Lateral Scler Frontotemporal Degener 2015;17:93–100. [DOI] [PubMed] [Google Scholar]
- 34.Eaglehouse YL, Talbott EO, Chang Y, Kuller LH. Participation in physical activity and risk for amyotrophic lateral sclerosis mortality among postmenopausal women. JAMA Neurol 2016;73:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henriques A, Blasco H, Fleury MC, et al. Blood cell palmitoleate-palmitate ratio is an independent prognostic factor for amyotrophic lateral sclerosis. PLoS One 2015;10:e0131512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariosa D, Beard JD, Umbach DM, et al. Body mass index and amyotrophic lateral sclerosis: a study of US Military Veterans. Am J Epidemiol 2017;185:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 2011;44:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997–2011. Neurol Clin Pract 2013;3:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology 2002;59:773–775. [DOI] [PubMed] [Google Scholar]
- 40.Sutedja NA, van der Schouw YT, Fischer K, et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2011;82:638–642. [DOI] [PubMed] [Google Scholar]
- 41.Doyle P, Brown A, Beral V, Reeves G, Green J. Incidence of and risk factors for motor neurone disease in UK women: a prospective study. BMC Neurol 2012;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huisman MH, Seelen M, van Doormaal PT, et al. Effect of presymptomatic body mass index and consumption of fat and alcohol on amyotrophic lateral sclerosis. JAMA Neurol 2015;72:1155–1162. [DOI] [PubMed] [Google Scholar]
- 43.Kioumourtzoglou MA, Rotem RS, Seals RM, Gredal O, Hansen J, Weisskopf MG. Diabetes mellitus, obesity, and diagnosis of amyotrophic lateral sclerosis: a population-based study. JAMA Neurol 2015;72:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calvo A, Moglia C, Lunetta C, et al. Factors predicting survival in ALS: a Multicenter Italian Study. J Neurol 2017;264:54–63. [DOI] [PubMed] [Google Scholar]
- 45.Mathis S, Couratier P, Julian A, Vallat JM, Corcia P, Le Masson G. Management and therapeutic perspectives in amyotrophic lateral sclerosis. Expert Rev Neurother 2017;17:263–276. [DOI] [PubMed] [Google Scholar]
- 46.Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol 2014;76:643–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atassi N, Berry J, Shui A, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology 2014;83:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed RM, Caga J, Devenney E, et al. Cognition and eating behavior in amyotrophic lateral sclerosis: effect on survival. J Neurol 2016;263:1593–1603. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed RM, Irish M, Piguet O, et al. Amyotrophic lateral sclerosis and frontotemporal dementia: distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol 2016;15:332–342. [DOI] [PubMed] [Google Scholar]
- 50.Timmins HC, Saw W, Cheah BC, et al. Cardiometabolic health and risk of amyotrophic lateral sclerosis. Muscle Nerve 2017;56:721–725. [DOI] [PubMed] [Google Scholar]
- 51.Beghi E, Pupillo E, Bonito V, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for ALS. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:397–405. [DOI] [PubMed] [Google Scholar]
- 52.Margaritis M, Mentis AF, Schulpis KH, Karikas G, Tsakiris S. The role of L-carnitine in neurodegenerative disorders. Pharmakeftiki 2009;22:39–45. [Google Scholar]
- 53.Li W, Lee MH, Henderson L, et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med 2015;7:307ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katzourakis A, Magiorkinis G, Lim AG, Gupta S, Belshaw R, Gifford R. Larger mammalian body size leads to lower retroviral activity. PLoS Pathog 2014;10:e1004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond) 2008;32(suppl 3):S56–S59. [DOI] [PubMed] [Google Scholar]