Abstract
Objective
To examine the association between maternal HIV infection and pregnancy outcomes controlling for potential confounding factors among a cohort of HIV-uninfected and HIV-infected pregnant women in Dar es Salaam, Tanzania.
Design
Prospective cohort study.
Methods
A cohort of 1078 HIV-infected and 502 HIV-uninfected pregnant women between 12 and 27 weeks of gestation were enrolled and followed up until delivery. Multiple regression models were used to compare the risk of adverse pregnancy outcomes among HIV-uninfected women with those among HIV-infected women overall, and separately among asymptomatic or symptomatic HIV-infected women.
Results
No significant differences between HIV-uninfected women and HIV-infected women were observed in risks of fetal loss or low birthweight or in the weight, head circumference and gestational age of infants at birth. HIV-infected women were more likely to have severe immature infants (<34 weeks) than HIV-uninfected women (multivariate RR 1.54 [95% CI 0.90–2.48]; P = 0.05). There was a significantly higher risk of low birthweight (RR 2.29, 95% CI 1.34–3.92; P = 0.03) and prematurity (<37 weeks) (RR 1.93, 95% CI 1.35–2.77; P = 0.0003) among symptomatic HIV-infected women when compared with HIV-uninfected women.
Conclusion
HIV-infected women, particularly those who are symptomatic, are at a higher risk of adverse pregnancy outcomes.
INTRODUCTION
HIV-1 infection is a major public health problem in developing countries, particularly in sub-Saharan Africa and South and Southeast Asia. According to the June 2000 UNAIDS AIDS epidemic update, 24.5 million of the world’s 34.3 million HIV-infected people live in sub-Saharan Africa. About 12.9 million infected women aged 15 to 49 years are from this region 1. A large volume of literature addressing HIV infection in women has provided conflicting reports on the association between HIV infection and pregnancy outcomes2–15. Factors such as differences in clinical settings, methodological quality of the studies, and study population characteristics could be responsible for the discrepancy in the reported associations. Many of the studies conducted on this topic are limited either because of the lack of an appropriate control group, relatively small sample size, or because of failure to control for factors such as health habits, co-infections, stage of HIV disease, nutritional and other potential confounding variables. In these published studies it was not possible to examine whether the stage of maternal HIV disease modified the association with pregnancy outcomes. In a recent meta-analysis based on 31 prospective studies, the estimated magnitudes of association between HIV infection and perinatal outcomes were modest16. The authors suggested that a more complete control for confounding factors might have eliminated the observed associations and called for further examination of this question using large prospective studies with more complete assessment of such factors.
It is the aim of this paper to first determine whether HIV infection does have an effect on pregnancy outcome. The biological mechanisms for HIV as a cause of adverse pregnancy outcomes are not clear. However, it is possible that low CD4 count may result in an increase in fetal infection and intrauterine growth restriction. High viral load in the mother is also likely to increase the risk of transmission to the baby, thus resulting in low birth-weight and other adverse outcomes.
In this report, we examined the association between maternal HIV infection and pregnancy outcomes, overall and within maternal disease stage, by controlling for potential confounding due to socio-demographic, socioeconomic, sexual, reproductive and anthropometric factors, among a well-characterised cohort of HIV-infected (n = 540) and HIV-uninfected (n = 502) pregnant women in Dar es Salaam, Tanzania.
METHODS
Study population and design
About 12.2 million Sub-Saharan African women, aged 15 to 49 years, are infected with HIV1. In this area, HIV-1 prevalence in women is highest in the peak of childbearing years. In Tanzania the prevalence of HIV infection among pregnant women ranges from 7.3% to 44% in rural areas and 22% to 36% in urban areas17. Tanzania has a gross national product (GNP) per capita of US$210.00 and an average annual expenditure per person for health care of US$4.00. Fifty percent of Tanzanians live below the locally defined poverty line and 36% live in abject poverty. Private health services are rare and unaffordable for the general population, thus more than 90% of Tanzanians seek health care in the public sector. There is high coverage of antenatal services across the country and in Dar es Salaam more than 85% of pregnant women seek antenatal care by 12 to 27 weeks of gestation. Antenatal clinics thus provide the most readily accessible, cross section of healthy sexually active women in the general population and have become the most common site for sentinel surveillance of HIV and other sexually transmitted diseases (STDs).
Between April 1995 and July 1997 a cohort consisting of pregnant women between 12 and 27 weeks of gestation who were infected and uninfected with HIV-1 was enrolled. The HIV-infected group was part of a randomised trial to examine the efficacy of vitamin supplements on HIV transmission and progression among pregnant women while the HIV-uninfected group was part of a psychosocial study. For our study 14,049 pregnant women between 12 and 27 weeks of gestation and receiving prenatal care at four prenatal care sites (e.g. Temeke, Mwanayamala and Ilala hospitals and Mwenge Clinic in Dar es Salaam) received information about the study and were asked to participate. After HIV pre-test counselling 13,781 women gave verbal informed consent for HIV testing. Ninety-nine percent of the women returned to the clinic to receive their HIV results and of these, 61% consented to participate in the studies. HIV-1 prevalence was 13.1%. Criteria for participation in the study included intention to reside in Dar es Salaam until delivery and for at least 18 months thereafter. Recruitment and enrolment occurred simultaneously at all sites. The women infected with HIV (n = 1078) were enrolled at the four prenatal care sites (Temeke, Mwanayamala and Ilala hospitals and the Mwenge clinic) while the HIV-uninfected women (n = 502) were enrolled at the Mwanayamala clinic only.
Details of the randomised trial among HIV-infected women have been published elsewhere18. Similar recruitment and assessment procedures were used among HIV-infected and HIV-uninfected women. Consenting HIV-infected women were randomised in a placebo-controlled study to examine the efficacy of vitamin supplementation on pregnancy outcomes. The women were randomised to receive daily doses of one of four regimens: 272 were given vitamin A (30mg beta-carotene plus 5000iu of preformed vitamin A); 270 were given multivitamins excluding vitamin A (20mg B1, 20mg B2, 25mg B6, 100mg niacin, 50mg B12, 500mg C, 30mg E, and 0.8mg folic acid); 268 were given multivitamins including vitamin A; and 268 were given a placebo. HIV-infected and uninfected women received daily doses of ferrous sulphate and folate tablets, and weekly prophylactic chloroquine phosphate, as per standard prenatal care.
At inclusion into the study, trained interviewers administered a standardised questionnaire to collect information about socio-demographic characteristics; sexual behaviour; gynaecological and medical history; contraceptive use; sexually transmitted diseases; and obstetric history. Laboratory tests for sexually transmitted diseases (syphilis, gonorrhoea, trichomoniasis), intestinal parasites and malaria were done; women with evidence of sexually transmitted diseases or parasitism were given the appropriate medical treatment in accordance with the guidelines of the Tanzania Ministry of Health. Counts of CD4, CD8 and CD3 T-lymphocyte were done on all HIV-infected women and a sub-sample of HIV-uninfected women (n = 198). HIV-infected women had a detailed clinical assessment at baseline, which we used for assignment of HIV disease stage using the WHO staging system19. At delivery, midwives recorded the newborn’s survival status and anthropometric measurements. Anthropometric measurements included birth-weight to the nearest 10g, and length and head circumference to the nearest 0.1cm.
As part of their participation in the trial, HIV-infected women were followed monthly during pregnancy and after delivery for at least a year at the study clinic at Muhimbili Medical Centre. At monthly follow up patients received appropriate routine antenatal care at which time symptomatic cases of STDs, malaria and other infectious diseases were laboratory diagnosed and appropriately treated. Women who missed their monthly appointments were visited at home. HIV-uninfected women were encouraged to continue attending antenatal care regularly at Mwanayamala and were examined at 36 weeks of gestation and at delivery. For women in both groups who left Dar es Salaam, we collected information on the outcome of pregnancy from their relatives and neighbours.
Laboratory tests were done at the Muhimbili Medical Centre laboratories. Sera from all women were screened for HIV-1 antibodies by enzyme-linked immunosorbent assay (Wellcozyme, Murex Biotech Ltd, Dartford, UK). Confirmatory Western blot analysis was done on all the enzyme-linked immunsorbent assay positive sera (BioRad Laboratories Ltd, Hertfordshire, UK). Absolute T-lymphocyte counts of CD4, CD8 and CD3 were performed using the FACScount system (Becton Dickinson, San Jose, California, USA) or the FACScan system (Becton Dickinson, San Jose, California, USA). Syphilis tests were done using standard laboratory procedures of venereal disease research laboratory test (Murex Biotech, Dartford, UK) and Treponema pallidum haemagglutination assay (Fujirebio Inc, Tokyo, Japan). Standard laboratory procedures were used to test endocervical and high vaginal swabs for Neisseria gonorrhoea, Candida albicans and Trichomonas vaginalis.
Statistical analyses
The study endpoints included the occurrence of fetal loss (defined as either miscarriage or stillbirth). A miscarriage was defined as delivery before 28 completed weeks of gestation. A stillbirth was defined as delivery of a dead baby at or after 28 completed weeks of gestation. We also examined the following endpoints: low birthweight (<2500g), severe low birthweight (<2000g), prematurity (<37 completed weeks of gestation at birth), severe prematurity (<34 completed weeks of gestation at birth). The following continuous endpoints were examined: weight, length and head circumference of infants at birth.
We previously reported that among HIV-infected women, multivitamins significantly reduced the risk of the adverse pregnancy outcomes that were examined in the paper18. Thus HIV-infected women who received multivitamins were excluded and only HIV-infected women who did not receive multivitamins are included in the analyses. Twins were excluded from the analyses of birthweight, length, or head circumference. If either twin was a fetal loss, we took that pregnancy to have had that outcome. The two-sample Wilcoxon rank sum test (for continuous variables) and χ2 test (for categorical variables) were used to compare the socio-demographic and background characteristics of the HIV-uninfected women to HIV-infected women. To assess the association between HIV status and categorical outcomes we estimated the relative risks and their 95% confidence intervals by binomial regression with the log link function. Linear regression was used to examine the mean difference in the birth outcomes measured by continuous variables, and the robust variance was used to compute the confidence interval20. Observations with missing values were retained in the analyses by the use of the missing indicator method21. Multiple regression models assessed the independent relations of the endpoint after adjusting for potential confounding by socio-economic and demographic characteristics (including those of the partner), presence of infections during current pregnancy (STDs and malaria), medical history (STDs, including those of the partner, tuberculosis, hypertension, eclampsia and smoking), obstetric and reproductive characteristics (type of delivery), sexual behaviours (number of sexual partners in the last five years, age at first pregnancy and contraceptive use), signs and symptoms indicative of STDs (genital ulcer, genital itch, foul genital smell and abnormal genital discharge), and anthropometric measurements at enrolment. Covariates for inclusion in final linear or binomial regression models were selected using a stepwise approach with P ≤ 0.2 level as the criterion for inclusion. A value of P ≤ 0.05 was used to define statistical significance.
We compared the risk of categorical outcomes and continuous measurements among HIV-uninfected women with HIV-infected women who had not received multivitamins. We investigated the presence of a recruitment centre effect on birth outcomes by comparing pregnancy outcomes among HIV-infected women from the four centres. Since there was no significant difference among the centres, they were pooled together and presented as a single group. We also assessed whether birth outcomes of HIV-infected women were modified by HIV disease stage: HIV-uninfected women were compared with two groups of HIV-infected women, namely asymptomatic (WHO Stage 1), and symptomatic (WHO Stages 2 or higher) women. We also assessed whether birth outcomes of HIV-infected women were modified by baseline CD4 cell count in five strata (<200 × 106 cells/L, 200–499 × 106 cells/L, ≥500 × 106 cells/L, <350 × 106 cells/L, ≥350 × 106 cells/L). Data were analysed using the Statistical Analysis Software (SAS Institute Inc, Cary, North Carolina, USA).
The study protocol was approved by the Research and Publication Committee of Muhimbili University College of Health Sciences, the Ethical Committee of the National AIDS Control Programme of the Tanzanian Ministry of Health, and the Institutional Review Board of the Harvard School of Public Health.
RESULTS
Of the 1078 HIV-infected women enrolled in the study, 540 did not receive multivitamins. Of the 540 HIV positive women, four died before delivery, 14 were lost to follow up before delivery, and the remaining 522 women (96.7%) had known pregnancy outcomes. Of the 502 HIV-uninfected women, one died before delivery, 16 were lost before delivery and the remaining 485 women (96.6%) had known pregnancy outcomes. Irrespective of HIV status, women with known pregnancy outcomes had the same socio-demographic, socio-economic and background obstetric characteristics as the overall sample. Socio-economically, HIV-infected women did not differ from HIV-uninfected women and socio-economic status was representative of that of women in the general population. Both groups of women spent a median of 1500 Tanzanian shillings (US$1.99) per day on food with a median of three persons eating (i.e. 500 (US$0.66) Tanzanian shillings per person per day). Of the HIV-infected women, 75.2% were totally supported, 23.9% partially supported and the remaining 0.99% had selfsupport. Of the HIV-uninfected, 78.2% were totally supported and 21.8% were partially supported. Background socio-demographic and obstetric characteristics of the HIV-uninfected and HIV-infected women for whom pregnancy outcomes were known are presented in Table 1. Since some women were delivered at home (3.6%) or other medical facility other than our follow up site (1.7%), or other facilities other than a medical facility (2.88%), we could not ascertain all of the dates of pregnancy outcomes and anthropometric measurements at birth. However, irrespective of HIV status, women with known and unknown dates of pregnancy outcome and anthropometric measures at birth had the same sociodemographic, socio-economic, background obstetric characteristics as the original sample. The date of pregnancy outcomes could be ascertained for 501 singleton pregnancies among HIV-infected women (98.6%) and 485 singleton pregnancies among HIV-uninfected women (100%).
Table 1.
Selected socio-demographic and baseline obstetric characteristics of pregnant women by HIV-1 infection status. GA = gestational age; STD = sexually transmitted disease; BMI = body mass index.
| Characteristics | HIV-uninfected n = 485 | HIV-infected n = 540 | Pa |
|---|---|---|---|
| Age (years): mean (SD) | 22.5 (4.9) | 24.6 (4.9) | <0.001 |
| GA (weeks): mean (SD) | 18.8 (3.1) | 18.0 (3.2) | <0.001 |
| Education (years) (%) | |||
| 0 | 7.5 | 7.7 | 0.21 |
| 1-4 | 5.6 | 5.1 | |
| 5-8 | 73.7 | 78.1 | |
| ≥9 | 13.3 | 9.1 | |
| Occupation (%) | |||
| Housewife | 78.0 | 72.3 | 0.03 |
| Professional | 2.1 | 1.6 | |
| Business | 13.9 | 15.6 | |
| Public house | 0.6 | 1.2 | |
| Wage employment | 4.9 | 6.3 | |
| Other | 0.4 | 3.0 | |
| Marital status (%) | |||
| Married | 65.5 | 63.0 | 0.33 |
| Cohabiting | 21.2 | 25.1 | |
| Unmarried | 13.3 | 11.9 | |
| Height (cm): mean (SD) | 155.2 (6.2) | 155.7 (5.8) | 0.31 |
| Weight (kg): mean (SD) | 56.4 (8.9) | 56.8 (9.0) | 0.39 |
| Arm circumference (cm): mean (SD) | 25.2 (2.7) | 25.2 (2.7) | 0.61 |
| BMI (kg/sq-m): mean (SD) | 23.4 (3.4) | 23.5 (3.2) | 0.38 |
| CD4 count (/mm3): mean (SD) | 780.5 (236.4) | 423.9 (212.2) | <0.001 |
| Previous pregnancies (%) | |||
| 0 | 52.4 | 26.7 | 0.001 |
| 1 | 20.7 | 27.4 | |
| 2 | 11.0 | 18.3 | |
| 3 | 7.8 | 11.0 | |
| 4+ | 8.2 | 16.6 | |
| History of STD in 5 yrs (%) | 4.5 | 19.2 | 0.001 |
| Genital itching in current pregnancy (%) | 13.2 | 29.1 | 0.001 |
| Foul genital smell in current pregnancy (%) | 2.2 | 7.6 | 0.001 |
| Abnormal vaginal discharge in current pregnancy (%) | 3.0 | 11.2 | 0.001 |
| Vaginal bleeding in current pregnancy (%) | 2.2 | 4.8 | 0.03 |
| Genital ulcers in current pregnancy (%) | 0.9 | 7.6 | 0.001 |
| Symptoms of STDsb (%) | 14.7 | 34.3 | 0.001 |
| History of tuberculosis (%) | 0.4 | 3.2 | 0.002 |
| Malaria in current pregnancy (%) | 32.1 | 35.8 | 0.23 |
Continuous variables were compared by the Wilcoxon rank – sum test. Categorical variables were compared by χ2 test.
Symptoms of sexually transmitted disease include either genital itch or foul genital smell or abnormal vaginal discharge or genital ulcer.
The mean gestational age at enrolment was 18 weeks (standard deviation 3.2) for HIV-infected women and 18.8 weeks (SD 3.1) for HIV-uninfected women. HIV-infected women had 14 (2.7%) sets of twins, while HIV-uninfected women had 17 (3.5%) sets. The HIV-infected women were significantly older than HIV-uninfected women with mean ages of 24.6 years (SD 4.9) and 22.5 years (SD 4.9), respectively (P < 0.001). The percentage of women with one or more previous pregnancies was significantly higher in HIV-infected women (73.3%), than HIV-uninfected women (47.7%) (P = 0.001). HIV-infected women did not differ significantly from HIV-uninfected women in terms of education and marital status. HIV-infected women were more likely to have reported history of STDs in the previous five years than HIV-uninfected women (19.2% versus 4.5%, respectively, P = 0.001). Similarly, HIV-infected women were more likely to present symptoms suggestive of STDs (34.3%) than HIV-uninfected women (14.7%) (P = 0.001). Compared with HIV-uninfected women, HIV-infected women were significantly more likely to have a history of tuberculosis (P = 0.002) and a significantly lower mean CD4 cell count at the time of enrolment (P < 0.001).
The frequency and basic data on pregnancy outcomes are presented in Table 2 while Tables 3 and 4 present the association between HIV-1 and pregnancy outcomes. There was no significant association between maternal HIV status and fetal loss. HIV-infected women had 51 fetal losses (9.8%), while HIV-uninfected women had 42 fetal losses (8.7%). When we compared HIV-uninfected women with HIV infected women, there was no significant difference in the risk of fetal loss between the two groups of women (RR 0.84, 95% CI 0.52–1.37; P = 0.49). There were no statistically significant relationships between maternal HIV status and the risks of stillbirth or miscarriage.
Table 2.
Univariate analysis of pregnancy outcomesa among HIV-infected women compared with HIV-uninfected women. GA = gestational age
| Pregnancy outcomes | HIV-uninfected |
HIV-infected |
|
|---|---|---|---|
| Categorical outcome variables | n/n (%) | n/n (%) | P |
| Miscarriage | 11 / 485 (2.3) | 17 / 520 (3.3) | 0.335 |
| Stillbirth | 31 / 485 (6.4) | 34 / 520 (6.5) | 0.925 |
| Fetal loss | 42 / 485 (8.7) | 51 / 520 (9.8) | 0.530 |
| Weight <2500 g at birth | 54 / 367 (14.7) | 66 / 433 (15.2) | 0.835 |
| Weight <2000 g at birth | 16 / 367 (4.4) | 20 / 433 (4.6) | 0.860 |
| Gestation <37 wks at birth | 102 / 412 (24.8) | 114 / 420 (27.1) | 0.433 |
| Gestation <34 wks at birth | 33 / 412 (8.0) | 48 / 420 (11.4) | 0.096 |
| Weight <2500 g and gestation <37 wks | 27 / 352 (7.7) | 38 / 405 (9.4) | 0.402 |
| Weight <2500 g and gestation ≥37 wks | 25 / 352 (7.1) | 25 / 405 (6.2) | 0.608 |
| Continuous outcome variables | Mean g ± SD (n) | Mean g ± SD (n) | P |
|---|---|---|---|
| Birthweight (g) | 2906 ± 546 (367) | 2966 ± 562 (433) | 0.096 |
| Birth length (cm) | 47.3 ± 3.1 (275) | 48.3 ± 2.8 (335) | <0.001 |
| Head circumference (cm) | 34.9 ± 1.97 (275) | 34.7 ± 2.1 (339) | 0.048 |
| GA (wks) | 38.5 ± 3.0 (412) | 38.2 ± 3.3 (420) | 0.549 |
Miscarriage, delivery at <28 weeks of gestation; Stillbirth, delivery of a dead baby at ≥28 weeks gestation; Fetal loss includes both miscarriage and stillbirth.
Table 3.
Age-adjusted and multivariate analysis of categorical pregnancy outcomesa and HIV-1 status.
| Pregnancy outcomes | HIV-infected women compared with HIV-uninfected women |
|||
|---|---|---|---|---|
| Age-adjusted RRb (95%CI) | P | Multivariate RR (95%CI) | P | |
| Miscarriage | 1.45 (0.66 - 3.20) | 0.36 | 0.67 (0.24 - 1.82) | 0.43 |
| Stillbirth | 1.04 (0.62 - 1.73) | 0.89 | 0.88 (0.50 - 1.56) | 0.66 |
| Fetal loss | 1.15 (0.75 - 1.74) | 0.52 | 0.84 (0.52 - 1.37) | 0.49 |
| Weight < 2500g at birth | 1.21 (0.87 - 1.69) | 0.26 | 1.25 (0.88 - 1.79) | 0.21 |
| Weight < 2000g at birth | 1.11 (0.60 - 2.06) | 0.73 | 1.23 (0.64 - 2.35) | 0.54 |
| Gestation <37 weeks at birth | 1.16 (0.92 - 1.48) | 0.21 | 1.11 (0.87 - 1.42) | 0.39 |
| Gestation <34 weeks at birth | 1.57 (1.02 - 2.41) | 0.04 | 1.54 (0.90 - 2.48) | 0.05 |
| Weight <2500g and gestation <37 weeks | 1.33 (0.83 - 2.14) | 0.23 | 1.25 (0.77 - 2.03) | 0.36 |
| Weight <2500g and gestation≥37 weeks | 1.13 (0.66 - 1.95) | 0.65 | 1.16 (0.64 - 2.07) | 0.63 |
Miscarriage, delivery at <28 weeks of gestation; Stillbirth, delivery of a dead baby at ≥28 weeks gestation; Fetal loss includes both miscarriage and stillbirth.
Relative risk, confidence interval (CI), and P-value by multiple binomial regression with log link function. Multivariate estimates adjusted for women’s age (<20 yrs, 20-24 yrs, 25-29 yrs, ≥30 yrs); education (0-4 yrs, 5-8 yrs, ≥9 yrs); occupation (housewife, business, others (professional, public house, wage employment, all other employment)); marital status (married, cohabiting, single/divorce/widowed); height (<150 cm, 150–159 cm, ≥160 cm); arm circumference (<22 cm, 22-23 cm, ≥24 cm); number of previous pregnancies (0, 1, 2, 3, ≥4); history of tuberculosis (yes, no); malaria in current pregnancy (yes, no); and symptoms of sexually transmitted disease this pregnancy, (i.e. either genital itch or foul genital smell or abnormal vaginal discharge or genital ulcer (yes, no)).
Table 4.
Age-adjusted and multivariate analysis of continuous pregnancy outcomesa and HIV-1 status.
| Pregnancy Outcomes | HIV-infected women compared with HIV-uninfected women |
|||
|---|---|---|---|---|
| Age-adjusted MDb (95%CI) | P | Multivariate MD (95%CI) | P | |
| Birthweight (g) | 19.87 (−54.89 to 94.64) | 0.60 | 19.16 (−56.43 to 94.76) | 0.62 |
| Birth length (cm) | 0.95 (0.45 to 1.44) | <0.001 | 1.03 (0.53 to 1.53) | < 0.001 |
| Head circumference (cm) | −0.17 (−0.50 to 0.16) | 0.32 | −0.09 (−0.43 to 0.25) | 0.600 |
| Gestational age (weeks) | −0.34 (−0.78 to 0.09) | 0.12 | −0.29 (−0.74 to 0.16) | 0.20 |
Measurees were taken at birth.
Mean difference determined by linear regression, p-values by Wald test using the robust variance. Multivariate estimates adjusted for women’s age (<20 yrs, 20-24 yrs, 25-29 yrs, $30 yrs); education (0-4 yrs, 5-8 yrs, ≥9 yrs); occupation (housewife, business, others (professional, public house, wage employment, all other employment)); marital status (married, cohabiting, single/divorce/widowed); height (<150 cm, 150-159 cm, ≥160 cm); arm circumference (<22 cm, 22-23 cm, ≥24 cm); number of previous pregnancies (0, 1, 2, 3, ≥4); history of tuberculosis infection (yes, no); malaria infection this pregnancy (yes, no); and symptoms of sexually transmitted disease this pregnancy, (i.e. either genital itch or foul genital smell or abnormal vaginal discharge or genital ulcer (yes, no)).
HIV-infected women had 471 live births (90.2%) (including 11 sets of twins) while HIV-uninfected women had 443 live births (91.3%) (including 12 sets of twins). Among the live born singleton infants, birth-weights were available for 421 infants (91.5%) born to HIV-infected women and 367 infants (85.2%) born to HIV-uninfected women. Among HIV-infected women, 15.2% had low birthweight infants compared with 14.7% of the HIV-uninfected women (RR = 1.25, 95% CI 0.88–1.79; P = 0.21). There was no significant association between maternal-HIV status and severe low birthweight infants (RR = 1.23, 95% CI 0.64–2.35; P = 0.54) (Table 3). There was no mean difference in birthweight of infants born to the HIV-infected women compared with HIV-uninfected women (mean difference 19.2g, 95% CI −56.4–94.8; P = 0.62) (Table 4).
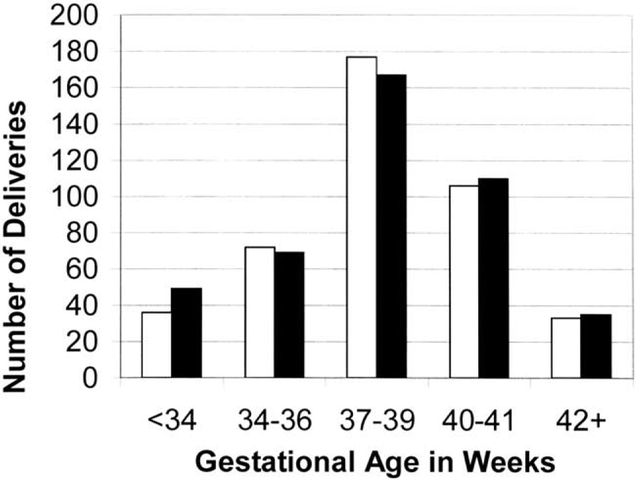
Gestational age at birth was known for 496 infants (95.0%) born to HIV-infected women and 412 infants (95.6%) born to HIV-uninfected women (Fig. 1). There was no significant association between maternal-HIV status and the gestational age of the infants at birth. There were 114 preterm births (27.1%) among HIV-infected women and 102 preterm births (24.8%) among HIV-uninfected women. The relative risk for having a preterm birth was 1.11 (95% CI 0.87–1.42; P = 0.39) for HIV-infected women compared with HIV-uninfected women. However, HIV-infected women compared with HIV-uninfected women, had a higher risk of severe preterm birth (RR 1.54, 95% CI 0.90–2.48; P = 0.05).
Fig. 1.
Gestational age at birth and HIV-1 status. ■ HIV-infected; □ HIV-uninfected.
Birth lengths were known for 347 infants (66.5%) born to HIV-infected women and 275 infants (62.1%) born to HIV-uninfected women. Head circumferences were known for 353 infants (67.6%) born to HIV-infected women and 275 infants (62.1%) born to HIV-uninfected women. There was no significant multivariate mean difference in head circumference of the infants born to HIV-infected women compared with HIV-uninfected women.
We compared the occurrence of adverse pregnancy outcomes between asymptomatic or symptomatic HIV-infected and HIV-uninfected women. The incidence of adverse pregnancy outcomes was not different between asymptomatic HIV-infected women and HIV-uninfected women. However, compared with HIV-uninfected women, HIV-infected women who were in Stage 2 or higher according to the WHO staging system had a significantly higher risk of low birthweight (multivariate, RR 2.29, 95% CI 1.34–3.93; P = 0.03) and prematurity (RR 1.93, 95% CI 1.35–2.77; P = 0.0003) (Table 5).
Table 5.
Multivariate analysis of pregnancy outcomes among HIV-infected asymptomatic (WHO Stage 1) and symptomatic (WHO Stage ≥2) women compared to HIV-uninfected women.
| HIV(−) | Stage 1 | Stage 23 | Stage 1 | Stage 23 | |||
|---|---|---|---|---|---|---|---|
| Pregnancy outcomes | n/n (%) | n/n (%) | n/n (%) | RR (95%CI) | P | RR (95%CI) | P |
| Fetal Loss | 42/485 (8.7) | 44/433 (10.2) | 6/86 (7.0) | 0.86 (0.53-1.39) | 0.54 | 0.61 (0.20-1.84) | 0.38 |
| Weight <2500 g at birth | 54/367 (14.7) | 47/361 (13.0) | 19/72 (26.4) | 0.98 (0.67-1.45) | 0.94 | 2.29 (1.34-3.92) | 0.03 |
| Gestation <37 wks at birth | 102/412 (24.8) | 84/350 (24) | 30/70 (42.9) | 1.05 (.81-1.37) | 0.72 | 1.93 (1.35-2.77) | 0.0003 |
| Weight <2500 g and gestation <37 wks | 27/352 (7.7) | 26/337 (7.7) | 12/68 (17.7) | 1.10 (0.66-1.83) | 0.72 | 3.70 (1.81-7.56) | 0.0003 |
| Weight <2500 g and gestation ≥37 wks | 25/352 (7.1) | 20/337 (5.9) | 5/68 (7.4) | 0.83 (0.42-1.64) | 0.59 | 1.48 (0.58-3.78) | 0.416 |
DISCUSSION
Our findings suggest that while overall there was no association between HIV infection and fetal loss, low birthweight or low birthweight full term infants. Severe prematurity occurred more among infected women compared with uninfected women. When HIV-infected women were categorised according to HIV disease stage, greater risks of low birthweight and prematurity were observed among symptomatic HIV-infected women when compared with HIV-uninfected women. Similar studies conducted in other regions of sub-Saharan Africa (e.g. Rwanda4,7, Kenya5,6, Zambia3, Congo8 and Malawi9) have found HIV-1 infection to be generally associated with adverse pregnancy outcomes of low birthweight, prematurity and intrauterine growth retardation, while we found HIV-1 infection to be significantly associated with adverse pregnancy outcomes of low birthweight and prematurity among symptomatic HIV-infected women only.
The association between maternal HIV infection and pregnancy outcomes was examined in several studies. In their meta-analysis of these studies, Brocklehurst and French16 reported that a modest relationship was found between HIV infection and fetal loss, low birthweight and prematurity. However, they suggested that these findings might have resulted from inadequate adjustment for confounding variables. In this study we do not find HIV infection to be associated with fetal loss, and we found low birthweight and prematurity to be associated with symptomatic HIV infection only. A major strength of this study is its large sample size of pregnant women, low loss to follow up, and ability to adjust for several potential confounders of the relationships that were examined. For example, sexually transmitted diseases have been shown to be associated with low birthweight and immature infants and are more likely to occur among HIV-infected women 4,6,7,9,22,23. We minimised confounding by STDs by controlling for the number of sexual partners, age at first sexual intercourse, number of previous pregnancies, as well as more direct proxy indicators for current STDs including history of STDs among women and their sexual partners and symptoms of STDs during pregnancy. We also adjusted for malaria that has been shown to be associated with adverse pregnancy outcomes among HIV-infected women7,9,24. Another confounder that we considered was socio-economic status, which is associated with adverse pregnancy outcomes25 and likely to be related to HIV status as well. To control for this potential, we included the following variables in our multivariate analyses: education, occupation, marital status, the amount of money spent on food per day, and the number of persons eating from the money spent on food per day. Efforts were also made to control for other pregnancy complications which may adversely affect pregnancy outcomes, such as types of delivery, hypertension and eclampsia.
In addition, we recruited women early in the prenatal period (12 to 27 weeks of gestation) thus allowing for the possibility of examining miscarriages as an outcome. Adverse pregnancy outcome of fetal loss, that is miscarriage or stillbirths, was similar among HIV-uninfected women and HIV-infected women regardless of the stage of HIV disease among women who were infected with HIV. Also there was no difference in full term low birth-weight infants among HIV-infected and uninfected women. There maybe be some other causative factor to fetal loss and full term low birthweight infants, rather than HIV infection per se, in this population. Micronutrient deficiency is a major public health problem in pregnant women, particularly in developing countries, and this deficiency has an adverse impact on pregnancy outcomes regardless of HIV status26–32. Maternal micronutrient deficiency may be the major role player in the adverse pregnancy outcome of fetal loss and full term low birthweight infants observed in this cohort of pregnant women infected and uninfected with HIV. We found that in this population, women who were infected with HIV and who received multivitamins experienced significantly less fetal losses, stillbirths, low birthweight and full term low birthweight infants, compared with HIV-uninfected women. Also previous findings show that HIV-infected women who received multivitamins experienced less fetal losses, low birthweight, severe preterm birth and small for gestational age at birth compared with HIV-infected women who did not receive multivitamins18.
The detailed clinical assessment of women in our study at baseline afforded us the opportunity to examine the relationships between HIV-uninfected women separately with symptomatic or asymptomatic HIV-infected women. We observed that compared with HIV-uninfected women, HIV-infected women who were in Stage 2 or higher according to the WHO staging system had significantly higher risk of low birthweight and immature infants. This finding is in accord with the results of the meta-analyses that reported an apparently stronger association between HIV infection and adverse pregnancy outcomes in studies from developing countries compared with those from developed countries. HIV-infected women in developing countries are likely to consist of a higher proportion of women with relatively more advanced clinical and immunological stage given the higher prevalence of under-nutrition and opportunistic infections, and the poorer access to treatment when compared with developed countries. These conditions have an adverse impact on pregnancy outcomes regardless of HIV status26–32 and may be major players in the adverse pregnancy outcomes observed in HIV-infected women, particularly among asymptomatic women.
The biological mechanisms for HIV as a cause of adverse pregnancy outcomes are not clear. However, low CD4 count may result in an increase in fetal infection and intrauterine growth restriction. High viral load in the mother is also likely to increase the risk of HIV transmission to the baby thus resulting in low birthweight and other adverse outcomes. In addition a possible explanation for HIV infection, its viral load and the resulting low CD4 count being a risk factor for adverse pregnancy outcome might be the cumulative immunosuppressive effect of the HIV infection and the susceptibility to infections. The overall immunosuppressed condition and infective antigen may interfere with the production of steroids, hormones and metabolites which are involved in parturition hence triggering the onset of labour prematurely.
In conclusion, our findings suggest that HIV-infected women in sub-Saharan Africa, particularly those who are symptomatic, are at a higher risk of giving birth to low birthweight and immature infants.
Acknowledgements
This work was supported in part by the National Institutes of Child Health and Human Development (NICHD R0132257), National Institutes of Mental Health (NIH MH 55 451), and the Fogarty International Centre (NIH D43 TWOOOO4). The authors are grateful to the mothers who participated in this study without whom this study would not have been possible. The authors would like to thank the field team of nurses, midwives, supervisors, laboratory staff and administrative staff who made this work possible, particularly Illuminata Ballonzi, Gretchen Antelman, Davis Mwakagile, Charles Kagoma, Ernest Urassa, and Jesse Mbwambo. We also thank Muhimbili Medical Centre, Muhimbili University College of Health Sciences, and the National AIDS Control Programme in Dar es Salaam for their institutional support.
References
- 1.UNAIDS. Joint United Nation Programme on HIV/AIDS: AIDS epidemic update, June 2000. http://www.unaids.org/publication/documents/epidemiology/surveillance/wad1999/Una 99e53.doc. [Google Scholar]
- 2.Kumar RM, Uduman SA, Khurranna AK. Impact of maternal HIV-1 infection on perinatal outcome. Int J Obstet Gynecol 1995;49:137–143. [DOI] [PubMed] [Google Scholar]
- 3.Sukwa TY, Bakketeig L, Kanyama I, Samdal HH. Maternal human immunodeficiency virus infection and pregnancy outcome. Cent Afr J Med 1996;42:233–235. [PubMed] [Google Scholar]
- 4.Bulterys M, Chao A, Munyemana S, et al. Maternal human immunodeficiency virus 1 infection and intrauterine growth: a prospective cohort study in Butare, Rwanda. Pediatr Infect Dis J 1994;13:94–100. [DOI] [PubMed] [Google Scholar]
- 5.Braddick MR, Kreiss JK, Embree JE, et al. Impact of maternal HIV infection on obstetrical and early neonatal outcome. AIDS 1990;4:1001–1005. [DOI] [PubMed] [Google Scholar]
- 6.Temmerman M, Chomba EN, Ndinya-Achola J, Plummer FA, Coppens M, Piot P. Maternal human immunodeficiency virus-1 infection and pregnancy outcome. Obstet Gynecol 1994;83:495–501. [DOI] [PubMed] [Google Scholar]
- 7.Leroy V, Ladner J, Nyiraziraje M, et al. Effects of HIV-1 infection on pregnancy outcome in women in Kigali, Rwanda, 1992-1994. AIDS 1998;12:643–650. [DOI] [PubMed] [Google Scholar]
- 8.Lallemant M, Lellemant-Le-Coeur S, Cheyneir D. Mother-child transmission of HIV-1 and infant survival in Brazzaville, Congo. AIDS 1989;3:643–646. [DOI] [PubMed] [Google Scholar]
- 9.Bloland PB, Wirima JJ, Steketee RW, Chilima B, Hightower A, Breman JG, Maternal HIV. infection and infant mortality in Malawi: evidence of increased mortality due to placental malaria infection. AIDS 1995;9:721–772. [DOI] [PubMed] [Google Scholar]
- 10.Ryder RW, Nsa W, Hassig SE, et al. Perinatal transmission of the human immunodeficiency virus type 1 to infants of seropositive women in Zaire. N Eng J Med 1989;320:1637–1642. [DOI] [PubMed] [Google Scholar]
- 11.D’Ubaldo C, Pezzotti P, Rezza G, Branca M, Ippolito G. Association between HIV-1 infection and miscarriage: a retrospective study. AIDS 1998;12:1087–1093. [DOI] [PubMed] [Google Scholar]
- 12.Selwyn P, Schoenbaum E, Davenny K, et al. Prospective study of HIV infection and pregnancy outcomes in intravenous drug users. JAMA 1989;261:1289–1294. [PubMed] [Google Scholar]
- 13.Geary F, Lindsay M, Graves W, Klein L. HIV infection as a risk for adverse perinatal outcome [abstract]. Am J Obstet Gynecol 1994;170:277. [Google Scholar]
- 14.Alger L, Farley J, Robinson B, Hines S, Berchin J, Johnson J. Interactions of human immunodeficiency virus infection and pregnancy. Obstet Gynecol 1993;82:787–796. [PubMed] [Google Scholar]
- 15.Minkoff HL, Henderson C, Mendez H, et al. Pregnancy outcomes among mothers infected with human immunodeficiency virus and uninfected control subjects. Am J Obstet Gynecol 1990;163:1598–1604. [PubMed] [Google Scholar]
- 16.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol 1998;105:836–848. [DOI] [PubMed] [Google Scholar]
- 17.National AIDS Control Programme: United Republic of Tanzania. HIV/AIDS/STD Surveillance Report No. 13, Dar es Salaam, Tanzania, 1998. [Google Scholar]
- 18.Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomized trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1 infected women in Tanzania. Lancet 1998;351:1477–1482. [DOI] [PubMed] [Google Scholar]
- 19.WHO International Collaborating Group for the Study of the WHO Staging System. Proposed World Health Organization Staging System for HIV infection and disease: preliminary testing by an international collaborative cross sectional study. AIDS 1993;7:711–718. [PubMed] [Google Scholar]
- 20.White H A heteroskedasticity: consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrics 1980;48:817–838. [Google Scholar]
- 21.Miettinen OS. Theoretical Epidemiology. New York: John Wiley, 1985. [Google Scholar]
- 22.Goldenberg RL, Andrews WW, Yuan AC, Mackay HT, St Louis ME. Sexually transmitted diseases and adverse outcomes of pregnancy. Clin Perinatol 1997;24:23–41. [PubMed] [Google Scholar]
- 23.Temmerman M, Njagi E, Nagelkerke N, Ndinya-Achola J, Plummer FA, Meheus A. Mass antimicrobial treatment in pregnancy. A randomized, placebo-controlled trial in a population with high rates of sexually transmitted diseases. J Reprod Med 1995;40:176–180. [PubMed] [Google Scholar]
- 24.Taha T, el T, Gray RH, Mohamedani AA. Malaria and low birthweight in central Sudan. Am J Epidemiol 1993;138:318–325. [DOI] [PubMed] [Google Scholar]
- 25.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull WHO 1997;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 26.Semba RD, Miotti PG, Chiphangwi JD, et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. Lancet 1994;343:1593–1597. [DOI] [PubMed] [Google Scholar]
- 27.Scholl TO, Hediger ML, Bendich A, Scholl JL, Smith WK, Krueger PM. Use of multivitamin/mineral prenatal supplements: influence on the outcome of pregnancy. Am J Epidemiol 1997;146:134–141. [DOI] [PubMed] [Google Scholar]
- 28.Semba RD, Miotti PG, Chiphangwi JD, et al. Infant mortality and maternal vitamin A deficiency during human immunodeficiency virus infection. Clin Infect Dis 1995;21:966–972. [DOI] [PubMed] [Google Scholar]
- 29.Tang AM, Graham NMH, Saah AJ. Effects of micronutrients intake on survival in human immunodeficiency virus type 1 infection. Am J Epidemiol 1996;143:1244–1256. [DOI] [PubMed] [Google Scholar]
- 30.Semba RD. Overview of the potential role of vitamin A in mother-to-child transmission of HIV-1. Acta Paediatrics 1997;421(Suppl):1075–1125. [DOI] [PubMed] [Google Scholar]
- 31.Beach RS, Mantero-Atienza E, Shor-Posner G, et al. Specific nutrient abnormalities in asymptomatic HIV-1 infection. AIDS 1992;6:701–708. [DOI] [PubMed] [Google Scholar]
- 32.Rolschau J, Date J, Kristoffersen K. Folic acid supplementation and intrauterine growth. Acta Obstet Gynecol Scand 1979;58:343–346. [DOI] [PubMed] [Google Scholar]