Abstract
Purpose:
To review the salient features of the diagnosis and management of the most common skin and soft tissue infections (SSTI). This review focuses on severe SSTIs that require care in an intensive care unit (ICU), including toxic shock syndrome, myonecrosis/gas gangrene, and necrotizing fasciitis.
Methods:
Guidelines, expert opinion, and local institutional policies were reviewed.
Results:
Severe SSTIs are common and their management complex due to regional variation in predominant pathogens and antimicrobial resistance patterns, as well as variations in host immune responses. Unique aspects of care for SSTIs in the ICU are discussed, including the role of prosthetic devices, risk factors for bacteremia, and the need for surgical consultation. SSTI mimetics, the role of dermatologic consultation, and the unique features of SSTIs in immunocompromised hosts are also described.
Conclusions:
We provide recommendations for clinicians regarding optimal SSTI management in the ICU setting.
Keywords: Skin and soft tissue infections in the intensive care unit, Necrotizing fasciitis, Gas gangrene
Introduction
Skin and soft tissue infections (SSTI), also known as acute bacterial skin and skin structure infections, are a common reason for patients seeking inpatient and out-patient medical care. In the US, SSTI are responsible for at least 14 million outpatient visits a year [1], and almost 900,000 inpatient admissions [2]. SSTI are similarly problematic across Europe, though with regional variation in predominant pathogens, antimicrobial resistance patterns, duration of hospitalization, and rates of readmission [3–7]. Pathogen isolation from SSTI is limited by currently available diagnostics and is influenced by host and geographic factors, making empiric therapy selection complicated [4, 8, 9]. In this review, we summarize the salient features of diagnosis and treatment of SSTI, with a particular focus on those necessitating management in intensive care settings.
Epidemiology of SSTI in the USA, Europe, and worldwide
Severity of illness due to SSTI loosely correlates with depth of skin structure involvement. Table 1 provides a summary of some of the common skin infections along with predominant pathogens, characteristic skin features, and requisite treatment. Any assessment of a patient with a possible SSTI should include assessment of immune status, exposure history (animals, water, trauma), and travel history to inform empiric antimicrobial decisions [9, 10]. Travel history is important for all patients with infections, as knowledge of local pathogens in recent travel destinations may be useful in directing empiric therapy. As an example, travelers to areas endemic for multidrug resistant (MDR) Enterobacteriaceae have an increased risk of acquisition of an MDR Enterobacteriaceae colonizer and patients who are colonized with carbapenem-resistant Enterobacteriaceae (CRE) have a 16.5 % risk of infection with CRE [11].
Table 1.
Skin and soft tissue infections—types, pathogens, features, and treatment
| Infection type | Predominant pathogens | Characteristic features | Treatment |
|---|---|---|---|
| Impetigo | Staphylococcus aureus, Streptococcus pyogenes | Honey-crusted lesions, less common bullous variant | PO penicillins, 1 st generation cephalosporins, or clindamycin |
| Ecthyma | S. aureus, S. pyogenes | Dry crusted lesions that involve the dermis and lead to scarring, predilection for the lower extremities | PO penicillins, 1 st generation cephalosporins, or clindamycin. If MRSA suspected, doxycycline, TMP-SMX, or clindamycin |
| Ecthyma gangrenosum | Pseudomonas aeruginosa, S. aureus, S. pyogenes, less commonly other Gram-negative rods, fungi, mold | Cutaneous vasculitis, typically seen between umbilicus and knees, have potential for rapid increases in size. Erythematous nodules that evolve into necrotic ulcers with eschar | Broad spectrum antibiotics, pathogen directed therapy when culture results available |
| Purulent SSTI—abscesses, furuncles, carbuncles | S. aureus | Pustules surrounded by erythema. Furuncles and carbuncles centered on hair follicles. May exhibit 5 cardinal signs of infection—calor, rubor, dolor, tumor, fluor | Incision and drainage. Antibiotic therapy for MRSA in patients meeting SIRS criteria or immunocompromised |
| Cellulitis | Beta-hemolytic streptococci, S. aureus | Diffuse, superficial spreading erythema. May be associated with lymphangitis | Mild: PO therapy directed against MSSA and streptococci. Moderate: PO or IV therapy directed against MSSA and streptococci. Severe: surgical consultation, broad spectrum antibiotics directed against MRSA, Pseudomonas, and anaerobes |
| Pyomyositis | S. aureus | Localized pain in a single muscle group with fever. Overlying skin may have”woody”feel | Surgical consultation, vancomycin. Addition of gram-negative agents if immunocompromised or penetrating trauma |
| Surgical site infections | Dependent on surgical site | Wound drainage, local inflammation | Surgical consultation, antimicrobials dependent on surgical site and severity of illness |
| Toxic shock syndrome | S. aureus, S. pyogenes, rarely other streptococci | Staphylococcal disease: erythroderma that starts on the trunk and spreads to extremities (including palms and soles). Streptococcal disease: scarlitinform rash may be seen | Vancomycin PLUS clindamycin for toxin production OR linezolid monotherapy (limited studies) |
| Gas gangrene/myonecrosis | Clostridium spp., C. perfringens—trauma related, C. septicum—non-traumatic | Bullae, crepitus | Immediate surgical consultation, broad spectrum agents—vancomycin PLUS piperacillin-tazo- bactam, an anti-pseudomonal carbapenem OR cefepime PLUS metronidazole |
| Necrotizing fasciitis | Polymicrobial aerobes and anaerobes (type 1), Group A streptococcus or S. aureus (type II) | Classic finding of pain out of proportion to exam. Spectrum from normal external appearance to woody feeling subcutaneous tissues with obliterated fascial planes/muscle groupings | Immediate surgical consultation, vancomycin or linezolid PLUS cefepime and metronidazole OR an anti-pseudomonal carbapenem OR pipera- cillin/tazobactam |
Impetigo and ecthyma
Impetigo is the most superficial of the SSTI and causes a mild illness that can be managed as an outpatient with topical or oral antibiotics directed against Staphylococcus aureus and Streptococcus pyogenes. The classic appearance is honey-crusted lesions, though a less common bullous form is also possible. Impetigo is primarily a disease of children, representing one of the most common SSTI in this group. Affected adults are typically those in contact with afflicted children [12]. Ecthyma is a scarring form of non-bullous impetigo which involves the dermis, predominantly on the lower extremities, and can be confused with ecthyma gangrenosum [12]. Impetigo or ecthyma due to S. pyogenes can result in post-streptococcal glomerulonephritis, with or without antimicrobial treatment [12].
Ecthyma gangrenosum
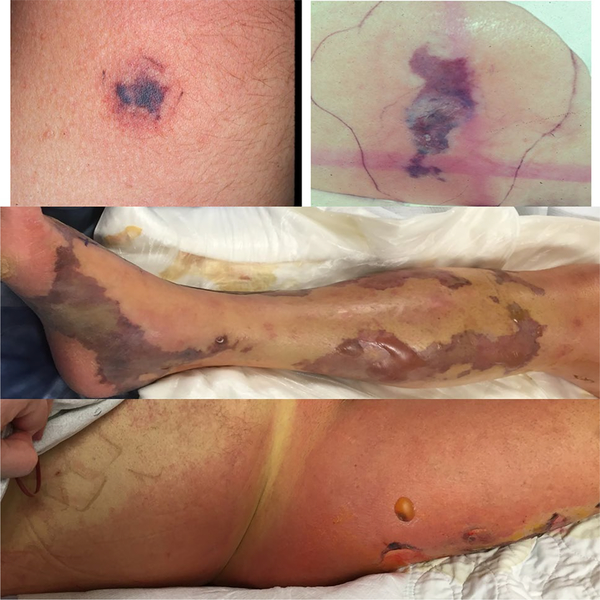
Ecthyma gangrenosum (see Fig. 1) is an uncommon necrotizing, hemorrhagic cutaneous vasculitis that is classically associated with Pseudomonas aeruginosa septicemia, though many other pathogens have been implicated, including other Gram-negative rods, S. aureus, S. pyogenes, fungi, molds, atypical mycobacteria, and viruses [12–14]. The lesions typically begin as painless erythematous nodules that evolve into painful necrotic ulcers with eschar. Necrosis results from invasion of the medial and adventitial blood vessel walls by the implicated microbe. A recent review found only 167 published cases of ecthyma gangrenosum from 1975 to 2014 [14], though this is likely an underestimate as not all cases are published. When suspected, patients with ecthyma gangrenosum should be given broad spectrum antimicrobials, particularly agents with anti-pseudomonal activity.
Fig. 1.
Severe skin and soft tissue infections—ecthyma gangrenosum secondary to Pseudomonas aeruginosa infection (top left and right— images courtesy of Dr. Arthur Z. Eisen) and necrotizing fasciitis of the lower extremity (middle and bottom) with retiform purpura, bullae formation, and spreading erythema
Purulent SSTI
Purulent SSTI—abscesses, furuncles, and carbuncles— are predominantly caused by S. aureus. Furuncles and carbuncles are centered on hair follicles. In the US in 2005, the combined category of abscess/cellulitis was responsible for ~10 million outpatient and emergency room visits, an increase from 1997 that correlated with an increasing frequency of community-acquired MRSA [1]. Polymicrobial infections are possible depending on the site of infection. Infections that include anaerobes are more likely in the cervical, pelvic, and lower extremity regions (particularly in those with peripheral vascular disease and/or diabetes). Enterobacteriaceae from the gut play a role in polymicrobial milieu of pelvic abscesses.
All critically ill patients with purulent SSTI require source control with incision and drainage. Source control of abscesses, furuncles, and carbuncles can be accomplished via surgical debridement or percutaneous drainage if the location is favorable without any interceding vital structures. The need for antibiotic therapy is dictated by severity of illness, which takes into consideration the immune status of the host and lab and clinical parameters. Antibiotic therapy is generally required for patients with severe illness, with empiric therapy dictated by anatomical site of the abscess and local antibiogram. Readers should refer to the treatment section of this manuscript for a discussion on grading SSTI severity in light of the recently updated sepsis definitions.
Cellulitis
Cellulitis is predominantly caused by beta-hemolytic streptococci (BHS), though staphylococci also play an important role [15]. The most common presentation is a superficial spreading erythema that may be associated with lymphangitis [15]. In its early stages, cellulitis may appear clinically similar to necrotizing skin and soft tissue infection. The two can be difficult to differentiate, but bullae, crepitus, renal failure, shock, lactic acidosis, progressive spread of infection despite appropriate antibiotics, and systemic toxicity are all suggestive of a necrotizing infection. In patients with cellulitis and no evidence of BHS infection, the vast majority nonetheless respond to therapy directed against BHS [15]. It is reassuring that most cases respond to treatment directed against BHS, as our ability to detect pathogens with currently available technology is still limited, despite our advanced molecular testing [8].
Similarly to abscesses, there is anatomical variation in predominant pathogens in cellulitis. In the cervical region, oral flora, including anaerobes, tend to be more problematic as in Ludwig’s angina. Infections in the pelvic region more commonly result from Enterobacteriaceae from the genitourinary or gastrointestinal tracts. Anaerobes and gram-negative organisms also play a role in cellulitis of the lower extremities, particularly in patients with diabetes or peripheral vascular disease.
Pyomyositis
Pyomyositis is classically thought of as a tropical disease, though the incidence in temperate climates is increasing. Staphylococcus aureus is the predominant pathogen, and certain staphylococcal genotypes may predominate [16]. Physical examination findings tend to be limited, but skin overlying affected muscle groups may have a “woody” feel. MRI is the imaging study of choice for diagnosis, though bedside ultrasound may be a useful rapid diagnostic [17, 18]. Source control and empiric antimicrobials directed against MRSA are paramount. Antimicrobials directed against MDR Gram-negative organisms should be added in immunocompromised patients.
Surgical site infections
Surgical site infections (SSI) are common, occurring after up to 9 % of operations depending on surgical site [19–21]. The likelihood of developing a SSI is dependent on multiple factors, including patient, hospital, surgeon, operation, and operation site characteristics, among others [21–23]. Risk prediction modeling may help individualize risk factors for SSI [24] and will hopefully guide future trials in discovery of interventions that can reduce the incidence of SSI. Pathogens implicated in surgical site infections vary by country and type of surgery [25]. Recent guidelines provide a helpful management algorithm for SSI [9]. Wound infection with systemic toxicity in the 4 days immediately following surgery should prompt consideration of streptococcal or clostridial infection, both of which require surgical management and penicillin PLUS clindamycin therapy [9]. Other infections within 4 days post-surgery are possible, though they tend to be less fulminant. In patients that are more than 4 days post-operative, antibiotic therapy along with surgical exploration should be considered in patients with signs of systemic illness and wounds with >5 cm of erythema or induration [9]. When SSI is present along with systemic toxicity, source control via surgery is essential, particularly in patients with infected mesh who are at higher risk of bacteremia [26]. In patients who are not systemically ill, opening the wound at bedside or via percutaneous drainage in combination with close attention to dressing changes may be sufficient for resolution without antibiotics.
ICU‑specific infections
Purulent SSTI, cellulitis, pyomyositis, and SSI can all result in severe illness, but the most severe end of the SSTI spectrum is composed of toxic shock syndrome, gas gangrene, and necrotizing fasciitis.
Toxic shock syndrome
Toxic shock syndrome (TSS) is a fulminant Gram positive infection, typically due to S. aureus or S. pyogenes, though small series have described similar syndromes in group B, C, and G streptococci, as well as Clostridium species. The annual incidence of staphylococcal TSS (SaTSS) is ~0.5/100,000 and ~0.4/100,000 for streptococcal TSS (SeTSS), though local rates may vary [27]. Mortality rates are <5 % for menstrual SaTSS, 5–22 % for non-menstrual SaTSS, and 30–70 % for SeTSS [27]. Clostridial toxic shock is rare and its incidence is uncertain [28, 29].
When TSS is suspected, empiric therapy must cover for drug-resistant infections. Expert opinion based on retrospective studies and in vitro data highlight vancomycin and clindamycin or linezolid alone as possible treatment regimens [30–33]. Nafcillin or oxacillin are good choices for methicillin-sensitive staphylococcal TSS, but must be used in combination with clindamycin as nafcillin alone can increase toxin production [32]. Clindamycin or linezolid are essential in treatment as they reduce superantigen production in both staphylococcal and streptococcal TSS [31–33]. When susceptibilities are available, antibiotics should be de-escalated while still including an agent that suppresses toxin production. In the rare event of clostridial toxic shock syndrome, clindamycin and penicillin should be used, though there is limited data on this syndrome.
Intravenous immunoglobulin (IVIG) nonspecifically binds and inactivates superantigens, limiting cytokine storm, though the clinical benefits are controversial. Due to the rarity of TSS, recruitment for randomized controlled trials of IVIG has been difficult [34]. A recent prospective observational study found a significantly improved mortality in patients that received IVIG or clindamycin for SeTSS [35]. IVIG is even less studied in SaTSS, though in a recent study, five patients with confirmed SaTSS received IVIG and none expired [36]. IVIG can be considered in patients with TSS, though specific dosing regimens have not been well studied.
Necrotizing skin and soft tissue infections: gas gangrene/ myonecrosis and necrotizing fasciitis
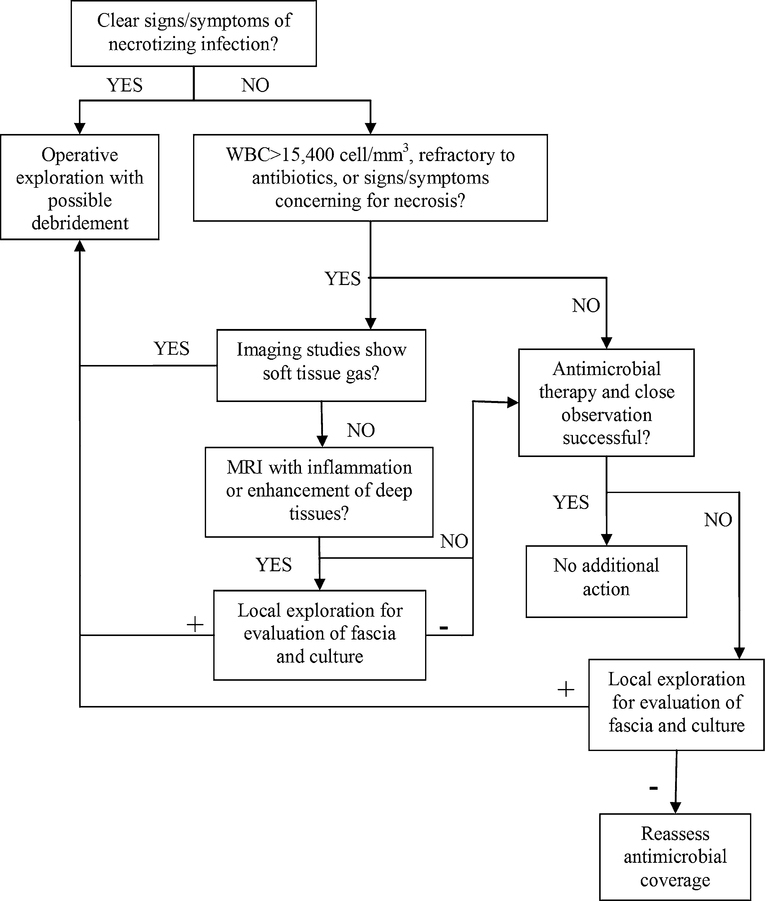
Necrotizing skin and soft tissue infections are difficult to treat and require aggressive surgical debridement, broad-spectrum antimicrobials, and intensive care. Tables 2, 3, and Fig. 2 provide guidance on factors associated with increased likelihood of necrotizing infection, common pathogens, and a proposed surgical decision tree. Source control of infection is paramount and serial surgical debridements are generally required. The frequency and number of required debridements varies based on aggressiveness of infection, but generally patients should return to the operating room for debridement every 24–48 h until there is no evidence of continued or progressive skin and soft tissue necrosis. Wound dressing changes should be carried out at least daily to look for evidence of ongoing infection (e.g. bullae, devitalized tissue, spreading erythema) that would require repeat debridement. In addition to wound appearance, clinical deterioration as measured by increased requirements for intensive care support or laboratory parameters suggestive of worsening infection (e.g. progressive renal failure, increasing leukocytosis, increasing lactate) should prompt discussion of repeat debridement.
Table 2.
Characteristics associated with increased likelihood of necrotizing infection
| Clinical parameters | Laboratory parameters |
|---|---|
| Pain out proportion to examination | Serum sodium <135 mmmol/L |
| Bullae | White blood cell count >15,400 cell/mm3 |
| Tenderness beyond area of erythema | Renal failure |
| Crepitus | Progressive lactic acidosis |
| Cutaneous anesthesia | |
| Cellulitis refractory to antibiotic therapy | |
| Rapid progression of cellulitis | |
| Dusky appearance of skin | |
| Systemic toxicity |
Table 3.
Necrotizing fasciitis—pathogens and treatments by anatomical site
| Anatomical location | Predominant pathogens | Empiric antimicrobial therapy |
|---|---|---|
| Head/neck | Anaerobes | Ampicillin/sulbactam usually sufficient, though MRSA coverage should be considered, particularly in immunosuppressed or IV drug abusers |
| Abdomen/perineal | Gram negative, anaerobes | Cefepime + metronidazole OR an anti-pseudomonal carbapenem OR piperacillin- tazobactam |
| Lower extremity | Gram negative, anaerobes, Gram positive | In MRSA prevalent areas vancomycin PLUS cefepime + metronidazole OR an anti- pseudomonal carbapenem OR piperacillin-tazobactam |
| Surgical site | Variable depending on surgical site | In addition to anatomic location pertinent antimicrobials, if not already included, MRSA coverage should be considered in regions with high incidence |
Fig. 2.
Proposed decision tree for surgical management of suspected necrotizing soft tissue infections
Surgical control of infection is particularly important because diffusion of antimicrobials into affected tissues is limited due to significant tissue edema, necrosis, inflammation, and penetrating vessel thromboses [37]. These anaerobic environments are particularly crucial for the proliferation of clostridial species in gas gangrene/ myonecrosis and anaerobes in type I necrotizing fasciitis. Additionally, bacteria can invade blood vessel walls and result in direct vascular injury that worsens tissue perfusion. In type II necrotizing fasciitis, streptococcal superantigens result in cytokine cascades that cause systemic vasodilation and inflammation, leading to tissue hypoxia that precludes effective tissue antimicrobial concentrations.
Gas gangrene/myonecrosis
Gas gangrene or myonecrosis is caused by Clostridium species. Clostridium perfringens is classically associated with traumatic injuries; C. septicum with neutropenic patients or those with gastrointestinal malignancies or abnormalities; C. sordellii with childbirth and “home” abortions; and C. perfringens, C. novyi, and C. sordellii with drug users who “skin pop” [38–41]. Gas gangrene and myonecrosis are primarily surgical diseases and should be managed emergently as such, in combination with broad-spectrum antibiotics while awaiting culture results. Clostridium sordellii infections are relatively rare, with only 45 cases found on review of the literature in 2006 [41]. Though rare, C. sordellii infections are notable as they can be associated with a toxic-shock like syndrome, particularly in patients with recent parturition or abortion [28, 29, 42]. The toxic shock syndrome associated with clostridial infection is mediated by two clostridial cytotoxins, making it pathophysiologically dissimilar to streptococcal or staphylococcal toxic shock, both of which are mediated by superantigens [28, 29, 42].
Necrotizing fasciitis
Necrotizing fasciitis (see Fig. 1) is a rare SSTI that involves the deep fascia and always requires surgical intervention and broad-spectrum intravenous antimicrobials. Rates of necrotizing fasciitis vary widely based on region (0.18–15.5 per 100,000) and seem to be increasing over time [43, 44]. Type I necrotizing fasciitis is polymicrobial, including aerobic and anaerobic organisms. Type II necrotizing fasciitis is classically caused by S. pyogenes, though S. aureus also falls into this category.
Two rare causes of necrotizing fasciitis merit special mention due to their well described exposure histories— Vibrio vulnificus and Aeromonas hydrophila. Vibrio vulnificus is a cause of necrotizing fasciitis in patients with exposure to warm coastal waters (particularly the Gulf of Mexcio), penetrating injuries from seafood, or ingestion of uncooked/undercooked seafood. Once identified in culture, V. vulnificus is best treated with doxycycline and ceftriaxone or cefotaxime. Aeromonas hydrophila necrotizing fasciitis occurs after exposure of wounds to fresh or brackish water or contaminated soil. Leech use can also result in A. hydrophila infections. Treatment is typically doxycycline PLUS ciprofloxacin, though ciprofloxacin resistance has been reported, which may necessitate empiric cefepime use while awaiting susceptibilities (Table 1 Electronic Supplementary Material).
There are a variety of case reports and case series of less frequently encountered agents causing necrotizing fasciitis, making it important for practitioners to realize the importance of surgical debridement with attendant bacterial cultures in combination with broad-spectrum antimicrobials as the first lines of therapy [45, 46]. Though the classic teaching for necrotizing fasciitis is pain out proportion to physical examination findings, it is important to remember that superficial nerves can undergo necrosis, resulting in anesthesia of affected areas. Eliciting a history may be problematic due to the severity of illness and alterations in sensorium, requiring maintenance of a high degree of suspicion for necrotizing SSTI. Due to unacceptably low sensitivity, imaging findings cannot rule out necrotizing fasciitis and may delay surgical intervention, which is associated with poor outcomes [47]. However, in patients that are clinically stable, MRI may be helpful in distinguishing necrotizing infection from non-necrotizing infection [48].
Necrotizing fasciitis predominates on the lower extremity and predisposing conditions reflect this localization—diabetes, abnormalities of venous return or arterial insufficiency, and intravenous drug use. Due to the relative rarity, heterogeneity of microbiologic causes, and severity of disease, no clinical trials are available to guide duration of therapy, though guidelines based on expert opinion suggest continuation of therapy directed against cultured organisms for at least 48–72 h after patients are clinically stable and require no further operative interventions [9].
Bacteremia
The probability of bacteremia in patients with SSTI is greater in those with device or prosthesis infection, having healthcare exposure, and more advanced age [26]. Risk scores can be used to help predict those patients at highest risk of bacteremia with SSTI (Table 2 Electronic Supplementary Material) [26]. Of ICU acquired secondary bacteremia, SSTI are implicated as the source in ~4 % [4]. In general, blood cultures are not recommended for patients with SSTI, but critically ill and immunocompromised patients with SSTI should have blood cultures performed due to the increased yield.
Role of prosthetic materials
Knowledge of surgical history and presence of any prosthetic materials is important for all patients requiring intensive care as prosthetic materials increase the risk for infection. Patients with synthetic mesh placed after abdominal surgeries have a higher risk of subsequent SSI [49]. Ventricular assist devices are associated with high rates of infections, the majority of which are driveline infections that present as SSTI at the driveline exit site [50]. Similarly, cardiac implantable electronic devices (CIED) can present with SSTI at the device site. Rates of CIED infections vary based on device type, but in general seem to be increasing in incidence [51]. In addition to septicemia, short and long term intravascular catheters can present with cellulitis or abscesses at insertion sites or along the tract of the catheter. Updated guidelines are in progress, but in general, it is preferable for patients with catheter infections to undergo removal if at all possible [52].
Dermatologic findings and dermatology consultants
Dermatology consultation can be an important tool to help with the diagnosis of dermatologic findings in critically ill patients and reduce antimicrobial use in those with non-infectious conditions [53, 54]. Many dermatologic conditions can mimic infections, for which dermatologist expertise can be helpful in distinguishing; including pyoderma gangrenosum and pustular psoriasis, among others (Table 4) [55]. Pyoderma gangrenosum should be suspected in cases where non-healing wounds undergo progressive necrosis with each debridement, particularly in patients with associated autoimmune conditions or malignancy. Dermatologic findings can be present in up to 42 % of patients requiring ICU care, though they are the primary or secondary diagnosis for ICU admission in only ~0.5 % [56, 57]. Infections are the predominant etiology of skin changes in the ICU, with regional variations in the most common pathogens [56–59]. Cutaneous manifestations as a whole do not confer an increased risk of mortality in ICU patients when adjusted for severity of illness [56].
Table 4.
SSTI mimetics
| Syndrome | Cause(s) | Characteristic features |
|---|---|---|
| Pyoderma gangrenosum | Autoimmune conditions, hematologic malignancies, idiopathic in up to 50 % | Tender papulopustules with surrounding erythema that develop into ulcers with purulent base.Typically on lower extremities, tend to be painful with undermined edges. Heaped up borders with gun-metal gray color. Necrosis extends with surgical intervention |
| Lymphedema/chronic venous stasis | Multiple—cardiac dysfunction, renal failure, lymph node surgeries, lymph node destruction (filariasis), varicose veins | In volume overload states, often symmetric and bilateral |
| Deep venous thrombosis | Thrombosis | Can be hard to differentiate on exam alone, may be suggested by history—i.e. prolonged immobility, genetic risk factors |
| Loxoscelism | Brown recluse spider bite | Retiform purpura |
Treatment
Surgical and general considerations
For all patients with SSTI requiring ICU care, general resuscitative measures should be followed in accordance with individual institutions’ protocols for management of sepsis and septic shock. Aggressive source control is paramount, which may include surgical debridement, removal of invasive devices, or vaginal examination in the case of menstrual TSS. Urgent surgical consultation and debridement may be required. For necrotizing SSTI, serial debridements every 24–48 h are necessary until there is no evidence of continued necrosis and clinical stability has been achieved. In all cases of necrotizing soft tissue infections, one of the goals of surgery should be to seek out portals of entry for bacteria that could have established the infection, either from indwelling devices, the external environment/foreign bodies, or other organs (e.g. gastrointestinal or genitourinary systems). Pro-longed time from presentation to first surgical intervention are associated with increased mortality [47]. Delays in diagnosis of necrotizing soft tissue infections were felt to be one of the highest impact risk factors for delayed time to surgical intervention in a recent survey of ICU practitioners in Europe [60]. In conjunction with serial debridements, vacuum assisted closure of wounds may contribute to healing [61]. For cases of necrotizing infection involving the perineum or other sites with potential for stool contamination, temporary colostomy may be required to assist in wound healing. Rates of amputation in lower extremity necrotizing fasciitis vary from 15 to 72 % based on comorbidities, with diabetes being a strong risk factor for amputation [62]. While potentially life-saving, it is important to recognize that amputations, among other factors, may be associated with significant functional limitations after discharge [63].
Antimicrobial considerations
SSTI in patients that are immunocompromised should be treated with broad-spectrum antibiotic therapy [9]. In the most recent IDSA guidelines, the presence of any SIRS criteria resulted in classification as a severe SSTI [9]. The recently released update of sepsis definitions has not yet been studied for or incorporated into SSTI management [64]. In the face of the new sepsis definitions, a prudent approach would be to define SSTI as severe if the patient meets either of the following criteria: (1) ICU patients with an acute change in Sequential Organ Failure Assessment (SOFA) score ≥2 points due to infection, (2) non-ICU patients meeting 2/3 quick SOFA (qSOFA) criteria (altered mental status, systolic blood pressure ≤100 mmHg, or respiratory rate ≥22/min) [64]. Patients without baseline organ dysfunction can be assumed to have a baseline SOFA score of 0 [64].
As a general rule, all severe SSTI should be treated empirically with broad-spectrum antibiotics directed against typical pathogens, specifically MRSA, resistant Gram-negatives, and anaerobes (see Table 3 for a breakdown by anatomic site). However, when selecting empiric therapy, all practitioners should consider their local antibiograms as these can vary significantly from institution to institution. In regions such as Northern Europe with low rates of MRSA [65], it may be prudent to exclude MRSA coverage from empiric therapy in patients at low risk of MRSA infections. Risk factors for mixed grampositive and gram-negative SSTI include admission to an ICU, residence in a nursing home, and SSTI other than an abscess [66]. Reasonable empiric therapies meeting these criteria include vancomycin or linezolid PLUS piperacillin/tazobactam, meropenem or imipenem, or cefepime PLUS metronidazole.
De-escalation of antibiotic therapy should be based on clinical improvement and cultured pathogens from blood or surgical specimens. Once patients have improved and are ready for discharge, transition to oral antibiotic therapy is possible, though non-adherence to prescribed antibiotics is common and is a risk factor for treatment failure [67].
Dosing and caveats for selected antimicrobials
A full dosing algorithm for all antibiotics is outside the scope of this review, but below we provide information on some of the most commonly used empiric antimicrobials relevant for severe SSTI and caveats for certain drugs.
Ceftaroline
The USFDA approved dose of ceftaroline is 600 mg intravenously (IV) Q12 h for patients with normal creatinine, though a dose escalation strategy for patients with severe infections or those with BMI >40 or >100 kg may be beneficial [68, 69]. Practitioners should be aware that the duration of ceftaroline therapy seems to correlate with risk of neutropenia, with rates as high as 21 % reported in those patients receiving ≥21 days of therapy [70].
Cefepime
For severe, life threatening infections, or in those with morbid obesity, we use an increased dose of cefepime, based on CrCl [68].
Clindamycin
Clindamycin can be used to reduce toxin production, treat cervical cellulitis/abscesses, and may be used as step down therapy for susceptible S. aureus strains. Practitioners should be aware that clindamycin increases the risk of subsequent Clostridium difficile infection.
Dalbavancin/Oritavancin
Dalbavancin and oritavancin are long-acting semisynthetic lipoglycopeptides with approval for SSTIs that cover a wide range of gram-positive organisms. However, further studies are needed before their use can be recommended in critically ill patients.
Daptomycin
For patients >120 % of their ideal body weight, we used an adjusted body weight to dose daptomycin, rounded to the nearest 25 mg [68]. Doses are adjusted for CrCl <30 mL/ min and in those on intermittent hemodialysis [68, 71]. Daptomycin use may be contraindicated in some patients with necrotizing fasciitis if their CK is above five times the upper limit of normal.
Linezolid
Caution is advised as use of linezolid for MRSA bacteremia may be associated with worse outcomes in patients with APACHE II scores ≥14 [72]. Use is also not recommended in patients on serotonin reuptake inhibitors due to the risk of serotonin syndrome. Tidezolid may be an alternative to linezolid and as it has been reported to have less risk of serotonin syndrome, though clinical data are still limited [73].
Telavancin
Telavancin is a lipoglycopeptide that blocks peptidoglycan cross-linking and disrupts bacterial cell membrane potential. It is associated with higher rates of toxicity than other available agents for SSTI and we therefore do not recommend its use when other agents can be employed.
Tigecycline
Though approved for SSTIs, tigecycline has been linked with worse outcomes in patients with severe illness. Tigecycline may also be a risk factor for treatment failure in patients with drug resistant infections. As such, we recommend avoiding tigecycline therapy when other options are available.
Vancomycin
For all patients, we use an initial dosing regimen of 15 mg/kg of vancomycin, with a maximum single dose of 2.25 g, and a maximum daily dose of 4.5 g [68]. Use of vancomycin alternatives is favored (e.g. ceftaroline, daptomycin, linezolid) in patients with progressive renal failure, those with CrCl <30 mL/min, or in those who therapeutic levels cannot be rapidly achieved. An in depth discussion of therapeutic vancomycin level maintenance is outside the scope of this review and practitioners should refer to their institutional protocols.
Special considerations
There are several special considerations in SSTI that merit further mention. Certain exposures put patients at risk for unusual pathogens as causes of SSTI. Table 1 in the Electronic Supplementary Material and Table 5 mention some of these, but a complete listing is outside the scope of this review, particularly as most are rare and not associated with severe illness. Table 5 includes several endemic mycoses, which Europeans are most likely to acquire as a result of travel [74]. Readers should refer to reviews on exposure-related causes of SSTI for rare pathogens. Of particular importance due to the increasing prevalence of immunosuppressed patients, special considerations in the immunocompromised host are detailed below.
Table 5.
Severe infections with skin manifestations
| Pathogen/disease entity | Epidemiologic clues | Skin findings |
|---|---|---|
| Bacterial | ||
| Rickettsia rickettsii/rocky mountain spotted fever | Late spring to early fall. Travel to United States predominantly southeast of Rocky Mountains, Central America, South America | Typically appears between day 3 and 6 of illness. Erythematous macules on wrists and ankles that spread centripetally, but spare the face. Includes palms/soles. May also see petechiae that develop into purpura |
| Francisella tularensis/tularemia | Rabbit, tick, or deer fly exposure. Travel to US, Eastern Europe, China, Japan | No skin findings in most severe typhoidal form. In ulceroglandular form, can see ulcer at site of tick bite with associated regional lymphadenopathy |
| Yersinia pestis/bubonic plague | Flea or rodent exposure.Travel to Southeast Asia, Western/Southwestern United States, South America, predominantly Southeast Africa including Madagascar, but also Libya and Algeria | Bubonic: inoculation site may have pustule or ulcer. Painful regional lymphadenopathy with suppuration and discharge from lymph nodes. Septicemic: vesicles, carbuncles, petechiae, and purpura all possible |
| Neisseria meningitidis/meningococcemia | Worldwide distribution, most cases in winter and spring. Patients with asplenia or terminal complement deficiency | Petechiae that may progress to retiform purpura and ischemic necrosis. Bullous hemorrhagic lesions also possible |
| Mycobacterial | ||
| Mycobacterium tuberculosis/miliary TB | Travel or residence in TB endemic areas | Small blue/red papules topped by vesicles that develop umbilication and crust formation |
| Viral | ||
| Variola major virus/Smallpox | Agent of bioterror | Synchronous firm, deep-seated, well-circumscribed vesiculo-pustules. Car involve palms and soles, though tend to be concentrated on face/limbs |
| Varicella zoster virus | Immunocompromised hosts more likely to have disseminated disease | Multi-dermatome asynchronous vesicles, can have hemorrhagic and purpuric lesions |
| Fungal/mold | ||
| Aspergillus | Immunocompromised hosts | Necrotic papulonodules, nodules |
| Candidiasis | Immunocompromised hosts | Multiple possibilities including ecthyma gangrenosum, firm erythematous papules or nodules with pale or hemorrhagic centers |
| Mucormycoses | Immunocompromised hosts | Ecthyma gangrenosum, necrotic papulonodules, hemorrhagic crusts |
| Cryptococcus | Bird dropping exposure | Umbilicated papules (similar in appearance to molluscum contagiosum) |
| Histoplasmosis | Travel to US, Central or South America, Africa. Bird dropping exposure, activities that aerosolize soil, chicken coop exposure, spelunkers | Variable: Oral ulcers, mucocutaneous erosions or ulcers, erythematous papules or nodules with scale or crust |
| Blastomycosis | Travel to US or Canada with spore inhalation from soil | Papulopustules and verrucous plagues with scale/crust. Advanced disease may mimic pyoderma gangrenosum |
| Trichosporon | Immunocompromised hosts | Papulovesicles, purpura, necrotic papulonodules |
| Fusariosis | Immunocompromised hosts | Often has a periungual focus. Multiple possibilities: umbilicated or necrotic papules, pustules, subcutaneous nodules, ecthyma gangrenosum |
| Penicilliosis | Southeast Asia, China | Umbilicated papules (similar in appearance to molluscum contagiosum). Can also see necrotic nodules, acneiform lesions.Typically involves face, trunk, arms |
| Parasitic | ||
| Strongyioides stercoraiis | Worldwide, particularly tropical areas. Can occur decades after exposure if host becomes immunosuppressed | Localized perianal urticarial possible, but can involve thighs, abdomen (larva currens). Can also see retiform purpura |
Immunocompromised hosts
Immunodeficiency changes the physical examination findings of SSTI, the putative pathogens, and the diagnostic and treatment plans. In addition to the SSTI mimetics in Table 4, the differential diagnosis for dermatologic findings in the immunocompromised host should include drug eruptions (especially patients on chemotherapy), skin metastases, local invasion of tumor burden, leukocytoclastic vasculitis, graft-versus-host disease in stem cell transplant patients, and a broader infectious differential including invasive fungal and mold infections, mycobacterial infections, and parasitic infections such as disseminated strongyloidiasis [9]. Given the broader differential diagnosis and greater potential for decompensation, early dermatologic consultation with biopsy and culture may be beneficial [9, 53]. All immunocompromised patients that are critically ill should undergo thorough cutaneous examination as immunosuppression tends to reduce physical exam findings of SSTI. Immunosuppressed patients are also more likely to have dissemination of pathogens to the skin.
A unique situation to consider in generation of a differential diagnosis for immunosuppressed patients is the use of anti-infective prophylaxis, which can affect the types and resistance profiles of potential pathogens. Immunosuppressed patients may be reservoirs for the development of antimicrobial resistance. As is important for all patients with SSTI, travel and exposure history can guide differential diagnosis and workup for immunocompromised hosts. When possible, reduction of immunosuppression should be considered for severe infections. For patients with febrile neutropenia, MASCC score is important for predicting complication rates [75]. The types of pathogens are also dependent on the type of immunosuppression—cell mediated versus neutropenia. In neutropenic patients, factors to consider when contemplating surgery are probable duration of neutropenia and severity of infection. Patients with shorter durations of neutropenia have a higher likelihood of recovering from surgical interventions and are likely better candidates for surgery. Unfortunately, patients with prolonged duration of neutropenia and severe infections have poor prognoses. However, data are limited on management of necrotizing soft tissue infections in neutropenic patients, and strategies should be individualized on a case-by-case basis. There is insufficient evidence to recommend for or against granulocyte transfusions in this population.
Conclusion
Skin and soft tissue infections have a variety of presentations and can be severe enough to require intensive care. Practitioners should be familiar with the spectrum of clinical presentations for SSTI that require urgent surgical debridement to avoid delays in surgery as this can lead to worsened outcomes. Aggressive source control and broad spectrum antimicrobials are essential for all severe SSTI, with empiric therapy guided by knowledge of patient risk factors and the local antibiogram.
Supplementary Material
Footnotes
Compliance with ethical standards Conflicts of interest
Dr. J.P. Burnham has no conflicts of interest to report. Dr. J.P. Kirby has no conflicts of interest to report. Dr. Kollef was supported by the Barnes-Jewish Hospital Foundation.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134–016-4576–0) contains supplementary material, which is available to authorized users.
References
- 1.Hersh AL, Chambers HF, Maselli JH, Gonzales R (2008) National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 168:1585–1591 [DOI] [PubMed] [Google Scholar]
- 2.Edelsberg J, Taneja C, Zervos M, Haque N, Moore C, Reyes K, Spalding J, Jiang J, Oster G (2009) Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 15:1516–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR (2007) Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 57:7–13 [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control (2013) Annual Epidemiological Report 2012 Reporting on 2010 surveillance data and 2011 epidemic intelligence data. ECDC, Stockholm [Google Scholar]
- 5.Itani KMF, Sorensen S, Stokes M, Shelbaya A, McKinnon P (2009) A regional comparison of resource utilization in patients with methicillin-resistant Staphylococcus aureus (MRSA) complicated skin and soft tissue infections (CSSTI) treated with linezolid vs vancomycin. In: [Presentation O-1791] 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco [Google Scholar]
- 6.Mullins CD, Yang K, Onukwugha E, Eisenberg DF, Myers DE, Huang DB, Lodise T (2013) Rehospitalizations and direct medical costs for cSSSI: linezolid versus vancomycin. Am J Pharm Benefits 5:258–267 [Google Scholar]
- 7.Garau J, Ostermann H, Medina J, Avila M, McBride K, Blasi F (2013) Current management of patients hospitalized with complicated skin and soft tissue infections across Europe (2010–2011): assessment of clinical practice patterns and real-life effectiveness of antibiotics from the REACH study. Clin Microbiol Infect 19:E377–E385 [DOI] [PubMed] [Google Scholar]
- 8.Crisp JG, Takhar SS, Moran GJ, Krishnadasan A, Dowd SE, Finegold SM, Summanen PH, Talan DA (2015) Inability of polymerase chain reaction, pyrosequencing, and culture of infected and uninfected site skin biopsy specimens to identify the cause of cellulitis. Clin Infect Dis 61:1679–1687 [DOI] [PubMed] [Google Scholar]
- 9.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC (2014) Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59:e10–e52 [DOI] [PubMed] [Google Scholar]
- 10.Stevens DL (2015) Reply to Gonzalez del Castillo et al and Rashid and Kravitz. Clin Infect Dis 60:172–174 [DOI] [PubMed] [Google Scholar]
- 11.Tischendorf J, de Avila RA, Safdar N (2016) Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control 44:539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolognia JL, Jorizzo J, Schaffer JV (2012) Dermatology. Elsevier Saunders, Philadelphia [Google Scholar]
- 13.Reich HL, Fadeyi DW, Naik NS, Honig PJ, Yan AC (2004) Nonpseudomonal ecthyma gangrenosum. J Am Acad Dermatol 50:S114–S117 [DOI] [PubMed] [Google Scholar]
- 14.Vaiman M, Lazarovitch T, Heller L, Lotan G (2015) Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis 34:633–639 [DOI] [PubMed] [Google Scholar]
- 15.Jeng A, Beheshti M, Li J, Nathan R (2010) The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine 89:217–226 [DOI] [PubMed] [Google Scholar]
- 16.Garcia C, Hallin M, Deplano A, Denis O, Sihuincha M, de Groot R, Gotuzzo E, Jacobs J (2013) Staphylococcus aureus causing tropical pyomyositis, Amazon Basin, Peru. Emerg Infect Dis 19:123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theodorou SJ, Theodorou DJ, Resnick D (2007) MR imaging findings of pyogenic bacterial myositis (pyomyositis) in patients with local muscle trauma: illustrative cases. Emerg Radiol 14:89–96 [DOI] [PubMed] [Google Scholar]
- 18.Kumar MP, Seif D, Perera P, Mailhot T (2014) Point-of-care ultrasound in diagnosing pyomyositis: a report of three cases. J Emerg Med 47:420–426 [DOI] [PubMed] [Google Scholar]
- 19.Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, Hebert L, Newhouse JP, Weiler PC, Hiatt H (1991) The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med 324:377–384 [DOI] [PubMed] [Google Scholar]
- 20.Mangram AJ, Horan TC, Pearson ML, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20:250–278 (quiz 279–280) [DOI] [PubMed] [Google Scholar]
- 21.Wilson J, Ramboer I, Suetens C (2007) Hospitals in Europe Link for Infection Control through Surveillance (HELICS). Inter-country comparison of rates of surgical site infection–opportunities and limitations. J Hosp Infect 65(Suppl 2):165–170 [DOI] [PubMed] [Google Scholar]
- 22.Winfield RD, Reese S, Bochicchio K, Mazuski JE, Bochicchio GV (2016) Obesity and the risk for surgical site infection in abdominal surgery. Am Surg 82:331–336 [PubMed] [Google Scholar]
- 23.Pedroso-Fernandez Y, Aguirre-Jaime A, Ramos MJ, Hernandez M, Cuervo M, Bravo A, Carrillo A (2016) Prediction of surgical site infection after colorectal surgery. Am J Infect Control 44:450–454 [DOI] [PubMed] [Google Scholar]
- 24.Olsen MA, Nickel KB, Margenthaler JA, Fox IK, Ball KE, Mines D, Wallace AE, Colditz GA, Fraser VJ (2016) Development of a risk prediction model to individualize risk factors for surgical site infection after mastectomy. Ann Surg Oncol 23:2471–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Centre for Disease Prevention and Control (2013) Surveillance of surgical site infections in Europe 2010–2011. ECDC, Stockholm [Google Scholar]
- 26.Lipsky BA, Kollef MH, Miller LG, Sun X, Johannes RS, Tabak YP (2010) Predicting bacteremia among patients hospitalized for skin and skin-structure infections: derivation and validation of a risk score. Infect Control Hosp Epidemiol 31:828–837 [DOI] [PubMed] [Google Scholar]
- 27.Burnham JP, Kollef MH (2015) Understanding toxic shock syndrome. Intensive Care Med 41:1707–1710 [DOI] [PubMed] [Google Scholar]
- 28.Cohen AL, Bhatnagar J, Reagan S, Zane SB, D’Angeli MA, Fischer M, Killgore G, Kwan-Gett TS, Blossom DB, Shieh WJ, Guarner J, Jernigan J, Duchin JS, Zaki SR, McDonald LC (2007) Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet Gynecol 110:1027–1033 [DOI] [PubMed] [Google Scholar]
- 29.Fischer M, Bhatnagar J, Guarner J, Reagan S, Hacker JK, Van Meter SH, Poukens V, Whiteman DB, Iton A, Cheung M, Dassey DE, Shieh WJ, Zaki SR (2005) Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med 353:2352–2360 [DOI] [PubMed] [Google Scholar]
- 30.Stevens DL, Wallace RJ, Hamilton SM, Bryant AE (2006) Successful treatment of staphylococcal toxic shock syndrome with linezolid: a case report and in vitro evaluation of the production of toxic shock syndrome toxin type 1 in the presence of antibiotics. Clin Infect Dis 42:729–730 [DOI] [PubMed] [Google Scholar]
- 31.Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R (2014) Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis 59:358–365 [DOI] [PubMed] [Google Scholar]
- 32.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE (2007) Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 195:202–211 [DOI] [PubMed] [Google Scholar]
- 33.Coyle EA, Cha R, Rybak MJ (2003) Influences of linezolid, penicillin, and clindamycin, alone and in combination, on streptococcal pyrogenic exotoxin a release. Antimicrob Agents Chemother 47:1752–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darenberg J, Ihendyane N, Sjolin J, Aufwerber E, Haidl S, Follin P, Andersson J, Norrby-Teglund A (2003) Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, doubleblind, placebo-controlled trial. Clin Infect Dis 37:333–340 [DOI] [PubMed] [Google Scholar]
- 35.Linner A, Darenberg J, Sjolin J, Henriques-Normark B, Norrby-Teglund A (2014) Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis 59:851–857 [DOI] [PubMed] [Google Scholar]
- 36.Matsushima A, Kuroki Y, Nakajima S, Sakai T, Kojima H, Ueyama M (2015) Low level of TSST-1 antibody in burn patients with toxic shock syndrome caused by methicillin-resistant Staphylococcus aureus. J Burn Care Res 36:e120–e124 [DOI] [PubMed] [Google Scholar]
- 37.Umbert IJ, Winkelmann RK, Oliver GF, Peters MS (1989) Necrotizing fasciitis: a clinical, microbiologic, and histopathologic study of 14 patients. J Am Acad Dermatol 20:774–781 [DOI] [PubMed] [Google Scholar]
- 38.Bangsberg DR, Rosen JI, Aragon T, Campbell A, Weir L, Perdreau-Remington F (2002) Clostridial myonecrosis cluster among injection drug users: a molecular epidemiology investigation. Arch Intern Med 162:517–522 [DOI] [PubMed] [Google Scholar]
- 39.Ahmed S, Gruer L, McGuigan C, Penrice G, Roberts K, Hood J, Redding P, Edwards G, Black M, McFarlane J, Cromie D, Howie H, Leonard A, Goldberg D, Taylor A, Hutchinson S, Roy K, Wadd S, Andraghetti R, Barry J, Sayers G, Cronin M, O’Connell T, Ward M, O’Sullivan P, O’Herlihy B, Keenan E, O’Connor J, Mullen L, Sweeney B, O’Flanagan D, Igoe D, Bergin C, O’Briain S, Keane C, Mulvihill E, Plunkett P, McMahon G, Boyle T, Clarke S, Leen E, Cassidy M, Djuretic T, Gill N, Hope V, Jones J, Nichols G, Weild A, George R, Borriello P, Brazier J, Salmon J, Lightfoot N, Roberts A (2000) Update: Clostridium novyi and unexplained illness among injecting-drug users-Scotland, Ireland, and England, April–June 2000. MMWR Morb Mortal Wkly Rep 49:543–545 [PubMed] [Google Scholar]
- 40.Kimura AC, Higa JI, Levin RM, Simpson G, Vargas Y, Vugia DJ (2004) Out-break of necrotizing fasciitis due to Clostridium sordellii among black-tar heroin users. Clin Infect Dis 38:e87–e91 [DOI] [PubMed] [Google Scholar]
- 41.Aldape MJ, Bryant AE, Stevens DL (2006) Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin Infect Dis 43:1436–1446 [DOI] [PubMed] [Google Scholar]
- 42.Sinave C, Le Templier G, Blouin D, Leveille F, Deland E (2002) Toxic shock syndrome due to Clostridium sordellii: a dramatic postpartum and post-abortion disease. Clin Infect Dis 35:1441–1443 [DOI] [PubMed] [Google Scholar]
- 43.Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, Lyon JL (2006) Cellulitis incidence in a defined population. Epidemiol Infect 134:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das DK, Baker MG, Venugopal K (2011) Increasing incidence of necrotizing fasciitis in New Zealand: a nationwide study over the period 1990–2006. J Infect 63:429–433 [DOI] [PubMed] [Google Scholar]
- 45.Shaked H, Samra Z, Paul M, Madar-Shapiro L, Cohen J, Pitlik S, Bishara J (2012) Unusual “flesh-eating” strains of Escherichia coli. J Clin Microbiol 50:4008–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng NC, Yu YC, Tai HC, Hsueh PR, Chang SC, Lai SY, Yi WC, Fang CT (2012) Recent trend of necrotizing fasciitis in Taiwan: focus on monomicrobial Klebsiella pneumoniae necrotizing fasciitis. Clin Infect Dis 55:930–939 [DOI] [PubMed] [Google Scholar]
- 47.Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO (2003) Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 85–a:1454–1460 [PubMed] [Google Scholar]
- 48.Kim KT, Kim YJ, Won Lee J, Kim YJ, Park SW, Lim MK, Suh CH (2011) Can necrotizing infectious fasciitis be differentiated from nonnecrotizing infectious fasciitis with MR imaging? Radiology 259:816–824 [DOI] [PubMed] [Google Scholar]
- 49.El-Gazzaz GH, Farag SH, El-Sayd MA, Mohamed HH (2012) The use of synthetic mesh in patients undergoing ventral hernia repair during colorectal resection: risk of infection and recurrence. Asian J Surg 35:149–153 [DOI] [PubMed] [Google Scholar]
- 50.Nienaber JJ, Kusne S, Riaz T, Walker RC, Baddour LM, Wright AJ, Park SJ, Vikram HR, Keating MR, Arabia FA, Lahr BD, Sohail MR (2013) Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 57:1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB, Bolger AF, Estes NA 3rd, Gewitz M, Newburger JW, Schron EB, Taubert KA (2010) Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 121:458–477 [DOI] [PubMed] [Google Scholar]
- 52.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strazzula L, Cotliar J, Fox LP, Hughey L, Shinkai K, Gee SN, Kroshinsky D (2015) Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol 73:70–75 [DOI] [PubMed] [Google Scholar]
- 54.Arakaki RY, Strazzula L, Woo E, Kroshinsky D (2014) The impact of dermatology consultation on diagnostic accuracy and antibiotic use among patients with suspected cellulitis seen at outpatient internal medicine offices: a randomized clinical trial. JAMA Dermatol 150:1056–1061 [DOI] [PubMed] [Google Scholar]
- 55.Falagas ME, Vergidis PI (2005) Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 142:47–55 [DOI] [PubMed] [Google Scholar]
- 56.Agrawal P, Peter JV, George R (2013) Dermatological manifestations and relationship to outcomes of patients admitted to a medical intensive care unit: a study from a tertiary care hospital in India. Postgrad Med J 89:501–507 [DOI] [PubMed] [Google Scholar]
- 57.George SM, Harrison DA, Welch CA, Nolan KM, Friedmann PS (2008) Dermatological conditions in intensive care: a secondary analysis of the Intensive Care National Audit and Research Centre (ICNARC) Case Mix Programme database. Crit Care 12(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emre S, Emre C, Akoglu G, Demirseren DD, Metin A (2013) Evaluation of dermatological consultations of patients treated in intensive care unit. Dermatology 226:75–80 [DOI] [PubMed] [Google Scholar]
- 59.Badia M, Servia L, Casanova JM, Montserrat N, Vilanova J, Vicario E, Rodriguez A, Trujillano J (2013) Classification of dermatological disorders in critical care patients: a prospective observational study. J Crit Care 28:220 (e221–228) [DOI] [PubMed] [Google Scholar]
- 60.de Prost N, Sbidian E, Chosidow O, Brun-Buisson C, Amathieu R (2015) Management of necrotizing soft tissue infections in the intensive care unit: results of an international survey. Intensive Care Med 41:1506–1508 [DOI] [PubMed] [Google Scholar]
- 61.de Geus HR, van der Klooster JM (2005) Vacuum-assisted closure in the treatment of large skin defects due to necrotizing fasciitis. Intensive Care Med 31:601. [DOI] [PubMed] [Google Scholar]
- 62.Chen IW, Yang HM, Chiu CH, Yeh JT, Huang CH, Huang YY (2015) Clinical characteristics and risk factor analysis for lower-extremity amputations in diabetic patients with foot ulcer complicated by necrotizing fasciitis. Medicine 94:e1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pham TN, Moore ML, Costa BA, Cuschieri J, Klein MB (2009) Assessment of functional limitation after necrotizing soft tissue infection. J Burn Care Res 30:301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.European Centre for Disease Prevention and Control (2016) Proportion of methicillin resistant Staphylococcus aureus (MRSA) isolates in participating countries in 2014. ECDC, Stockholm: Accessed 19 July 2016 [Google Scholar]
- 66.Zilberberg M, Micek ST, Kollef MH, Shelbaya A, Shorr AF (2012) Risk factors for mixed complicated skin and skin structure infections to help tailor appropriate empiric therapy. Surg Infect 13:377–382 [DOI] [PubMed] [Google Scholar]
- 67.Eells SJ, Nguyen M, Jung J, Macias-Gil R, May L, Miller LG (2016) Relationship between adherence to oral antibiotics and postdischarge clinical outcomes among patients hospitalized with staphylococcus aureus skin infections. Antimicrob Agents Chemother 60:2941–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casabar E, Portell J The tool book: drug dosing and treatment guidelines. Barnes-Jewish Hospital, Department of Pharmacy, St. Louis: https://www.dorsata.com/client#. Accessed 19 May 2016 [Google Scholar]
- 69.Canut A, Isla A, Rodriguez-Gascon A (2015) Pharmacokinetic/pharmaco-dynamic analysis to evaluate ceftaroline fosamil dosing regimens for the treatment of community-acquired bacterial pneumonia and complicated skin and skin-structure infections in patients with normal and impaired renal function. Int J Antimicrob Agents 45:399–405 [DOI] [PubMed] [Google Scholar]
- 70.Furtek KJ, Kubiak DW, Barra M, Varughese CA, Ashbaugh CD, Koo S (2016) High incidence of neutropenia in patients with prolonged ceftaroline exposure. J Antimicrob Chemother 71:2010–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel N, Cardone K, Grabe DW, Meola S, Hoy C, Manley H, Drusano GL, Lodise TP (2011) Use of pharmacokinetic and pharmacodynamic principles to determine optimal administration of daptomycin in patients receiving standardized thrice-weekly hemodialysis. Antimicrob Agents Chemother 55:1677–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burnham JP, Burnham CA, Warren DK, Kollef MH (2016) Impact of time to appropriate therapy on mortality in patients with vancomycin intermediate Staphylococcus aureus infection. Antimicrob Agents Chemother 60:5546–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flanagan S, Bartizal K, Minassian SL, Fang E, Prokocimer P (2013) In vitro, in vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. Antimicrob Agents Chemother 57:3060–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panackal AA, Hajjeh RA, Cetron MS, Warnock DW (2002) Fungal infections among returning travelers. Clin Infect Dis 35:1088–1095 [DOI] [PubMed] [Google Scholar]
- 75.Uys A, Rapoport BL, Anderson R (2004) Febrile neutropenia: a prospective study to validate the Multinational Association of Supportive Care of Cancer (MASCC) risk-index score. Support Care Cancer 12:555–560 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.