Abstract
Cysticercosis is a cosmopolitan zoonotic parasitic disease infected by larval of Taenia solium (T. solium). Several drugs for the treatment of cysticercosis, such as praziquantel, albendazole, and mebendazole, have certain toxicity and side effects. Considering that there is no vaccine available, we studied a new vaccine for cysticercosis in this study. The complete TSOL18 gene and the optimized SP-TSOL18 gene fragments were obtained using PCR-based accurate synthesis method. The secretory and intracellular recombinant pMG36e-SP-TSOL18/Lactococcus lactis (L. lactis) and pMG36e-TSOL18/L. lactis vaccines of T. solium were prepared. Immune responses in mice orally immunized with these two recombinant L. lactis vaccines were analyzed by the determination of specific antibodies (IgG, IgG1, IgG2a, and sIgA) in serum, spleen lymphocyte proliferation, and cytokines (IL-2, IFN-γ, IL-4, and IL-10) in spleen lymphocyte culture supernatant. Our results showed that, after the first immunization, in these two recombinant L. lactis vaccine groups, the levels of serum specific IgG, IgG2a, and IgG1 increased on 14–56 d and reached the highest level on days 42, 42, and 28, respectively. The level of specific sIgA of intestinal mucosa also increased on 14–56 d and reached the highest level on day 42. The level of spleen lymphocyte proliferation increased on 14–56 d and reached the highest level on day 42. The levels of IL-2, IFN-γ, IL-4, and IL-10 in spleen lymphocyte culture supernatant increased on 14–56 d and reached the highest level on days 42, 42, 28, and 28, respectively. These results indicated that the recombinant pMG36e-SP-TSOL18/L. lactis and pMG36e-TSOL18/L. lactis vaccines can induce specific cellular, humoral, and mucosal immune responses in mice with oral vaccination. More importantly, the recombinant pMG36e-SP-TSOL18/L. lactis vaccine has a better immune effect. In summary, these results demonstrated the possibility of using L. lactis strain as a vector to deliver protective antigens of T. solium.
1. Introduction
Cysticercosis is a zoonotic parasitic disease that seriously harms human health and is distributed in many developing countries or areas in Latin America, Africa, and Asia [1–3]. A large number of sporadic cases with cysticercosis have been reported in the Southeast and Southern of Guizhou province, such as Kaili, Congjiang, Duyun, and Luodian [4–8]. Surgery and chemotherapy treatment of the disease have several problems, including the limited efficacy, serious side effects, and drug resistance. It is very necessary to develop a safe and effective vaccine against cysticercosis, which can be used in China and other cysticercosis endemic countries [9–12].
TSOL18 is a specific antigen of Taenia solium (T. solium) oncosphere, which has good immunogenicity and immunoprotection. The TSOL18 gene is considered to be the most promising candidate vaccine gene and has been studied extensively [13, 14]. Lactococcus lactis (L. lactis) is an important probiotic in intestine of human and animal. It is generally recognized as safe (GRAS) food grade microorganism and naturally present in milk foods, which has functions of regulating microecological balances, inhibiting tumor growth, reducing cholesterol, delaying aging, and improving immunity [15]. With the development of genetic engineering technology, it has been recently used as a new foreign antigen delivery system and applied to the field of food, vaccines, medicines, health products, and domestic animal breeding industries [16–21]. The objective of this study was to prepare the recombinant pMG36e-SP-TSOL18/L. lactis and pMG36e-TSOL18/L. lactis vaccines and investigate their induced immune responses in mice. Kunming mice were immunized orally with these two recombinant L. lactis vaccines, and then antibodies of serum and intestinal mucosa, proliferation and cytokines of spleen lymphocytes were determined at different time points of postvaccination.
2. Materials and Methods
2.1. Construction and Identification of Recombinant Plasmids pMG36e-TSOL18 and pMG36e-SP-TSOL18
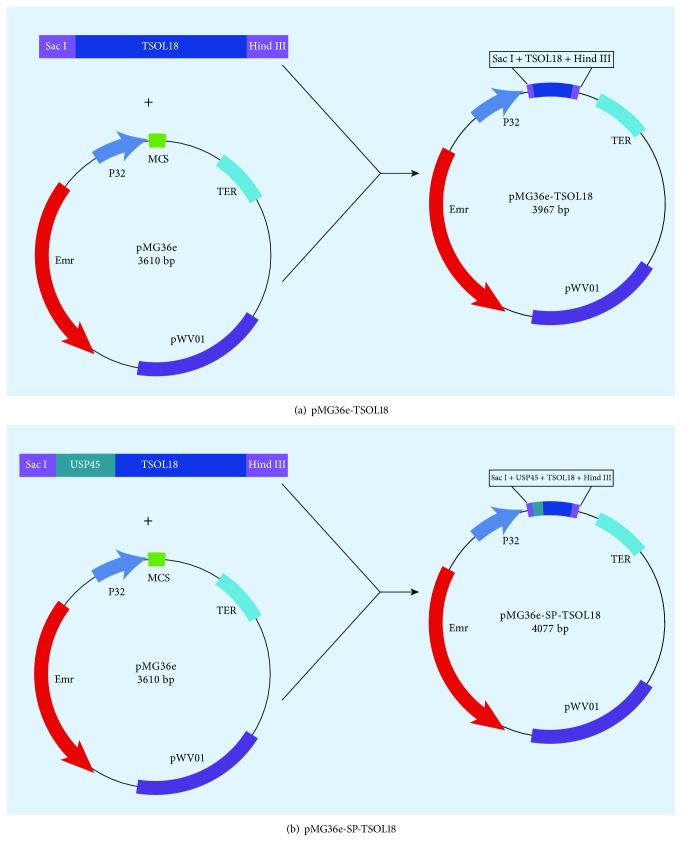
According to the TSOL18 gene sequence (Accession No. AF017788), using the L. lactics as a host system for gene optimization, the TSOL18 gene was synthesized using a PAS (PCR-based accurate synthesis) method. The signal secretion protein SPUSP45 was added at its N-terminus to synthesize the SP-TSOL18 target gene. Restriction enzyme digestion was performed using SacI and HindIII for the TSOL18 gene fragment and plasmid pMG36e to construct recombinant plasmids pMG36e-TSOL18 and pMG36e-SP-TSOL18. And then transferred them into Top10 competent cells, respectively. Positive clones were selected to perform restriction enzyme digestion and sequencing identification.
2.2. Activation of L. lactis and Preparation of Competent Cells. L. lactis
MG1363 bacteria solution was inoculated in 1 mLG/L-SGM17 (M17 medium + 0.5 M sucrose + 2.5% glycine + 0.5% glucose) liquid culture medium and cultured at 30°C for 72 hours. After obvious turbidity appeared, this culture was inoculated into 5 mLG/L-SGM17 liquid culture medium, incubated at 30°C for 24 hours, and 5 mL of this culture was diluted into 50 mLG/L-SGM17 culture medium and cultivated for 24 hours. Then, 50 mL of the culture was diluted in 400 mL of G/L-SGM17 medium and continually cultured for 3 to 5 hours until the optical density (OD600) value of the bacteria solution reached 0.2 to 0.3.
The culture was transferred into a 50 mL centrifuge tube and centrifuged at 4000 rpm at 4°C for 20 minutes, and the supernatant was discarded. The pellet was resuspended in 400 mL of 4°C precooled 0.5 M sucrose containing 10% glycerol, thoroughly shaked, centrifuged, and then discarded the supernatant. Then, the pellet was resuspended in 200 mL of 4°C precooled 0.5 M sucrose containing 10% glycerol and 0.05 M ethylenediaminetetraacetic acid (EDTA), placed in ice water for 15 minutes. The cooled culture was centrifuged again at 4000 rpm at 4°C for 20 minutes, and the supernatant was discarded. The pellet was resuspended by adding 100 mL of 4°C precooled 0.5 M sucrose containing 10% glycerol and shaken well. The culture was centrifuged again, and the supernatant was discarded. The pellet was resuspended in 4 mL of 0.5 M sucrose containing 10% glycerol. After shaking, the final culture was separated into 100 tubes (each containing 40 μL) and placed in an −80°C freezer.
2.3. Electrotransformation of L. lactis MG1363
The previously obtained plasmids pMG36e-TSOL18 and pMG36e-SP-TSOL18 were separately mixed with competent L. lactis MG1363. Both were bathed in ice for 10 minutes and treated with an electronic current. The following electrotransformation parameters were used: a voltage of 2000 V, capacitance of 25 μF, and resistance of 200 Ω. After an initial first pulse, 900 μL of low-temperature GMMC recovery medium (M17 medium + 0.5% glucose + 20 mM MgCl2 + 2 mM CaCl2) was immediately added. The cultures were placed on ice untouched for 10 minutes, then allowed to resuscitate at 30°C for 2–3 hours. The bacteria solution were centrifuged at 4000 rpm and the supernatant was discarded, and the pellet was concentrated in 100 μL GMMC recovery medium. The solution was then spreaded on 10 μg/mL Erythromycin GM17 agar plates, cultured at 30°C for 2–3 days. Plates were kept in a relatively closed environment, observed for colony growth, and small circular white opaque colonies formed in about one week.
Positive single colony was picked and placed into 1 mL of G/L-SGM17+ 5 μg/mL Erythromycin liquid culture medium, which was then incubated at 30°C for 72 hours until the solution appeared cloudy.
2.4. Identification of Recombinant pMG36e-SP-TSOL18/L. lactis and pMG36e-TSOL18/L. lactis Vaccines
The abovementioned cultured bacteria solution was centrifuged at 10000 rpm for 10 minutes, and the supernatant fluid was discarded. The bacteria solution was centrifuged and washed three times with double-distilled water, and the supernatant fluid was discarded each time. The pellets were resuspended in 30 μL of double-distilled water, placed in a boiling water bath for 10 minutes, then placed in an ice bath for 2 minutes, centrifuging again, and the supernatant was retained for extracting genomic DNA. A 579 bp region of the T. solium activated oncosphere TSOL18 gene, based on the sequence reported by Gauci et al. (1998), was amplified using the forward primer 5′-ATGGTTTGTCGTTTTGCTT-3′ and the reverse primer 5′-TTATGAACGACGAACCTTTTTA-3′. After a positive clone was confirmed, it was prepared for use as an expression strain.
2.5. Expression and Identification of TSOL18 Protein
Untransformed L. lactis MG1363 bacteria were cultured in GM17 liquid medium. Colonies that were identified as positive were separately selected and inoculated in GM17 liquid medium containing Erythromycin. After stationary culturing at 30°C for 72 hours, the culture was centrifuged at 6000 rpm and 4°C for 15 minutes together with the transformed bacterial solution. The precipitate and supernatant were collected separately for later use. Precooled phosphate-buffered saline solution (PBS) was used to resuspend the precipitate. This culture was then placed in an ice bath and ultrasonicated (300 watts) for 20 minutes, alternating 4 s of ultrasonication with 8 s wait intervals. An equal volume of 2x SDS loading buffer (0.1 mol/L Tris-Cl, pH 6.8, 10% dithiothreitol, 4% SDS, 0.2% bromophenol blue, 20% glycerol) was added, and the culture was then placed in a boiling water bath for 4–8 minutes. The total 20 μL samples were prepared after cooling and then were loaded in SDS-PAGE and Western blot gel electrophoresis plates to separately detect the expression of supernatant and intracellular components.
2.6. Animals and Immunity
Eighty specific-pathogen-free (SPF) Kunming mice (40 males and 40 females) were purchased from Experimental Animal Center, Daping Hospital, Third Military Medical University, China (number of animal license SCXK(YU)2012-0005). All mice were 6–8 weeks old, each weighed about 20 g. All experimental procedures involving the mice were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of People's Republic of China.
The Kunming mice were randomly divided into four groups with each group containing twenty mice. The mice in group 1 were immunized with recombinant pMG36e-SP-TSOL18/L. lactis vaccine. In group 2, the mice were immunized with recombinant pMG36e-TSOL18/L. lactis vaccine. In group 3 and 4, the mice were immunized only with L. lactis bacteria and PBS as control groups, respectively. The immunization doses were 3 × 109 CFU [22] and given orally three times with two-week intervals.
2.7. Antibody Detection
Four mice were taken from each group on days 0, 14, 28, 42, and 56 after the first immunization. Blood was collected from the orbital vein and let stand for 12 hours at 4°C, then centrifuged at 2000 rpm for 10 minutes to separate the serum. At the same time, mice colons were aseptically removed, cut into pieces, and placed in ice saline solution then ground to homogenate and centrifuged at 3500 rpm and 4°C for 10 minutes. The supernatant was collected and frozen at −20°C for future investigation. The serum specific IgG, IgG1, IgG2a, and intestinal mucosa sIgA [23] were evaluated using the enzyme-linked immunosorbent assay (ELISA) method. The 96-well ELISA plate was coated with 10 μg/mL recombinant TSOL18 antigen. The horseradish peroxidase (HRP) conjugated goat anti-mouse IgG (1 : 100000 dilution), IgG1 (1 : 10000 dilution), IgG2a (1 : 10000 dilution), IgA (1 : 10000 dilution). A diaminobenzidine (DAB) chromogenic substrate was used for staining, and absorbance (OD450) values were measured by a microplate reader. Each assay was performed in duplicate.
2.8. Preparation of Spleen Lymphocytes
Spleens were also aseptically removed from the four mice per group on days 0, 14, 28, 42, and 56 after the first immunization. The spleen lymphocytes were isolated according to the instructions of the mouse spleen lymphocyte separation kit. Spleen lymphocyte suspension was prepared and adjusted to 5 × 106 cells/mL in RPMI 1640 containing 10% fetal bovine serum. After the number of viable cells was above 90%, penicillin (100 U/mL) and streptomycin (100 U/mL) were added.
2.9. Spleen Lymphocyte Proliferation Assay
The cell counting kit CCK-8 detection method was used. Spleen lymphocytes (2 × 106 cells/mL) were dispensed in 24-well culture plates. Three wells were set for each specimen and contained 1 mL stock solution, 1 mL stock solution combining recombinant TSOL18 antigen (10 μg/mL), and 1 mL stock solution combining ConA (10 μg/mL). The cells were incubated in a 5% CO2 incubator at 37°C for 48 hours. Two hours before the end of the incubation period, 100 μL of CCK-8 solution was added to each well. Then, absorbance values (OD450) were measured for each well with a microplate reader. Each assay was performed in duplicate.
2.10. Detection of Spleen Lymphocyte Culture Supernatant IL-2, INF-γ, IL-4, and IL-10
Spleen lymphocytes (5 × 106 cells/mL) were dispensed in 24-well culture plates using the method as described in Section 2.9. After the 48-hour incubation, the samples were centrifuged in 4000 rpm for 5 minutes. The supernatant was then collected and assessed for IL-2, INF-γ, IL-4, and IL-10 cytokines using a commercial ELISA kit according to the manufacturer's manual. Each assay was performed in duplicate.
2.11. Statistical Analysis
Measured data were shown as the mean ± standard deviation (SD). ANOVA models were used for multigroup comparisons, and comparison between groups was performed using the least significant difference method (LSD). Values of p < 0.05 were considered to represent statistically significant differences.
3. Results
3.1. Construction of Recombinant Plasmids pMG36e-TSOL18 and pMG36e-SP-TSOL18
Recombinant plasmids pMG36e-TSOL18 and pMG36e-SP-TSOL18 were constructed following Figures 1(a) and 1(b).
Figure 1.
Construction of recombinant plasmids pMG36e-TSOL18 (a) and pMG36e-SP-TSOL18 (b).
3.2. Identification of Recombinant Plasmids pMG36e-TSOL18 and pMG36e-SP-TSOL18
The amplified TSOL18 gene fragment and pMG36e vector fragment were digested by restriction enzymes SacI and HindIII. The results of 1% agarose gel electrophoresis were shown in Figures 2(a) and 2(b), which were conformed to be the theoretical length. Gene sequencing was performed for recombinant plasmids pMG36e-TSOL18 and pMG36e-SP-TSOL18, which were proved to contain the complete sequences of TSOL18 gene and pMG36e vector.
Figure 2.
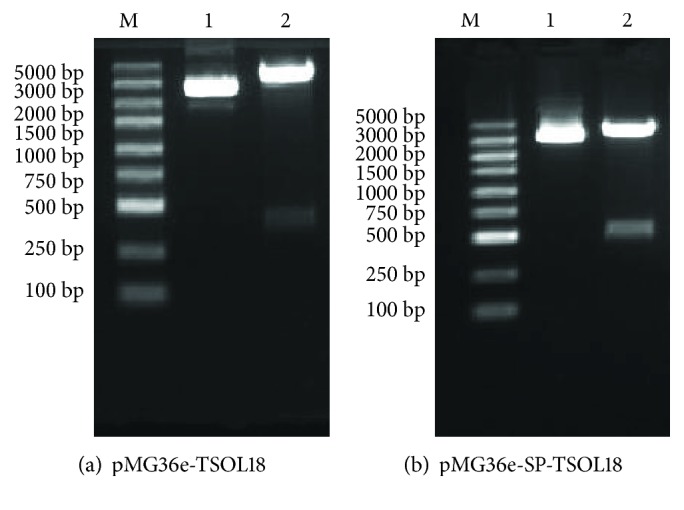
Identification of recombinant plasmid (pMG36e-TSOL18 and pMG36e-SP-TSOL18) by restriction enzyme digestion (SacI and HindIII). Lane M, DNA marker; lane 1, plasmid pMG36e; lane 2, production of restriction enzyme of recombinant plasmid pMG36e-TSOL18 (a) and pMG36e-SP-TSOL18 (b).
3.3. Identification of Recombinant pMG36e-TSOL18/L. lactis and pMG36e-SP-TSOL18/L. lactis Vaccines
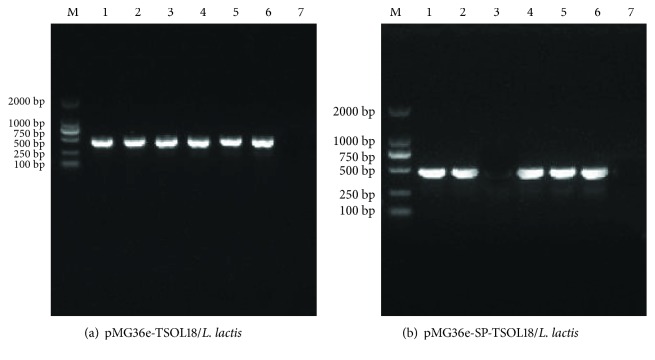
The culture supernatant of L. lactis MG1363 bacteria containing pMG36e-TSOL18 and pMG36e-SP-TSOL18 was used to perform PCR identification. The results showed that lanes 1–6 were the PCR products of L. lactis MG1363-positive bacteria containing pMG36e-TSOL18 and pMG36e-SP-TSOL18. Both are consistent with the expected results (see Figures 3(a) and 3(b)).
Figure 3.
PCR identification of recombinant pMG36e-TSOL18/L. lactis and pMG36e-SP-TSOL18/L. lactis vaccines. Lane M, DNA marker; Lanes 1–6, PCR products of L. lactis MG1363-positive bacteria containing pMG36e-TSOL18 (a) and pMG36e-SP-TSOL18 (b); lane 7, PCR products of L. lactis MG1363-negative bacteria.
3.4. SDS-PAGE Analysis
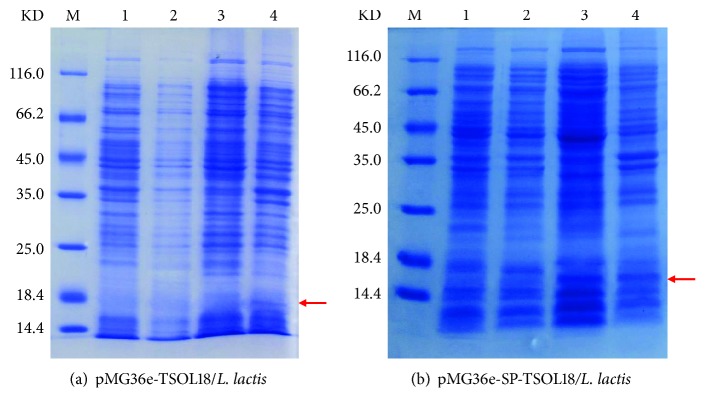
Positive colonies were selected and inoculated into GM17 liquid medium containing Erythromycin. The colony was cultured for 72 hours at 30°C, then supernatant and precipitation were collected for SDS-PAGE electrophoresis. The results showed that the target protein expression of recombinant pMG36e-TSOL18/L. lactis can be observed in around 15 KD of intracellular precipitation. However, no expression has yet been observed in the extracellular supernatant. Recombinant pMG36e-SP-TSOL18/L. lactis showed the corresponding target protein expression in both extracellular supernatant and intracellular precipitation (see Figures 4(a) and 4(b)).
Figure 4.
SDS-PAGE analysis of recombinant pMG36e-TSOL18/L. lactis and pMG36e-SP-TSOL18/L. lactis vaccines. Lane M, protein marker; lane 1, supernatant of MG1363 strain cultured for 72 h; lane 2, precipitation of MG1363 strain cultured for 72 h; lane 3, supernatant of transformation bacteria pMG36e-TSOL18/L. lactis (a) and pMG36e-SP-TSOL18/L. lactis (b) cultured for 72 h; lane 4, precipitation of transformation bacteria pMG36e-TSOL18/L. lactis (a) and pMG36e-SP-TSOL18/L. lactis (b) cultured for 72 h.
3.5. Western Blot Identification
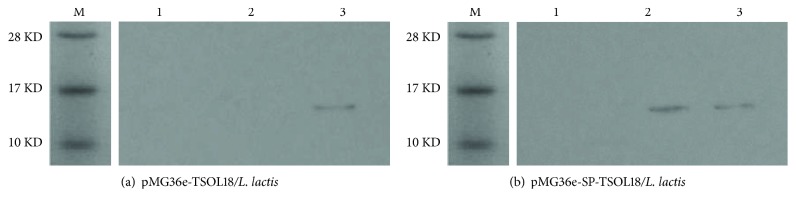
After expression of TSOL18 recombinant protein combined with TSOL18 recombinant rabbit antiserum protein, only the recombinant pMG36e-TSOL18/L. lactis appeared around 15 KD reflection band in intracellular precipitation. The recombinant pMG36e-SP-TSOL18/L. lactis showed corresponding reaction bands in both extracellular supernatant and intracellular precipitation (see Figures 5(a) and 5(b)).
Figure 5.
Western blot identification of TSOL18 and SP-TSOL18 protein expression in L. lactis. Lane M, protein marker; lane 1, L. lactis MG1363-negative bacteria; lane 2, TSOL18 (a) and SP-TSOL18 (b) protein in extracellular supernatant reacted with rabbit-anti-TSOL18; lane 3, TSOL18 (a) and SP-TSOL18 (b) protein in intracellular precipitation reacted with rabbit-anti-TSOL18.
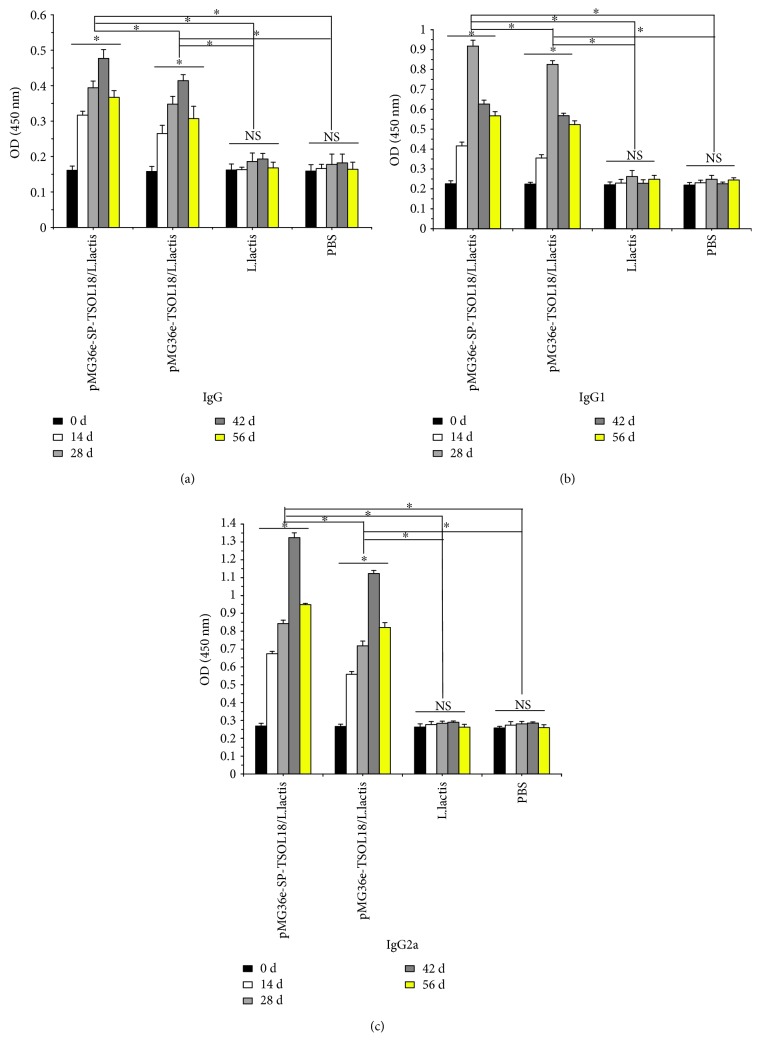
3.6. Serum Specific IgG, IgG1, and IgG2a Levels in Immunized Mice
As compared to the level on day 0, in both recombinant pMG36e-TSOL18/L. lactis and pMG36e-SP-TSOL18/L. lactis groups, the serum specific IgG, IgG1, and IgG2a levels in mice were increased from 14 to 56 days after the first immunization. Each antibody reached the highest level on days 42, 28, and 42, respectively, which was significantly higher than L. lactis and PBS control group (p < 0.05). The level of each antibody in recombinant pMG36e-SP-TSOL18/L. lactis group was significantly higher than that of recombinant pMG36e-TSOL18/L. lactis group (p < 0.05) (see Figures 6(a)–6(c)).
Figure 6.
The level of serum specific IgG (a), IgG1 (b), and IgG2a (c) in immunized mice as measured by ELISA, respectively. Serum was obtained on days 0, 14, 28, 42, and 56 after the first immunization. Inset shows the absorbance values of four groups at different time points. ∗Represents the difference between groups. p < 0.05. NS = nonsignificant.
3.7. Intestinal Mucosa-Specific sIgA Levels in Immunized Mice
As compared to the level on day 0, in both recombinant pMG36e-TSOL18/L. lactis and pMG36e-SP-TSOL18/L. lactis groups, the intestinal mucosa-specific secretory IgA (sIgA) levels in mice were increased from 14 to 56 days after the first immunization. sIgA reached the highest level on day 42 which was significantly higher than L. lactis and PBS control group (p < 0.05). The level of sIgA in recombinant pMG36e-SP-TSOL18/L. lactis group was significantly higher than that of recombinant pMG36e-TSOL18/L. lactis group (p < 0.05) (see Figure 7).
Figure 7.
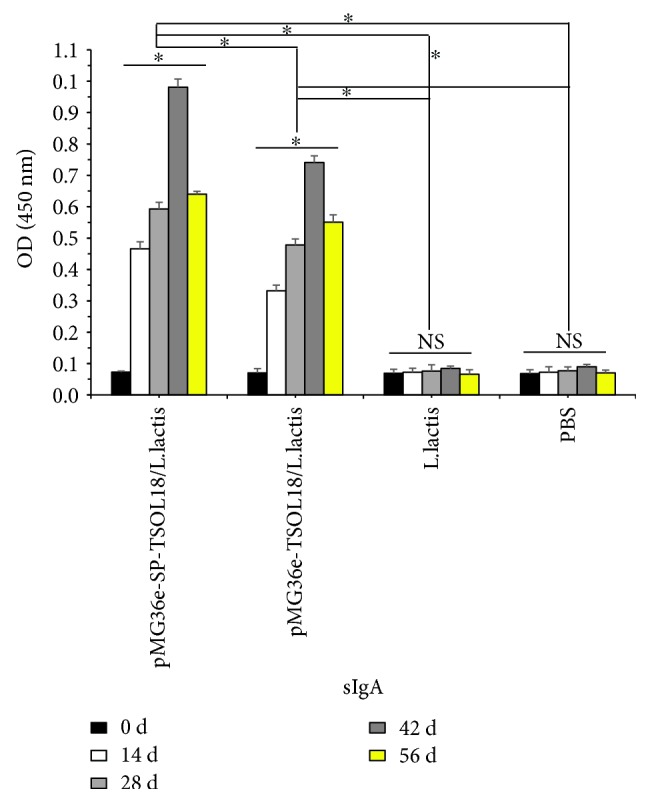
The level of intestinal mucosa-specific sIgA in immunized mice as measured by ELISA. Intestinal mucosa was obtained on days 0, 14, 28, 42, and 56 after the first immunization. Inset shows the absorbance values of four groups at different time points. ∗Represents the difference between groups. p < 0.05. NS = nonsignificant.
3.8. Spleen Lymphocyte Proliferation Levels in Immunized Mice
As compared to the level on day 0, in both recombinant pMG36e-TSOL18/L. lactis and pMG36e-SP-TSOL18/L. lactis groups, the spleen lymphocyte proliferation levels in mice were increased from 14 to 56 days after the first immunization. Spleen lymphocyte proliferation reached the highest level on day 42, which was significantly higher than L. lactis and PBS control group (p < 0.05). The level of spleen lymphocyte proliferation in recombinant pMG36e-SP-TSOL18/L. lactis group was significantly higher than that of recombinant pMG36e-TSOL18/L. lactis group (p < 0.05) (see Figure 8).
Figure 8.
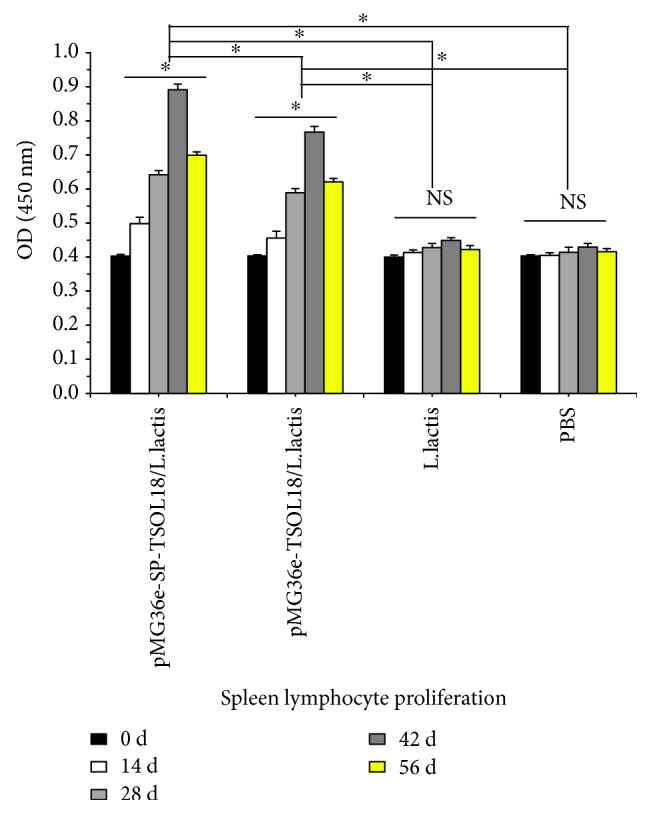
The level of spleen lymphocyte proliferation in immunized mice as measured by CCK-8. Spleen lymphocytes were obtained on days 0, 14, 28, 42, and 56 after the first immunization. Inset shows the absorbance values of four groups at different time points. ∗Represents the difference between groups. p < 0.05. NS = nonsignificant.
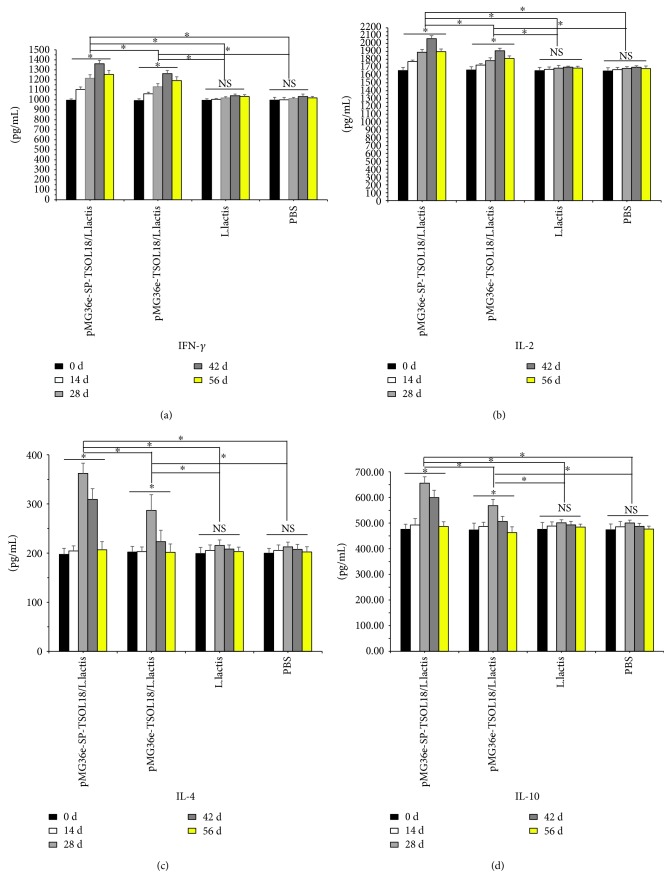
3.9. Spleen Lymphocyte Culture Supernatant IFN-γ, IL-2, IL-4, and IL-10 Levels in Immunized Mice
As compared to the level on day 0, in both recombinant pMG36e-TSOL18/L. lactis and pMG36e-SP-TSOL18/L. lactis groups, the levels of IFN-γ, IL-2, IL-4, and IL-10 in spleen lymphocyte culture supernatant were increased from 14 to 56 days after the first immunization. Each cytokine reached the highest level on days 42, 42, 28, and 28, respectively, which was significantly higher than L. lactis and PBS control group (p < 0.05). The level of each cytokine in recombinant pMG36e-SP-TSOL18/L. lactis group was significantly higher than that of recombinant pMG36e-TSOL18/L. lactis group (p < 0.05) (see Figures 9(a)–9(d)).
Figure 9.
The level of spleen lymphocyte culture supernatant IFN-γ (a), IL-2 (b), IL-4 (c), and IL-10 (d) in immunized mice as measured by ELISA. Spleen lymphocytes were obtained on days 0, 14, 28, 42, and 56 after the first immunization. Inset shows the concentration of four groups at different time points. ∗Represents the difference between groups. p < 0.05. NS = nonsignificant.
4. Discussion
Cysticercosis is a zoonotic parasitic disease caused by the larvae of T. solium in the humans and pigs and led to serious health and economic consequences [24, 25]. There were limitations to medication and surgical treatment [12]. Therefore, it was the best way to eliminate this disease by developing an effective vaccine against T. solium infection [26, 27]. Because the eggs of T. solium primarily infect hosts through ingestion, L. lactis as an oral vaccine for T. solium infection may be a more effective as well as practical new vaccine for the prevention and control of cysticercosis [28]. Cysticercosis is caused by T. solium eggs or gravid proglottid contamination, and oncospheres are hatched and developed into cysticerci, which would bring great harm to the host. Oncosphere was the key stage in the invasion of host, thus, developing an effective candidate vaccine from oncosphere antigens may be an economic and effective means.
Several recombinant antigens have been expressed and evaluated as potential vaccine candidates such as 45 W, 18 ku, and 16 ku [29–31]. Among these, TSOL18 was the primary vaccine candidate [13, 32], and the TSOL18 gene was successfully cloned from the T. solium oncosphere for the first time. Its coding sequence was highly homologous to other corresponding protective antigens in the tapeworm family, and it was highly conserved among different strains and between different clones [33, 34]. Subsequently, the Chinese researchers Luo et al. successfully cloned the TSO18 gene of T. solium, and the TSO18-GST protein was successfully expressed in E. coli [35]. There have been numerous studies on its vaccine potentialities as a recombinant protein, a DNA vaccine, a recombinant yeast vaccine, a recombinant Bacillus Calmette-Guerin vaccine, and a recombinant Bifidobacterium vaccine [36–40], but none of these vaccines has been successfully developed into available ones. L. lactis is a good candidate for the delivery of heterologous proteins in foods, which have many advantages such as safety, simplicity, affordability, easiness to prepare, and practicality [41]. As far as the field of parasite is concerned, there has been no report of a recombinant L. lactis vaccine of T. solium.
The data showed that the signal peptide SPUSP45 derived from L. lactis was a major secretory protein and was currently a signal peptide which improved the efficiency of exogenous protein secretion [42, 43]. The secretory expression scheme of this study introduced the fusion of signal peptide SP and the fusion of propeptide fusion LEISSTCDA into the TSOL18 gene to obtain the SP-TSOL18. The intracellular and extracellular (secretory) expressions were designed and expressed in full length, respectively. All of the expected TSOL18 proteins were obtained, and the specific binding to the rabbit antiserum of the recombinant protein of TSOL18 was found. The above results indicate that recombinant pMG36e-SP-TSOL18/L. lactis and pMG36e-TSOL18/L. lactis were successfully prepared and the TSOL18 protein expressed in extracellular supernatant and intracellular precipitation has specific antigenicity. It is proved that the signal peptide SP and propeptide fusion sequence LEISSTCDA can effectively realize the extracellular expression of TSOL18 and increase the success rate of protein secretion [44, 45]. This lays the experiment foundation for further research on immune responses induced in mice immunized with these two vaccines.
It is important to investigate the immune responses generated in mice by recombinant pMG36e-TSOL18/L. lactis and pMG36e-SP-TSOL18/L. lactis vaccines. Our results demonstrate that these two recombinant vaccines can induce significant immune responses compared to the levels at the time of nonvaccination at day 0, including antibody isotypes, cytokines associated with activation of both CD4+ Th 1 and Th 2 cells, and CD4+ T-cell proliferation.
The lymphocyte proliferation test is an important indicator of cellular immunity. As our results showed, spleen lymphocytes showed a strong proliferative response upon stimulation with antigen or mitogen, and the responsiveness in orally immunized mice peaked at day 42, suggesting that recombinant pMG36e-SP-TSOL18/L. lactis and pMG36e-TSOL18/L. lactis might induce a MHC classII restricted CD4+ T cell response. The CD4+ T cell may play a role in B-cell differentiation, proliferation, and isotype regulation [46]. Activated CD4+ T cells proliferate and differentiate into effector Th cells. This is consistent with the generation of specific cellular immune responses we observed by recombinant L. lactis vaccination [47–49]. In addition, lymphocyte proliferation in response to ConA was enhanced substantially. This may be attributed to the fact that ConA produces polyclonal activation of T lymphocytes. Therefore, it is possible that lymphocytes by ConA stimulation showed stronger proliferation response than that for antigen stimulation.
Cytokines and expression of specific isotypes have important role besides regulating the balance between Th1 and Th2 responses [50]. It is known that IL-2, IFN-γ, and TNF-α are indicators of Th1 response, which promote the production of IgG2a and IgG2b [51, 52], whereas IL-4, IL-5, and IL-10 are indicators of Th2 response, which promote the generation of IgG1, IgG3, and IgE [53–55]. As shown in Figure 9, the stimulation with TSOL18 produced high levels of IFN-γ, IL-2, IL-4, and IL-10 in spleen lymphocytes from all immunized groups. As shown in Figure 6, the antibody responses showed a significantly great increase in IgG, IgG1, and IgG2a in orally vaccinated mice than those in nonvaccinated mice. These results demonstrated that cytokines in spleen lymphocytes and antibody isotype in serum from all immunized mice showed that immunization with these two recombinant L. lactis vaccines resulted in stimulation of both Th1 and Th2 immune responses. Data showed that the intestinal mucosa is a site that induces an effective mucosal immune response, and the immunization method is simple and easy to operate [56]. sIgA is the main effector molecule of the mucosal immune system, which has a higher content in intestinal mucosa [57]. Our results showed that the specific sIgA level was significantly increased in the intestinal mucosa and these two recombinant L. lactis vaccines could induce a mucosal immune response in all immunized mice.
In conclusion, we have demonstrated that an oral live vaccine prepared in this study is capable of inducing specific humoral immune responses, cellular immune responses, and mucosal immune responses in mice and that L. lactis is a potential vaccine vehicle to deliver T. solium antigens.
Acknowledgments
This study was supported by Outstanding Youth Science and Technology Talent Cultivation Support Project of Guizhou Province Grant no. Qian Sci Con Platform Talent [2017] 5629, Health and Family Planning Commission Research Project of Guizhou Province Grant no. Gzwjkj 2016-1-048 and Science and Technology Cooperation Special Fund Project of Province and City, Grant no. Prov City Sci Con [2015]52.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Trevisan C., Devleesschauwer B., Schmidt V., Winkler A. S., Harrison W., Johansen M. V. The societal cost of Taenia solium cysticercosis in Tanzania. Acta Tropica. 2017;165:141–154. doi: 10.1016/j.actatropica.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 2.da Guarda K. X., Costa-Cruz J. M., da Costa Barcelos I. S. Seroprevalence of human cysticercosis in Jataí, Goiás State, Brazil. The Brazilian Journal of Infectious Diseases. 2018;22(2):146–149. doi: 10.1016/j.bjid.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen M. V., Trevisan C., Gabriël S., Magnussen P., Braae U. C. Are we ready for Taenia solium cysticercosis elimination in sub-Saharan Africa. Parasitology. 2017;144(1):59–64. doi: 10.1017/s0031182016000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotelo J. Clinical manifestations, diagnosis, and treatment of neurocysticercosis. Current Neurology and Neuroscience Reports. 2011;11(6):529–535. doi: 10.1007/s11910-011-0226-7. [DOI] [PubMed] [Google Scholar]
- 5.Sciutto E., Fragoso G., Fleury A., et al. Taenia solium disease in humans and pigs: an ancient parasitosis disease rooted in developing countries and emerging as a major health problem of global dimensions. Microbes and Infection. 2000;2(15):1875–1890. doi: 10.1016/S1286-4579(00)01336-8. [DOI] [PubMed] [Google Scholar]
- 6.Xu A. J., Gu J. C. The current situation and the prevalent trend of cysticercosis in China. China Tropical Medicine. 2010;10(2):239–240. [Google Scholar]
- 7.Li T., Craig P. S., Ito A., et al. Taeniasis/cysticercosis in a Tibetan population in Sichuan Province, China. Acta Tropica. 2006;100(3):223–231. doi: 10.1016/j.actatropica.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Liu G., Li Y., Cui Y., et al. Cysticercosis in Shandong Province, Eastern China. Emerging Infectious Diseases. 2018;24(2):384–385. doi: 10.3201/eid2402.151253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diseases T. L. I. Treatment of neurocysticercosis. The Lancet Infectious Diseases. 2014;14(8):p. 657. doi: 10.1016/S1473-3099(14)70857-6. [DOI] [PubMed] [Google Scholar]
- 10.Sciutto E., Fragoso G., de Aluja A., Hernandez M., Rosas G., Larralde C. Vaccines against cysticercosis. Current Topics in Medicinal Chemistry. 2008;8(5):415–423. doi: 10.2174/156802608783790839. [DOI] [PubMed] [Google Scholar]
- 11.Flisser A., Lightowlers M. W. Vaccination against Taenia solium cysticercosis. Memórias do Instituto Oswaldo Cruz. 2001;96(3):353–356. doi: 10.1590/s0074-02762001000300012. [DOI] [PubMed] [Google Scholar]
- 12.Ma C. X., Wang H. W., Yang Y. X. Research progress on the genomics of Taenia solium and candidate vaccines for cysticercosis. Chinese Journal of Parasitology and Parasitic Diseases. 2016;34(2):161–165. [PubMed] [Google Scholar]
- 13.Ding J., Zheng Y., Wang Y., et al. Immune responses to a recombinant attenuated Salmonella typhimurium, strain expressing a Taenia solium, oncosphere antigen TSOL18. Comparative Immunology Microbiology and Infectious Diseases. 2013;36(1):17–23. doi: 10.1016/j.cimid.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B. Y., Yang F. J., Liu M. C., et al. Protective immune responses induced by a recombinant BCG-TSOL18 vaccine of Taenia solium in piglets. Current Immunology. 2017;37(5):399–403. [Google Scholar]
- 15.Kun L. Y., Nomoto K., Salminen S., Gorbach S. L. Handbook of probioties. International Journal of Food Science and Technology. 2010;36(2):p. 224. [Google Scholar]
- 16.Szatraj K., Szczepankowska A. K., Chmielewska-Jeznach M. Lactic acid bacteria—promising vaccine vectors: possibilities, limitations, doubts. Journal of Applied Microbiology. 2017;123(2):325–339. doi: 10.1111/jam.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao H.-P., Wang H.-N., Yang X., et al. Lactococcus lactis anchoring avian infectious bronchitis virus multi-epitope peptide EpiC induced specific immune responses in chickens. Bioscience, Biotechnology, and Biochemistry. 2013;77(7):1499–1504. doi: 10.1271/bbb.130157. [DOI] [PubMed] [Google Scholar]
- 18.Lee P. Biocontainment strategies for live lactic acid bacteria vaccine vectors. Bioengineered Bugs. 2010;1(1):75–77. doi: 10.4161/bbug.1.1.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parlane N. A., Grage K., Lee J. W., Buddle B. M., Denis M., Rehm B. H. A. Production of a particulate hepatitis C vaccine candidate by an engineered Lactococcus lactis strain. Applied and Environmental Microbiology. 2011;77(24):8516–8522. doi: 10.1128/AEM.06420-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Y.-B., Yang W.-T., Yang G.-L., Wang C.-F. Progress in application of recombinant genetic engineering LAB. Chinese Journal of Microecology. 2012;24(9):850–852. [Google Scholar]
- 21.Tarahomjoo S. Development of vaccine delivery vehicles based on lactic acid bacteria. Molecular Biotechnology. 2012;51(2):183–199. doi: 10.1007/s12033-011-9450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J., Zhong X. Z., Li W. S. Evaluation of the optimum immune dose for recombinant Lactococcus lactis expressing P30 of Toxoplasma gondii orally immunized to mice. Journal of Wenzhou Medical College. 2007;37(4):350–353. [Google Scholar]
- 23.Pereira V. B., Saraiva T. D. L., Souza B. M., et al. Development of a new DNA vaccine based on mycobacterial ESAT-6 antigen delivered by recombinant invasive Lactococcus lactis FnBPA+ Applied Microbiology and Biotechnology. 2015;99(4):1817–1826. doi: 10.1007/s00253-014-6285-3. [DOI] [PubMed] [Google Scholar]
- 24.Wu W., Jia F., Wang W., Huang Y., Huang Y. Antiparasitic treatment of cerebral cysticercosis: lessons and experiences from China. Parasitology Research. 2013;112(8):2879–2890. doi: 10.1007/s00436-013-3459-3. [DOI] [PubMed] [Google Scholar]
- 25.Del Brutto O. H., Nash T. E., White A. C., Jr, et al. Revised set of diagnostic criteria for neurocysticercosis (in reply to Garg and Malhotra) Journal of the Neurological Sciences. 2017;373:350–351. doi: 10.1016/j.jns.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Liu M. C., He L. F., Zhou B. Y. Progress in genetic engineering vaccine research of Taenia solium. Chinese Journal of Endemiology. 2014;33(2):233–236. [Google Scholar]
- 27.Lightowlers M. W. Vaccines for prevention of cysticercosis. Acta Tropica. 2003;87(1):129–135. doi: 10.1016/S0001-706X(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 28.Li W. G., Chen Y. T. Current condition of researches in recombinant bacteria mediated by lactococcus. Foreign Medical Science Section of Medgeography. 2016;37(1):1–12. [Google Scholar]
- 29.Plancarte Agustín, Flisser A., Gauci C. G., Lightowlers M. W. Vaccination against Taenia solium cysticercosis in pigs using native and recombinant oncosphere antigens. International Journal for Parasitology. 1999;29(4):643–647. doi: 10.1016/S0020-7519(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhou B. Y. Research progress on recombinant antigens of Taenia solium. Chinese Journal of Zoonoses. 2014;30(4):418–422. [Google Scholar]
- 31.Russel M., Linderoth N. A., Sali A. Filamentous phage assembly: variation on a protein export theme. Gene. 1997;192(1):23–32. doi: 10.1016/S0378-1119(96)00801-3. [DOI] [PubMed] [Google Scholar]
- 32.Guo A.-J., Cai X.-P., Fang Y.-X., Jia W.-Z., Luo X.-N., Liu H.-X. Construction of gene vaccine vector carrying TSOL18 gene of Taenia solium oncosphere and its immunogenicity. Acta Veterinaria et Zootechnica Sinica. 2008;39(7):945–949. [Google Scholar]
- 33.Gauci C. G. P., Flisser A., Lightowlers M. W. Research note a Taenia solium oncosphere protein homologous to host-protective Taenia ovis and Taenia saginata 18 kDa antigens. International Journal for Parasitology. 1998;28(5):757–760. doi: 10.1016/S0020-7519(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 34.Gauci C. G., Ito A., Lightowlers M. W. Conservation of the vaccine antigen gene, TSOL18, among genetically variant isolates of Taenia solium. Molecular and Biochemical Parasitology. 2006;146(1):101–104. doi: 10.1016/j.molbiopara.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Luo X. N., Zheng Y. D., Wu X. G., Dou Y. X., Jing Z. Z., Cai X. P. Cloning and expression of TSO18 gene of Taenia solium oncosphere. Chinese Journal of Zoonoses. 2005;21(3):262–264. [Google Scholar]
- 36.Flisser A., Gauci C. G., Zoli A., et al. Induction of protection against porcine cysticercosis by vaccination with recombinant oncosphere antigens. Infection and Immunity. 2004;72(9):5292–5297. doi: 10.1128/IAI.72.9.5292-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y.-Y., Chang X.-L., Tao Z.-Y., et al. Optimized codon usage enhances the expression and immunogenicity of DNA vaccine encoding Taenia solium oncosphere TSOL18 gene. Molecular Medicine Reports. 2015;12(1):281–288. doi: 10.3892/mmr.2015.3387. [DOI] [PubMed] [Google Scholar]
- 38.Cai X., Yuan G., Zheng Y., et al. Effective production and purification of the glycosylated TSOL18 antigen, which is protective against pig cysticercosis. Infection and Immunity. 2008;76(2):767–770. doi: 10.1128/IAI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang F., Jiang N., Zhou L., Liu H., Wang L., Zhou B. Dynamic observation of immune responses induced in mice by immunization with a recombinant BCG-TSOL18 vaccine of Taenia solium. Chinese Journal of Endemiology. 2015;34(12):878–883. [Google Scholar]
- 40.Zhou B., Liu M., Zhou L., et al. Protective effect of a mixed recombinant Bifidobacterium vaccine of Taenia solium in piglets. Chinese Journal of Endemiology. 2017;36(8):552–556. [Google Scholar]
- 41.Wyszyńska A., Kobierecka P., Bardowski J., Jagusztyn-Krynicka E. K. Lactic acid bacteria—20 years exploring their potential as live vectors for mucosal vaccination. Applied Microbiology and Biotechnology. 2015;99(7):2967–2977. doi: 10.1007/s00253-015-6498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Loir Y., Nouaille S., Commissaire J., Bretigny L., Gruss A., Langella P. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Applied and Environmental Microbiology. 2001;67(9):4119–4127. doi: 10.1128/aem.67.9.4119-4127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Loir Y., Azevedo V., Oliveira S. C., et al. Protein secretion in Lactococcus lactis: an efficient way to increase the overall heterologous protein production. Microbial Cell Factories. 2005;4(1):p. 2. doi: 10.1186/1475-2859-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim P. Y., Tan L. L., Ow D. S. W., Wong F. T. A propeptide toolbox for secretion optimization of Flavobacterium meningosepticum endopeptidase in Lactococcus lactis. Microbial Cell Factories. 2017;16(1):p. 221. doi: 10.1186/s12934-017-0836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong Z., Zhang J., Li H., du G., Chen J., Lee B. Codon and propeptide optimizations to improve the food-grade expression of bile salt hydrolase in Lactococcus lactis. Protein and Peptide Letters. 2015;22(8):727–735. doi: 10.2174/0929866522666150610094829. [DOI] [PubMed] [Google Scholar]
- 46.Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 47.Asensi G. F., de Sales N. F. F., Dutra F. F., et al. Oral immunization with Lactococcus lactis secreting attenuated recombinant staphylococcal enterotoxin B induces a protective immune response in a murine model. Microbial Cell Factories. 2013;12(1):32–38. doi: 10.1186/1475-2859-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bermúdez-Humarán L. G., Langella P., Cortes-Perez N. G., et al. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infection and Immunity. 2003;71(4):1887–1896. doi: 10.1128/IAI.71.4.1887-1896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steidler L., Robinson K., Chamberlain L., et al. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infection and Immunity. 1998;66(7):3183–3189. doi: 10.1128/iai.66.7.3183-3189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finkelman F. D., Holmes J., Katona I. M., et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annual Review of Immunology. 1990;8(1):303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 51.Kuribayashi K., Tsukiyama M., Takenaka T. Secretion patterns of Th1- and Th2-type cytokines in immune deviation caused by dendritic cells. International Archives of Allergy and Immunology. 1997;114(1):30–37. doi: 10.1159/000237639. [DOI] [PubMed] [Google Scholar]
- 52.Stevens T. L., Bossie A., Sanders V. M., et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334(6179):255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 53.Brière F., Servet-Delprat C., Bridon J. M., Saint-Remy J. M., Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. Journal of Experimental Medicine. 1994;179(2):757–762. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of Immunology. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 55.Ortona E., Riganò R., Buttari B., et al. An update on immunodiagnosis of cystic echinococcosis. Acta Tropica. 2003;85(2):165–171. doi: 10.1016/S0001-706X(02)00225-5. [DOI] [PubMed] [Google Scholar]
- 56.Wittig B. M., Zeitz M. The gut as an organ of immunology. International Journal of Colorectal Disease. 2003;18(3):181–187. doi: 10.1007/s00384-002-0444-1. [DOI] [PubMed] [Google Scholar]
- 57.Newberry R. D., Lorenz R. G. Organizing a mucosal defense. Immunological Reviews. 2005;206(1):6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.