Abstract
Hydroquinone (HQ) is widely used in food stuffs and is an occupational and environmental pollutant. Although the hepatotoxicity of HQ has been demonstrated both in vitro and in vivo, the prevention of HQ-induced hepatotoxicity has yet to be elucidated. In this study, we focused on the intervention effect of aqueous extracts of Flos lonicerae Japonicae (FLJ) on HQ-induced cytotoxicity. We demonstrated that HQ reduced cell viability in a concentration-dependent manner by administering 160 μmol/L HQ for 12 h as the positive control of cytotoxicity. The aqueous FLJ extracts significantly increased cell viability and decreased LDH release, ALT, and AST in a concentration-dependent manner compared with the corresponding HQ-treated groups in hepatic L02 cells. This result indicated that aqueous FLJ extracts could protect the cytotoxicity induced by HQ. HQ increased intracellular MDA and LPO and decreased the activities of GSH, GSH-Px, and SOD in hepatic L02 cells. In addition, aqueous FLJ extracts significantly suppressed HQ-stimulated oxidative damage. Moreover, HQ promoted DNA double-strand breaks (DSBs) and the level of 8-hydroxy-2′-deoxyguanosine and apoptosis. However, aqueous FLJ extracts reversed HQ-induced DNA damage and apoptosis in a concentration-dependent manner. Overall, our results demonstrated that the toxicity of HQ was mediated by intracellular oxidative stress, which activated DNA damage and apoptosis. The findings also proved that aqueous FLJ extracts exerted protective effects against HQ-induced cytotoxicity in hepatic L02 cells.
1. Introduction
Hydroquinone (HQ) is a ubiquitous environmental chemical in cosmetics, medicines, the environment, and human diet; HQ can be metabolized from benzene as potentially hematotoxic, genotoxic, and carcinogenic compounds [1]. Humans are exposed to HQ through various channels, including oral administration, inhalation, and through the skin [2, 3]. Although the effects of HQ exposure on human health have been extensively studied and reported, the actual mechanisms of such effects remain unclear. The involved mechanisms trigger oxidative stress, which causes DNA damage, mutation in cellular transformation, in vivo tumorigenesis, gene toxicity, and epigenetic changes [1, 4–8]. In our previous experiments, HQ induced apoptosis in hepatic L02 cells by changing the cellular redox status by reducing the cellular thiol level and increasing the cellular reactive oxygen species (ROS) level. In addition, ROS can lead to DNA damage by breaking DNA or producing lipid peroxidation in the membrane, thereby increasing the degrees of apoptosis and necrosis of L02 hepatocytes [1, 9–11]. These findings indicated that HQ can damage L02 hepatocytes through a series of oxidative stress reactions, so it is possible to use some antioxidants for reducing HQ toxicity.
Flos lonicerae Japonicae (FLJ) is the flower of Lonicera japonica Thunb, which is widely planted in China [12]. FLJ is used worldwide as a popular traditional herbal medicine with various pharmacological activities [13, 14]. In traditional Chinese medicine, FLJ is typically used to treat common colds and fevers. FLJ exerts various effects, such as antioxidant, anti-inflammatory, antihyperlipidemic, and anticancer [15–18]. In addition, FLJ protects cells against hydrogen peroxide-induced apoptosis by phosphorylating MAPKs and PI3K/Akt. FLJ is believed to dispel noxious heat from the blood and neutralize poisonous effects. FLJ significantly increases blood neutrophil activity and promotes neutrophil phagocytosis at appropriate concentrations [12]. Some investigators posited that the methanol extract of FLJ induces protective effects against rat hepatic injuries caused by carbon tetrachloride and aqueous extracts of FLJ flowers may act as therapeutic agents for inflammatory disease through the selective regulation of NF-κB activation in rat liver [19]. FLJ is characterized by high biomass, easy cultivation, extensive competitive ability, wide geographic distribution, and strong resistance to environmental stresses, including bacterial, viral, and oxidative stresses [20]. However, the protective effects of aqueous FLJ extracts against HQ-induced cytotoxicity have not been demonstrated.
In this study, we examined the protective effects of aqueous FLJ extracts against HQ-induced cytotoxicity and their involvement in oxidative stress, DNA damage, and apoptosis. For this reason, we investigated the MDA and LPO levels as indexes of lipid peroxidation; the activities of SOD, GSH, and GSH-Px as antioxidant enzymes; DNA double-strand breaks (DSBs) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) level as specific markers of oxidative damage of DNA; HQ-induced apoptosis; and the cytoprotective effect of aqueous FLJ extracts in hepatic L02 cells.
2. Materials and Methods
2.1. Chemicals
HQ, 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT), Hoechst33258, low-melting agarose (LMA), normal-melting agarose (NMA), 8-OHdG, deoxyguanosine (dG), and propidium iodide (PI) were purchased from Sigma (St. Louis, MO, USA). Fetal bovine serum (FBS) and RPMI-1640 medium were acquired from HyClone (Logan, UT, USA). Penicillin-streptomycin for the cell culture and trypsin were procured from Gibco/Invitrogen (Carlsbad, CA, USA). Other chemicals and reagents were of the highest analytical grade and bought from Sangon Biotech Co. Ltd. (Shanghai, China).
2.2. Plant Material and Preparation of Extract
The aqueous FLJ extracts were prepared by adopting the standard method used for treating patients with liver disease in traditional Chinese medicine. In brief, dried FLJ fruits (100 g) were boiled in 500 mL of distilled water for 3 h. The total extract was centrifuged at 5000 rcf for 30 min. The supernatant was filtered with filter paper, and the residue was further extracted twice under the same conditions. The filtrates were evaporated to dryness under vacuum and weighed. The final yield was 12.5% (w/w). The lyophilized extract was dissolved in distilled water to produce a final concentration of 100 g/mL FLJ extract, which was stored at −20°C.
2.3. Cell Lines and Culture
The immortalized human normal hepatocyte L02 cell line was provided by Dr. Zhixiong Zhuang (Shenzhen Center for Disease Control and Prevention, Guangdong, China). L02 cells were cultured in RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated FBS and antibiotic supplement (100 U/mL of penicillin and 100 μg/mL of streptomycin) in a humidified incubator at 37°C under 95% air and 5% CO2. After each specified treatment time of incubation, the cells were harvested for further analysis.
2.4. Assessment of L02 Cell Viability
The cell viability of L02 cells was determined by MTT assay. The cells were seeded into 96-well flat-bottomed plates overnight at 37°C under 5% CO2 and immediately treated with HQ (5, 10, 20, 40, 80, and 160 μM) for 6, 12, 24, and 48 h; 160 μmol/L HQ was administered for 12 h as the positive control of oxidative damage. The samples were classified under five groups, namely, control (without HQ and FLJ), 160 μmol/L HQ, 0.25 g/mL FLJ + HQ, 0.50 g/mL FLJ + HQ, and 1.00 g/mL FLJ + HQ for 12 h. Subsequently, 20 μL of MTT solution (5 mg/mL) was added to the culture medium for 4 h before the end of the treatment time to allow the formation of formazan crystals. The supernatant was discarded, and 100 μL of cell lysis buffer (50% DMF, 20% SDS, pH 4.6–4.7) was added to dissolve the intracellular crystalline formazan products. After constant and gentle shaking for 10 min at room temperature, the absorbance was recorded at 570 nm. Cell viability was calculated using the equation cellular relative viability = (ODtreated wells − ODblank)/(ODcontrol wells − ODblank).
2.5. Measurement of Intracellular Cytotoxicity and Oxidative Damage
The toxic effect of HQ in hepatic L02 cells was estimated in terms of LDH release and the activities of ALT and AST by using commercial kits (Nanjing Jiancheng Bioengineering Institute, China) in accordance with the manufacturer's protocol.
Commercial kits (Nanjing Jiancheng Bioengineering Institute, China) were utilized to assess the activities of SOD, GSH-Px, and GSH and the levels of MDA and LPO. The activities of SOD, GSH-Px, and GSH were expressed as units/milligram of protein. MDA and LPO contents were expressed as nmol/milligram of protein. The protein concentration was estimated using a BCA kit.
2.6. DNA Damage Assay
DNA damage was determined by the following assays. (i) DNA damage was evaluated using alkaline single-cell gel electrophoresis (comet assay). In brief, after 24 h of exposure, followed by washing with PBS, a 1.2 × 105 cell suspension was mixed with 0.8% LMA at 37°C and spread on a fully frosted microscope slide precoated with 0.65% NMA. After the agarose solidified, the slide was covered with another 75 μL of 0.8% LMA and then immersed in lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris base, 1% Triton-X 100, 10% DMSO, pH 10) for 2 h at 4°C. The slides were placed in a gel-electrophoresis apparatus containing 300 mM NaOH and 1 mM Na2EDTA (pH 13) for 30 min at 4°C to allow DNA unwinding and alkali labile damage. Electrophoresis was performed at 25 V (300 mA) and 4°C for 20 min. Subsequently, all slides were washed three times with a neutralizing buffer (0.4 M Tris, pH 7.5) for 5 min each time and stained with 80 μL of PI (5 μg/mL). A total of 100 randomly chosen cells (comets) were visually scored using a fluorescence microscope (Nikon Eclips TE-S, Japan) equipped with an excitation filter of 515–560 nm and a barrier filter of 590 nm. The “tail length and tail moment” of each comet were calculated using casp-1.2.2 analysis software. (ii) 8-OHdG was evaluated by high-performance liquid chromatography with electrochemical detection (HPLC-ECD) [21]. In brief, genomic DNA was extracted using a Genomic DNA Purification Kit in 500 μg hydrolyzed DNA samples through the nuclease P1 and alkaline phosphatase hydrolysis of DNA. The samples were then filtered through 0.22 μm nylon filters. 8-OHdG and dG levels were measured by using HPLC-ECD and HPLC with variable wavelength detector (HPLC-UV) systems as previously described. About 100 μL of final hydrolysates was analyzed by HPLC-ECD with reverse phase-C18 (RP-C18) analytical column as the column. The mobile phase consisted of 50 mM KH2PO4 buffer (pH 5.5 and containing 10% methanol). The separations were performed at a flow rate of 1 mL/min. The amount of 8-OHdG in DNA was calculated as the number of 8-OHdG molecules/106 unmodified dG molecules.
2.7. Apoptosis Assay
Apoptosis was assessed by flow cytometry using PI assay. In brief, the cells were incubated in RPMI 1640 with 10% FBS. The cells consisted of the control group, the group treated with 160 μM HQ, and the group coincubated in the absence or presence of FLJ (0.25, 0.50, and 1.00 g/mL) for 12 h. Subsequently, the cells were washed with cold PBS and incubated with PI (0.5 μg) for 20 min at room temperature in the dark. The cells were immediately analyzed on a FACSCanto II flow cytometer (Becton and Dickinson, San Jose, CA, USA), and data from 10,000 events were obtained. The results were expressed as the percentage of cells labeled with PI (apoptotic).
2.8. Statistical Analysis
All data are presented as the mean ± standard deviation (SD). Statistical evaluation of data analysis was performed using SPSS 16.0 for Windows. The differences between the mean values of multiple groups were analyzed by one-way ANOVA, followed by SNK test. P < 0.05 was considered statistically significant.
3. Results
3.1. Protective Effects of Aqueous FLJ Extracts against HQ-Induced Cytotoxicity
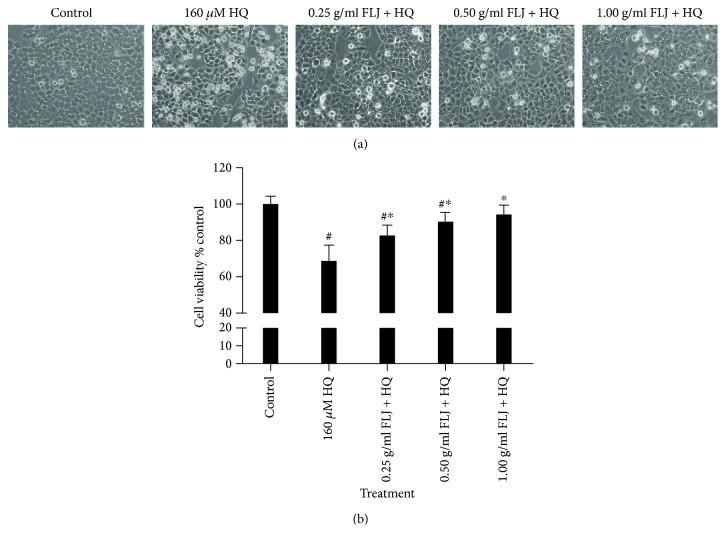
As the first step in determining the necessary HQ concentration to induce cytotoxicity, the viability of L02 cells was assessed by MTT assay. HQ induced a concentration- and time-dependent reduction in L02 cell viability compared with untreated control cells. We administered 160 μmol/L HQ for 12 h as the positive control of cytotoxicity. After 12 h of coexposure to aqueous FLJ extracts at the specified concentrations, the cell significantly increased compared with that in the corresponding HQ-treated groups (Figures 1(a) and 1(b)). These results revealed that aqueous FLJ extracts intervened the HQ-induced cytotoxicity.
Figure 1.
Protective effects of aqueous FLJ extracts against HQ-induced cytotoxicity (a) L02 cells treated with 0 and 160 μM HQ and 0.25, 0.50, and 1.00 g/mL FLJ + HQ for 12 h. Morphological features of L02 cells were observed by inverted microscope. (b) L02 cells treated with 0 and 160 μM HQ and 0.25, 0.50, and 1.00 g/mL FLJ + HQ for 12 h. Cell proliferation was detected by using the MTT proliferating reagent. All data were representative of at least three independent experiments. #P < 0.05 compared with the control. ∗P < 0.05 compared with 160 μM HQ treatment.
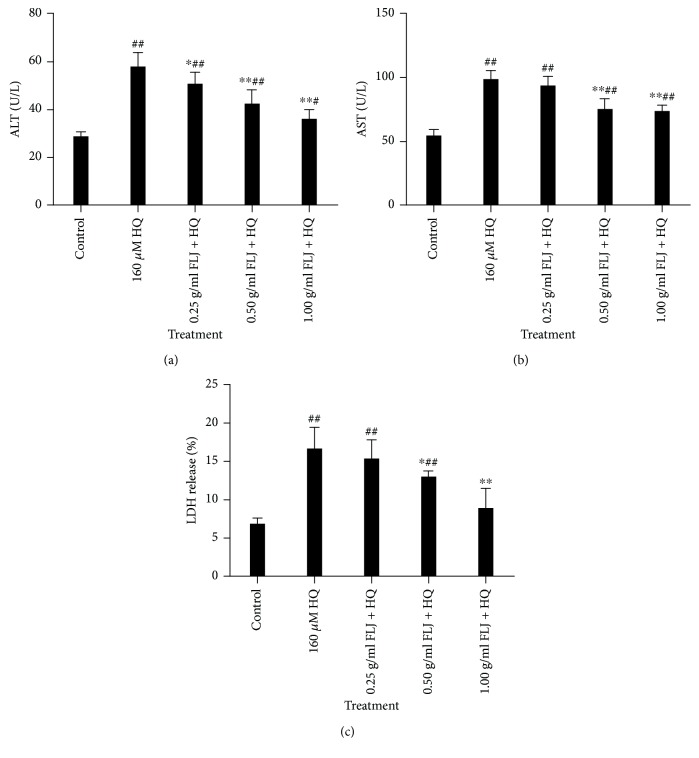
To evaluate the protective effects of aqueous FLJ extracts against HQ, we examined cytotoxicity in terms of the LDH release and the ALT and AST levels. The results showed that aqueous FLJ extracts significantly downregulated LDH release, ALT, and AST in a concentration-dependent manner compared with the corresponding HQ-treated groups (Figure 2). Thus, aqueous FLJ extracts could reverse HQ-induced cytotoxicity.
Figure 2.
Protective effects of aqueous FLJ extracts against HQ-induced cytotoxicity L02 cells treated with 0 and 160 μM HQ and 0.25, 0.50, and 1.00 g/mL FLJ + HQ for 12 h. LDH release and the activities of aminotransferase (ALT) and aspartate aminotransferase (AST) were detected by using commercial kits. All data were representative of at least three independent experiments. #P < 0.05 compared with the control. ∗P < 0.05 compared with 160 μM HQ treatment.
3.2. Protective Effects of Aqueous FLJ Extracts against HQ-Induced Cellular Oxidative Damage
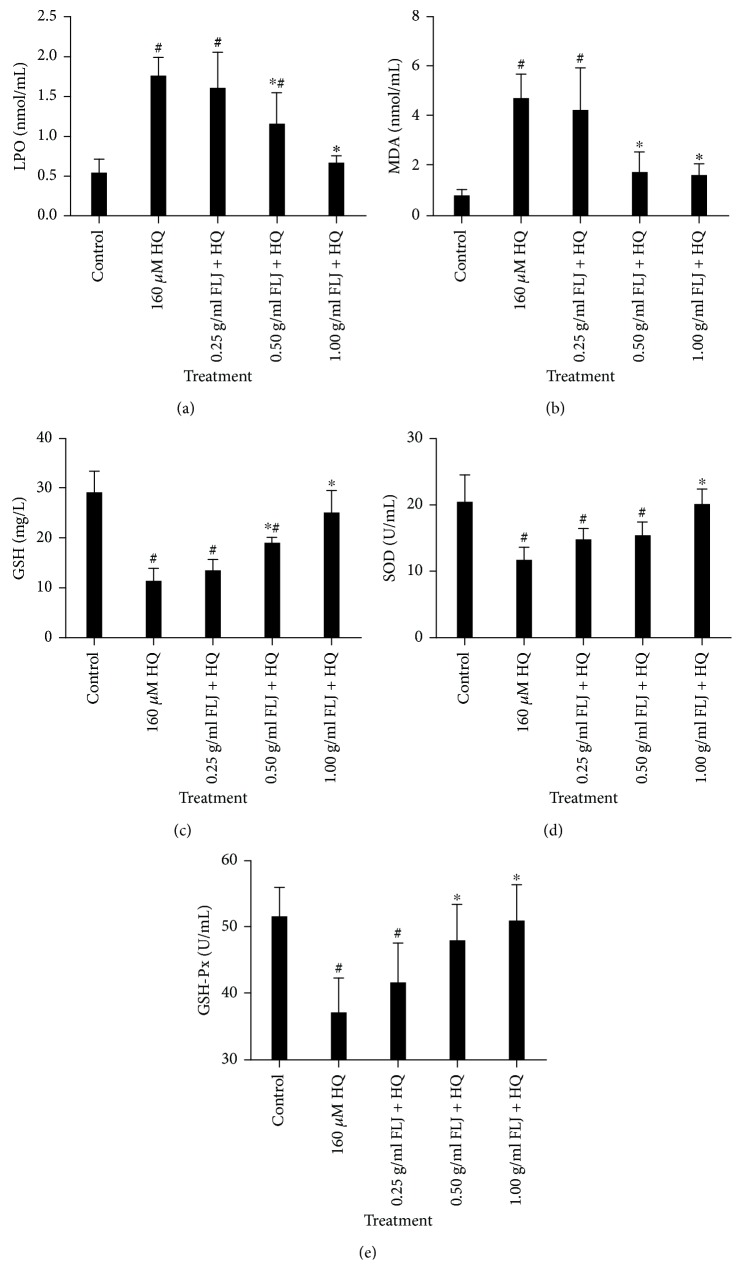
Oxidative stress can mediate apoptosis in various cell models and is considered an important apoptotic signal. Therefore, we investigated whether intracellular oxidative damage is involved in HQ-induced cell death and found that HQ exposure increased MDA and LPO production, but aqueous FLJ extracts reduced the production of MDA and LPO in a concentration-dependent manner. In addition, aqueous FLJ extracts reversed the production of antioxidant enzymes (such as GSH, SOD, and GSH-Px), indicating that aqueous FLJ extracts exerted a protective effect against HQ-induced oxidative stress in L02 cells (Figure 3).
Figure 3.
Protective effects of aqueous FLJ extracts against HQ-induced oxidative damage L02 cells treated with 0 and 160 μM HQ and 0.25, 0.50, and 1.00 g/mL FLJ + HQ for 12 h. MDA and LPO levels as the indexes of lipid peroxidation and the SOD, GSH, and GSH-Px activities as antioxidant enzymes were detected by using commercial kits for oxidative stress. All data were representative of at least three independent experiments. #P < 0.05 compared with the control. ∗P < 0.05 compared with 160 μM HQ treatment.
3.3. Protective Effects of Aqueous FLJ Extracts against HQ-Induced DNA Damage
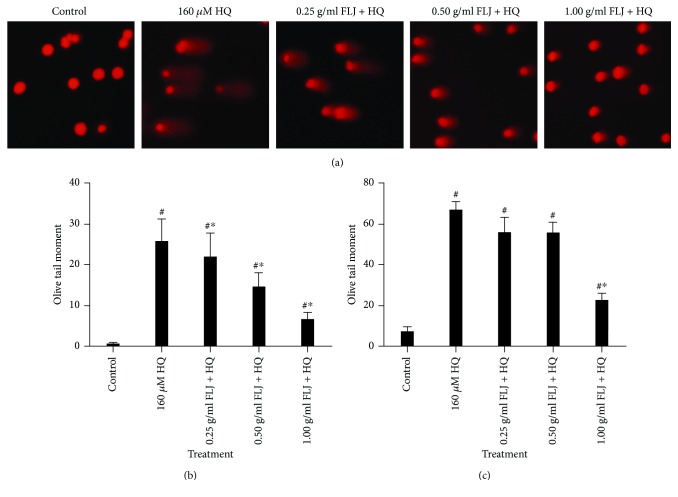
To elucidate the protective effects of aqueous FLJ extracts against HQ-induced DNA damage, L02 cells were exposed to various treatments: 0 and 160 μM HQ and 0.25, 0.5, and 1.0 g/mL FLJ + HQ for 12 h. DNA damage was assessed. We performed comet assay, which is a commonly used indicator of genomic instability and genotoxic exposure. As shown in Figure 4(a), HQ induced a marked increase in DNA damage. However, aqueous FLJ extracts significantly decreased DNA damage compared with the corresponding HQ-treated groups as measured in terms of the tail moment and tail length. To further validate our findings regarding the protective effects of aqueous FLJ extracts against HQ-induced DNA damage, we used HLPC-ECD to measure the level of 8-OHdG, which is a widely used marker of oxidative DNA damage. The results revealed a concentration-dependent decrease in 8-OHdG levels in the samples treated with aqueous FLJ extracts (Figure 4(b)), implying that aqueous FLJ extracts exerted a protective effect against HQ-induced DNA damage.
Figure 4.
Protective effects of aqueous FLJ extracts against HQ-induced DNA damage (a, b) L02 cells treated with 0 and 160 μM HQ and 0.25, 0.50, and 1.00 g/mL FLJ + HQ for 12 h. DNA damage was evaluated by alkaline single-cell gel electrophoresis (comet assay). (c) L02 cells treated with 0 and 160 μM HQ and 0.25, 0.50, and 1.00 g/mL FLJ + HQ for 12 h. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) level as a specific marker of oxidative damage of DNA. 8-OHdG was evaluated using high-performance liquid chromatography with electrochemical detection (HPLC-ECD). All data were representative of at least three independent experiments. #P < 0.05 compared with the control. ∗P < 0.05 compared with 160 μM HQ treatment.
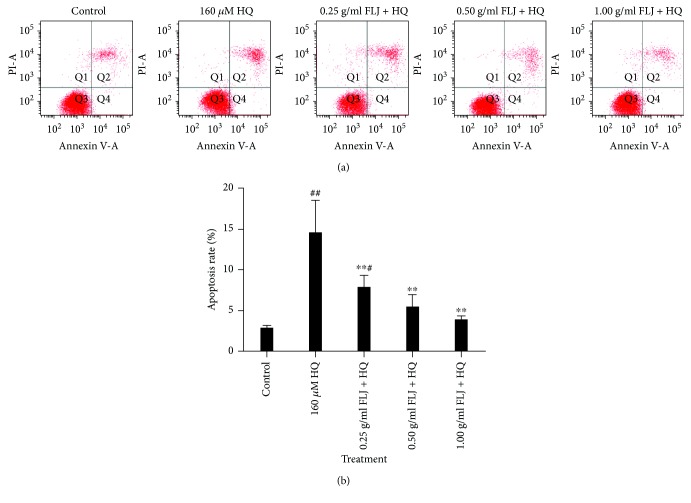
3.4. Protective Effects of Aqueous FLJ Extracts against HQ-Induced Apoptosis
Given that apoptosis is regulated by oxidative stress and DNA damage, the apoptosis rate in the L02 cells was also investigated by flow cytometry using PI assay. The apoptosis rate significantly decreased in the HQ-intoxicated L02 cells compared with that in the control. To further evaluate whether the protective effect of aqueous FLJ extracts on the L02 cells involves cell apoptosis, the L02 cells were incubated with aqueous FLJ extracts and HQ, and the apoptosis rate was then assessed. The results revealed that the apoptosis rate of the group exposed to both aqueous FLJ extracts and HQ was lower than that of the HQ group, indicating that aqueous FLJ extracts played an important role in reducing apoptosis in HQ-exposed L02 cells (Figure 5).
Figure 5.
Protective effects of aqueous FLJ extracts against HQ-induced apoptosis L02 cells treated with 0 and 160 μM HQ and 0.25, 0.50, and 1.00 g/mL FLJ + HQ for 12 h. Apoptosis was assessed by flow cytometry using propidium iodide (PI) assay. All data were representative of at least three independent experiments. #P < 0.05 compared with the control. ∗P < 0.05 compared with 160 μM HQ treatment.
4. Discussion
HQ is a well-known toxicant of liver and induces many effects on the hepatic system. Although many studies have explored HQ, the actual mechanisms underlying these effects remain poorly understood. FLJ, which is also known as Jinyinhua or Japanese honeysuckle, is the dried flower bud or open flower of Lonicera japonica Thunb [22]. It is one of the most popular traditional Chinese medicines, and it has been applied in the healthcare of China and other East Asian countries for a long time. It has been proven to display antioxidant, antiviral, anticarcinogenic, anti-inflammatory, analgesic, antipyretic, and antimicrobial functions [23]. This study focused on the protective effects of aqueous FLJ extracts against HQ-induced cytotoxicity.
Serum ALT and AST levels are used as biochemical markers of liver damage, as the membrane destruction of hepatocytes releases hepatic enzymes, such as ALT and AST, into blood circulation [24]. The water extracts of FLJ containing 20% chlorogenic acid are protective against alcohol-induced chemical liver injury in mice [12, 24], which was similar to the protection conferred by aqueous FLJ extracts to the L02 hepatic cell model induced by HQ.
Oxidative stress plays a critical role in the development of drug-induced liver damage [25] and in HQ-induced toxicity. Intercellular ROS may cause detrimental alterations in cell membranes, DNA, and other cellular structures. ROS are critical intermediates under normal physiological conditions that contribute to pathophysiological events in liver injury. Intracellular oxidative stress balance is crucial in maintaining normal cellular function in response to exogenous and endogenous factors [26]. MDA is a key marker of lipid peroxidation. The progression of liver damage was correlated with oxidative stress, as confirmed by MDA measurements [27]. FLJ polyphenol extracts were protective against the lipid peroxidation of erythrocyte and lipid membranes [28]. Consistent with the literature, the levels of MDA and LPO in our study were significantly higher in the HQ-treated group than in the controls. The levels of MDA and LPO concentrations were lower in aqueous FLJ extracts than in the HQ group.
The primary antioxidant defense systems include SOD and the GSH redox cycle [29]. SOD, which is produced from the mitochondrion electron transfer chain and scavenges superoxide anions, is an important antioxidant enzyme. SOD can transform superoxide anions into H2O2 and catalase and then continuously detoxify them to H2O [30]. The GSH redox cycle, which mainly includes GSH and GPx, modulates the redox-mediated responses of hepatic cells induced by external or intracellular stimulation. GSH is the main nonenzymatic regulator of intracellular redox homeostasis. GSH directly scavenges hydroxyl radicals and is a cofactor in detoxifying hydrogen peroxide, lipid peroxides, and alkyl peroxides. GPx, which is a selenocysteine-containing enzyme, reduces lipid hydroperoxides to their corresponding alcohols and hydrogen peroxide to water in the liver. Therefore, enhancing the hepatic antioxidant system capacity may be an effective therapeutic strategy for alleviating and treating liver damage [25]. Our study showed that the cytotoxicity of HQ is mediated by intracellular oxidative stress and confirmed the protective effects exerted by aqueous FLJ extracts as an antioxidant against HQ-induced cytotoxicity in L02 cells.
DNA damage is caused by multiple factors, including oxidative stress, vitamin B12 deficiency, and ischemia-reperfusion injury [31]. The ratio of 8-OHdG/dG is correlated with the severity of oxidative stress. The high activity of an antioxidant enzyme may be a compensatory regulation in response to increased oxidative stress. In our previous study, we demonstrated that DNA damage is related to HQ-induced hepatotoxicity [11]. ROS causes oxidative stress, which leads to DNA damage. Cells have evolved elaborate mechanisms to respond to DNA damage, at the core of which is the signaling pathway known as the DNA damage checkpoint [32]. This pathway initiates many aspects of the DNA damage response (DDR), including activation of DNA repair and induction of apoptosis [33, 34]. DNA damage is an early event in DDR. Thus, we examined DNA damage through the comet assay and 8-OHdG level, which is a specific marker of oxidative damage of DNA. In different kinds of cells, HQ induces the production of superoxides and hydroperoxides, which are implicated in the initiation and promotion stages of apoptosis and DNA damage. In the present study, although HQ induced DNA damage and apoptosis, we observed a concentration-dependent reverse in 8-OHdG levels, DDBs in the nucleus, and apoptosis in the group treated with aqueous FLJ extracts.
In conclusion, the results of this study strongly suggested that aqueous FLJ extracts played a role in protecting against HQ-induced cytotoxicity. On the basis of our results, we suggest a possible mechanism involved in the protective effects of aqueous FLJ extracts against HQ-induced cytotoxicity. HQ induced MDA and LPO formation, which activated antioxidant enzyme production. Intracellular oxidative stress balance was disturbed, thereby inducing DNA damage and apoptosis and promoting HQ-induced cytotoxicity. However, aqueous FLJ extracts could increase the activation of the antioxidant, which may reduce DNA damage and apoptosis and exert a protective effect against HQ-induced cytotoxicity. The molecular mechanism could further elucidate the antioxidant role of aqueous FLJ extracts.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81460504, 81260434, 81273116, and 81060241), the Traditional Chinese Medicine Scientific Research Project funded by the Health Department of Jiangxi Province (2009A074), the Science and Technology Project funded by the Education Department of Jiangxi Province (GJJ13689, GJJ170862), the Natural Science Foundation of Jiangxi Province (20132BAB205071, 20114BAB205041, and 20142BAB215014), and supported by Doctor Initial Funding for Gannan Medical University (QD201302 and QD201701).
Abbreviations
- DDR:
DNA-damage response
- HQ:
Hydroquinone
- MDA:
Malondialdehyde
- LPO:
Lipid peroxidation
- ROS:
Reactive oxygen species
- FLJ:
Flos lonicerae Japonicae
- LDH:
Lactic dehydrogenase
- ALT:
Alanine transaminase
- AST:
Aspartate aminotransferase
- PI:
Propidium iodide
- HPLC-EC:
High-performance liquid chromatography with electrochemical
- DSBs:
DNA double-strand breaks
- 8-OHdG:
8-hydroxy-2′-deoxyguanosine.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
Yanfang Gao, Huanwen Tang, and Liang Xiong contributed equally to this work.
References
- 1.Chen S., Liang H., Hu G., et al. Differently expressed long noncoding RNAs and mRNAs in TK6 cells exposed to low dose hydroquinone. Oncotarget. 2017;8(56):95554–95567. doi: 10.18632/oncotarget.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebeda C. B., Pinedo F. J., Bolonheis S. M., et al. Intracellular mechanisms of hydroquinone toxicity on endotoxin-activated neutrophils. Archives of Toxicology. 2012;86(11):1773–1781. doi: 10.1007/s00204-012-0886-3. [DOI] [PubMed] [Google Scholar]
- 3.Inayat-Hussain S. H., Ibrahim H. A., Siew E. L., et al. Modulation of the benzene metabolite hydroquinone induced toxicity: evidence for an important role of fau. Chemico-Biological Interactions. 2010;184(1-2):310–312. doi: 10.1016/j.cbi.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Enguita F. J., Leitao A. L. Hydroquinone: environmental pollution, toxicity, and microbial answers. BioMed Research International. 2013;2013:14. doi: 10.1155/2013/542168.542168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z., Wang C., Zhu J., et al. The possible role of liver kinase B1 in hydroquinone-induced toxicity of murine fetal liver and bone marrow hematopoietic stem cells. Environmental Toxicology. 2016;31(7):830–841. doi: 10.1002/tox.22094. [DOI] [PubMed] [Google Scholar]
- 6.Liu J., Yuan Q., Ling X., et al. PARP-1 may be involved in hydroquinone-induced apoptosis by poly ADP-ribosylation of ZO-2. Molecular Medicine Reports. 2017;16(6):8076–8084. doi: 10.3892/mmr.2017.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J., Wang H., Yang S., et al. Comparison of toxicity of benzene metabolite hydroquinone in hematopoietic stem cells derived from murine embryonic yolk sac and adult bone marrow. PLoS One. 2013;8(8, article e71153) doi: 10.1371/journal.pone.0071153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahadar H., Maqbool F., Mostafalou S., et al. The molecular mechanisms of liver and islets of Langerhans toxicity by benzene and its metabolite hydroquinone in vivo and in vitro. Toxicology Mechanisms and Methods. 2015;25(8):628–636. doi: 10.3109/15376516.2015.1053650. [DOI] [PubMed] [Google Scholar]
- 9.Hu G. H., Zhuang Z. X., Huang H. Y., Yu L., Yuan J. H., Yang L. Q. Effect of hydroquinone on expression of ubiquitin-ligating enzyme Rad18 in human L-02 hepatic cells. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2009;27(4):222–225. [PubMed] [Google Scholar]
- 10.Hu G. H., Zhuang Z. X., Huang H. Y., Yu L., Yang L. Q., Ji W. D. Relationship between polymerase eta expression and DNA damage-tolerance in human hepatic cells by hydroquinone. Zhonghua Yu Fang Yi Xue Za Zhi. 2009;43(1):56–60. [PubMed] [Google Scholar]
- 11.Hu G., Huang H., Yang L., et al. Down-regulation of Polη expression leads to increased DNA damage, apoptosis and enhanced S phase arrest in L-02 cells exposed to hydroquinone. Toxicology Letters. 2012;214(2):209–217. doi: 10.1016/j.toxlet.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Nam Y., Lee J. M., Wang Y., Ha H. S., Sohn U. D. The effect of Flos Lonicerae Japonicae extract on gastro-intestinal motility function. Journal of Ethnopharmacology. 2016;179:280–290. doi: 10.1016/j.jep.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y. P., Lin L. G., Wang Y. T. Chemistry and pharmacology of the herb pair Flos Lonicerae japonicae-Forsythiae fructus. Chinese Medicine. 2015;10(1):p. 16. doi: 10.1186/s13020-015-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W., Yin A., Shan J., Wang S., Cai B., Di L. Study on the rationality for antiviral activity of Flos Lonicerae Japonicae-Fructus Forsythiae herb couple preparations improved by chito-oligosaccharide via integral pharmacokinetics. Molecules. 2017;22(4):p. 654. doi: 10.3390/molecules22040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y., Wang L., Wu Y., et al. On-line monitoring of extraction process of Flos Lonicerae Japonicae using near infrared spectroscopy combined with synergy interval PLS and genetic algorithm. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2017;182:73–80. doi: 10.1016/j.saa.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhou W., Shan J., Wang S., Cai B., di L. Transepithelial transport of phenolic acids in Flos Lonicerae Japonicae in intestinal Caco-2 cell monolayers. Food & Function. 2015;6(9):3072–3080. doi: 10.1039/C5FO00358J. [DOI] [PubMed] [Google Scholar]
- 17.Kao S. T., Liu C. J., Yeh C. C. Protective and immunomodulatory effect of flos Lonicerae japonicae by augmenting IL-10 expression in a murine model of acute lung inflammation. Journal of Ethnopharmacology. 2015;168:108–115. doi: 10.1016/j.jep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Cai W., Weng X., et al. Lonicerae Japonicae Flos and Lonicerae Flos: a systematic pharmacology review. Evidence-Based Complementary and Alternative Medicine. 2015;2015:16. doi: 10.1155/2015/905063.905063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng B. C. Y., Yu H., Guo H., et al. A herbal formula comprising Rosae Multiflorae Fructus and Lonicerae Japonicae Flos, attenuates collagen-induced arthritis and inhibits TLR4 signalling in rats. Scientific Reports. 2016;6(1, article 20042) doi: 10.1038/srep20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang J. W., Yun N., Han H. J., Kim J. Y., Kim J. Y., Lee S. M. Protective effect of Flos Lonicerae against experimental gastric ulcers in rats: mechanisms of antioxidant and anti-inflammatory action. Evidence-Based Complementary and Alternative Medicine. 2014;2014:11. doi: 10.1155/2014/596920.596920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaya Y., Çebi A., Söylemez N., Demir H., Alp H. H., Bakan E. Correlations between oxidative DNA damage, oxidative stress and coenzyme Q10 in patients with coronary artery disease. International Journal of Medical Sciences. 2012;9(8):621–626. doi: 10.7150/ijms.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Wang Y., Xue J., Wang P., Shi S. Dietary exposure risk assessment of flonicamid and its effect on constituents after application in Lonicerae Japonicae Flos. Chemical and Pharmaceutical Bulletin. 2018;66(6):608–611. doi: 10.1248/cpb.c17-00985. [DOI] [PubMed] [Google Scholar]
- 23.Hu Z., Lausted C., Yoo H., et al. Quantitative liver-specific protein fingerprint in blood: a signature for hepatotoxicity. Theranostics. 2014;4(2):215–228. doi: 10.7150/thno.7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fomenko S. E., Kushnerova N. F., Sprygin V. G., Momot T. V. Hepatorotective activity of honeysuckle fruit extract in carbon tetrachloride intoxicated rats. Eksperimental'naia i Klinicheskaia Farmakologiia. 2014;77(10):26–30. [PubMed] [Google Scholar]
- 25.Arauz J., Ramos-Tovar E., Muriel P. Redox state and methods to evaluate oxidative stress in liver damage: from bench to bedside. Annals of Hepatology. 2016;15(2):160–173. doi: 10.5604/16652681.1193701. [DOI] [PubMed] [Google Scholar]
- 26.Wang C., Harwood J. D., Zhang Q. Oxidative stress and DNA damage in common carp (Cyprinus carpio) exposed to the herbicide mesotrione. Chemosphere. 2018;193:1080–1086. doi: 10.1016/j.chemosphere.2017.11.148. [DOI] [PubMed] [Google Scholar]
- 27.Ullah S., Li Z., Hasan Z., Khan S. U., Fahad S. Malathion induced oxidative stress leads to histopathological and biochemical toxicity in the liver of rohu (Labeo rohita, Hamilton) at acute concentration. Ecotoxicology and Environmental Safety. 2018;161:270–280. doi: 10.1016/j.ecoenv.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Bonarska-Kujawa D., Pruchnik H., Cyboran S., Żyłka R., Oszmiański J., Kleszczyńska H. Biophysical mechanism of the protective effect of blue honeysuckle (Lonicera caerulea L. var. kamtschatica Sevast.) polyphenols extracts against lipid peroxidation of erythrocyte and lipid membranes. The Journal of Membrane Biology. 2014;247(7):611–625. doi: 10.1007/s00232-014-9677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukai T., Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants & Redox Signaling. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nojima Y., Ito K., Ono H., et al. Superoxide dismutases, SOD1 and SOD2, play a distinct role in the fat body during pupation in silkworm Bombyx mori. PLoS One. 2015;10(2, article e0116007) doi: 10.1371/journal.pone.0116007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakabeppu Y., Sakumi K., Sakamoto K., Tsuchimoto D., Tsuzuki T., Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biological Chemistry. 2006;387(4):373–379. doi: 10.1515/BC.2006.050. [DOI] [PubMed] [Google Scholar]
- 32.Park C., Hong S., Shin S., et al. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of Sargassum serratifolium extract against oxidative stress-induced DNA damage and apoptosis in SW1353 human chondrocytes. International Journal of Environmental Research and Public Health. 2018;15(6, article 1173) doi: 10.3390/ijerph15061173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan S., Zafar A., Naseem I. Copper-redox cycling by coumarin-di(2-picolyl)amine hybrid molecule leads to ROS-mediated DNA damage and apoptosis: a mechanism for cancer chemoprevention. Chemico-Biological Interactions. 2018;290:64–76. doi: 10.1016/j.cbi.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Liu D., Li R., Guo X., et al. DNA damage regulated autophagy modulator 1 recovers the function of apoptosis-stimulating of p53 protein 2 on inducing apoptotic cell death in Huh7.5 cells. Oncology Letters. 2018;15(6):9333–9338. doi: 10.3892/ol.2018.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.