Abstract
Purpose
To summarize the characteristics and the relevant factors and to give references for preventing adverse drug reactions (ADRs) associated with xiyanping (XYP), we provide a systematic review of adverse case reports about XYP.
Methods
Seven medical databases were searched from inception to January 2018. Case reports detailing ADRs associated with XYP were included. Data were extracted independently by two reviewers. After the assessment of causality and severity, we carried out a descriptive analysis for the relevant ADRs.
Results
Forty-three articles involving a total number of 55 cases were included. Eight cases were off-label drug use. In the remaining 47 cases, 26 (55.3%) had probable causality and 23 (48.9%) were serious cases. XYP used in children (≤14 years old) accounted for 66.0%. Respiratory diseases (83.0%) were major primary diseases. No allergic history mentioned (55.3%) and unspecific drug combination (59.6%) were common in these reports. As for ADR types, anaphylaxis and anaphylactic shock were up to 97.9%. ADRs happened mostly when applying XYP within 30 minutes (70.2%) and the majority (95.7%) were cured when treated in time.
Conclusions
Clinicians and patients are supposed to obey the package insert of XYP in clinical application. Through the results of XYP, normalization of ADR reports is also worthy of attention. High-quality researches are required to improve the drug instruction and evaluate the safety of XYP in effective diseases and different age groups. Mechanism of ADRs aiming at the hypersensitivity and the drug combination should still be further identified.
1. Introduction
Xiyanping (XYP), a traditional Chinese medicine available in injection form, is used for the treatment of bronchitis, hand, foot, and mouth disease, bacillary dysentery, and other infectious diseases in China [1–4]. The sole active ingredient of XYP is the water-soluble andrographolide [5], which is a diterpenoid lactone from the traditional Chinese medicinal herb Andrographis paniculata [3]. Data have showed the effects of this sulfonated andrographolide in sepsis, acute lung injury, the associated antimicrobial, antiviral, and other anti-inflammatory actions in representative studies [5–8]. In China, XYP has been a topic [9, 10] since the safety impacted factors are not completely clear and the mechanism of ADRs is not validated. Therefore, it is imperative to conduct comprehensive assessment of ADRs associated with XYP using rigorous systematic review of case reports [11] to fully understand the scope of safety of XYP and the prevention measures in clinical use.
2. Methods
2.1. Study Identification
We searched PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP), Chinese Biomedical Literature Database (CBM), and Wanfang Data from database inception to 9 January 2018 for eligible case reports with information regarding ADRs related to XYP in humans published peer-reviewed journals. No restrictions on language were imposed. The search terms, in both English and Simplified Chinese, included “andrographolide sulfonate”, “xiyanping”, “adverse drug reaction”, “adverse effect”, “side-effect”, “anaphylaxis”, “allergic reaction”, “safety”, “toxicity”.
2.2. Study Selection and Data Extraction
Two reviewers (Shiqi Chen and Rui Zheng) independently conducted the study selection and data extraction. Firstly, we removed the duplicates of all papers through searching. Then, we examined the titles and abstracts according to the inclusion criteria. Finally, the full texts were retrieved for detailed assessment. A standardized data extraction form was used to collect data of included studies, including title, first author, year of publication, drug batch number, admission time, age, gender, primary disease, allergic history, solvent, dosage, drug combination and mixture, dripping speed, ADR type, ADR symptom, occurrence time, ADR treatment, and prognosis. Disagreements were resolved through discussion or by consulting a third reviewer (Hongcai Shang).
2.3. Criteria of ADR, Causality, and Severity
Two investigators (Shiqi Chen and Rui Zheng) evaluated ADRs of included studies according to the criteria of ADR, causality, and severity. The definition of ADR by China Food and Drug Administration is “an unintended and unfavorable outcome that occurs during or after the normal use of a qualified pharmaceutical” [12]. Further investigation was required to clarify any casual relationship between the case report findings and XYP. The reports that were not coincident with the definition of ADR or irrelevant with XYP were not analyzed.
The causality categories described by the WHO Collaborating Centre for International Drug Monitoring (The Uppsala Monitoring Centre) [13, 14] are classified into 6 levels: certain, probable, possible, unlikely, conditional/unclassified, and unassessable/unclassifiable ADRs related to the drug. The results consist of 5 aspects: (1) the clinical events with laboratory test abnormality; (2) the plausible time between drug use and ADR; (3) the plausible explanations provided by other drugs, chemicals, or underlying diseases; (4) the response to withdrawal of the drug (dechallenge); and (5) the rechallenge information. The detailed classification standard is showed in Table 1 [14].
Table 1.
The causality categories described by the WHO Collaboration Center for International Drug Monitoring (The Uppsala Monitoring Centre) [14].
| Degrees | Definitions |
|---|---|
| Certain | A clinical event, including laboratory test abnormality, occurring in a plausible time relationship to drug administration, and which cannot be explained by concurrent disease or other drugs or chemicals. The response to withdrawal of the drug (dechallenge) should be clinically plausible. The event must be definitive pharmacologically or phenomenologically, using a satisfactory rechallenge procedure if necessary. |
|
| |
| Probable/Likely | A clinical event, including laboratory test abnormality, with a reasonable time sequence to administration of the drug, unlikely to be attributed to concurrent disease or other drugs or chemicals, and which follows a clinically reasonable response on withdrawal (dechallenge). Rechallenge information is not required to fulfil this definition. |
|
| |
| Possible | A clinical event, including laboratory test abnormality, with a reasonable time sequence to administrations of the drug, but which could also be explained by concurrent disease or other drugs or chemicals. Information on drug withdrawal may be lacking or unclear. |
|
| |
| Unlikely | A clinical event, including laboratory test abnormality, with a temporal relationship to drug administration which makes a causal relationship improbable, and in which other drugs, chemicals or underlying disease provide plausible explanations. |
|
| |
| Conditional/Unclassified | A clinical event, including laboratory test abnormality, reported as an adverse reaction, about which more data is essential for a proper assessment, or the additional data is under examination. |
|
| |
| Unassessable/Unclassifiable | A report suggesting an adverse reaction which cannot be judged because information is insufficient or contradictory, and which cannot be supplemented or verified. |
Serious ADRs can be identified as one of the following 6 reasons [12]: (1) lethality; (2) life-threatening; (3) carcinogenesis, teratogenesis, or birth defects; (4) conspicuous or permanent injuries or organ dysfunctions; (5) prolonged length of hospitalization; and (6) other serious medical incidents which can progress to (1)-(5) consequences above.
2.4. Data Analysis
Descriptive analysis was applied to the included studies involving 5 aspects: (1) age and gender; (2) primary diseases and allergic history; (3) mixture and combination of drugs; (4) occurrence time and type of ADR; and (5) prognosis of ADR.
3. Results
3.1. Studies Identified and Characteristics
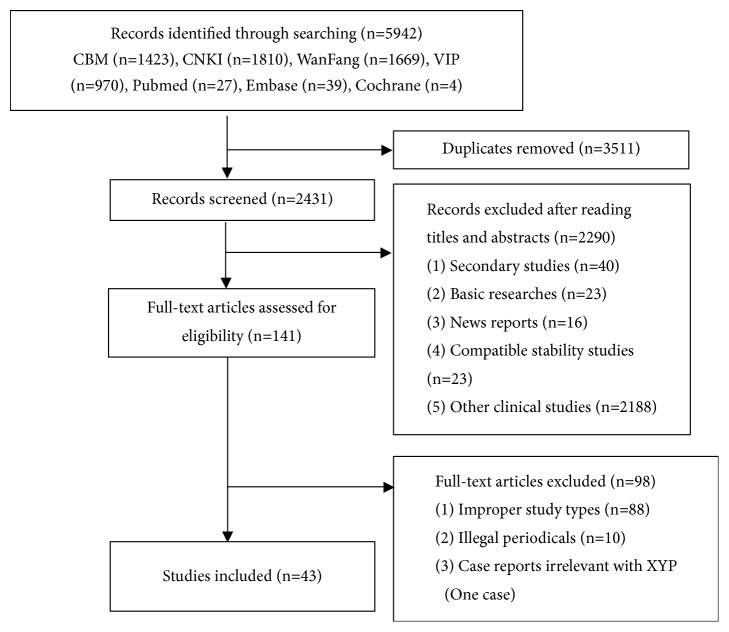
The initial database search yielded 5942 records. After removing 3511 duplicates, 2431 records were screened for eligibility. Of these, full-text articles of 141 studies were obtained for further assessment. We eventually included 43 reports (involving 55 cases). Our study selection process is illustrated in Figure 1. The characteristics of the case reports are shown in Table 2.
Figure 1.
Process of searching and screening studies.
Table 2.
Patient details recorded from the included studies.
| Case report | Drug batch number | Occurrence time (year/month/ day) | Age/Gender (years/female or male) | Primary disease | Dosage (mL) | Dripping speed (drops per minute) | Drug Delivery | Solvent | Combination | Mixture | Allergic history | Medication interval | ADR type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Danzhu Huang et al. [15] | NA | 2008.6.19 | 0.75/M | Diarrhea | NA | NA | i.v.gtt. | NA | NA | No | NA | NA | Anaphylactic shock |
| Wenlan Huang et al. [16] | NA | 2015.2.1 | 65/F | Upper respiratory tract infections | 10 | 25 | i.v.gtt. | ② | NA | No | No | 2min | Anaphylactic shock |
| Bo Chen et al. [17] | 20070606 | NA | 28/F | Acute bacillary dysentery | 12 | NA | i.v.gtt. | ① | No | No | Cephalosporin | 8min | Anaphylactic shock |
| Xiangyu Di et al. [18] | NA | NA | 7/M | Mycoplasma pneumonia | 4 | NA | i.v.gtt. | ③ | NA | No | NA | NA | Anaphylaxis |
| Hong Lai et al. [19] | NA | 2012.7 | 6/M | Upper respiratory tract infections | NA | NA | i.v.gtt. | NA | Ceftezole | No | No | NA | Anaphylaxis |
| Zhongying Jian et al. [20] | NA | NA | 80/M | Chronic bronchitis | 8 | 50 | i.v.gtt. | ② | NA | No | No | 2min | Anaphylaxis |
| Zhong Miao et al. [21] | 2014031103 | NA | 1/M | Bronchitis | 2 | NA | i.v.gtt. | ① | Mezlocillin sodium | No | No | After the infusion | Anaphylactic shock |
| Shujun Hu et al. [22] | NA | NA | 8/M | Upper respiratory tract infections | 4 | 40 | i.v.gtt. | ① | NA | No | NA | 5min | Anaphylactic shock |
| Yingyun Shen et al. [23] | 20081203 | NA | 8/M | Upper respiratory tract infections | 4 | NA | i.v.gtt. | ① | NA | No | NA | 50mL remained | Anaphylaxis |
| Yanli Li et al. [24] | 20120904 | 2012.10.15 | 2/M | Asthmatic bronchitis | 3 | NA | i.v.gtt. | NA | No | No | NA | 20min | Anaphylactic shock (vegetative state) |
| Hongmei Li et al. [25] | NA | 2012.11.23 | 9/M | Upper respiratory tract infections | 3 | NA | i.v.gtt. | ① | NA | No | NA | 50mL remained | Anaphylactic shock |
| Xiangping Li et al. [26] | 20050708 | 2005.12.18 | 6/M | Upper respiratory tract infections | 2 | NA | i.m. | No | No | No | NA | 5min | Anaphylaxis |
| Wenhua Zhang et al. [27] | NA | NA | 15/F | Upper respiratory tract infections | 6 | NA | i.v.gtt. | ① | NA | No | NA | 1/4 infused | Anaphylaxis |
| Yuzhi Zuo et al. [28] | 051133708 | 2007.10.12 | 10/M | Fever of unknown origin | 6 | NA | i.v.gtt. | ① | Penicillin, energy mixture | No | NA | 30min | Anaphylaxis |
| Xinhua An et al. [29] | 2013122303 | 2014.2.7 | 3/F | Acute bronchitis | 2 | 30 | i.v.gtt. | ① | Cefuroxime sodium, ribavirin injection | No | No | 5min | Anaphylactic shock |
| Jihong Sun et al. [30] | NA | NA | 11/M | Amygdalitis | 4 | NA | i.v.gtt. | NA | Cefotaxime, Xingnaojing injection | No | No | Time of using Xingnaojing injection (after XYP) | Anaphylaxis |
| Yuxin Liu et al. [31] | NA | 2014.3.20 | 53/F | Amygdalitis | 10 | 60 | i.v.gtt. | ① | Azithromycin | No | No | NA | Convulsion of unknown origion |
| Kunna Xiu et al. [32] | NA | NA | NA/F | Upper respiratory tract infections | 5 | NA | i.v.gtt. | ① | NA | No | NA | 2min | Anaphylaxis |
| Hong Yu et al. [33] | NA | 2002.12.31 | 60/M | Scald with infections | 12 | NA | i.v.gtt. | NA | NA | No | Sulfonamides | 3h | Anaphylactic shock (death) |
| Xiaolei Chen et al. [34] | 20090914 | 2010.1.17 | 34/F | Upper respiratory tract infections | 4 | NA | i.v.gtt. | ① | No | No | No | 5min | Anaphylactic shock |
| Xiwei Feng et al. [35] | NA | NA | 2/F | Bronchopneumonia | 2 | NA | i.v.gtt. | ① | NA | No | NA | 15min | Anaphylaxis |
| Hualing Qiao et al. [36] | NA | NA | 16/F | Upper respiratory tract infections | 8 | NA | i.v.gtt. | ① | NA | No | NA | 10min | Anaphylactic shock |
| Taiyu Wan et al. [37] | NA | 2001.7.3 | 30/F | Upper respiratory tract infections | 10 | 40 | i.v.gtt. | ④ | Dexamethasone, VC | No | NA | 2min | Anaphylactic shock |
| Xiaojie Hao et al. [38] | NA | NA | 5/F | Upper respiratory tract infections | 3 | NA | i.v.gtt. | ③ | NA | No | No | 5min | Anaphylactic shock |
| Xiaoru He et al. [39] | 20100603 | NA | 10/F | Mycoplasma pneumonia | 6 | NA | i.v.gtt. | ① | Azithromycin, cefoperazone | No | NA | 20min | Anaphylaxis |
| Cuiling Wang et al. [40] | NA | 2005.6.5 | 3.5/M | Adenomesenteritis | 2 | NA | i.v.gtt. | NA | No | No | Cephalosporin | 5min | Anaphylaxis |
| Liqiang Wu et al. [41] | 2013020602 | 2013.9.19 | 39/M | Upper respiratory tract infections | 12 | NA | i.v.gtt. | ① | NA | No | No | 5min | Anaphylactic shock |
| Xiaoqiang Xiong et al. [42] | 20111024 | 2012.2.16 | 3/M | Amygdalitis | 2 | NA | i.v.gtt. | ① | NA | No | NA | 15min | Anaphylactic shock |
| Fengshu Zhang et al. [43] | 20090911 | 2009.2.6 | 1/F | Upper respiratory tract infections | 2 | 45 | i.v.gtt. | ① | NA | No | No | 8min | Anaphylactic shock |
| Guixin Zhang et al. [44] | NA | 2008.2.18 | 3.5/F | Amygdalitis | 4 | NA | i.v.gtt. | ① | NA | No | No | 5min | Anaphylaxis |
| Yunying Zhang et al. [45] | 20090715 | NA | 51/F | Pneumonia | 8 | NA | i.v.gtt. | ① | NA | No | No | 20min | Anaphylactic shock |
| Fang Dong et al. [46] | NA | NA | 12/M | Amygdalitis | 8 | NA | i.v.gtt. | ① | NA | No | NA | 10min | Anaphylactic shock |
| Fang Dong et al. [47] | 060417 | NA | 0.83/M | Bronchitis | 2 | NA | i.v.gtt. | ① | NA | No | NA | 5min | Anaphylaxis |
| Xuejin Zhao et al. [48] | 20040918 | 2004.10.18 | 58/F | Chronic bronchitis | 8 | 50 | i.v.gtt. | ② | No | No | No | 5min | Anaphylaxis |
| Li Zheng et al. [49] | 20131015 | NA | 55/F | Pneumonia | 8 | NA | i.v.gtt. | ① | NA | No | No | 20min | Anaphylactic shock |
| Li Zheng et al. [49] | 20140105 | NA | 4/M | Bronchitis | 4 | NA | i.v.gtt. | ① | NA | No | Yanhuning injection | 10min | Anaphylactic shock |
| Liwei Yang et al. [50] | NA | 2012.3.29 | 4/F | Bronchitis | 2 | NA | i.v.gtt. | ① | NA | No | NA | NA | Anaphylaxis |
| Liwei Yang et al. [50] | NA | 2012.8.6 | 7/M | Pneumonia | 4 | NA | i.v.gtt. | ① | NA | No | NA | 20min | Anaphylaxis |
| Xiuju Wang et al. [51] | NA | 2014.6.9 | 64/F | Pneumonia | 20 | NA | i.v.gtt. | ① | NA | No | Penicillins | 15min | Anaphylaxis |
| Xiuju Wang et al. [51] | NA | 2014.6.23 | 35/F | Bronchiectasia with infections | 20 | NA | i.v.gtt. | ① | NA | No | Cephalosporin | 20min | Anaphylaxis |
| Lijuan Wang et al. [52] | NA | 2015.4 | 2/F | Bronchopneumonia | 2 | NA | i.v.gtt. | ① | NA | No | No | 20min | Anaphylaxis |
| Lijuan Wang et al. [52] | NA | 2015.5 | 1.5/M | Upper respiratory tract infections | NA | NA | i.v.gtt. | NA | NA | No | No | 5min | Anaphylaxis |
| Xing Du et al. [53] | 20050721 | NA | 31/F | Upper respiratory tract infections | 4 | NA | i.v.gtt. | ① | Azithromycin | No | NA | 35min | Anaphylaxis |
| Xing Du et al. [53] | 20050721 | NA | 42/M | Acute bronchitis | 4 | NA | i.v.gtt. | ① | Penicillin | No | NA | 3min | Anaphylaxis |
| Xing Du et al. [53] | 20050721 | NA | 4.5/F | Upper respiratory tract infections | 2 | NA | i.v.gtt. | ① | Azithromycin | No | NA | 3min | Anaphylaxis |
| Tian Gao et al. [54] | NA | NA | 55/F | Severe pneumonia | 10 | 30 | i.v.gtt. | ④ | Cefpirome | No | No | 70min | Abdominal distension |
| Tian Gao et al. [54] | NA | NA | 16/M | Psoriasis | 8 | 30 | i.v.gtt. | ① | 10% calcium gluconate injection, VC | No | No | 100mL remained | Dizziness of unknown origin |
| Zhongli Zhang et al. [55] | NA | NA | 4/F | Emesis and hypogastralgia | 4 | NA | i.v.gtt. | ② | NA | No | NA | 30s | Anaphylaxis |
| Zhongli Zhang et al. [55] | NA | NA | 2/F | Fever of unknown origin | 4 | NA | i.v.gtt. | ① | NA | No | NA | 1min | Anaphylaxis |
| Wei Zhu et al. [56] | 20121028 | 2013.3.25 | 40/M | Upper respiratory tract infections | 8 | NA | i.v.gtt. | ② | NA | No | No | 150mL infused | Anaphylaxis |
| Wei Zhu et al. [56] | 20130202 | 2013.5.25 | 10/F | Bronchial asthma | 2 | NA | i.v.gtt. | ① | NA | No | Shrimp | 60mL infused | Anaphylaxis |
| Renze Yang et al. [57] | 070118 | NA | 6/M | Upper respiratory tract infections | 2 | NA | i.v.gtt. | ① | Azithromycin | No | NA | 4min | Anaphylaxis |
| Renze Yang et al. [57] | 070118 | NA | 5/M | Upper respiratory tract infections | 2 | NA | i.v.gtt. | ① | Ceftriaxone sodium | No | NA | 40mL infused | Anaphylaxis |
| Renze Yang et al. [57] | 070118 | NA | 9/M | Amygdalitis | 2 | NA | i.v.gtt. | ① | Cefuroxime axetil | No | NA | 10min | Anaphylaxis |
| Renze Yang et al. [57] | 070118 | NA | 8/F | Bronchitis | 2 | NA | i.v.gtt. | ① | Roxithromycin | No | NA | 3min | Anaphylaxis |
①5% glucose solution (5% GS); ②normal saline (N.S.); ③10% glucose solution (10% GS); ④5% glucose and sodium chloride injection.
NA: not available; i.v.gtt.: injectio venosa gutta; i.m.: intramuscular; VC: vitamin C injection.
3.2. Assessment Results of ADR, Causality, and Severity
Administration of XYP in 8 cases was found to not be in line with the package inserts of XYP: 4 were outside of the recommended dripping speed and 4 used improper solvents; thus they were excluded from our ADR analysis. After the assessment of causality and severity for the remaining 47 cases (reported in 36 articles), the results of causality showed that 26 (26/47, 55.3%) were probable and 21 (21/47, 44.7%) were possible. For the severity, it showed that 23 (23/47, 48.9%) were serious ADRs while 24 (24/47, 51.1%) were general ADRs.
3.2.1. Primary Diseases and Allergic History
Respiratory diseases (83.0%, 39/47) were major primary diseases, including upper respiratory tract infections, acute/chronic bronchitis, bronchopneumonia, amygdalitis, pneumonia, bronchial asthma, bronchiectasia with infections, and mycoplasma pneumonia. 4.3% (2/47) were fever of unknown origin. The rest are digestive and skin diseases, comprising about 12.8% (6/47). The details were given in Table 3.
Table 3.
The primary disease of taking XYP of ADR case reports.
| Primary disease | Number of patients | Percentage |
|---|---|---|
| Upper respiratory tract infections | 17 | 36.2% |
| Acute/chronic bronchitis | 8 | 17.0% |
| Bronchopneumonia | 2 | 4.3% |
| Amygdalitis | 5 | 10.6% |
| Pneumonia | 4 | 8.5% |
| Bronchial asthma | 1 | 2.1% |
| Bronchiectasia with infections | 1 | 2.1% |
| Mycoplasma pneumonia | 1 | 2.1% |
| Acute/chronic bacillary dysentery | 1 | 2.1% |
| Diarrhea | 1 | 2.1% |
| Emesis and hypogastralgia | 1 | 2.1% |
| Adenomesenteritis | 1 | 2.1% |
| Scald with infections | 1 | 2.1% |
| Psoriasis | 1 | 2.1% |
| Fever of unknown origin | 2 | 4.3% |
| Total | 47 | 100% |
In 47 cases, 26 were unspecific in allergic history, making up 55.3% (26/47). The patients of nonallergic history were 14 (14/47, 29.8%). The other 7 cases (7/47, 14.9%) had the allergic history. Detailed data were shown in Table 4.
Table 4.
The allergic history of taking XYP of ADR case reports.
| Allergic history | Number of patients | Percentage |
|---|---|---|
| Not available | 26 | 55.3% |
| No allergic history | 14 | 29.8% |
| Cephalosporin | 3 | 6.4% |
| Penicillins | 1 | 2.1% |
| Sulfonamides | 1 | 2.1% |
| Yanhuning injection † | 1 | 2.1% |
| Shrimp | 1 | 2.1% |
| Total | 47 | 100% |
† Yanhuning injection and XYP injection are both andrographis preparations.
3.2.2. Mixture and Combination of Drugs
All included ADR cases of XYP failed to report the mixture of drugs. There were 28 (28/47, 59.6%) cases which were unclear of the combination. In terms of the combination use, 3 drugs were 5 (5/47, 10.6%) and 2 drugs were 9 (9/47, 19.1%). The remaining 5 cases (5/47, 10.6%) were single use with XYP injection. Details of other combined drugs were shown in Table 5.
Table 5.
The drug combination of taking XYP of ADR case reports.
| Drug combination | Number/percentage | |
|---|---|---|
| Antibiotics | Azithromycin | 4(21.1%) |
| Penicillin | 2(10.5%) | |
| Cefuroxime | 2(10.5%) | |
| Cefoperazone | 1(5.3%) | |
| Ceftriaxone sodium | 1(5.3%) | |
| Ceftezole | 1(5.3%) | |
| Roxithromycin | 1(5.3%) | |
| Mezlocillin sodium | 1(5.3%) | |
| Cefotaxime | 1(5.3%) | |
| Antiviral drug | Ribavirin Injection | 1(5.3%) |
| Nutrient | Energy mixture | 1(5.3%) |
| Vitamin C Injection | 1(5.3%) | |
| Antiallergic drug | 10% calcium gluconate injection | 1(5.3%) |
| Other traditional Chinese medicine injection (TCMI) | Xingnaojing injection | 1(5.3%) |
| Total | 19(100%) | |
3.2.3. Occurrence Time and Types of ADR
Amongst the included case reports, ADRs of 17 cases (17/47, 36.2%) occurred in 5 minutes, and 16 (16/47, 34.0%) were between 5 minutes and half an hour. Two cases (2/47, 4.3%) were between half an hour and 3 hours, and the remaining 12 cases (12/47, 25.5%) did not report the occurrence time.
For the ADR type, anaphylaxis and anaphylactic shock were common types, up to 97.9%. 29 (29/47, 61.7%) cases were treated as anaphylaxis and 17 (17/47, 36.2%) were anaphylactic shock. Patients of anaphylaxis may break out in a rash, together with itch, or they may have symptoms such as cyanosis, cough, abdominal pain, dizziness, chest congestion, short of breath, chills, or fever. Anaphylactic shock was more serious than anaphylaxis. Besides the symptoms above, it had a sharp drop of blood pressure, dyspnea, and disturbance of consciousness. In addition to these cases, 1 (1/47, 2.1%) was just reported to have dizziness of unknown origin. More details were in Table 6.
Table 6.
Details of the symptoms of ADR cases.
| Systems | Symptoms (occurrence number and percentage) |
|---|---|
| Skin structure | Cyanosis of lips (15, 6.7%); cyanosis (10, 4.4%); itch (10, 4.4%); rash (9, 4.0%); flush (6, 2.7%); maculopapule (4, 1.8%); urticaria (1, 0.5%); ecchymosis (1, 0.5%) |
| Systemic symptoms | Cold limbs (13, 5.8%); pallor (11, 4.9%); hyperhidrosis (9, 4.0%); Chills (8, 3.6%); feebleness (3, 1.3%); fever (1, 0.5%) |
| Digestive system | Nausea (6, 2.7%); emesis (3, 1.3%); abdominal pain (2, 0.9%) |
| Respiratory system | Dyspnea (13, 5.8%); short of breath (10, 4.4%); cough (4, 1.8%); polypnea (4, 1.8%); throat itching (1, 0.5%); nasal congestion (1, 0.5%) |
| Cardiovascular system | Chest congestion (17, 7.6%); drop of blood pressure (15, 6.7%); tachycardia (12, 5.3%); palpitation (5, 2.2%); bradycardia (3, 1.3%) |
| Nervous system | Irritability (7, 3.1%); coma (4, 1.8%); tremor (4, 1.8%); confusion of consciousness (3, 1.3%); dizziness (3, 1.3%); numbness (2, 0.9%) |
| Urinary system | Hydrocele (1, 0.5%); oliguria (1, 0.5%) |
| Application site | Local swelling (2, 0.9%); headache (1, 0.5%) |
3.2.4. Prognosis of ADR
Oxygen uptake, epinephrine, dopamine, dexamethasone, diphenhydramine, promethazine, and 10% calcium gluconate were frequently used in anaphylaxis and anaphylactic shock as described in the case reports. After the treatment, 45 cases (45/47, 95.7%) recovered soon.
The case of ADR resulting in death was having an allergy and had primary disease of scald with infections [33]. After the first-time treatment, the right upper limb was swelling and painful to exercise. The next day after the second-time use of XYP, the patient developed into anaphylactic shock, showing dyspnea, disturbance of consciousness, and a sharp drop of blood pressure. After the urgent anti-shock treatment, the patient died finally. The report had recorded that sulfonamide was his allergen and at the same time had a 10-year history of diabetes mellitus. However, it was unclear of the allergic constitution and the disease progression. With the rational relationship of time between drug use and ADR, the causality can be classified as possible. The other case of anaphylactic shock leading to the vegetative state had the primary disease of pediatric asthmatic bronchitis [24]. The symptoms of shock came out at the first time when using XYP. After the anti-shock treatment, the patient was in a coma and finally in the persistent vegetative state. Allergic history was indistinct. Considering rational relationship of time, absence of both the provocation test (rechallenge), and the clinically reasonable response on withdrawal (dechallenge), the causality can be also classified as possible. The cases which are classified possible need further investigating in the concurrent diseases or other drugs or chemicals. Other factors like these may be more possible than XYP itself.
4. Discussion
4.1. Normalization of the Drug Use and the ADR Reports
According to the results of a prospective, postmarketing, and large-scale centralized hospital monitoring study [58], a total of 30759 patients employing XYP were collected from 21 hospitals; as a result, a number of 23 patients developed mild ADRs related to XYP, and the ADR incidence rate was 0.75‰ (95% confidence interval: 0.47‰ ~ 1.12‰). Another prospective randomized controlled trial [2], which contained 114 patients of severe hand, foot, and mouth disease in XYP combination group, observed no ADRs during the period of study. Considering the rare ADR incidence of XYP, we should pay more attention to the rational drug use in clinical practice.
Our comprehensive literature search revealed 8 cases where the use of XYP fell outside of the recommendations as provided in the package inserts of XYP, including fast dripping speed and the use of improper solvents. Formalization of the drug use should be emphasized for fear of an increased risk of adverse events. Overdose and overspeed would quicken blood circulation and increase cardiac burden, leading to heart failure and serious anaphylactic shock [59]. Clinicians and patients should strictly follow the instruction to assure the safety of XYP [60].
We intended to identify detailed information about ADRs through the findings in case reports. However, they were far from content without explicit description and critical appraisal of evidence. Compared with adverse effects and adverse events, information regarding ADRs should be more specific to a drug [61]. In the published literature, assessment of ADR, causality, and severity should be evaluated aforehand and described normatively according to the standard [12–14].
4.2. Attention to the Patients Employing XYP
XYP is widely used in various age groups, especially in pediatrics [62]. Kids (≤14 years old) accounted for 66.0% among cases of ADRs. Clinicians and patients should attach importance to the children in early growth with incomplete functional organs, which have individual limits and narrow efficacy of threshold [63].
XYP is an injectable traditional Chinese medicine, with mechanisms of actions in clearing heat and detoxicating for bronchitis, hand, foot, and mouth disease, bacillary dysentery, and other infectious diseases. In these reported cases, it applied not only to some other diseases such as bronchial asthma, adenomesenteritis, and psoriasis, but also to emesis, hypogastralgia, and fever which were not diagnosed definitively. The application of TCMI should be distinguished from the western medicine: treatment based on syndrome differentiation should integrate with the treatment of disease differentiation [64]. According to TCM theory and high-quality research findings, clinical indications of employing XYP should be identified clearly in the package insert.
The unknown information of allergic history in ADR cases accounted for 55.3%. The types of ADRs were mainly allergic reactions, involving diseases in respiratory system, integumentary system, digestive system, and so on. With the high rates of anaphylaxis and anaphylactic shock (97.9%), it is significant to inquire the detailed allergic history from the patients and consider the allergic constitution before taking XYP [65]. Moreover, we need to pay more attention to the allergic histories of andrographis preparations such as lianbizhi injection [66], yanhuning injection [67], and chuanhuning injection [68].
4.3. The Mechanism and Prognosis of Allergic Reaction
Allergic reactions without specific proof of diagnosis accounted for 97.9% in these cases. These ADRs happened mostly in 30 minutes (70.2%) and can be defined as immediate hypersensitivity reactions (IHRs) [69]. Previous research has identified that only a small part of those IHRs are immune-mediated (IgE or T cell) IHRs, thus true drug allergies, and the majority are non-immune-mediated IHRs, hence pseudo drug allergies [70]. Studies regarding XYP demonstrated that the results of active systemic anaphylaxis (ASA) test and passive cutaneous anaphylaxis (PCA) test were both negative on guinea pigs [71], while rats receiving directly intracutaneous injection showed positive outcomes with the assay of Evans blue spots [72]. IHRs induced by XYP usually occurred without the previous exposure, conforming to the features of pseudo-allergic reactions [73]. Another TCMI named Shuanghuanglian (SHL) has indicated that during sensitization the specific IgE was not elicited and the pseudo-allergic reactions were directly motivated by the activation of RhoA/ROCK signal pathway [74]. In-depth mechanism of the hypersensitivity reaction for XYP merits future researches to seek potential therapeutic strategy to prevent or treat with the associated ADRs.
After the expectant treatment for ADRs, 95.7% cases were improved and recovered. It has been warned in the header of the package insert that people should employ XYP in hospitals with emergency equipment in case of the anaphylactic shock.
4.4. Tube-Flushing and Systematic Researches in Combination of Drugs
The package insert of XYP has showed that mixture of drugs should be forbidden [75], and combination of drugs should be adopted prudently because the possible insoluble particles precipitated out would produce ADRs [76]. The drug instruction has also informed that appropriate intervals associated with tube-flushing should be vigilant in case of drug interactions. However, a study [77] concerning 3 TCMIs (including XYP, reduning injection, and danhong injection) showed that only 9.6% of the 2,045 investigating cases were flushed or replaced with infusion tubes at the time of intervals. Due to XYP-use in infectious diseases [1–3], most of the combined medications were antibiotics and antiviral drugs in clinical practice. In this study, more than a half of the cases did not even report the combinations. Researchers should record detailed information about combined medication in the case reports and strengthen the systematic researches in the incompatibility of antibiotics and antiviral drugs.
4.5. Limitation
The systematic review of case reports has some limitations itself. In a systematic review of RCTs, we can conduct a meta-analysis to identify the efficacy of a drug or an intervention. However, in a systematic review of case reports, we cannot use the combined data to obtain advantages or disadvantages for the missing number of drug users and comparisons. So, we just carried out a descriptive analysis and list all the factors for the relevant ADRs in different states. Afterwards, we expect more prospective clinical studies and experiment researches to obtain the accurate ADR outcomes in every state.
5. Conclusions
As for the rare incidence of ADRs of XYP in the prospective study, clinicians and patients should strictly obey the drug instruction in clinical practice, including indications, allergic history, solvent, dosage, drug combination, dripping speed, and tube-flushing.
ADRs in the case reports merit close attention and detailed description. Assessment of ADR, causality, and severity are all necessary in reporting an ADR case, and more valid information from the literatures are also required to construct the integral postmarketing security evaluation system [78].
Considering its misleading indications and unclear age groups in the package insert, high-quality clinical studies and pharmaceutical experiments are demanded to supplement the drug instruction. Attention should be paid when XYP is used in children and people with allergic constitution. To prevent and treat with serious anaphylactic shock, mechanism of the hypersensitivity reaction and the drug combination should still be fully identified.
Acknowledgments
This study was conducted under a grant from the National Science Fund for Distinguished Young Scholars (81725024) and a grant from the Second Batch of “Ten Thousand Plan”, National High Level Talents Special Support Plan (W02020052).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
All authors were responsible for the study design: Shiqi Chen and Rui Zheng took part in the literature search; Joey S.W. Kwong and Yanping Wang were responsible for the analysis and interpretation of the data; Shiqi Chen and Joey S.W. Kwong drafted the paper; Hongcai Shang guided the study and critically reviewed the paper; all authors approved the final version of the article.
References
- 1.Wen T., Xu W., Liang L., et al. Clinical Efficacy of Andrographolide Sulfonate in the Treatment of Severe Hand, Foot, and Mouth Disease (HFMD) is Dependent upon Inhibition of Neutrophil Activation. Phytotherapy Research. 2015;29(8):1161–1167. doi: 10.1002/ptr.5361. [DOI] [PubMed] [Google Scholar]
- 2.Li X., Zhang C., Shi Q., et al. Improving the Efficacy of Conventional Therapy by Adding Andrographolide Sulfonate in the Treatment of Severe Hand, Foot, and Mouth Disease: A Randomized Controlled Trial. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/316250.316250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee M., Parai D., Chattopadhyay S., Mukherjee S. K. Andrographolide: antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiologica. 2017;62(3):237–244. doi: 10.1007/s12223-017-0496-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z.-F., Chen X., Zhang W., Xie Y.-M. Literature review on premarketing and postmarketing evidence of Xiyanping injection. Zhongguo Zhongyao Zazhi. 2014;39(18):3637–3640. doi: 10.4268/cjcmm20141842. [DOI] [PubMed] [Google Scholar]
- 5.Li M., Yang X., Guan C., et al. Andrographolide sulfonate reduces mortality in Enterovirus 71 infected mice by modulating immunity. International Immunopharmacology. 2018;55:142–150. doi: 10.1016/j.intimp.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Peng S., Hang N., Liu W., et al. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-κB pathways. Acta Pharmaceutica Sinica B (APSB) 2016;6(3):205–211. doi: 10.1016/j.apsb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo W., Liu W., Chen G., et al. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-κB pathways. International Immunopharmacology. 2012;14(4):613–619. doi: 10.1016/j.intimp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Tan W. S. D., Liao W., Zhou S., Wong W. S. F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochemical Pharmacology. 2017;139:71–81. doi: 10.1016/j.bcp.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 9. China Drug and Food Administration (CFDA), Notification of adverse drug reaction (phase 48): take notice of the serious allergic reaction of xiyanping injection and mailuoning injection, 2012, http://samr.cfda.gov.cn/WS01/CL1989/72891.html.
- 10. China Drug and Food Administration (CFDA), Notice of the general administration on the quality of honghua injection and xiyanping injection (no. 153), 2017, http://samr.cfda.gov.cn/WS01/CL1278/177901.html.
- 11.Lei X., Chen J., Ren J., et al. Liver Damage Associated with Polygonum multiflorum Thunb.: A Systematic Review of Case Reports and Case Series. Evidence-Based Complementary and Alternative Medicine. 2015;2015:9. doi: 10.1155/2015/459749.459749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Health Commission of the People’s Republic of China. Reports and monitoring management methods of adverse drug reaction (ministry of health no. 81), 2011, http://www.moh.gov.cn/mohzcfgs/pgz/201105/51770.shtml.
- 13.Li B., Gao R., Li R., et al. Causal determination of the adverse events and adverse drug reactions in drug clinical trials. Chinese Journal of New Drugs. 2014;23(12):1465–1470. [Google Scholar]
- 14.The Uppsala Monitoring Centre (the UMC) The use of the WHO-UMC system for standardised case causality assessment. 2018. https://www.who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf. [Google Scholar]
- 15.Huang D. Z. One case of clinical care of anaphylactic shock induced by xiyanping injection. Shi Yong Yi Gi Za Zhi. 2008;15(31):p. 4518. [Google Scholar]
- 16.Huang W. L. One case of anaphylactic shock induced by xiyanping injection. Chinese Community Doctors. 2017;33(6):p. 160. [Google Scholar]
- 17.Chen B. One case of anaphylaxis induced by xiyanping injection. Zhongguo Zhong Yi Ji Zheng. 2008;17(4):p. 561. [Google Scholar]
- 18.Di X. Y., Tan Q. M., Mi M. Y. One case of clinical care of generalized skin eruption induced by xiyanping injection. Clinical Journal of Medical Official. 2004;32(5):p. 46. [Google Scholar]
- 19.Lai H., Wang H. H. One case of clinical care of anaphylaxis induced by xiyanping injection. Xin Li Yi Sheng. 2016;22(6):p. 220. [Google Scholar]
- 20.Guan Z. Y., Wang Y. S., Wang H. One case for emergency and clinical care of anaphylactic shock induced by xiyanping injection. Medical Information. 2011;24(8):p. 5059. [Google Scholar]
- 21.Miao Z., Zhang J., Shi X. K. One case of clinical care of anaphylactic shock induced by xiyanping injection combined with mezlocillin. Journal of Pediatric Pharmacy. 2016;22(8):p. 66. [Google Scholar]
- 22.Hu S. J. One case of immediate hypersensitivity induced by intravenously infusing xiyanping injection. Hebei Zhong Yi. 2012;34(11):p. 1702. [Google Scholar]
- 23.Shen Y. Y., Lin H. L. One case of acute urticaria induced by xiyanping injection. Chinese Journal of Pharmacoepidemiology. 2012;21(7):p. 360. [Google Scholar]
- 24.Li Y. L., Zhao M. One case of anaphylactic shock leading to the vegetative state induced by xiyanping injection. Hainan Medical Journal. 2014;25(3):p. 454. [Google Scholar]
- 25.Li H. M. One case of anaphylaxis induced by xiyanping injection. Shaanxi Medical Journal. 2013;42(6):p. 622. [Google Scholar]
- 26.Li X. P., Guo T. J., Li D. M., Wang H. One case of serious anaphylaxis induced by xiyanping injection. Journal of China Pharmacy. 2007;10(6):p. 543. [Google Scholar]
- 27.Zhang W. H. One case of adverse drug reaction induced by xiyanping injection. Chinese Journal of Clinical Rational Drug Use. 2013;6(26):p. 41. [Google Scholar]
- 28.Zuo Y. Z., Yue H. X. One case of anaphylaxis induced by xiyanping injection. Zhongguo Zhong Yi Ji Zheng. 2008;17(7):p. 1013. [Google Scholar]
- 29.An X. H. One case of anaphylactic shock induced by xiyanping injection. Qinghai Medical journal. 2014;44(7):p. 25. [Google Scholar]
- 30.Sun J. H., Huang L. One case of severe tetter induced by xiyanping injection combined with xingnaojing injection. Evaluation Analysis of Drug-Use in Chinese Hospitals. 2014;14(10):p. 960. [Google Scholar]
- 31.Liu Y. X. Analysis of involuntary convulsion induced by xiyanping injection combined with azithromycin. Zhongguo Cheng xiang Qi ye Wei Sheng. 2014;29(6):101–102. [Google Scholar]
- 32.Xiu K. N. One case of rare immediate hypersensitivity reactions. Journal of Medical Theory and Practice. 2011;24(9):p. 1110. [Google Scholar]
- 33.Yu H. Fatal allergy caused by xiyanping injection. Adverse Drug Reactions Journal. 2003;(6):401–402. [Google Scholar]
- 34.Chen X. L. One case of clinical care of anaphylactic shock induced by xiyanping injection. China Pharmaceuticals. 2010;19(22):p. 87. [Google Scholar]
- 35.Feng X. W., Ren Y., Li X. P., Wu J. One case of anaphylaxis induced by xiyanping injection. Clinical Journal of Medical Official. 2013;41(6):p. 564. [Google Scholar]
- 36.Qiao H. L., Yu X. Y. A case of allergic shock induced by xiyanping injection. Chinese Nursing Research. 2004;18(15):p. 1406. [Google Scholar]
- 37.Wan T. Y., Liao Y. M., Jin K. G. A case of allergic shock induced by xiyanping injection. People's Military Surgeon. 2002;45(9):555–556. [Google Scholar]
- 38.Hao X. J., Liu G. G. One case of allergic shock induced by xiyanping injection. China Medical Herald. 2006;3(26):p. 159. [Google Scholar]
- 39.He X. R., He L. M., Liang H. M. A case of lump in the transfusion side induced by xiyanping injection. Chinese Journal of Pharmacoepidemiology. 2011;20(7):p. 339. [Google Scholar]
- 40.Wang C. L., Zhang N. One case of allergy induced by xiyanping injection. Clinical Focus. 2006;21(6):p. 392. [Google Scholar]
- 41.Wu L. Q., Li X. L., Zhou X. Q., Zhao Q. One case of anaphylactic shock caused by xiyanping injection. Journal of Logistics University of PAPF (Medical Sciences) 2014;23(8):p. 691. [Google Scholar]
- 42.Xiong X. Q. One case of anaphylactic shock caused by xiyanping injection. China Healthcare and Nutrition. 2014;24(4):p. 2402. [Google Scholar]
- 43.Zhang F. S. One case of anaphylactic shock caused by xiyanping injection. Chinese Journal of Hospital Pharmacy. 2010;30(23):p. 2050. [Google Scholar]
- 44.Zhang G. X. One case of acute laryngeal edema caused by xiyanping injection. Chinese Journal of Misdiagnostics. 2009;9(25):p. 6298. [Google Scholar]
- 45.Zhang Y. Y. One case of serious anaphylaxis induced by xiyanping injection. China Practical Medical. 2010;5(35):p. 152. [Google Scholar]
- 46.Dong F. A case of anaphylactic shock caused by xiyanping injection. Xinjiang Journal of Traditional Chinese Medicine. 2004;22(4):p. 21. [Google Scholar]
- 47.Dong F., Liu G. Z. A case of allergic reactions caused by xiyanping injection. Chinese Practical Journal of Rural Doctor. 2008;15(7):p. 15. [Google Scholar]
- 48.Zhao X. J. A case of delayed hypersensitivity reaction induced by xiyanping injection. Chinese Journal of Practical Nursing. 2005;21(7):p. 56. [Google Scholar]
- 49.Zheng L. Two cases of serious anaphylaxis induced by xiyanping injection. Special Health. 2014;(6) [Google Scholar]
- 50.Yang L. W., Liu C. H. Two cases of adverse drug reactions induced by xiyanping injection. Special Health. 2014;(4) [Google Scholar]
- 51.Wang X. J. Two cases of adverse drug reactions induced by xiyanping injection. Zhi Hui Jian Kang. 2017;3(5):156–157. [Google Scholar]
- 52.Wang L. J., An J. B., Xia M. L. Two cases of Children's allergic reactions induced by xiyanping injection. Contemporary Medicine. 2016;22(19):135–136. [Google Scholar]
- 53.Du X., Shang J., Qu Y. J. Three cases of allergic reactions induced by andrographolide sulfonate. Herald of Medicine. 2006;25(10):p. 1034. [Google Scholar]
- 54.Gao T., Chen Q. Y., He Y., et al. Case analysis of adverse events related to the use of xiyanping injection. Pharmacy and Clinics of Chinese Materia Medical. 2014;5(3):33–34. [Google Scholar]
- 55.Zhang Z. L., Gu H. A report of two cases of rapid allergic reaction caused by xiyanping injection. Clinical Misdiagnosis and Mistherapy. 2004;17(8):p. 593. [Google Scholar]
- 56.Zhu W., Zhang L. Two cases of serious anaphylaxis induced by xiyanping injection and analysis of literatures. Strait Pharmaceutical Journal. 2016;28(11):268–270. [Google Scholar]
- 57.Yang R. Z., Li Y. The analysis of clinical use of anaphylaxis induced by xiyanping injection. China Pharmaceuticals. 2007;16(21):p. 61. [Google Scholar]
- 58.Deng J. X., Wang Z. F., Xie Y. M., et al. Post-marketing safety reevaluation of xiyanping injection. Adverse Drug Reactions Journal. 2018;20(1):15–22. [Google Scholar]
- 59.Ding L. J. The exploration of reasons for adverse drug reactions of xiyanping injection. Nei Menggu Journal of Traditional Chinese Medicine. 2015;34(7):99–100. [Google Scholar]
- 60.Yang X. L., Cheng F., Li Z. Y., Liu D. F. Study on the stability of xiyanping injection and solvent compatibility. China Journal of Traditional Chinese Medicine and Pharmacy. 2012;27(5):1415–1417. [Google Scholar]
- 61.Zorzela L., Loke Y. K., Ioannidis J. P., et al. PRISMA harms checklist: improving harms reporting in systematic reviews. British Medical Journal. 2016;352, article ID i157 doi: 10.1136/bmj.i157. [DOI] [PubMed] [Google Scholar]
- 62.Luo Y. J., Wen X. Y., Ni X. L., Xiao X. L. Efficacy and safety of Xiyanping injection combined with azithromycin in treating mycoplasma pneumonia of children: Meta-analysis. Zhongguo Zhong Yao Za Zhi. 2018;43(10):2153–2161. doi: 10.19540/j.cnki.cjcmm.2018.0068. [DOI] [PubMed] [Google Scholar]
- 63.Chen R., Zhuo L., Pan Y. T., Cai T., Cao Y., Wang S. F. Features of adverse drug reaction in post-marketing study about xiyanping injection based on literature. Chinese Journal of Pharmacoepidemiology. 2018;27(5):317–323. [Google Scholar]
- 64.He P., Li F. J., Li L. D., Li Y. K. Developing traditional Chinese medicine injection is the need for curing sickness to save patients. Zhongguo Zhong Yao Za Zhi. 2017;42(6):1011–1014. doi: 10.19540/j.cnki.cjcmm.2017.0031. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y. P., Jiao K., He Z. F., et al. Systematic evaluation of adverse drug reactions of xiyanping injection from documents. Chinese Journal of Experimental Traditional Medical Formulae. 2011;17(24):236–239. [Google Scholar]
- 66.Feng X., Fang S. N., Gao Y. X., Liu J. P., Chen W. Current research situation of nephrotoxicity of Chinese herbal medicine. Zhongguo Zhong Yao Za Zhi. 2018;43(3):417–424. doi: 10.19540/j.cnki.cjcmm.2018.0009. [DOI] [PubMed] [Google Scholar]
- 67.Shi G.-X., Yan Y.-Y., Shao J., et al. Effect of andrographolide derivative yanhuning on in vivo candida albicans biofilms in rats. Zhongguo Zhongyao Zazhi. 2014;39(15):2924–2929. doi: 10.4268/cjcmm20141525. [DOI] [PubMed] [Google Scholar]
- 68.Xiang D., Wang M. D., Wang W. Q., et al. Analysis and exploration on adverse reactions of four kinds of andrographolide injections. Zhongguo Zhong Yao Za Zhi. 2016;41(12):2350–2355. doi: 10.4268/cjcmm20161229. [DOI] [PubMed] [Google Scholar]
- 69.Gao Y., Hou R., Han Y., Fei Q., Cai R., Qi Y. Shuang-Huang-Lian injection induces an immediate hypersensitivity reaction via C5a but not IgE. Scientific Reports. 2018;8(1):p. 3572. doi: 10.1038/s41598-018-21843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hausmann O., Schnyder B., Pichler W. J. Etiology and pathogenesis of adverse drug reactions. Chemical Immunology and Allergy. 2012;97:32–46. doi: 10.1159/000335614. [DOI] [PubMed] [Google Scholar]
- 71.Xie J., Wang J. C., Wang A. W. Safety evaluation of three traditional Chinese medicine injections. Chinese Journal of Experimental Traditional Medical Formulae. 2013;19(16):263–267. [Google Scholar]
- 72.Kang L. J., Xie J. J., Yi J. J., Zhao L. Application of rat skin anaphylactoid test to several drugs. Chinese Traditional Patent Medicine. 2014;36(10):2033–2036. [Google Scholar]
- 73.McNeil B. D., Pundir P., Meeker S., et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han J., Zhao Y., Zhang Y., et al. RhoA/ROCK Signaling Pathway Mediates Shuanghuanglian Injection-Induced Pseudo-allergic Reactions. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang X.-L., Cheng F., Liu Y.-H., et al. Study on the compatible stability of Xiyanping injection with fifteen injections. Chinese Journal of New Drugs. 2013;22(20):2374–2378. [Google Scholar]
- 76.Du W., Zeng C. F. Application rationality of xiyanping injections in pediatric outpatients. Journal of Chinese Pharmaceutical Sciences. 2017;26(5):82–85. [Google Scholar]
- 77.Deng F. Y., Su Y. C., Wang J. F., Wei J. A survey on the use of 3 common traditional Chinese medicine injections. Chinese Journal of Clinical Rational Drug Use. 2016;9(3B):76–78. [Google Scholar]
- 78.Zhang X. Y., Wang Y. P., Lin L. K., Shang H. C., Wang Y. Y. Strategy of constructing post-market integral evaluation system of traditional Chinese medicine injection. Zhongguo Zhong Yao Za Zhi. 2017;42(16):3229–3232. doi: 10.19540/j.cnki.cjcmm.20170623.007. [DOI] [PubMed] [Google Scholar]