Abstract
Tuberculosis is one of the 10 leading causes of death in the world. The current treatment is based on a combination of antimicrobials administered for six months. It is essential to find therapeutic agents with which the treatment time can be shortened and strengthen the host immune response against Mycobacterium tuberculosis. M. tuberculosis needs cholesterol to infect and survive inside the host, but the progression of the infection depends to a large extent on the capacity of the immune response to contain the infection. Statins inhibit the synthesis of cholesterol and have pleiotropic effects on the immune system, which have been associated with better results in the treatment of several infectious diseases. Recently, it has been reported that cells treated with statins are more resistant to M. tuberculosis infection, and they have even been proposed as adjuvants in the treatment of M. tuberculosis infection. The aim of this review is to summarize the immunopathogenesis of tuberculosis and its mechanisms of evasion and to compile the available scientific information on the effect of statins in the treatment of tuberculosis.
1. Introduction
Tuberculosis is an infectious disease caused by the Mycobacterium tuberculosis complex, which includes the species M. tuberculosis, M. bovis, BCG strain of M. bovis, M. africanum, M. caprae, M. microti, M. canettii, and M. pinnipedii. Of these, M. tuberculosis is the cause of 98% of cases in humans. The most common site of infection is the lung, although it can affect other organs and systems (lymph nodes, bones, meninges, etc.) [1]. Tuberculosis is one of the 10 leading causes of death in the world, is the leading cause of death by a single infectious agent, and produces more deaths than HIV/AIDS. In 2016, the World Health Organization reported that there were 10.3 million new cases and 1.4 million deaths caused by tuberculosis [2].
The current treatment against tuberculosis is based on the administration of a combination of antimicrobials for six months. The purpose of this strategy is to cure the disease, eradicate the infection, prevent relapse, and prevent the development of resistance. This strategy has been used for the past 60 years; however, the lengthy treatment and its adverse effects favor poor adherence, failure, and the development of resistance. Despite clinical cure, approximately half of treated patients have permanent lung damage due to excess inflammation caused by this infection [3, 4]. Therefore, it is essential to find therapeutic agents with the potential to shorten treatment time and, eventually, with the capacity to strengthen the immune response against M. tuberculosis.
There are two different approaches in the search for new anti-TB drugs: bactericidal antimicrobials and host-directed therapies (HDT) that improve the immune response of the host to the infection. These approaches reduce excess inflammation, prevent damage to tissues, preserve lung function, and possibly improve the effectiveness of anti-TB treatment to eliminate infection [5]. Examples of this group are vitamin D, rapamycin, and statins.
Statins inhibit the main enzyme in the synthesis of cholesterol, called 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase. Its main use is in patients with hypercholesterolemia and in the prevention of cardiovascular diseases in patients with risk factors such as dyslipidemia, diabetes, hypertension, and smoking [6]. However, it has been reported that statins have pleiotropic effects on the immune system and have been associated with better outcomes in several infectious diseases. Recently, statins have been proposed as adjuvants in the treatment of M. tuberculosis infection [7]. Based on the above, the objective of this review is to summarize the immunopathogenesis of tuberculosis and to collect all available scientific information on the effect of statins in the treatment of tuberculosis.
1.1. Immunopathogenesis of Tuberculosis
Infection by M. tuberculosis begins with the inhalation of aerosols. Although most inhaled bacilli are trapped in the upper respiratory tract, approximately 10% of them reach the alveoli, where they are phagocytosed by cells of the innate immune response (macrophages, dendritic cells, and alveolar epithelial cells) [8]. Phagocytosis of M. tuberculosis involves the participation of complement receptors (CR1, CR3, and CR4), Fc, and mannose, among others.
It has been noted that the control of the infection depends to a large extent on the effective acidification of the phagosome, the adequate fusion of the phage lysosome, and the activation of processes such as autophagy and apoptosis in the abovementioned cells [9].
The recognition of M. tuberculosis during the innate immune response triggers cell activation and the production of cytokines and proinflammatory chemokines such as interferon- (IFN-) γ, tumor necrosis factor- (TNF-) α, interleukin- (IL-) 6, IL-12, IL-17, IL-23, C-C motif chemokine ligand (CCL)2, CCL3, CCL5, C-X-C motif chemokine ligand (CXCL)8, and CXCL10 [10, 11].
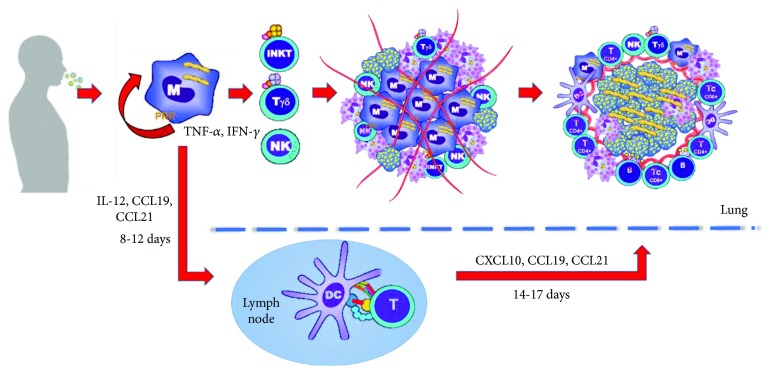
A key cytokine in limiting the intracellular growth of bacilli is IFN-γ, which is secreted in the initial stages by natural killer (NK) cells, gamma delta T cells, and natural killer T (NKT) cells (Figure 1). NK cells lyse monocytes and macrophages infected with M. tuberculosis through perforin-mediated cytotoxic activity, granzymes, and the Fas-FasL system [12]. Other cells that help to control the growth of M. tuberculosis and that contribute to the formation of the granuloma are the invariant natural killer T (iNKT) cells. The iNKT cells are important in the activation of macrophages and dendritic cells through the production of IFN-γ; additionally, they have been shown to be capable of killing macrophages infected with M. tuberculosis [13]. It has been reported that the specific activation of iNKT cells by the alpha-galactosylceramide ligand presented via CD1d protects inbred mouse strains susceptible to tuberculosis [14]. However, several studies have shown that subjects with active tuberculosis had a lower number of iNKT cells, which could indicate that they are more susceptible to M. tuberculosis infection [15]. IL-1β and IL-18 contribute to the containment of infection. They are produced by mononuclear phagocytes and promote the recruitment of neutrophils and monocytes to the site of infection [16]. Recent reports show that IL-1β increases the secretion and signaling of TNF, as well as the expression of TNFR1, which leads to the activation of caspase 3, apoptosis, and death of M. tuberculosis in macrophages [17].
Figure 1.
Immunopathogenesis of tuberculosis. The M. tuberculosis infection begins with the inhalation of airdrops that contain numerous bacilli that are phagocytosed. The initial stages of infection are directed by cells responsible for innate immunity, and the recruitment of inflammatory cells leads to the formation of an early granuloma. Antigen-presenting cells migrate to nearby lymph nodes and activate lymphocytes that return to the lung and generate the mature granulomas. The immune system contains the primary infection in almost 90% of patients, who will develop latent tuberculosis.
The recruitment of inflammatory cells leads to the formation of early granuloma (Figure 1), composed of macrophages, dendritic cells, neutrophils, apoptotic cells, and necrotic cells [18]. Several experiments have suggested that the mechanism of apoptosis more effectively favors the elimination of bacillus, while the mechanism of necrosis favors its dissemination [19]. Fratazzi et al. reported that infected macrophages that die by apoptosis are associated with decreased mycobacterium viability, whereas this decrease is not observed if macrophages die due to necrosis (Figure 2) [20].
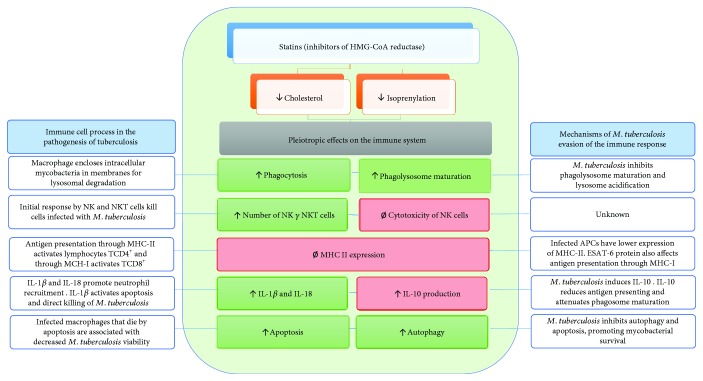
Figure 2.
Pleiotropic effect of statins. Statins exert diverse effects on the immune response. It has been reported that statins can promote autophagy (in macrophages) and apoptosis (in tumor cells). Statins increase the number of NK and NKT cells. Statins may inhibit cytotoxicity of NK cells. Statins inhibit MHC-II expression (in CPA) and promote discrete secretion of IL-1β, IL-18, and IFN-γ (in mononuclear cells). Statins increase serum levels of IL-10. The blue boxes show the function of the immune cells in the pathogenesis and the mechanisms of M. tuberculosis evasion of the immune response; at the center, the blue lines demonstrate that the effect of statins is related to these processes; the green boxes show the statin effects that potentially favor the immune response against M. tuberculosis; and the red boxes indicate the statin effects that could modulate the immune response against M. tuberculosis.
The development of the effector immune response by T lymphocytes requires the processing and presentation of bacterial antigens through major histocompatibility complex (MHC) molecules expressed by antigen-presenting cells (APCs). The presentation of antigens to naïve T cells occurs through MHC class II (MHCII) for CD4+ T lymphocytes and class I (MHCI) for TCD8+ lymphocytes (Figure 1) [21]. The main effector mechanism of CD4+ T cells is the production of IFN-γ, which induces the maturation and activation of macrophages to control or eliminate the bacillus; it has been observed that the depletion of CD4+ T cells in animal models causes reactivation of the infection and death of the host, with high bacterial loads in the lung [22]. Another function of CD4+ T cells is the direct production of other cytokines, such as IL-2 and TNF-α [23].
CD8+ T lymphocytes also play an important role in the cellular response against mycobacteria since they are capable of killing infected cells or of directly eliminating bacilli [24, 25].
Likewise, nonconventional T cells (γδ T cells) are activated and contribute to fight M. tuberculosis infection. For example, a marked expansion of γδ T cells in the blood of patients with tuberculosis has been reported. These cells contribute to the secretion of cytokines (IFN-γ and TNF-α), cytotoxic effector function, and cellular contact-dependent signaling [26].
Recently, the involvement of B cells in the process of developing immunity during tuberculosis infection has been described. These cells favor antigenic presentation and through the production of antibodies opsonize the bacilli, activate the complement, and promote the formation of memory cells and plasma cells [27].
With the formation of the granuloma (Figure 1), the immune system controls more than 90% of the bacterial load of the primary infection. In this way, most infected subjects develop latent tuberculosis (defined as a state where the individual is infected but remains free of clinical manifestations); however, if the infecting bacterial inoculum is very large, if the subject has HIV, or if the individual has some primary immunodeficiency, the infection may evolve to progressive active tuberculosis [28]. In subjects who develop latent tuberculosis, when an immunosuppressive condition appears and alters the homeostasis of the immune system, such as the coexistence of diabetes mellitus, the administration of immunosuppressive drugs, or a state of malnutrition, the bacillus is reactivated and initiates its replication, the granulomas are broken, the levels of proinflammatory cytokines increase, and the clinical manifestations of the disease appear. Therefore, active tuberculosis is defined precisely by the appearance of signs and symptoms of the disease (chronic cough, sputum, and/or hemoptysis) confirmed by the isolation of M. tuberculosis in the cultures [29].
1.2. Mechanisms of Mycobacterium tuberculosis Evasion of the Immune Response
M. tuberculosis is able to evade both innate and adaptive immune responses. In macrophages, bacteria inhibit phagosome maturation by different mechanisms, such as the retention of the protein TACO (tryptophan aspartate-containing protein) and the Rab5 protein in the phagosome (Figure 2) [30]. This retention prevents the process called “Rab conversion,” through which Rab5 is exchanged for Rab7, which inhibits phagolysosome fusion [31]. To perform this function, the phosphatidylinositol 3-phosphate molecule produced by hVPS34 kinase is necessary. Another mechanism of inhibition of phagolysosome maturation occurs through the glycosylated lipoarabinomannan of the M. tuberculosis cell wall, which decreases the activity of hVPS34 and inhibits the recruitment of the early endosomal antigen 1 (EEA1) [32].
Under normal conditions, phagocytic cells, such as neutrophils, monocytes, macrophages, and eosinophils, destroy phagocytosed microorganisms by producing reactive oxygen and nitrogen species (NO-, O2-, and ONOO-). However, M. tuberculosis can inhibit the recruitment of iNOS into the phagosome and prevent the formation of reactive oxygen and nitrogen species [33]. In addition, M. tuberculosis secretes methionine sulfoxide reductase enzymes A and B that reduce peroxynitrite (ONOO-) in nonionic molecules, which do not destroy mycobacteria [34].
Another form of immune response evasion is the inhibition of antigenic presentation. In vitro models have shown that macrophages infected with M. tuberculosis reduce the expression of MHCII [35]. Recent data from in vivo experiments demonstrated that APCs infected with M. tuberculosis have lower expression of MHCII compared with uninfected APCs [36]. It has also been reported that the ESAT-6 protein interacts with beta-2-microglobulin, which affects the function of antigen presentation through MHCI (Figure 2) [37], and it has been shown that the suboptimal presentation of the antigen contributes to the persistence of M. tuberculosis in vivo [38]. In addition, the inhibition of antigenic presentation has been associated with the virulence of the strains, since, for example, M. tuberculosis H37Rv has a greater capacity to inhibit antigenic presentation compared with H37Ra [39].
The antigenic presentation of the dendritic cells is also affected in the normal maturation process, which is essential for the proper activation of the cell [40]; in contrast, they have a lower expression of integrins, which decreases the capacity of the dendritic cells to migrate to the lymph nodes [41]. There is evidence that M. tuberculosis infection alters the presentation of lipid antigens by CD1 molecules. Mycobacterial wall alpha-glucan negatively regulates the expression of CD1 in APCs [42, 43]. Recent data indicate that immune evasion can occur not only through a blockade of the maturation of dendritic cells but also by facilitating poorly coordinated maturation. According to this model, M. tuberculosis induces the movement of MHC-II molecules to the cell membrane and inhibits the synthesis of new MHC-II-type molecules. As a consequence, the presentation of antigenic peptides of M. tuberculosis is affected (Figure 2) [44].
In the adaptive immune response, Mahon et al. have shown that the glycolipids of the M. tuberculosis cell wall, including the mannosylated lipoarabinomannan (LAM), directly inhibit the activation of polyclonal CD4+ T cells by blocking the phosphorylation of ZAP-70 [45], and Sande et al. reported that LAM induces CD4+ T cell anergy by inducing the overexpression of GRAIL (the receptor associated with the induction and maintenance of anergy in CD4+ T cells) [46]. Saavedra et al. showed that the activation of CD8+ T lymphocytes is also inhibited by the mycobacterial glycolipid 2,3-di-O-acyl-trehalose (DAT) since it reduces the cellular proliferation induced by the antigen [47].
Another way that M. tuberculosis evades the immune response is by altering the production of cytokines. The production of proinflammatory cytokines, such as TNF-α, IL-6, and interleukin-1 beta (IL-1β), and the chemokine MCP-1 is inhibited by phenolic glycolipids present in the cell wall of M. tuberculosis [48]. M. tuberculosis inhibits the production of IL-12, and the mmA4 gene has been identified as a key locus for such inhibition [49]. It has also been reported that signaling induced through TLR2 by M. tuberculosis in macrophages induces the secretion of IL-10, suppresses IL-12, and attenuates the Th1 response, which is critical for controlling infection [50]. Wang et al. reported that M. tuberculosis induces the expression of the IL-10 gene and that IL-10 reduces antigen presentation and attenuates phagosome maturation, which prevents bacterial death and induces infection by M. tuberculosis over the long term in the lung (Figure 2) [51].
The survival of M. tuberculosis within the host macrophages implies apoptosis resistance dependent on overexpression of the antiapoptotic protein Mcl-1 (Figure 2) [52]. It has also been described that M. tuberculosis causes a significant alteration of the inner membrane of the mitochondria of macrophages, which favors cell death by necrosis, a mechanism that promotes the spread of the pathogen and the appearance of the disease [53]. M. tuberculosis can evade the autophagy mechanism through the ectopic expression of ESAT-6, which inhibits the formation of autophagosomes in infected macrophages [53] and reduces the expression of Atg8 (ubiquitin-like protein) in human dendritic cells (Figure 2) [54].
1.3. The Role of Cholesterol in Mycobacterium tuberculosis Infection
The cell wall of M. tuberculosis contains an abundant amount of lipids, and a relatively large fraction of its genes encode proteins for their synthesis. It has been shown that mycobacteria can accumulate and use cholesterol as a carbon source and for the synthesis of some virulence factors, such as phthiocerol dimycocerosate and sulpholipid-1 [55]. It has been reported that M. tuberculosis dysregulates the metabolism of lipids in the host and the progression of the granuloma until the caseation correlates with the high expression of the genes for lipid metabolism and sequestration [56]; in an apoE -/- mouse model, Martens et al. studied the effect of hypercholesterolemia in tuberculosis infection, observing that hypercholesterolemic mice infected with M. tuberculosis have a greater bacillary load and an accentuated pulmonary pathology [57]. In another human study, it was reported that dietary cholesterol is dose-dependently associated with an increased risk of having active tuberculosis [58].
Cholesterol is essential for the internalization of mycobacteria in host cells [30]. M. tuberculosis enters cells through high-cholesterol domains, and by eliminating cholesterol from the cell membrane, phagocytosis is inhibited [59, 60]. Within the macrophages, the mycobacterium can inhibit the maturation of the phagosome, along with its hydrolytic and microbicidal capacities, and cholesterol also plays an important role in arresting the phagosome's maturation. For example, the accumulation of cholesterol causes abnormal retention of the TACO protein [30] and inhibits the dissociation of Rab7 from the phagosome membrane [61].
The foamy macrophages of the granulomas of patients with tuberculosis are reservoirs rich in nutrients, have altered their phagocytic capacity, and favor the persistence of mycobacteria [62]. One study shows that M. tuberculosis infection induces the accumulation of oxidized low-density lipoprotein in alveolar macrophages, and when alveolar macrophages are exposed to oxidized low-density lipoproteins in vitro, the survival and persistence of intracellular bacilli are promoted; the mechanism is unknown but could be related to the use of host cholesterol as a source of energy [63].
2. Pleiotropic Effect of Statins
The main mechanism of the action of statins is the inhibition of the enzyme HMG-CoA reductase, which regulates the synthesis of cholesterol and has been used mainly in patients with hypercholesterolemia. Recently, pleiotropic effects of statins on the immune system and some bactericidal effects have been reported [64] (Figure 2).
It has been documented that statins have the capacity to act as immunomodulators. For example, statins induce the phagocytic activity of macrophage J774 [65]. It has also been reported that they act as inhibitors of the expression of MHCII induced by IFN-γ in primary endothelial cells, monocytes, and human macrophages, which in turn inhibits the activation of T lymphocytes [66]. The treatment of mononuclear cells with fluvastatin produced the discrete activation of caspase 1 and moderated the secretion of IL-1β, IL-18, and IFN-γ [67]. It has also been demonstrated that statins upregulate IL-10 in the serum of patients with acute coronary syndrome [68].
Another study showed that the in vitro treatment of mononuclear cells with atorvastatin increases the number of NK and NKT cells in peripheral venous blood [69]. It has also been reported that treatment with simvastatin and IL-2 promotes the activation of NK cells [70]. In contrast, other studies have reported that statins inhibit the cytotoxicity of NK cells [71] and the function of activating receptors [72]. It has also been shown that simvastatin therapy in patients with hypercholesterolemia for six months increases the iNKT cells in peripheral venous blood [73].
Other studies show that statins can induce apoptosis in human cells from tumors through the inhibition of Ras signaling pathways [74, 75]. Statins also promote autophagy through the activation of the AMPK-TOR signaling pathway in cells from rhabdomyosarcoma [76]. Treatment with lovastatin increases the expression of Rab7 mRNA by decreasing the synthesis of isoprenyl groups and promoting phagosome maturation [77].
2.1. Effect of Statins on Infectious Diseases
With respect to statins and their antimicrobial effect, it has been reported that statin therapy reduces mortality in patients with bacteremia and multiple organ failure [78]. Several studies have evaluated the benefit of the use of statins in the prevention or treatment of sepsis, although some results are contradictory. In different meta-analyses, promising results have been observed in which treatment with statins significantly reduced the progression of the disease and/or mortality associated with sepsis [79–81].
In vitro studies have shown some antimicrobial effects against gram-positive and gram-negative bacteria and on some viruses and fungi [82]. The addition of atorvastatin or lovastatin reduces the in vitro growth of Chlamydophila pneumoniae [83] and of Salmonella enterica [84]. Simvastatin demonstrated a significant antimicrobial effect against methicillin-sensitive Staphylococcus aureus (average MIC, 15.65 μg/mL) and, to a lesser extent, against methicillin-resistant S. aureus (MIC 31.25 μg/mL), inhibiting adhesion and formation of biofilm of the microorganism [85].
It has been observed that lovastatin interferes with the replication of hepatitis C virus RNA through the inhibition of geranylgeranylation protein of the host [86]. Statins also inhibit the assembly of dengue virus virions through a mechanism independent of cholesterol levels [87]. Statins have also shown antiviral effects on cytomegalovirus [88], the Epstein-Barr virus [89] and HIV infection [90]. The antiviral mechanism that statins exert is not clear; however, the metabolite rescue experiments suggest participation of the nonsteroidal isoprenoid arm of the mevalonate pathway as a possible mechanism of action [91, 92].
Statins inhibit the formation of biofilms of Candida albicans [93] and, in C. glabrata, reduce ergosterol levels, inhibit their growth, and cause the loss of mtDNA [94]. Statins also inhibit the growth of Aspergillus fumigatus; in addition, lovastatin strengthens the activity of caspofungin against A. fumigatus in an in vitro model [95, 96].
Together, these in vitro studies show that statins slow the growth of some microorganisms, including some resistant bacteria, and also show that they can interfere with biofilm formation. These effects have clinical relevance because within the biofilm, bacteria are protected against the action of antibodies, the attack of phagocytic cells, and the effect of antimicrobials. It is also known that biofilm bacteria can be up to 1000 times more resistant to antibiotics than the same bacteria grown in liquid medium [97].
Therefore, it is very important to note that statins have the potential to inhibit the growth of resistant bacteria and interfere with the biofilm formation process. However, it is necessary to emphasize that the concentrations used in in vitro studies that have antimicrobial properties are 100 to 1000 times higher than the plasma concentrations reached in patients undergoing statin therapy, which is why it is still necessary to clarify whether these effects are transferable and if they have any therapeutic benefit in humans.
2.2. In Vitro Effect of Statins on Mycobacterium tuberculosis Infection
The first study on the potential effect of statins on infection by M. tuberculosis was performed 18 years ago by Montero et al., where it was observed that fluvastatin slightly induces the release of TH1 cytokines and promotes the activation of caspase 1; by infecting peripheral blood mononuclear cells (PBMCs) with M. tuberculosis and treating them with fluvastatin, this stimulation was synergistic, yielding concentrations up to 10 times higher than those of TH1 cytokines and caspase 1. This result suggested that statins could strengthen the host response against M. tuberculosis [67]. In 2009, another study reported that lovastatin and fluvastatin inhibit the activation of γδ T cells induced by M. tuberculosis antigens [98]; however, none of these studies evaluated the effect of statins on mycobacterial growth or the influence of these effects on the immune response of the host against infection (Table 1).
Table 1.
In vitro effects of statins in tuberculosis.
| Author | Cell type | Treatment | Strain | Effect |
|---|---|---|---|---|
| Montero et al. | PBMC | Fluvastatin 5 μm | Heat-inactivated M. tuberculosis H37Ra 10 μg/mL | Promotes release of TH1 cytokines and promotes the activation of caspase 1 |
| Lu et al. | PBMC | Lovastatin 10 μm Fluvastatin 2 μm | M. tuberculosis antigens | Inhibits the activation of γδ T cells |
| Parihar et al. | PBMC and MDM from patients with hypercholesterolemia receiving statin therapy | Simvastatin 50 μM | M. tuberculosis H37Rv MOI 5 | Significant reduction of mycobacterial growth |
| Parihar et al. | Murine bone marrow-derived macrophages | Simvastatin 50 μM | M. tuberculosis H37Rv MOI 5 | Significant reduction of mycobacterial growth, simvastatin treatment promotes phagosomal maturation and autophagy |
| Lobato et al. | THP-1 macrophages | Rifampin 1 μg/mL plus 0.2 μM atorvastatin | M. tuberculosis H37Rv MOI 10 BCG strain of M. bovis MOI 50 | Atorvastatin has an additive effect with rifampin, reducing intracellular mycobacterial viability |
| Skerry et al. | J774 macrophage-like cells | Isoniazid 0.05 μg/mL plus 5 μM simvastatin | M. tuberculosis CDC1551 MOI 10 | Simvastatin treatment enhanced the bacterial killing activity of isoniazid at day 3 after infection |
| Dutta et al. | THP-1 macrophages | 0.011 μM isoniazid, 0.012 μM rifampicin, and 162.5 μM pyrazinamide plus 0.1 μM simvastatin | Bioluminescent M. tuberculosis H37Rv MOI 0.05 | Simvastatin treatment significantly increased the bactericidal effect of isoniazid, rifampicin, and pyrazinamide alone or in combination |
PBMC: peripheral blood mononuclear cells; MDM: monocyte-derived macrophages.
In 2014, Parihar et al. [99] found that mononuclear cells and monocyte-derived macrophages from patients with familial hypercholesterolemia who had received statin therapy for at least six months were more resistant to infection with M. tuberculosis with a multiplicity of infection (MOI) of 5 (number of bacteria per number of human/mammal cells) compared with cells from healthy subjects who had never taken statins. In the same study, they performed an in vitro model with murine bone marrow-derived macrophages that were exposed to simvastatin at a concentration of 50 μM and were infected with M. tuberculosis at an MOI of 5. The results showed a significant reduction in mycobacterial growth, without adverse effects on cell viability. In addition, they performed Western blot and confocal microscopy experiments, where it was observed that simvastatin promotes maturation in phagosomes and autophagy in macrophages infected by M. tuberculosis. The effect of simvastatin on the inhibition of M. tuberculosis growth was reversed by mevalonate supplementation (Table 1). This was the first study where the use of statins as HDT was proposed, and the study opens the possibility of studying the additive effect of statins with first-line drugs in drug therapy against tuberculosis.
Lobato et al. investigated the activity and the possible additive effects of treatment with rifampicin (1 μg/mL), atorvastatin (0.2 μM-2 μM), and simvastatin (0.2 μM-2 μM) on THP-1 macrophages infected with M. tuberculosis, BCG strain of M. bovis, and M. leprae at an MOI 10. After 72 hours, both statins had dose-dependent bactericidal effects on all strains. For M. tuberculosis, both statins (2 μM atorvastatin and 2 μM simvastatin) reduced the viability of the mycobacteria by approximately 75% and showed an additive effect with rifampicin (1 μg/mL rifampicin plus 0.2 μM atorvastatin or 0.2 μM simvastatin). For the BCG strain of M. bovis and M. leprae, only 0.2 μM atorvastatin had an additive effect with rifampicin. To determine the mechanism involved in the inhibition of mycobacterial growth, they only tested the effect of atorvastatin on THP-1 macrophages infected with M. leprae at an MOI of 10; the results confirmed that statins promote phagosome maturation (Table 1) [100].
Another study investigated whether statins could strengthen the bactericidal effect of isoniazid. They tested J774 macrophages, which were infected with M. tuberculosis CDC1551 at an MOI of 10, and treated them with 5 μM simvastatin and 0.05 μg/mL isoniazid. The results confirmed that cells cultured in the presence of simvastatin and infected with M. tuberculosis CDC1551 had a lower intracellular bacillary load on day five after infection, and this effect was additive when the cells were treated simultaneously with simvastatin and isoniazid on the third day after infection. On the fifth day, the additive effect lost statistical significance [101].
In 2016, Dutta et al. studied the possible adjuvant activity of simvastatin with isoniazid, rifampicin, and pyrazinamide. They performed experiments with the M. tuberculosis H37Rv expressing the lux operon and THP-1 macrophages infected at an MOI of 0.05. They used drugs at concentrations used in experiments to reduce 50% of the relative light units (RLU) of the mycobacterium (0.011 μM isoniazid, 0.012 μM rifampicin, and 162.5 μM pyrazinamide); the simvastatin concentration was 0.1 μM. The results confirmed that simvastatin without antibiotic inhibited the growth of mycobacteria in the THP-1 macrophages compared with the control without drugs, and the effect of simvastatin was equivalent to the activity of 0.011 μM of isoniazid. In addition, simvastatin significantly increased the bactericidal effect when the three drugs were added simultaneously [7]. They also evaluated whether simvastatin could affect the intracellular accumulation of rifampicin using liquid chromatography-mass spectrometry. It was observed that although simvastatin (0.1-1 μM) increased the bactericidal activity of rifampicin, it did not alter its intracellular accumulation (Table 1) [7].
The different studies used different strains of mycobacteria (reference and clinical isolates) and different MOIs (from 0.05 to 10); however, the effect of statins has been consistent, and the studies have shown that statins activate several cellular mechanisms (autophagy and maturation of the lysosome phage) by which control of infection in infected cells is favored in vitro. However, identification of the possible direct antimicrobial effect of statins has not been performed.
Skerry et al. reported the first study on the direct antimicrobial effect of simvastatin against M. tuberculosis tested at different concentrations (0-320 μM). The results obtained showed that simvastatin had no inhibitory effect, even at the highest concentration (320 μM, a concentration 60 times higher than those used in in vitro experiments performed in cells) [101].
Another study also evaluated the direct antimicrobial effect of statins on M. tuberculosis H37Rv and the BCG strain of M. bovis using the agar proportion method, with different concentrations of simvastatin. The MIC found was 100 μg/mL (238.91 μM), defined as the lowest concentration of the statin that inhibited more than 99% of the bacterial population. In addition, this study reported that simvastatin decreases the amount of phosphatidylinositol mannosides and triacylglycerols present in the mycobacterial cell wall (Table 1) [102].
The MICs reported by the CLSI for the first-line drugs are as follows: isoniazid, 0.2 or 1.0 μg/mL; rifampin, 1.0 μg/mL; pyrazinamide, 100 μg/mL; and ethambutol, 5.0 μg/mL [103]. In contrast, the MIC for simvastatin is elevated and similar to that of pyrazinamide, well above the therapeutic concentrations recommended for the treatment of hyperlipidemia (0.0209 μg/mL) [104]. Thus, the direct antimicrobial effect in vitro appears to be weak, which supports the idea that simvastatin favors or triggers the cellular mechanisms necessary to eliminate the bacillus.
2.3. Treatment of Tuberculosis with Statins in Animal Models
All in vivo and prospective studies of the effect of statins against tuberculosis have been performed in mice (Table 2). The first study was performed in C57BL/6 mice, 8-12 weeks of age, treated intraperitoneally with simvastatin or rosuvastatin (20 mg/kg) or with phosphate-buffered saline (PBS) as a control every two days for two weeks; the mice were infected with M. tuberculosis by exposure to aerosols. Statin therapy was continued until four weeks after infection. Both statins showed a protective response in the infected mice, with up to 10-fold reductions in the bacillary load in the spleen, liver, and lungs of mice infected and treated with simvastatin, in comparison with untreated control animals (Table 2) [99]. Lobato et al. investigated the effect of atorvastatin using the Shepard infection model [105], in which a suspension of 1 × 104M. leprae was injected into the plantar pads of BALB/c mice. After one month of infection, the mice were treated with atorvastatin (80 mg/kg/day) in combination with rifampicin (1 mg/kg) for five months. The results showed that atorvastatin reduced the replication of M. leprae and had an additive effect with rifampicin. They also showed that treatment with atorvastatin or the combination of rifampin plus atorvastatin did not increase muscle damage or hepatotoxicity in mice (Table 2) [100].
Table 2.
Statin treatment of tuberculosis in animal models.
| Author | Animal model | Treatment | Strain | Effect |
|---|---|---|---|---|
| Parihar et al. | C57BL/6 mice (age 8-12 weeks) | Simvastatin or rosuvastatin (20 mg/kg) i.p. every second day for 6 weeks | Low-dose aerosol-based M. tuberculosis H37Rv | Up to a 10-fold reduction in bacilli burden in spleen, liver, and lungs |
| Lobato et al. | Mouse foot pads of BALB/c mice (Shepard's mouse model) | Atorvastatin (80 mg/kg/day added daily to food) alone or combined with rifampin (1 mg/kg by gavage weekly) for five months | 1 × 104 live M. leprae in 10 μL inoculated in each footpad | Reduced replication, additive effect with rifampin. Neither atorvastatin treatment nor combination treatment increased muscle damage or induced hepatotoxicity |
| Dutta et al. | BALB/c mice (age 4-6 weeks) | Rifampicin (10 mg/kg), isoniazid (10 mg/kg), and pyrazinamide (25 mg/kg), plus simvastatin (25 mg/kg) by gavage 5 days per week for 8 weeks | Aerosol infection with 3.7 log10 CFU of M. tuberculosis CDC1551 | The combination regimen with simvastatin enhanced mycobacterial killing and reduced the relapse rates in mice treated for 2.5 and 3.5 months |
Subsequently, another group tested whether the addition of statins to the standard first-line treatment regimen could increase bactericidal capacity. BALB/c mice four to six weeks of age were infected by aerosols with 3.7 log10 CFU of M. tuberculosis CDC1551. The infection was allowed to progress for six weeks before the start of treatment, after which the mice were treated by gavage with rifampicin (10 mg/kg), isoniazid (10 mg/kg), and pyrazinamide (25 mg/kg), with or without simvastatin (25 mg/kg), five days per week for eight weeks. After four and eight weeks of treatment, the standard regimen in combination with simvastatin showed a greater efficacy for the elimination of mycobacteria, reducing the number of lung CFU by one additional log10 on day 28 and 1.25 log10 on day 56 [101], suggesting that simvastatin could improve current treatment (Table 2).
Dutta et al. confirmed that simvastatin therapy as an adjuvant to standard treatment reduced the time to obtain a negative culture in BALB/c mice infected with M. tuberculosis H37Rv by aerosols. They also evaluated the relapse rates in mice treated with simvastatin (60 mg/kg) for 2.5, 3.5, and 4.5 months. Relapse was evaluated three months after stopping treatment. The results showed that treatment with anti-TB drugs plus simvastatin reduced the percentage of relapses by 50% compared with treatment with only anti-TB drugs (Table 2) [7].
These studies together propose statins as adjuvant treatment to first-line drugs for the treatment of active tuberculosis, as they show that treatment with statins reduces the bacillary load, shortens the duration of therapy, and decreases the relapse rate. However, these studies have some limitations. First, the doses used in mice are much higher than those used in humans. Second, some statins are prodrugs, and in mice, 100% of the prodrug is converted into the active metabolite, whereas the conversion reaches 50% of the administered dose in humans [106]. It is also necessary to determine the best time to start treatment with statins, as the treatment started before infecting the mice in the studies reported. In addition, it would be advisable to carry out studies in other animal models that have a drug metabolism more similar to that of humans and to determine the concentrations associated with better antimicrobial activity.
2.4. Retrospective Studies of Statin Use in Humans and the Risk of Developing Tuberculosis
Kang et al. reported in 2014 the first study in humans that evaluated the effect of statins on the risk of developing tuberculosis. This study was retrospective, and the results were obtained from the information of the database of the National Health Insurance of South Korea, which included 840,894 subjects with recently diagnosed diabetes mellitus type 2 (DM2) and who were 20-99 years of age. The use of statins was less frequent among patients with active tuberculosis (19.2% patients with tuberculosis vs. 33.6% without tuberculosis, p < 0.01). However, after adjustment for possible initial confounding factors (age, sex, history of silicosis, malignancy, HIV/AIDS, chronic kidney disease, use of systemic corticosteroids, comorbidities [e.g., dyslipidemia, hypertension, angina, myocardial infarction, cerebrovascular disease, peripheral artery disease, and retinopathy], and history of hospitalization), the use of statins in subjects with newly diagnosed DM2 was not associated with a lower or higher risk of developing tuberculosis (hazard ratio [HR] 0.98, 95% CI 0.89-1.07) [107]. The authors note that although comorbidities were adjusted as confounding variables, they could influence the HR for active tuberculosis development (Table 3).
Table 3.
Retrospective studies of statin use in humans and the risk of developing tuberculosis.
| Author | Year | Method | Patients | Conclusions |
|---|---|---|---|---|
| Kang et al. | 2014 | Retrospective cohort study | 840,894 newly diagnosed type 2 DM patients aged 20-99 years who were newly treated with antidiabetic drugs | Statin use in newly diagnosed type 2 diabetics was not associated with protection against or a higher risk of developing tuberculosis |
| Lee et al. | 2015 | Retrospective cohort study | 13,981 patients with type 2 diabetes aged more than 65 years | After adjusting for age, sex, other comorbidities, and medications, statin users had a lower independent association, with a risk ratio of 0.76 (95% CI, 0.60-0.97) |
| Lai et al. | 2016 | Retrospective nested case-control study | 8098 new TB cases and 809,800 control patients | Statin users had a decreased risk of active tuberculosis. Chronic use of statins (more than 90 days) was associated with the lowest risk (RR 0.62; 95% CI 0.53-0.72) |
| Su et al. | 2017 | Retrospective nested case-control study | 102,424 statin users, 202,718 patients aged 20 years or older, and 202,718 matched subjects | Statin use is an independent protective factor for tuberculosis development. There is a dose-dependent association between statin use and risk of active tuberculosis |
Subsequently, another retrospective study conducted by Lee et al. in Taiwan included 13,981 patients with DM2 older than 65 years. They used the Cox proportional hazards regression model to determine the independent effects of diabetes on the risk of developing active tuberculosis. After adjusting for age, sex, comorbidities (gout, hypertension, hyperlipidemia, asthma, COPD, acquired immunodeficiency syndrome (AIDS), connective tissue disease, end-stage renal disease, heart failure, and other cardiovascular diseases) and medications (antihyperglycemic, antihypertensive, and antihyperlipidemic agents), the investigators reported that Taiwanese diabetic subjects older than 65 treated with statins had a lower risk of developing active tuberculosis, with a risk of 0.76 (95% CI, 0.60-0.97) [108]. In this study, exposure to statins was based only on the prescription information compiled from the National Health Insurance Database; therefore, the level of adherence and dose received by patients is unknown (Table 3).
Lai et al. conducted a nested case-control study that included patients older than 18 years of age from 1999 to 2011, using the database from Taiwan's national health insurance program. They included 8098 new cases of tuberculosis and 809,800 control patients, and statin users were divided into four groups. The first group was called the current users (patients with prescription of statins within 30 days before the diagnosis of tuberculosis), the second group were the recent users (patients with prescription of statins between 31 and 90 days before the diagnosis of tuberculosis), the third group were patients with statin consumption between 91 days and one year before the date of tuberculosis diagnosis, and the fourth group were those with chronic use (patients with a cumulative prescription greater than 90 days). They used a sampling strategy to control cases of coincident time, and the relative risk (RR) of developing active tuberculosis was calculated with a confidence interval greater than 95%. The four types of statin users had a lower risk of active tuberculosis. For the first group, the RR of developing active tuberculosis was 0.64 (95% CI 0.54-0.76). The fourth group showed the lowest risk of developing active tuberculosis (RR: 0.62, 95% CI: 0.53 to 0.72) [109]. One of the methodological strengths of this study is the inclusion of a large sample of patients and controls and the performance of a conditional logistic regression analysis with more than 75 possible confounding factors (e.g., cardiovascular comorbidities, risk factors for developing tuberculosis, frailty indicators, and use of specific medications). However, some data related to lifestyle and its effects (e.g., diet, exercise, overcrowding, smoking, and body mass index) are not available; therefore, the effects of some residual confounding variables are unknown (Table 3).
Another case-control study also conducted in Taiwan included 8236 subjects older than 20 years of age with recently diagnosed pulmonary tuberculosis from 2000 to 2013. Each case was matched by age and sex with 8236 controls without pulmonary tuberculosis. The users of some type of statin were subjects who received medication 12 months before being diagnosed with pulmonary tuberculosis. Multivariable logistic regression analysis was performed to estimate the odds ratio (OR) with a confidence interval of 95%. After adjusting for lipid-lowering drugs that were not statins and for DM2, the OR adjusted for pulmonary tuberculosis was 0.67 for the subjects who used statins at some point (95% CI: 0.59 to 0.75). They also analyzed the estimated OR for each type of statin, and the results showed that the subjects taking atorvastatin had a lower probability of developing active tuberculosis (0.56, 95% CI 0.46, 0.68) [110]. The researchers confirmed that the use of statins is a protective factor for the development of pulmonary tuberculosis. However, like all studies conducted using a database, there are residual confounding factors due to a lack of information. In this study, we did not have access to socioeconomic status and lifestyle data (Table 3).
The most recent published study (also retrospective) included 102,424 statin users and 202,718 control subjects (no statin use). The use of statins was defined as a prescription record for ≥30 days of some type of statin. They calculated the cumulative defined daily dose (cDDD) of statins and defined three groups: low (<180), medium (180-365), and high (>365). The HR for the development of tuberculosis disease in patients taking statins was 0.53 (95% CI: 0.47 to 0.61, p < 0.001), suggesting that the use of statins is an independent protection factor for the development of tuberculosis. They also found a dose-dependent association between the use of statins and the risk of active tuberculosis (low, HR 1.06, p = 0.477; medium, HR 0.57, p < 0.001; high, HR 0.27, p < 0.001) [111]. In this study, the authors adjusted the possible confounding variables, including age, sex, level of urbanization, visits to the emergency department, comorbidities (such as DM2, coronary heart disease, heart failure, cerebrovascular disease, chronic kidney disease, cancer, lung disease, asthma, liver cirrhosis, rheumatoid arthritis and systemic lupus erythematosus), and the use of medications (biological agents, systemic glucocorticoids, and disease-modifying antirheumatic drugs). However, again, information regarding the diagnosis of tuberculosis and the use of statins depended on a database; thus, the study suffers from the lack of precision that can be achieved with a prospective cohort study (Table 3).
All the studies carried out on the effect of statins on the risk of developing tuberculosis have been performed in Korea and Taiwan, so the generalization of the results to other populations requires verification. In addition, since they are retrospective studies obtained from databases, no microbiological data are available to support the diagnosis of tuberculosis, and a causal relationship cannot be verified.
3. Perspectives and Conclusions
The information gathered in this review provides the basis for considering statins as a host-directed therapy in infection by M. tuberculosis. The studies described show that the cells (mostly macrophages) are more resistant to infection by M. tuberculosis in the presence of statins in in vitro models. The mechanism proposed to favor the immune response of the host is promoting phagolysosome maturation and autophagy. However, it is necessary to deepen the knowledge of the effect of statins on the immune response against M. tuberculosis. In this sense, our group recently reported that treatment with simvastatin increases the percentage of NKT cells and increases the expression of costimulatory molecules in monocytes in an infection model in vitro, and the increase in the expression of these molecules may favor the antigenic presentation that is inhibited by M. tuberculosis [112].
Studies in mice show that statin therapy shortens the duration of antituberculosis treatment and appears to decrease the risk of relapse. Therefore, investigations of the mechanism through which statins increase the antimicrobial effect of first-line antituberculosis drugs are still needed, although we can speculate that it is possible that statins weaken the mycobacterial wall making it more susceptible to first-line drugs, that they may only strengthen the immune response of the host that contributes to the most effective and rapid elimination of the bacillus, or even a combination of both effects; specifically, it has been shown that statins affect the lipids of the wall of some fungi and can also decrease the phosphatidylinositol mannosides and triacylglycerols of the cell wall of M. tuberculosis H37Rv.
Although the mechanism by which statins improve the immune response against M. tuberculosis is still not fully known, retrospective studies in humans show a protective effect of statins against the development of active tuberculosis. The evidence that exists to date is sufficient to test statins in prospective studies for the determination of whether these drugs have only protective effects against the reactivation of latent tuberculosis or if they could really be effective adjuvants in the pharmacological treatment of active tuberculosis.
Acknowledgments
The authors fully appreciate the collaboration of Dr. Alejandro Escobar Gutiérrez from Instituto de Diagnóstico y Referencia Epidemiológicos “Dr. Manuel Martínez Báez” for the support provided in doing Figure 1. This work was supported by a scholarship grant [276366] received by PCDGB, Consejo Nacional de Ciencia y Tecnología, https://www.conacyt.gob.mx/, and a grant [241754] received by APL, Consejo Nacional de Ciencia y Tecnología, https://www.conacyt.gob.mx. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
- 1.Russell D. G., Barry C. E., 3rd, Flynn J. L. Tuberculosis: what we don’t know can, and does, hurt us. Science. 2010;328(5980):852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report. Geneva: 2017. Licence: CC BY-NCSA3.0 IGO. [Google Scholar]
- 3.Wallis R. S., Hafner R. Advancing host-directed therapy for tuberculosis. Nature Reviews Immunology. 2015;15(4):255–263. doi: 10.1038/nri3813. [DOI] [PubMed] [Google Scholar]
- 4.Maguire G. P., Anstey N. M., Ardian M., et al. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. The International Journal of Tuberculosis and Lung Disease. 2009;13(12):1500–1506. [PubMed] [Google Scholar]
- 5.Zumla A., Chakaya J., Hoelscher M., et al. Towards host-directed therapies for tuberculosis. Nature Reviews Drug Discovery. 2015;14(8):511–512. doi: 10.1038/nrd4696. [DOI] [PubMed] [Google Scholar]
- 6.National Clinical Guideline Centre. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London: National Clinical Guideline Centre; 2014. [Google Scholar]
- 7.Dutta N. K., Bruiners N., Pinn M. L., et al. Statin adjunctive therapy shortens the duration of TB treatment in mice. The Journal of Antimicrobial Chemotherapy. 2016;71(6):1570–1577. doi: 10.1093/jac/dkw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin P. L., Flynn J. L. Understanding latent tuberculosis: a moving target. The Journal of Immunology. 2010;185(1):15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons J. D., Stein C. M., Seshadri C., et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nature Reviews Immunology. 2018;18(9):575–589. doi: 10.1038/s41577-018-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottenhoff T. H. M. The knowns and unknowns of the immunopathogenesis of tuberculosis. The International Journal of Tuberculosis and Lung Disease. 2012;16(11):1424–1432. doi: 10.5588/ijtld.12.0479. [DOI] [PubMed] [Google Scholar]
- 11.Etna M. P., Giacomini E., Severa M., Coccia E. M. Pro- and anti-inflammatory cytokines in tuberculosis: a two-edged sword in TB pathogenesis. Seminars in Immunology. 2014;26(6):543–551. doi: 10.1016/j.smim.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Allen M., Bailey C., Cahatol I., et al. Mechanisms of control of Mycobacterium tuberculosis by NK cells: role of glutathione. Frontiers in Immunology. 2015;6:p. 508. doi: 10.3389/fimmu.2015.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwander S., Dheda K. Human lung immunity against Mycobacterium tuberculosis: insights into pathogenesis and protection. American Journal of Respiratory and Critical Care Medicine. 2011;183(6):696–707. doi: 10.1164/rccm.201006-0963PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chackerian A., Alt J., Perera V., Behar S. M. Activation of NKT cells protects mice from tuberculosis. Infection and Immunity. 2002;70(11):6302–6309. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder-Cappione J. E., Nixon D. F., Loo C. P., et al. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d-restricted NKT cells. The Journal of Infectious Diseases. 2007;195(9):1361–1364. doi: 10.1086/513567. [DOI] [PubMed] [Google Scholar]
- 16.Domingo-Gonzalez R., Prince O., Cooper A., Khader S. A. Chapter 2: cytokines and chemokines in Mycobacterium tuberculosis infection. Tuberculosis and the Tubercle Bacillus. (Second Edition) 2017;4(5) doi: 10.1128/microbiolspec.TBTB2-0018-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayaraman P., Sada-Ovalle I., Nishimura T., et al. IL-1β promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. The Journal of Immunology. 2013;190(8):4196–4204. doi: 10.4049/jimmunol.1202688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozzano F., Marras F., De Maria A. Immunology of tuberculosis. Mediterranean Journal of Hematology and Infectious Diseases. 2014;6(1, article e2014027) doi: 10.4084/MJHID.2014.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behar S. M., Martin C. J., Booty M. G., et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunology. 2011;4(3):279–287. doi: 10.1038/mi.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fratazzi C., Arbeit R. D., Carini C., Remold H. G. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. The Journal of Immunology. 1997;158(9):4320–4327. [PubMed] [Google Scholar]
- 21.Zuñiga J., Torres-García D., Santos-Mendoza T., Rodriguez-Reyna T. S., Granados J., Yunis E. J. Cellular and humoral mechanisms involved in the control of tuberculosis. Clinical and Developmental Immunology. 2012;2012, article 193923:18. doi: 10.1155/2012/193923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scanga C. A., Mohan V. P., Yu K., et al. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon γ and nitric oxide synthase 2. The Journal of Experimental Medicine. 2000;192(3):347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper A. M. Cell-mediated immune responses in tuberculosis. Annual Review of Immunology. 2009;27(1):393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva C. L., Lowrie D. B. Identification and characterization of murine cytotoxic T cells that kill Mycobacterium tuberculosis. Infection and Immunity. 2000;68(6):3269–3274. doi: 10.1128/IAI.68.6.3269-3274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L., Yang B., Zhang X., Lao S., Changyou Wu Mycobacterium tuberculosis-specific polyfunctional cytotoxic CD8+ T cells express CD69. Tuberculosis. 2014;94(3):219–225. doi: 10.1016/j.tube.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Meraviglia S., El Daker S., Dieli F., Martini F., Martino A. γδ T cells cross-link innate and adaptive immunity in Mycobacterium tuberculosis infection. Clinical and Developmental Immunology. 2011;2011, article 587315:11. doi: 10.1155/2011/587315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao M., Valentini D., Poiret T., et al. B in TB: B cells as mediators of clinically relevant immune responses in tuberculosis. Clinical Infectious Diseases. 2015;61(Supplement 3):S225–S234. doi: 10.1093/cid/civ614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scriba T. J., Coussens A. K., Fletcher H. A. Human immunology of tuberculosis. Microbiology Spectrum. 2017;5(1) doi: 10.1128/microbiolspec.TBTB2-0016-2016. [DOI] [PubMed] [Google Scholar]
- 29.O'Garra A., Redford P. S., McNab F. W., Bloom C. I., Wilkinson R. J., Berry M. P. R. The immune response in tuberculosis. Annual Review of Immunology. 2013;31(1):475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 30.Gatfield J., Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288(5471):1647–1651. doi: 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 31.Via L. E., Deretic D., Ulmer R. J., Hibler N. S., Huber L. A., Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. The Journal of Biological Chemistry. 1997;272(20):13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 32.Fratti R. A., Backer J. M., Gruenberg J., Corvera S., Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. The Journal of Cell Biology. 2001;154(3):631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis A. S., Vergne I., Master S. S., Kyei G. B., Chua J., Deretic V. Mechanism of inducible nitric oxide synthase exclusion from mycobacterial phagosomes. PLoS Pathogens. 2007;3(12, article e186) doi: 10.1371/journal.ppat.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee W. L., Gold B., Darby C., et al. Mycobacterium tuberculosis expresses methionine sulphoxide reductases A and B that protect from killing by nitrite and hypochlorite. Molecular Microbiology. 2009;71(3):583–593. doi: 10.1111/j.1365-2958.2008.06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hmama Z., Gabathuler R., Jefferies W. A., de Jong G., Reiner N. E. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. The Journal of Immunology. 1998;161(9):4882–4893. [PubMed] [Google Scholar]
- 36.Pecora N. D., Fulton S. A., Reba S. M., et al. Mycobacterium bovis BCG decreases MHC-II expression in vivo on murine lung macrophages and dendritic cells during aerosol infection. Cellular Immunology. 2009;254(2):94–104. doi: 10.1016/j.cellimm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sreejit G., Ahmed A., Parveen N., et al. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (β2M) affecting antigen presentation function of macrophage. PLoS Pathogens. 2014;10(10, article e1004446) doi: 10.1371/journal.ppat.1004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grace P. S., Ernst J. D. Suboptimal antigen presentation contributes to virulence of Mycobacterium tuberculosis in vivo. The Journal of Immunology. 2016;196(1):357–364. doi: 10.4049/jimmunol.1501494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh C. R., Moulton R. A., Armitige L. Y., et al. Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. The Journal of Immunology. 2006;177(5):3250–3259. doi: 10.4049/jimmunol.177.5.3250. [DOI] [PubMed] [Google Scholar]
- 40.Hanekom W. A., Mendillo M., Manca C., et al. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. The Journal of Infectious Diseases. 2003;188(2):257–266. doi: 10.1086/376451. [DOI] [PubMed] [Google Scholar]
- 41.Roberts L. L., Robinson C. M. Mycobacterium tuberculosis infection of human dendritic cells decreases integrin expression, adhesion and migration to chemokines. Immunology. 2014;141(1):39–51. doi: 10.1111/imm.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagliardi M. C., Lemassu A., Teloni R., et al. Cell wall-associated alpha-glucan is instrumental for Mycobacterium tuberculosis to block CD1 molecule expression and disable the function of dendritic cell derived from infected monocyte. Cellular Microbiology. 2007;9(8):2081–2092. doi: 10.1111/j.1462-5822.2007.00940.x. [DOI] [PubMed] [Google Scholar]
- 43.Stenger S., Niazi K. R., Modlin R. L. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. The Journal of Immunology. 1998;161(7):3582–3588. [PubMed] [Google Scholar]
- 44.Fraga A. G., Barbosa A. M., Ferreira C. M., Fevereiro J., Pedrosa J., Torrado E. Immune-evasion strategies of mycobacteria and their implications for the protective immune response. Current Issues in Molecular Biology. 2018;25:169–198. doi: 10.21775/cimb.025.169. [DOI] [PubMed] [Google Scholar]
- 45.Mahon R. N., Sande O. J., Rojas R. E., Levine A. D., Harding C. V., Henry Boom W. Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cellular Immunology. 2012;275(1-2):98–105. doi: 10.1016/j.cellimm.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sande O. J., Karim A. F., Li Q., et al. Mannose-capped Lipoarabinomannan from Mycobacterium tuberculosis induces CD4+ T cell anergy via GRAIL. The Journal of Immunology. 2016;196(2):691–702. doi: 10.4049/jimmunol.1500710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saavedra R., Segura E., Tenorio E. P., López-Marín L. M. Mycobacterial trehalose-containing glycolipid with immunomodulatory activity on human CD4+ and CD8+ T-cells. Microbes and Infection. 2006;8(2):533–540. doi: 10.1016/j.micinf.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Elsaidi H. R. H., Barreda D. R., Cairo C. W., Lowary T. L. Mycobacterial phenolic glycolipids with a simplified lipid aglycone modulate cytokine levels through toll-like receptor 2. Chembiochem. 2013;14(16):2153–2159. doi: 10.1002/cbic.201300505. [DOI] [PubMed] [Google Scholar]
- 49.Dao D. N., Sweeney K., Hsu T., et al. Mycolic acid modification by the mmaA4 gene of M. tuberculosis modulates IL-12 production. PLoS Pathogens. 2008;4(6, article e1000081) doi: 10.1371/journal.ppat.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson E. T., Shukla S., Sweet D. R., et al. Toll-like receptor 2-dependent extracellular signal-regulated kinase signaling in Mycobacterium tuberculosis-infected macrophages drives anti-inflammatory responses and inhibits Th1 polarization of responding T cells. Infection and Immunity. 2015;83(6):2242–2254. doi: 10.1128/IAI.00135-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X., Wu Y., Jiao J., Huang Q. Mycobacterium tuberculosis infection induces IL-10 gene expression by disturbing histone deacetylase 6 and histonedeacetylase 11 equilibrium in macrophages. Tuberculosis. 2018;108:118–123. doi: 10.1016/j.tube.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Sly L. M., Hingley-Wilson S. M., Reiner N. E., McMaster W. R. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. The Journal of Immunology. 2003;170(1):430–437. doi: 10.4049/jimmunol.170.1.430. [DOI] [PubMed] [Google Scholar]
- 53.Chen M., Gan H., Remold H. G. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. The Journal of Immunology. 2006;176(6):3707–3716. doi: 10.4049/jimmunol.176.6.3707. [DOI] [PubMed] [Google Scholar]
- 54.Romagnoli A., Etna M. P., Giacomini E., et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012;8(9):1357–1370. doi: 10.4161/auto.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abuhammad A. Cholesterol metabolism: a potential therapeutic target in mycobacteria. British Journal of Pharmacology. 2017;174(14):2194–2208. doi: 10.1111/bph.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim M. J., Wainwright H. C., Locketz M., et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Molecular Medicine. 2010;2(7):258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martens G. W., Arikan M. C., Lee J., Ren F., Vallerskog T., Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infection and Immunity. 2008;76(8):3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soh A. Z., Chee C. B. E., Wang Y. T., Yuan J. M., Koh W. P. Dietary cholesterol increases the risk whereas PUFAs reduce the risk of active tuberculosis in Singapore Chinese. The Journal of Nutrition. 2016;146(5):1093–1100. doi: 10.3945/jn.115.228049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muñoz S., Rivas-Santiago B., Enciso J. A. Mycobacterium tuberculosis entry into mast cells through cholesterol-rich membrane microdomains. Scandinavian Journal of Immunology. 2009;70(3):256–263. doi: 10.1111/j.1365-3083.2009.02295.x. [DOI] [PubMed] [Google Scholar]
- 60.Fine-Coulson K., Reaves B. J., Karls R. K., Quinn F. D. The role of lipid raft aggregation in the infection of type II pneumocytes by Mycobacterium tuberculosis. PLoS One. 2012;7(9, article e45028) doi: 10.1371/journal.pone.0045028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huynh K. K., Gershenzon E., Grinstein S. Cholesterol accumulation by macrophages impairs phagosome maturation. The Journal of Biological Chemistry. 2008;283(51):35745–35755. doi: 10.1074/jbc.M806232200. [DOI] [PubMed] [Google Scholar]
- 62.Peyron P., Vaubourgeix J., Poquet Y., et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathogens. 2008;4(11, article e1000204) doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palanisamy G. S., Kirk N. M., Ackart D. F., et al. Uptake and accumulation of oxidized low-density lipoprotein during Mycobacterium tuberculosis infection in guinea pigs. PLoS One. 2012;7(3, article e34148) doi: 10.1371/journal.pone.0034148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hennessy E., Adams C., Reen F. J., O'Gara F. Is there potential for repurposing statins as novel antimicrobials? Antimicrobial Agents and Chemotherapy. 2016;60(9):5111–5121. doi: 10.1128/AAC.00192-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka N., Abe-Dohmae S., Iwamoto N., Fitzgerald M. L., Yokoyama S. HMG-CoA reductase inhibitors enhance phagocytosis by upregulating ATP-binding cassette transporter A7. Atherosclerosis. 2011;217(2):407–414. doi: 10.1016/j.atherosclerosis.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwak B., Mulhaupt F., Myit S., Mach F. Statins as a newly recognized type of immunomodulator. Nature Medicine. 2000;6(12):1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 67.Montero María T., Hernández O., Suárez Y., et al. Hydroxymethylglutaryl-coenzyme A reductase inhibition stimulates caspase-1 activity and Th1-cytokine release in peripheral blood mononuclear cells. Atherosclerosis. 2000;153(2):303–313. doi: 10.1016/S0021-9150(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y., Jiang H., Liu W., et al. Effects of fluvastatin therapy on serum interleukin-18 and interleukin-10 levels in patients with acute coronary syndrome. Acta Cardiologica. 2010;65(3):285–289. doi: 10.2143/AC.65.3.2050343. [DOI] [PubMed] [Google Scholar]
- 69.Sutherland J. S., Jeffries D. J., Donkor S., et al. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis. 2009;89(6):398–404. doi: 10.1016/j.tube.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Gruenbacher G., Gander H., Nussbaumer O., Nussbaumer W., Rahm A., Thurnher M. IL-2 costimulation enables statin-mediated activation of human NK cells, preferentially through a mechanism involving CD56+ dendritic cells. Cancer Research. 2010;70(23):9611–9620. doi: 10.1158/0008-5472.CAN-10-1968. [DOI] [PubMed] [Google Scholar]
- 71.Raemer P. C., Kohl K., Watzl C. Statins inhibit NK‐cell cytotoxicity by interfering with LFA‐1‐mediated conjugate formation. European Journal of Immunology. 2009;39(6):1456–1465. doi: 10.1002/eji.200838863. [DOI] [PubMed] [Google Scholar]
- 72.Poggi A., Boero S., Musso A., Zocchi M. R. Selective role of mevalonate pathway in regulating perforin but not FasL and TNFalpha release in human natural killer cells. PLoS One. 2013;8(5, article e62932) doi: 10.1371/journal.pone.0062932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakou E., Babageorgakas P., Bouchliou I., et al. Statin-induced immunomodulation alters peripheral invariant natural killer T-cell prevalence in hyperlipidemic patients. Cardiovascular Drugs and Therapy. 2012;26(4):293–299. doi: 10.1007/s10557-012-6387-z. [DOI] [PubMed] [Google Scholar]
- 74.Fujiwara D., Tsubaki M., Takeda T., et al. Statins induce apoptosis through inhibition of Ras signaling pathways and enhancement of Bim and p27 expression in human hematopoietic tumor cells. Tumor Biology. 2017;39(10) doi: 10.1177/1010428317734947. [DOI] [PubMed] [Google Scholar]
- 75.Jang H. J., Hong E., Park S. W., et al. Statin induces apoptosis of human colon cancer cells and downregulation of insulin-like growth factor 1 receptor via proapoptotic ERK activation. Oncology Letters. 2016;12(1):250–256. doi: 10.3892/ol.2016.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araki M., Maeda M., Motojima K. Hydrophobic statins induce autophagy and cell death in human rhabdomyosarcoma cells by depleting geranylgeranyl diphosphate. European Journal of Pharmacology. 2012;674(2-3):95–103. doi: 10.1016/j.ejphar.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 77.Laezza C., Bucci C., Santillo M., Bruni C. B., Bifulco M. Control of Rab5 and Rab7 expression by the isoprenoid pathway. Biochemical and Biophysical Research Communications. 1998;248(3):469–472. doi: 10.1006/bbrc.1998.9007. [DOI] [PubMed] [Google Scholar]
- 78.Kruger P., Fitzsimmons K., Cook D., Jones M., Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Medicine. 2006;32(1):75–79. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- 79.Wan Y. D., Sun T. W., Kan Q. C., Guan F. X., Zhang S. G. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Critical Care. 2014;18(2):p. R71. doi: 10.1186/cc13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Almog Y., Shefer A., Novack V., et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110(7):880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 81.Thomas G., Hraiech S., Loundou A., et al. Statin therapy in critically-ill patients with severe sepsis: a review and meta-analysis of randomized clinical trials. Minerva Anestesiologica. 2015;81(8):921–930. [PubMed] [Google Scholar]
- 82.Sun H. Y., Singh N. Antimicrobial and immunomodulatory attributes of statins: relevance in solid-organ transplant recipients. Clinical Infectious Diseases. 2009;48(6):745–755. doi: 10.1086/597039. [DOI] [PubMed] [Google Scholar]
- 83.Erkkila L., Jauhiainen M., Laitinen K., et al. Effect of simvastatin, an established lipid-lowering drug, on pulmonary Chlamydia pneumoniae infection in mice. Antimicrobial Agents and Chemotherapy. 2005;49(9):3959–3962. doi: 10.1128/AAC.49.9.3959-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Catron D. M., Lange Y., Borensztajn J., Sylvester M. D., Jones B. D., Haldar K. Salmonella enterica serovar typhimurium requires nonsterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Infection and Immunity. 2004;72(2):1036–1042. doi: 10.1128/IAI.72.2.1036-1042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Graziano T. S., Cuzzullin M. C., Franco G. C., et al. Statins and antimicrobial effects: simvastatin as a potential drug against Staphylococcus aureus biofilm. PLoS One. 2015;10(5, article e0128098) doi: 10.1371/journal.pone.0128098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ye J., Wang C., Sumpter R., Brown M. S., Goldstein J. L., Gale M. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martinez-Gutierrez M., Castellanos J. E., Gallego-Gomez J. C. Statins reduce dengue virus production via decreased virion assembly. Intervirology. 2011;54(4):202–216. doi: 10.1159/000321892. [DOI] [PubMed] [Google Scholar]
- 88.Potena L., Frascaroli G., Grigioni F., et al. Hydroxymethyl-glutaryl coenzyme a reductase inhibition limits cytomegalovirus infection in human endothelial cells. Circulation. 2004;109(4):532–536. doi: 10.1161/01.CIR.0000109485.79183.81. [DOI] [PubMed] [Google Scholar]
- 89.Cohen J. I. HMG CoA reductase inhibitors (statins) to treat Epstein-Barr virus-driven lymphoma. British Journal of Cancer. 2005;92(9):1593–1598. doi: 10.1038/sj.bjc.6602561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.del Real G., Jiménez-Baranda S., Mira E., et al. Statins inhibit HIV-1 infection by down-regulating rho activity. The Journal of Experimental Medicine. 2004;200(4):541–547. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ponroy N., Taveira A., Mueller N. J., Millard A. L. Statins demonstrate a broad anti-cytomegalovirus activity in vitro in ganciclovir-susceptible and resistant strains. Journal of Medical Virology. 2015;87(1):141–153. doi: 10.1002/jmv.23998. [DOI] [PubMed] [Google Scholar]
- 92.Marakasova E. S., Eisenhaber B., Maurer-Stroh S., Eisenhaber F., Baranova A. Prenylation of viral proteins by enzymes of the host: Virus-driven rationale for therapy with statins and FT/GGT1 inhibitors. BioEssays. 2017;39(10, article 1700014) doi: 10.1002/bies.201700014. [DOI] [PubMed] [Google Scholar]
- 93.Liu G., Vellucci V. F., Kyc S., Hostetter M. K. Simvastatin inhibits Candida albicans biofilm in vitro. Pediatric Research. 2009;66(6):600–604. doi: 10.1203/PDR.0b013e3181bd5bf8. [DOI] [PubMed] [Google Scholar]
- 94.Westermeyer C., Macreadie I. G. Simvastatin reduces ergosterol levels, inhibits growth and causes loss of mtDNA in Candida glabrata. FEMS Yeast Research. 2007;7(3):436–441. doi: 10.1111/j.1567-1364.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 95.Natesan S. K., Chandrasekar P. H., Alangaden G. J., Manavathu E. K. Fluvastatin potentiates the activity of caspofungin against aspergillus fumigatus in vitro. Diagnostic Microbiology and Infectious Disease. 2008;60(4):369–373. doi: 10.1016/j.diagmicrobio.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 96.Macreadie I. G., Johnson G., Schlosser T., Macreadie P. I. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiology Letters. 2006;262(1):9–13. doi: 10.1111/j.1574-6968.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 97.Stewart P. S., Costerton J. W. Antibiotic resistance of bacteria in biofilms. The Lancet. 2001;358(9276):135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 98.Lü H.-Z., Li B.-Q. Effect of HMG-CoA reductase inhibitors on activation of human γδT cells induced by Mycobacterium tuberculosis antigens. Immunopharmacology and Immunotoxicology. 2009;31(3):485–491. doi: 10.1080/08923970902806505. [DOI] [PubMed] [Google Scholar]
- 99.Parihar S. P., Guler R., Khutlang R., et al. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. The Journal of Infectious Diseases. 2014;209(5):754–763. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- 100.Lobato L. S., Rosa P. S., Ferreira J. . S., et al. Statins increase rifampin mycobactericidal effect. Antimicrobial Agents and Chemotherapy. 2014;58(10):5766–5774. doi: 10.1128/AAC.01826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skerry C., Pinn M. L., Bruiners N., Pine R., Gennaro M. L., Karakousis P. C. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. The Journal of Antimicrobial Chemotherapy. 2014;69(9):2453–2457. doi: 10.1093/jac/dku166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rens C., Laval F., Daffé M., et al. Effects of lipid-lowering drugs on vancomycin susceptibility of mycobacteria. Antimicrobial Agents and Chemotherapy. 2016;60(10):6193–6199. doi: 10.1128/AAC.00872-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.CLSI. Susceptibility Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes; Approved Standard–Second Edition. CLSI Document M24-A2. Wayne: Clinical and Laboratory Standards Institute; 2011. [PubMed] [Google Scholar]
- 104.Pentikainen P. J., Saraheimo M., Schwartz J. I., et al. Comparative pharmacokinetics of lovastatin, simvastatin and pravastatin in humans. Journal of Clinical Pharmacology. 1992;32(2):136–140. doi: 10.1002/j.1552-4604.1992.tb03818.x. [DOI] [PubMed] [Google Scholar]
- 105.Shepard C. C., Walker L. L., van Landingham R. Heat stability of Mycobacterium leprae immunogenicity. Infection and Immunity. 1978;22(1):87–93. doi: 10.1128/iai.22.1.87-93.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jemal M., Ouyang Z., Powell M. L. Direct-injection LC-MS-MS method for high-throughput simultaneous quantitation of simvastatin and simvastatin acid in human plasma. Journal of Pharmaceutical and Biomedical Analysis. 2000;23(2-3):323–340. doi: 10.1016/S0731-7085(00)00309-5. [DOI] [PubMed] [Google Scholar]
- 107.Kang Y. A., Choi N. K., Seong J. M., et al. The effects of statin use on the development of tuberculosis among patients with diabetes mellitus. The International Journal of Tuberculosis and Lung Disease. 2014;18(6):717–724. doi: 10.5588/ijtld.13.0854. [DOI] [PubMed] [Google Scholar]
- 108.Lee M. Y., Lin K. D., Hsu W. H., et al. Statin, calcium channel blocker and Beta blocker therapy may decrease the incidence of tuberculosis infection in elderly Taiwanese patients with type 2 diabetes. International Journal of Molecular Sciences. 2015;16(12):11369–11384. doi: 10.3390/ijms160511369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai C. C., Lee M. T. G., Lee S. H., et al. Statin treatment is associated with a decreased risk of active tuberculosis: an analysis of a nationally representative cohort. Thorax. 2016;71(7):646–651. doi: 10.1136/thoraxjnl-2015-207052. [DOI] [PubMed] [Google Scholar]
- 110.Liao K. F., Lin C. L., Lai S. W. Population-based case-control study assessing the association between statins use and pulmonary tuberculosis in Taiwan. Frontiers in Pharmacology. 2017;8:p. 597. doi: 10.3389/fphar.2017.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Su V. Y.-F., Su W.-J., Yen Y.-F., et al. Statin use is associated with a lower risk of TB. Chest. 2017;152(3):598–606. doi: 10.1016/j.chest.2017.04.170. [DOI] [PubMed] [Google Scholar]
- 112.Guerra-de Blas P. del C., Torres-González P., Ponce de León Garduño A., Bobadilla del Valle M., Sada Ovalle I., Sifuentes Osornio J. Insights into the effect of simvastatin on the immune response against Mycobacterium tuberculosis. International Journal of Tuberculosis and Lung Disease. 2017;21(11, Supplement 2) 48th World Conference of Lung Health and Tuberculosis. [Google Scholar]