Abstract
Background
Several researchers have investigated the relationship between ERCC2 rs13181 and rs1799793 polymorphisms and chemotherapy efficacy in terms of tumour response and prognosis in gastric patients. However, the published data have shown inconsistencies.
Methods
PubMed, Elsevier, and Chinese National Knowledge Infrastructure databases were searched for relevant articles published before August 1, 2017. Thirteen studies including 3096 gastric cancer patients treated with chemotherapy were included.
Results
For rs1799793, in the overall analyses, no relationships were found between four genetic models and clinical response (AA vs. GG: OR = 1.17, 95% CI, 0.70–1.95; GA vs. GG: OR = 0.94, 95% CI, 0.69–1.27; GA + AA vs. GG: OR = 1.12, 95% CI, 0.85–1.46; and AA vs. GG + GA: OR = 1.24, 95% CI, 0.81–1.92). In stratified analyses, the results remained negative. We also found no relationship between each of the genetic models and overall survival time in the overall analyses. In the stratified analyses, for Asians, the A carrier genotype might be more closely associated with shorter survival time and higher risk of death for patients than the GG genotype (AA vs. GG: HR = 1.77, 95% CI, 1.20–2.6; GA + AA vs. GG: HR = 1.62, 95% CI, 1.26–2.09), but the results were negative for Caucasians. No significant relationships were found between the rs13181 polymorphism and OR or OS.
Conclusions
This meta-analysis suggested that the ERCC2 rs1799793 polymorphism might be a predictor of prognosis in gastric cancer patients subjected to platinum-based chemotherapy.
1. Introduction
Gastric cancer is a serious public health problem worldwide, and its morbidity and mortality rates rank fourth and second, respectively, among all tumours [1]. Currently, surgery is the primary management modality for patients with early stage and locally advanced gastric cancer, but most patients with gastric cancer either are diagnosed at an advanced stage or develop a relapse after curative surgery [2]. Apart from supportive care and palliative radiotherapy for patients with advanced and localized metastasis, systemic chemotherapy is the only treatment option available [3]. Platinum (in the form of oxaliplatin, cisplatin, etc.) combined with fluoropyrimidines (5-fluorouracil, capecitabine, S-1, etc.) has been most commonly used in chemotherapy regimens for patients with gastric cancer so far [4–7]. However, the treatment response and prognosis in response to chemotherapy vary remarkably among individual patients. Some genetic factors have been speculated to affect the clinical outcomes of patients; one example of such a genetic factor is excision repair cross-complementing group 2 (ERCC2).
ERCC2, encoded by a gene located at chromosome 19q13.3, is an ATP-dependent helicase that mediates DNA unwinding for the initiation of nucleotide excision repair (NER) [8]. The NER pathway, one of the well-known DNA repair pathways, maintains genomic integrity by removing bulky DNA lesions or interstrand adducts induced by exogenous and/or endogenous factors [9]. The DNA repair system plays a crucial role in maintaining stable cellular functions and genomic integrity by reversing the DNA damage induced by various endogenous and/or exogenous factors including therapeutic agents; therefore, the host DNA repair capacity may contribute to the outcomes of cancer patients [10, 11]. For these reasons, whether there are connections between ERCC2 and the clinical outcomes of gastric cancer patients in response to platinum-based chemotherapy is a hot question.
In recent years, many researchers have investigated possible relationships between two ERCC2 polymorphisms, rs13181 and rs1799793, and treatment response and prognosis, which are indicators of chemotherapy efficacy, in patients with gastric cancer [12–24]; however, most of these studies have been inconclusive. Therefore, this meta-analysis was conducted to evaluate the abovementioned relationships.
2. Materials and Methods
2.1. Search Strategy
We conducted a comprehensive literature search of PubMed, Elsevier, and Chinese National Knowledge Infrastructure (CNKI) databases from inception until August 1, 2017, using the following terms: (“gastric cancer” or “stomach cancer” or “gastric carcinoma” or “stomach carcinoma” or “gastric Neoplasm” or “stomach Neoplasm”) and (“excision repair cross-complementing group 2” or “ERCC2” or “xeroderma pigmentosum group D” or “XPD”) and (“chemotherapy”). No restrictions on publication date or language were imposed. Furthermore, the bibliographies of the relevant reviews and articles were reviewed manually to identify additional eligible studies. The current study was conducted according to the PRISMA guidelines for systematic reviews and meta-analyses [25].
2.2. Inclusion and Exclusion Criteria
Studies that fulfilled all three of the following inclusion criteria were considered eligible: (1) the gastric cancer patients were treated with chemotherapy alone; (2) ERCC2 rs13181 or rs1799793 polymorphism was genotyped; and (3) the studies provided sufficient data of clinical outcomes (ORR, OS, and HR with corresponding to 95% CIs). The exclusion criteria were as follows: (1) repeated publications; (2) studies comprising reviews or meta-analyses; (3) obviously irrelevant studies; (4) studies not relevant to ERCC2; (5) studies including patients not treated with chemotherapy alone; and (6) studies with insufficient data.
2.3. Quality Assessment
Two investigators (Mengxi Li and Yan Zhao) assessed the quality of each study using the Newcastle-Ottawa Quality Assessment Scale in order to control the quality of this meta-analysis (Table 1). This scale provides scores according to patient selection, study comparability, follow-up, and outcome. NOS scores of 1–3, 4–6, and 7–9 were defined as low-, intermediate-, and high-quality studies, respectively. Any discrepancies were resolved by consensus.
Table 1.
Newcastle-Ottawa quality assessment scale.
| Selection |
| (1) Representativeness of the exposed cohort |
| (a) Truly representative of the average “GC patient” in the community (1 star) |
| (b) Somewhat representative of the average “GC patient” in the community (1 star) |
| (c) Selected group of users (e.g., nurses, volunteers) |
| (d) No description of the derivation of the cohort |
| (2) Selection of the nonexposed cohort |
| (a) Drawn from the same community as the exposed cohort (1 star) |
| (b) Drawn from a different source |
| (c) No description of the derivation of the nonexposed cohort |
| (3) Ascertainment of exposure (proof of GC and platinum-based chemotherapy) |
| (a) Secure record (e.g., chemotherapy records) (1 star) |
| (b) Structured interview |
| (c) Written self-report |
| (d) No description |
| (4) Demonstration that outcome of interest was not present at start of study |
| (a) Yes (1 star) |
| (b) No |
| Comparability |
| (1) Comparability of cohorts on the basis of the design or analysis |
| (a) Study controls for “chemotherapy regimens” (1 star) |
| (b) Study controls for any additional factor (age, stage, etc.) (1 star) |
| Outcome |
| (1) Assessment of outcome (death or recurrence) |
| (a) Independent blind assessment (1 star) |
| (b) Record linkage (1 star) |
| (c) Self-report |
| (d) No description |
| (2) Was follow-up long enough for outcomes to occur? (death or recurrence) |
| (a) Yes (sufficient follow-up time was selected to observe the outcome) (1 star) |
| (b) No |
| (3) Adequacy of follow-up of cohorts |
| (a) Complete follow-up all subjects accounted for (1 star) |
| (b) Subjection lost to follow-up unlikely to introduce bias-small number lost “25%” or description provided of those lost (1 star) |
| (c) Follow-up rate “75%” and no description of those lost |
| (d) No statement |
GC: gastric cancer.
2.4. Data Extraction
Two investigators (Li and Zhao) carried out the screening and extracted relevant data from each of the eligible studies independently. Discrepancies were resolved by consultation with a third investigator. For each study, the following data were collected: the first author's name, publication year, country, ethnicity of the study participants (Asian and Caucasian), number of patients, age, TNM stage, evaluation criterion (WHO and RECIST), outcomes (ORR, OS, and HR with corresponding 95% CIs), and the number of responders and nonresponders with different genotypes.
2.5. Statistical Analysis
Four genetic models were analysed for each gene site in this meta-analysis. For Lys751Gln (rs13181, A > C): we analysed homozygote genetic models (CC vs. AA), performed heterozygote comparison (AC vs. AA), and studied a dominant model (AC + CC vs. AA) and a recessive model (CC vs. AA+AC); for Asp312Asn (rs1799793, G > A): we analysed homozygote genetic models (AA vs. GG), performed heterozygote comparison (GA vs. GG), and studied a dominant model (GA + AA vs. GG) and a recessive model (AA vs. GG + GA). To evaluate the strength of the association between the ERCC2 rs13181 and rs1799793 polymorphisms and the rate of response to chemotherapy, the gastric cancer patients were classified into responders (complete response (CR) + partial response (PR)) and nonresponders (progressive disease (PD) + stable disease (SD)) according to RECIST [26] or WHO criteria [27]. The crude odds ratios (ORs) with 95% CIs were computed and compared between the responders and the nonresponders. For OS, the hazard risks (HRs) and CIs extracted from the raw data of the included articles were calculated to estimate the pooled HRs and 95% CIs in the homozygote genetic model, heterozygote comparison, and dominant comparison. The Chi-square-based Q-test and I2 statistics were used to estimate between-study heterogeneity. If the I2 index > 50% for the Q-test, the random effects model (DerSimonian-Laird method) was applied to estimate the pooled OR; else, the fixed-effects model (Mantel-Haenszel method) was used [28]. In addition, subgroup analyses were performed based on ethnicities (Caucasians and Asians). The potential publication bias of the literature was evaluated using funnel plots and Egger's linear regression method [29]. All statistical analyses were performed using the STATA software (version 12.0; STATA Corporation, College Station, TX, USA) with two-sided P values.
3. Results
3.1. Characteristics of the Studies
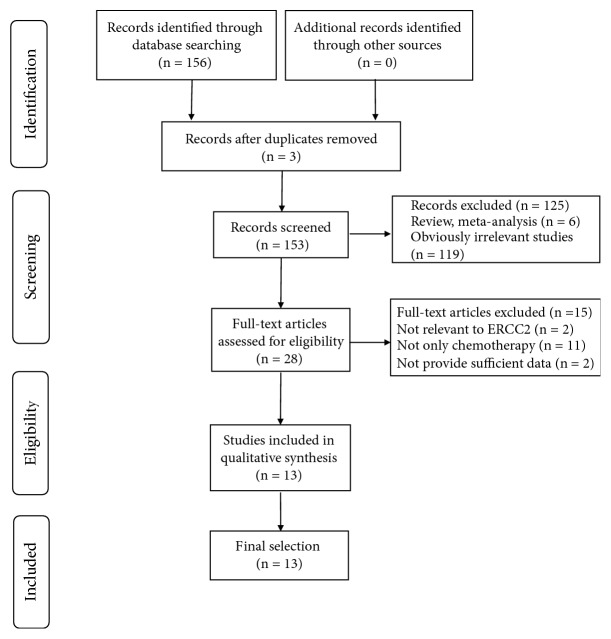
The primary search strategy yielded 156 potentially relevant publications. After excluding duplicated papers, reviews, meta-analyses, and irrelevant studies, the remaining 28 articles were assessed further. These articles underwent full-text review; two articles were excluded as they were not relevant to ERCC2; 11 were excluded as the patients were subjected to additional treatments other than chemotherapy, and two were excluded because of insufficient data. Finally, 13 articles including 3096 gastric cancer patients met the inclusion criteria and thus were included in this meta-analysis (Figure 1). Of them (Table 2), two studies were conducted on Caucasian patients and 11 on Asians. The sample sizes ranged from 73 to 415. Eleven studies reported ORR and ten reported the OS and HR.
Figure 1.
The flow chart of included studies in this meta-analysis.
Table 2.
Characteristics of eligible studies included in this meta-analysis.
| Number | Study | Year | Country | Ethnicity | Number of patients | Age | Cancer types | Chemotherapeutic | TNM stage | Evaluation criterion | Outcomes | ERCC2 rs13181 | ERCC2 rs1799793 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AC | CC | AC + CC | GG | GA | AA | GA + AA | ||||||||||||
| 1 | Zheng | 2016 | China | Asia | 224 | 57.4 ± 9.20 | Gastric cancer | Platinum-based chemotherapy | I-IV | WHO | ORR | 77/39a | 51/33a | 14/10a | _ | 82/42a | 46/30a | 14/10a | _ |
| 2 | Mo | 2015 | China | Asia | 228 | 56.65 ± 11.52 | Gastric cancer | Platinum-based chemotherapy oxaliplatin | I-IV | RECIST | ORR | 82/34a | 54/29a | 17/12a | 71/41a | 87/38a | 56/29a | 10/8a | 66/37a |
| 3 | Yu | 2015 | China | Asia | 346 | 64.5 ± 9.2 | Gastric cancer | Fluorouracil (5FU) and folinic acid chemotherapy | NR | WHO | ORR | 141/107a | _ | _ | 66/32a | 88/79a | _ | _ | 119/60a |
| 4 | Zhong | 2015 | China | Asia | 263 | 62.40 ± 9.50 | Gastric cancer | FOLFOX chemotherapy | I-IV | WHO | ORR | _ | _ | _ | _ | 86/57a | 57/45a | 9/9a | 66/54a |
| 5 | Ding | 2015 | China | Asia | 380 | 58.7 ± 16.3 | Gastric cancer | Platinum-based chemotherapy | I-IV | WHO | ORR | 92/67a | _ | _ | 131/90a | 86/79a | _ | _ | 137/78a |
| 7 | Xue | 2015 | China | Asia | 410 | 63.7 ± 11.4 | Gastric cancer | FOLFOX chemotherapy | I-IV | WHO | ORR | 89/69a | 123/52a | 57/20a | _ | 108/66a | 123/65a | 37/11a | _ |
| 8 | Yu | 2015 | China | Asia | 228 | 55.7 ± 13.8 | Gastric cancer | FOLFOX chemotherapy | I-IV | WHO | ORR | 86/50a | 46/32a | 7/7a | _ | 79/42a | 54/38a | 6/9a | _ |
| 9 | Zhou | 2014 | China | Asia | 415 | 56.2 ± 15.6 | Gastric cancer | Platinum-based chemotherapy | I-IV | WHO | ORR | 139/109a | 74/52a | 25/16a | _ | 130/119a | 67/46a | 41/12a | _ |
| 11 | Goekkurt | 2009 | Germany | Caucasian | 156 | 64 (27–86) | Gastric cancer | FU and platinum-based | NR | WHO | ORR | 66/91a | 60/90a | 36/88a | _ | 70/82a | 48/123a | 60/60a | _ |
| 12 | Keam | 2008 | South Korea | Asia | 73 | 59 (24–77) | Gastric cancer | FOLFOX chemotherapy | NR | WHO | ORR | 28/4a | _ | _ | 34/7a | 1/7a | 31/34a | _ | _ |
| 13 | Ruzzo | 2006 | Italy | Caucasian | 175 | 61 (38–79) | Gastric cancer | Fluorouracil/cisplatin palliative chemotherapy | I-IV | RECIST | ORR | 17/37a | 36/54a | 17/14a | _ | 13/34a | 36/52a | 21/19a | _ |
NR: not reported; ORR: objective response rate; OS: overall survival; RECIST: Response Evaluation Criteria in Solid Tumors; WHO: World Health Organization; anumber of patients for ORR; in front of oblique line is responders (complete response (CR) + partial response (PR)) and behind oblique line is nonresponder (stable disease (SD) + progressive disease (PD)).
3.2. ORR of ERCC2 rs13181 and rs1799793 Polymorphisms
Eleven studies (Table 2) including 2898 gastric cancer patients reported an association between ERCC2 rs13181 and rs1799793 polymorphisms and the clinical response to platinum-based chemotherapy.
For rs13181, the results of the meta-analysis are presented in Table 3 and Figure 2. In the overall analysis, no significant associations were observed between the ERCC2 rs13181 polymorphism and clinical response to platinum-based chemotherapy for any of the genetic models (P > 0.05, Table 3). Subgroup analysis by ethnicity also revealed a negative correlation with response to platinum-based chemotherapy in the Asian and Caucasian gastric cancer patients (P > 0.05, Table 3).
Table 3.
Meta-analysis of the association between ERCC2 rs13181 polymorphism and chemotherapy in objective response rate and overall survival for gastric cancer patients.
| Genetic comparisons | Subgroup analysis | No. of studies | Test of association | Model | Test of heterogeneity | P Egger | |||
|---|---|---|---|---|---|---|---|---|---|
| OR/HR (95% CI) | Z | P | P | I 2 (%) | |||||
| Objective response rate (OR) | |||||||||
| CC vs. AA | Total ethnicity | 7 | 1.01 (0.61–1.68) | 0.04 | 0.968 | R | 0.003 | 69.30 | 0.892 |
| Caucasian | 2 | 1.16 (0.26–5.27) | 0.20 | 0.845 | R | 0.004 | 88.20 | ||
| Asian | 5 | 1.00 (0.58–1.74) | 0.00 | 0.997 | R | 0.047 | 58.50 | ||
| AC vs. AA | Total ethnicity | 7 | 1.06 (0.83–1.37) | 0.47 | 0.641 | R | 0.143 | 37.40 | 0.517 |
| Caucasian | 2 | 1.06 (0.70–1.61) | 0.28 | 0.783 | R | 0.290 | 10.70 | ||
| Asian | 5 | 1.04 (0.74–1.46) | 0.25 | 0.806 | R | 0.077 | 52.60 | ||
| CC + AC vs. AA | Total ethnicity | 10 | 1.07 (0.84–1.36) | 0.52 | 0.600 | R | 0.017 | 55.40 | 0.661 |
| Caucasian | 2 | 1.07 (0.48–2.39) | 0.17 | 0.866 | R | 0.040 | 76.30 | ||
| Asian | 8 | 1.08 (0.83–1.41) | 0.58 | 0.562 | R | 0.037 | 53.20 | ||
| CC vs. AA + AC | Total ethnicity | 7 | 0.98 (0.65–1.46) | 0.12 | 0.907 | R | 0.031 | 56.70 | 0.760 |
| Caucasian | 2 | 1.06 (0.31–3.66) | 0.09 | 0.925 | R | 0.006 | 86.70 | ||
| Asian | 5 | 1.02 (0.70–1.50) | 0.12 | 0.907 | F | 0.270 | 22.60 | ||
|
| |||||||||
| Overall survival (HR) | |||||||||
| CC vs. AA | Asian | 5 | 0.99 (0.72–1.36) | 0.09 | 0.928 | F | 0.185 | 35.4 | 0.739 |
| AC vs. AA | Asian | 5 | 1.01 (0.78–1.30) | 0.07 | 0.947 | F | 0.468 | 0 | 0.651 |
| CC + AC vs. AA | Asian | 6 | 1.28 (0.85–1.91) | 1.18 | 0.238 | R | 0.073 | 50.4 | 0.814 |
OR: odds ratio; HR: hazard ratio; CI: confidence interval; vs.: versus; F: fixed effect model; R: random effect model.
Figure 2.
Forest plot for association of the ERCC2 rs13181 polymorphism with the treatment response to chemotherapy in gastric cancer patients (AC + CC VS. AA).
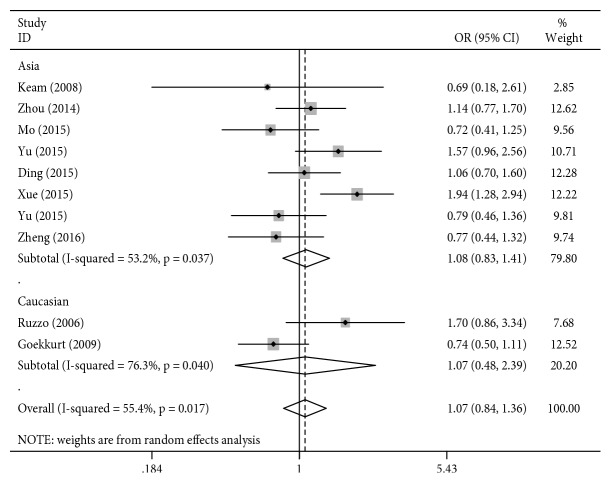
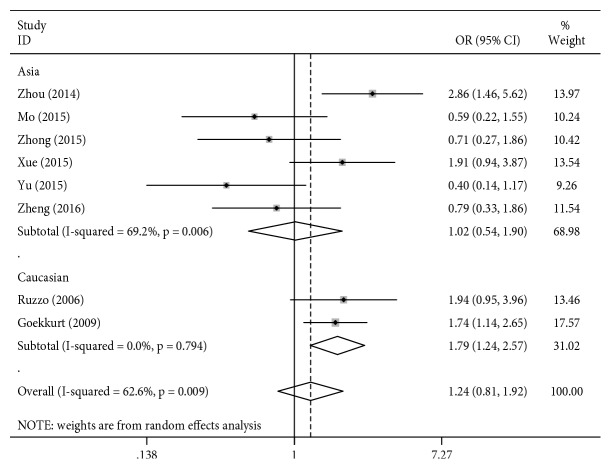
The results from the meta-analysis for rs1799793 are presented in Table 4 and Figure 3. In the overall analysis, no significant associations were observed between the ERCC2 rs1799793 polymorphism and clinical response to platinum-based chemotherapy for any of the genetic models (P > 0.05, Table 4). However, subgroup analysis by ethnicity revealed a positive correlation with clinical response to platinum-based chemotherapy in the Caucasian gastric cancer patients (for AA vs. GG + GA: OR = 1.79, 95% CI = 1.24–2.57, P = 0.002, Figure 3; all the other P values > 0.05, Table 4), but the results showed no associations between the ERCC2 rs1799793 polymorphisms and ORR in Asian patients (P > 0.05, Table 4).
Table 4.
Meta-analysis of the association between ERCC2 rs1799793 polymorphism and chemotherapy in objective response rate and overall survival for gastric cancer patients.
| Genetic comparisons | Subgroup analysis | No. of studies | Test of association | Model | Test of heterogeneity | P Egger | |||
|---|---|---|---|---|---|---|---|---|---|
| OR/HR (95% CI) | Z | P | P | I 2 (%) | |||||
| Objective response rate (ORR) | |||||||||
| AA vs. GG | Total ethnicity | 8 | 1.17 (0.70–1.95) | 0.59 | 0.556 | R | 0.002 | 69.2 | 0.348 |
| Caucasian | 2 | 1.70 (0.71–4.05) | 1.19 | 0.234 | R | 0.080 | 67.4 | ||
| Asian | 6 | 0.98 (0.48–1.98) | 0.06 | 0.950 | R | 0.002 | 74.1 | ||
| GA vs. GG | Total ethnicity | 9 | 0.94 (0.69–1.27) | 0.41 | 0.679 | R | 0.012 | 59.1 | 0.259 |
| Caucasian | 2 | 0.88 (0.23–3.38) | 0.19 | 0.805 | R | 0.003 | 89.0 | ||
| Asian | 7 | 1.01 (0.82–1.24) | 0.06 | 0.955 | F | 0.299 | 17.2 | ||
| AA + GA vs. GG | Total ethnicity | 10 | 1.12 (0.85–1.46) | 0.79 | 0.429 | R | 0.001 | 68.9 | 0.661 |
| Caucasian | 2 | 1.15 (0.39–3.42) | 0.26 | 0.797 | R | 0.009 | 85.5 | ||
| Asian | 8 | 1.13 (0.86–1.49) | 0.87 | 0.384 | R | 0.006 | 64.8 | ||
| AA vs. GG + GA | Total ethnicity | 8 | 1.24 (0.81–1.92) | 0.98 | 0.325 | R | 0.009 | 62.6 | 0.053 |
| Caucasian | 2 | 1.79 (1.24–2.57) | 3.13 | 0.002 | F | 0.794 | 0 | ||
| Asian | 6 | 1.02 (0.54–1.90) | 0.05 | 0.962 | R | 0.006 | 69.2 | ||
|
| |||||||||
| Overall survival (HR) | |||||||||
| GA vs. GG | Asian | 8 | 1.20 (0.96–1.51) | 1.61 | 0.108 | F | 0.577 | 0 | 0.900 |
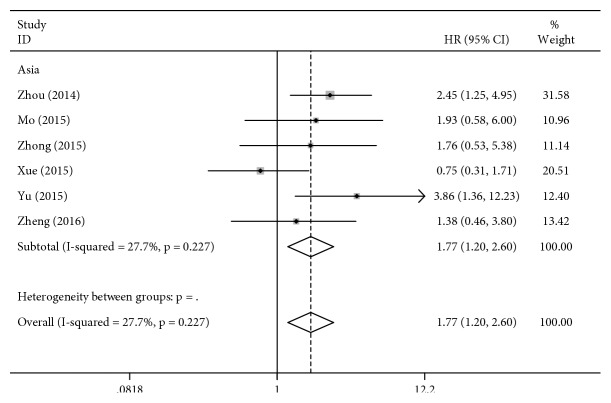
| AA vs. GG | Asian | 6 | 1.77 (1.20–2.60) | 2.89 | 0.004 | F | 0.227 | 27.7 | 0.959 |
| GA + AA vs. GG | Asian | 4 | 1.62 (1.26–2.09) | 3.76 | <0.001 | F | 0.479 | 0 | 0.032 |
OR: odds ratio; HR: hazard ratio; CI: confidence interval; vs.: versus; F: fixed effect model; R: random effect model.
Figure 3.
Forest plot for association of the ERCC2 rs1799793 polymorphism with the overall survival to chemotherapy in gastric cancer patients (AA VS. GG + GA).
3.3. OS of ERCC2 rs13181 and rs1799793 Polymorphisms
Ten studies (Table 5) including 2502 gastric cancer patients revealed an association between the ERCC2 rs13181 polymorphism and OS. The results of the meta-analysis showed no association between the ERCC2 rs13181 polymorphism and OS for three genetic models in the overall analysis. The overall analysis amounts to ethnicity-based subgroup analysis in this case because all the patients included in the studies involving OS were Asian (P > 0.05, Table 3).
Table 5.
Association between the ERCC2 rs13181 polymorphism and overall survival of chemotherapy in gastric cancer patients.
| Study | Year | Country | Outcomes | ERCC2 rs13181 | ERCC2 rs1799793 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AC | CC | AC + CC | GG | GA | AA | GA + AA | ||||
| Zheng | 2016 | China | OS | 1.0 (ref.) | 1.10 (0.56–2.15) | 1.37 (0.46–3.80) | — | 1.0 (ref.) | 1.12 (0.56–2.22) | 1.38 (0.46–3.80) | — |
| Mo | 2015 | China | OS | 1.0 (ref.) | 1.14 (0.58–2.28) | 1.35 (0.48–3.53) | 1.20 (0.64–2.25) | 1.0 (ref.) | 1.12 (0.57–2.20) | 1.93 (0.58–6.00) | 1.25 (0.66–2.34) |
| Yu | 2015 | China | OS | 1.0 (ref.) | — | — | 1.57 (0.93–2.65) | 1.0 (ref.) | — | — | 1.78 (1.13–2.81) |
| Zhong | 2015 | China | OS | — | — | — | — | 1.00 (ref) | 1.15 (0.63–2.10) | 1.76 (0.53–5.38) | 1.23 (0.69–2.18) |
| Ding | 2015 | China | OS | 1.0 (ref.) | — | — | 1.05 (0.68–1.62) | 1.0 (ref.) | — | — | 1.97 (1.28–3.03) |
| Liu | 2015 | China | OS | 1.0 (ref.) | — | — | 1.12 (0.44–2.88) | 1.0 (ref.) | 2.12 (0.89–5.08) | — | — |
| Xue | 2015 | China | OS | 1.0 (ref.) | 0.66 (0.39–1.11) | 0.44 (0.20–0.91) | — | 1.0 (ref.) | 0.82 (0.49–1.38) | 0.75 (0.31–1.71) | — |
| Yu | 2015 | China | OS | 1.0 (ref.) | 1.31 (0.73–2.36) | 1.75 (0.57–5.40) | — | 1.0 (ref.) | 1.52 (0.75–2.86) | 3.86 (1.36–12.23) | — |
| Keam | 2008 | South Korea | OS | 1.0 (ref.) | — | — | 0.49 (0.17–1.37) | 1.0 (ref.) | 0.80 (0.28–2.26) | — | — |
OS: overall survival.
Ten studies (Table 5) including 2675 gastric cancer patients were identified for the association between the ERCC2 rs1799793 polymorphism and OS. The results of the meta-analysis indicated that the ERCC2 rs1799793 polymorphism was associated with OS for three genetic models in the overall analysis (Table 4). The overall analysis amounts to ethnicity-based subgroup analysis in this case because all the patients included in the studies showing OS were Asian (for AA vs. GG: HR = 1.77, 95% CI = 1.20–2.60, P = 0.004, Figure 4; for GA + AA vs. GG: HR = 1.62, 95% CI = 1.26–2.09, P < 0.001, Figure 5; other P values > 0.05).
Figure 4.
Forest plot for association of the ERCC2 rs1799793 polymorphism with the overall survival to chemotherapy in gastric cancer patients (AA VS. GG).
Figure 5.
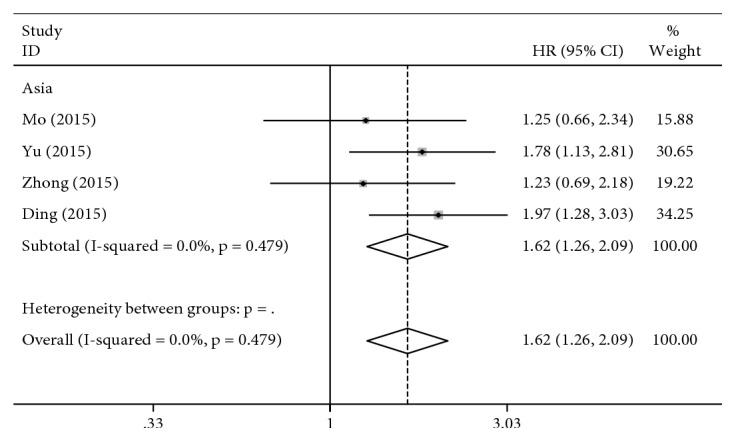
Forest plot for association of the ERCC2 rs1799793 polymorphism with the overall survival to chemotherapy in gastric cancer patients (GA + AA VS. GG).
3.4. Publication Bias
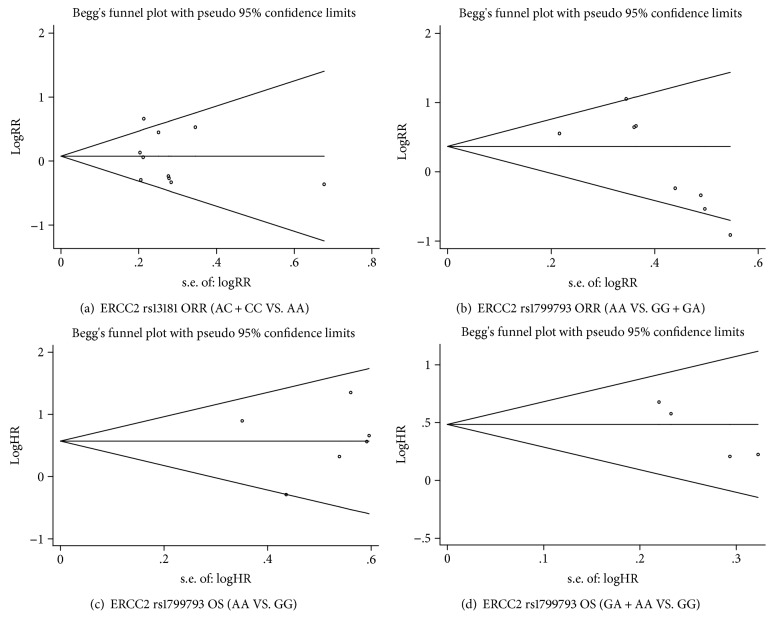
The potential publication bias of the literature was evaluated using funnel plot analysis and Egger's test. The shapes of the funnel plots were approximately symmetrical. Egger's test revealed almost no publication bias for any of the genetic models, except for the GA and AA genotypes in comparison with the GG genotype of rs1799793 for OS (Tables 3 and 4; Figure 6).
Figure 6.
Begg's funnel plot for publication bias analysis.
3.5. Sensitivity Analysis and Metaregression
We conduct a sensitivity analysis to evaluate whether the differences between studies induced instability in the meta-analysis or not. The results suggested that the meta-analysis was stable (Figures 7 and 8). We also performed a metaregression to assess potential heterogeneity of individual study; the results showed that the number of cases in each articles may be the source of potential heterogeneity (Figures 9 and 10).
Figure 7.
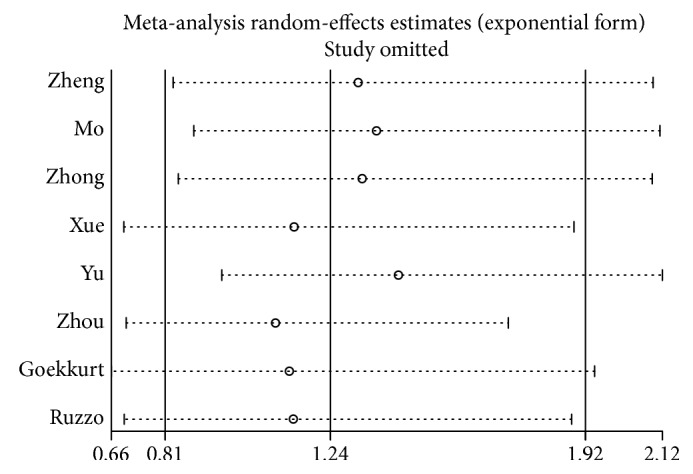
Sensitivity analysis of ERCC2 rs1799793 ORR (AA VS. GG + GA).
Figure 8.
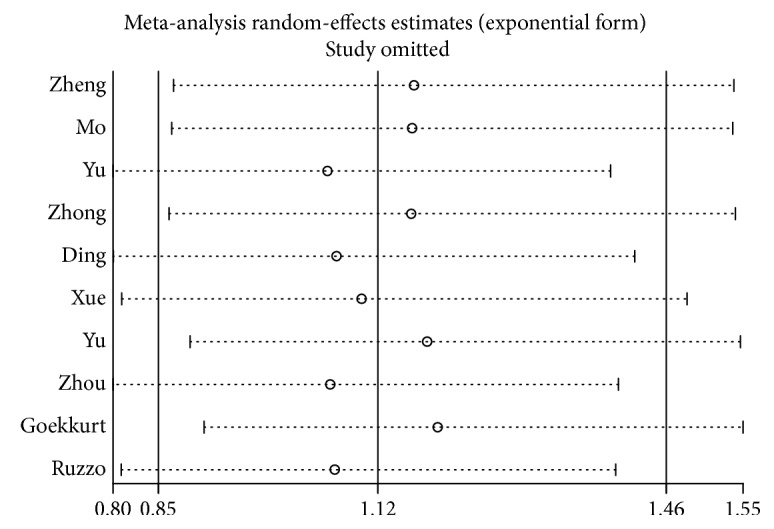
Sensitivity analysis of ERCC2 rs1799793 ORR (GA + AA VS. GG).
Figure 9.
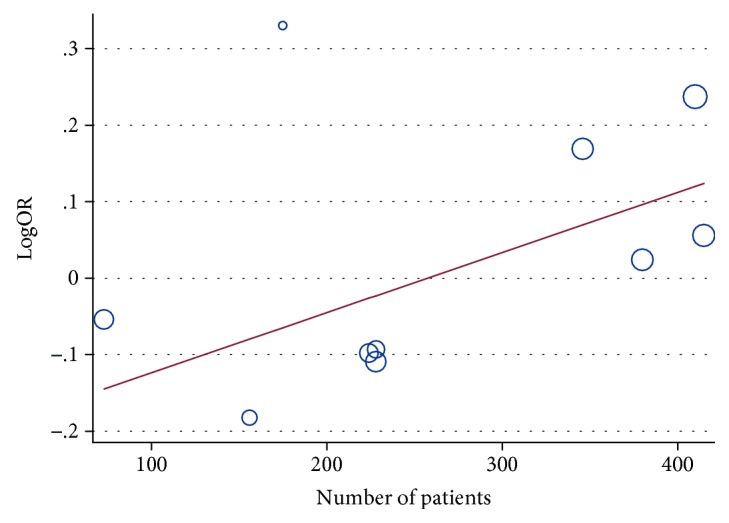
Metaregression of ERCC2 rs13181 ORR (AC + CC VS. AA).
Figure 10.
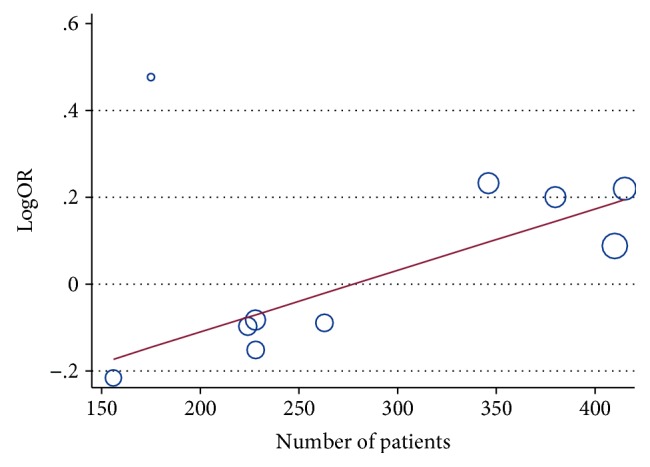
Metaregression of ERCC2 rs1799793 ORR (GA + AA VS. GG).
4. Discussion
Platinum-based chemotherapy agents represent the most active anticancer agents in clinical use, both in individual or in combination therapies with reasonable success, inducing DNA adducts. These platinum-DNA adducts cause distortion of the DNA double helix, activate a cellular DNA damage response, and lead to tumour cell death. ERCC2 is an important member of the NER pathway. It can remove platinum-DNA adducts with platinum-based chemotherapy. Polymorphisms in ERCC2 are closely related to the efficacy of chemotherapy drugs in gastric cancer patients.
Several researchers have reported a relationship between ERCC2 rs13181 and rs1799793 polymorphisms and chemotherapy efficacy in gastric cancer patients [12–24]. In 2014, a study by Yu et al. revealed that the AA genotype of the ERCC2 rs1799793 polymorphism is associated with better response to chemotherapy in gastric cancer patients [19]. Many other related findings were also reported in 2015. Zhong et al. found that the GA + AA genotypes of ERCC2 rs1799793 are associated with a significantly better response to chemotherapy compared with the GG genotype and that the GA + AA genotypes are significantly associated with a lower risk of mortality from gastric cancer compared with the GG genotype [15]. Ding et al. also showed that gastric cancer patients with the ERCC2 rs1799793 GA genotype tended to have shorter OS than those with the GG genotype [16]. However, Xue et al. found that the ERCC2 rs13181 and rs1799793 polymorphisms are not correlated with response to FOLFOX chemotherapy but revealed a significantly increased risk of death from gastric cancer among patients with the ERCC2 rs1799793 AA genotype compared with patients with the GG genotype in terms of overall survival [18]. Another study by Mo et al. found that patients with the ERCC2 rs1799793 GA + AA genotype exhibited longer survival times than did those with the GG genotype [13]. However, the meta-analysis by Zhang et al. found no statistically significant difference in the effective clinical response and overall survival between any of the genetic models of Lys751Gln (rs13181) [30]. In contrast, the meta-analysis conducted by Yin et al. showed that for ERCC2 rs13181 T > G, the G allele was associated with reduced objective response in all the patients, in all subgroups of Caucasians. For OS, the significance was observed only in Caucasian subgroups [31]. This study included patients with gastric and colorectal cancer, but the results for these two types of patients were not analysed separately. It is possible that the reason for the inconsistency between our findings and the results of this study was the inclusion of colorectal cancer patients. These studies by Zhang et al. and Yin et al. contained small sample sizes, and the patients were not treated with chemotherapy alone. In addition, neither of the two studies were focused on the relationship between ERCC2 rs1799793 and the clinical response of gastric cancer patients to platinum-based chemotherapy.
Thus, with a series of new studies published, we conducted a meta-analysis to derive a more precise and comprehensive assessment of the relationship of ERCC2 rs13181 and rs1799793 polymorphisms with the efficacy and clinical outcomes of gastric cancer patients treated with platinum-based chemotherapy. This is the first meta-analysis evaluating not only ERCC2 rs13181 but also rs1799793. Thirteen studies including 3096 gastric cancer patients identified the association between ERCC2 rs13181 or rs1799793 polymorphisms and clinical response to platinum-based chemotherapy drugs. All the patients included in our article were treated with chemotherapy alone, without surgery or radiotherapy. Our meta-analysis revealed some correlations between the recessive model of rs1799793 and response to platinum-based chemotherapy in Caucasian gastric cancer patients (AA vs. GG + GA: OR = 1.79, 95% CI 1.24–2.57). However, these results were not observed in patients with the other three genotypes. Therefore, the above data need to be handled with caution. Additionally, the A allele carrier of rs1799793 might be more closely associated with shorter survival time and higher risk of death than the GG genotype in Asian gastric cancer patients (AA vs. GG: HR = 1.77, 95% CI = 1.20–2.60; GA + AA vs. GG: HR = 1.62, 95% CI = 1.26–2.09) but not in Caucasian patients. This phenomenon may be attributable to the fact that the ERCC2 rs1799793 polymorphism causes an amino acid substitution from aspartic acid (asp) to asparagine (asn), which leads to increased activity of synthesis enzymes and increases their ability to detoxify and excrete platinum-based agents. This reduces the concentration of platinum-based agents in tumour cells, thereby decreasing the sensitivity of the cells. Thus, ERCC2 rs1799793 may be a useful biomarker in predicting the clinical outcomes of gastric cancer patients in response to platinum-based chemotherapy.
There are several limitations to our meta-analysis. Firstly, because of the differences in several characteristics of the design, histological type, tumour stage, gender, age, and follow-up time of the patients included in the studies, our results should be carefully evaluated. Secondly, in the subgroup analysis by ethnicity, the sample size was small for Caucasian patients, which may lead to insufficient power to assess the true correlation. Therefore, the results of the current meta-analysis should be applied with caution in Caucasian patients. Moreover, the ethnicity factor should be considered if a specific platinum-based chemotherapeutic regimen for gastric cancer patients is to be used in the future. Thirdly, because the patients included in the studies had received chemotherapy alone, without radiotherapy or surgical excision, the number of articles that could be included in our meta-analysis was greatly reduced, which may have led to some errors.
Nevertheless, this meta-analysis suggests that the ERCC2 rs1799793 polymorphism is a predictor of prognosis in Asian gastric cancer patients undergoing platinum-based chemotherapy. Furthermore, gastric cancer patients with the A allele (AA and GA) may be more likely to show shorter survival time and higher risk of death than those with GG genotypes. However, the use of the ERCC2 rs1799793 polymorphism as a predictive factor of prognosis in personalized chemotherapy treatment requires further verification from large well-designed pharmacogenetics studies.
5. Conclusion
This meta-analysis suggested that the ERCC2 rs1799793 polymorphism might be a predictor of prognosis in gastric cancer patients subjected to platinum-based chemotherapy.
Acknowledgments
We thank the corporation of Yideji, People's Republic of China (https://www.editage.cn/), for editing the English text of a draft of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest in this work.
Authors' Contributions
Mengxi Li and Yan Zhao contributed equally to this work.
References
- 1.Chen L., Zhao Q. H. Research advances in the association between polymorphism of ERCC2 and gastric cancer. Medical Recapitulate. 2013;19(2):288–290. [Google Scholar]
- 2.Macdonald J. S. Treatment of localized gastric cancer. Seminars in Oncology. 2004;31(4):566–573. doi: 10.1053/j.seminoncol.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Wagner A. D., Unverzagt S., Grothe W., et al. Chemotherapy for advanced gastric cancer. Cochrane Database of Systematic Reviews. 2010;3, article CD004064 doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Wohrer S. S., Raderer M., Hejna M. Palliative chemotherapy for advanced gastric cancer. Annals of Oncology. 2004;15(11):1585–1595. doi: 10.1093/annonc/mdh422. [DOI] [PubMed] [Google Scholar]
- 5.Xu R., Ma N., Wang F., et al. Results of a randomized and controlled clinical trial evaluating the efficacy and safety of combination therapy with endostar and S-1 combined with oxaliplatin in advanced gastric cancer. OncoTargets and Therapy. 2013;6:925–929. doi: 10.2147/ott.s46487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang Y. J., Kim Y. W., Yang H. K., et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 7.Cassidy J., Clarke S., Díaz-Rubio E., et al. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. British Journal of Cancer. 2011;105(1):58–64. doi: 10.1038/bjc.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spitz M. R., Wu X., Wang Y., et al. Modulation of nucleotide excision repair capacity by XPD polymorphisms in lung cancer patients. Cancer Research. 2001;61(4):1354–1357. [PubMed] [Google Scholar]
- 9.Wu Q., Christensen L. A., Legerski R. J., Vasquez K. M. Mismatch repair participates in error-free processing of DNA interstrand crosslinks in human cells. EMBO Reports. 2005;6(6):551–557. doi: 10.1038/sj.embor.7400418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann A. S., Sturgis E. M., Wei Q. Nucleotide excision repair as a marker for susceptibility to tobacco-related cancers: a review of molecular epidemio-logical studies. Molecular Carcinogenesis. 2005;42(2):65–92. doi: 10.1002/mc.20069. [DOI] [PubMed] [Google Scholar]
- 11.Liu L., Wu J., Zhong R., et al. Multi-loci analysis reveals the importance of genetic variations in sensitivity of platinum-based chemotherapy in non-small-cell lung cancer. Molecular Carcinogenesis. 2013;52(12):923–931. doi: 10.1002/mc.21942. [DOI] [PubMed] [Google Scholar]
- 12.Zheng D. L., Tang G. D., Chen Y. N., Zhang T., Qin M. B. Genetic variability of ERCC1 and ERCC2 genes involved in the nucleotide excision repair pathway influences the treatment outcome of gastric cancer. Genetics and Molecular Research. 2016;15(2) doi: 10.4238/gmr.15027384. [DOI] [PubMed] [Google Scholar]
- 13.Mo J., Luo M., Cui J., Zhou S. Prognostic value of ERCC1 and ERCC2 gene polymorphisms in patients with gastric cancer receiving platinum-based chemotherapy. International Journal of Clinical and Experimental Pathology. 2015;8(11):15065–15071. [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W. H., Wang Y. X., Guo J. Q., Wang W. L., Zheng J. S., Zhu K. X. Genetic variability of ERCC1 and ERCC2 influences treatment outcomes in gastric cancer. Genetics and Molecular Research. 2015;14(4):17529–17535. doi: 10.4238/2015.December.21.25. [DOI] [PubMed] [Google Scholar]
- 15.Zhong G., Li H. K., Shan T., Zhang N. Genetic variability of DNA repair mechanisms in chemotherapy treatment outcome of gastric cancer patients. Genetics and Molecular Research. 2015;14(4):17228–17234. doi: 10.4238/2015.December.16.22. [DOI] [PubMed] [Google Scholar]
- 16.Ding C., Zhang H., Chen K., Zhao C., Gao J. Genetic variability of DNA repair mechanisms influences treatment outcome of gastric cancer. Oncology Letters. 2015;10(4):1997–2002. doi: 10.3892/ol.2015.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu R., Zhao X., Liu X., et al. Influences of ERCC1, ERCC2, XRCC1, GSTP1, GSTT1, and MTHFR polymorphisms on clinical outcomes in gastric cancer patients treated with EOF chemotherapy. Tumour Biology. 2016;37(2):1753–1762. doi: 10.1007/s13277-015-3935-8. [DOI] [PubMed] [Google Scholar]
- 18.Xue M. H., Li G. Y., Wu X. J., Zhang C. X., Zhang C. F., Zhu K. X. Genetic variability of genes in NER pathway influences the treatment outcome of gastric cancer. International Journal of Clinical and Experimental Pathology. 2015;8(5):5563–5569. [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H., Wu X., Zhang Y., Jin Z., Li G., Zhao H. Genetic variability of DNA repair mechanisms influences chemotherapy outcome of gastric cancer. International Journal of Clinical and Experimental Pathology. 2015;8(4):4106–4112. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J., Liu Z. Y., Li C. B., et al. Genetic polymorphisms of DNA repair pathways influence the response to chemotherapy and overall survival of gastric cancer. Tumour Biology. 2015;36(4):3017–3023. doi: 10.1007/s13277-014-2936-3. [DOI] [PubMed] [Google Scholar]
- 21.Goekkurt E., Al-Batran S. E., Hartmann J. T., et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. Clinical Oncology. 2009;27(17):2863–2873. doi: 10.1200/JCO.2008.19.1718. [DOI] [PubMed] [Google Scholar]
- 22.Keam B., Im S. A., Han S. W., et al. Modified FOLFOX-6 chemotherapy in advanced gastric cancer: results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer. 2008;8(1):p. 148. doi: 10.1186/1471-2407-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A1 R., Graziano F., Kawakami K., et al. Pharmacogenetic profiling and clinical outcome of patients with advanced gastric cancer treated with palliative chemotherapy. Journal of Clinical Oncology. 2006;24(12):1883–1891. doi: 10.1200/jco.2005.04.8322. [DOI] [PubMed] [Google Scholar]
- 24.Li X., Jiang T., Ji Y. Z., Jia X. F., Liang J. The relationship between genetic polymorphism of ERCC1, XPD and prognosis of gastric cancer patients treated with oxaliplatin-based chemotherapy. Medical Journal of Qilu. 2011;26(2):95–97. [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., Altman D. G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P., Arbuck S. G., Eisenhauer E. A., et al. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 29.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Jiang L. P., Yin Y., Wang Y. D. XRCC1 and XPD genetic polymorphisms and clinical outcomes of gastric cancer patients treated with oxaliplatin-based chemotherapy: a meta-analysis. Tumor Biology. 2014;35(6):5637–5645. doi: 10.1007/s13277-014-1746-y. [DOI] [PubMed] [Google Scholar]
- 31.Yin M., Yan J., Martinezbalibrea E., Graziano F., Lenz H.-J. ERCC1 and ERCC2 polymorphisms predict clinical outcomes of oxaliplatin-based chemotherapies in gastric and colorectal cancer: a systemic review and meta-analysis. Clinical Cancer Research. 2011;17(6):1632–1640. doi: 10.1158/1078-0432.CCR-10-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]