Abstract
Arginine (Arg) has traditionally not been considered as a deficient nutrient in diets for gestating or lactating swine due to the assumption that these animals can synthesize sufficient amounts of Arg to meet their physiological needs. The lack of full knowledge about Arg nutrition has contributed to suboptimal efficiency of pork production. Over the past 25 yr, there has been growing interest in Arg metabolism in the pig, which is an agriculturally important species and a useful model for studying human biology. Arginine is a highly abundant amino acid in tissues of pigs, a major amino acid in allantoic fluid, and a key regulator of gene expression, cell signaling, and antioxidative reactions. Emerging evidence suggests that dietary supplementation with 0.5% to 1% Arg maintains gut health and prevents intestinal dysfunction in weanling piglets, while enhancing their growth performance and survival. Also, the inclusion of 1% Arg in diets is required to maximize skeletal muscle accretion and feed efficiency in growing pigs, whereas dietary supplementation with 1% Arg reduces muscle loss in endotoxin-challenged pigs. Furthermore, supplementing 0.83% Arg to corn- and soybean meal–based diets promotes embryonic/fetal survival in swine and milk production by lactating sows. Thus, an adequate amount of dietary Arg as a quantitatively major nutrient is necessary to support maximum growth, lactation, and reproduction performance of swine. These results also have important implications for improving the nutrition and health of humans and other animals.
Keywords: amino acids, function, growth, lactation, pregnancy
INTRODUCTION
Knowledge of Arginine (Arg) metabolism in pigs provides a foundation for understanding its nutritional value. Arginine has been known over 60 yr as a nutritionally essential amino acid (AA) for weanling pigs, because its absence from their diet results in poor growth (Mertz et al., 1952). Young pigs can grow (Southern and Baker, 1983) and sexually mature swine can gestate fetuses (Easter and Baker 1976) when their diets do not contain Arg. Those studies indicated the presence of endogenous Arg synthesis in young and adult pigs. The pathways for de novo synthesis of Arg from glutamine and proline in pigs were identified in the 1990s (Wu et al., 1994b; Wu, 1997). As a mechanism for the regulation of Arg homeostasis, Arg catabolism in pigs occurs primarily via the arginase pathway and increases gradually in response to dietary Arg intake (Wu et al., 2016).
Over 40 yr ago, Arg was considered to be sufficient in the diets of prepubertal or gestating swine based on results of nitrogen balance experiments (Easter and Baker, 1974; 1976). In 1998, relatively low concentrations of dietary Arg were recommended by the NRC (1998) for nursery, weanling, and growing-finishing pigs. Modern breeds of pigs grow faster, gain more lean tissues, and gestate more fetuses (Mateo et al., 2007, 2008) and, therefore, have greater physiological requirements for Arg, when compared with previous breeds (Wu et al., 2010a, 2013b). Currently, adequate provision of dietary Arg is particularly important in the pig industry because low-protein diets, which are currently used to reduce the production of nitrogenous wastes by swine farms, may not supply sufficient Arg or its AA precursors (Hou et al., 2016). Thus, there is a need to reevaluate the dietary requirements of modern breeds of pigs for Arg during their nursery, weaning, growing-finishing, gestating, and lactating periods. To achieve this goal, the present article highlights Arg metabolism and nutrition in pigs.
ARGININE METABOLISM IN PIGS
Arginine Synthesis in Pigs
It was recognized in the early 1990s that previous investigators had failed to determine all physiologically important AA (e.g., glutamine, citrulline, and ornithine) in sow’s milk; therefore, studies were initiated to quantify free and protein-bound AA in sow’s colostrum and milk on days 1 to 28 of lactation (Wu and Knabe, 1994). Strikingly, concentrations of free glutamine in milk increased progressively with advancing lactation and reached the highest mean value of 3.5 mM at day 28 of lactation, in comparison with 0.3 to 0.4 mM glutamine in the plasma of lactating sows (Wu and Knabe, 1994). In contrast, concentrations of Arg in sow’s milk (free plus peptide-bound) were much lower than those of glutamine plus glutamate, proline, lysine, and branched-chain AA on all days of lactation (Table 1). Concentrations of free Arg in sow’s colostrum or milk account for less than 0.7% of their total Arg content (Wu and Knabe, 1994). On a gram basis, Arg/lysine ratios were 0.35 and 0.97, respectively, in sow’s milk on day 7 of lactation and in the tissue proteins of 7-d-old piglets (Wu et al., 2004), suggesting a deficiency of Arg in sow’s milk for maximum growth of piglets.
Table 1.
Concentrations of amino acids in whole milk from sows between days 7 and 21 of lactation
| Amino acids1 | g/L | g/kg DM |
|---|---|---|
| Alanine | 1.97 | 10.6 |
| Arginine | 1.43 | 7.69 |
| Asparagine + Aspartate | 5.12 | 27.5 |
| Cysteine | 0.72 | 3.87 |
| Glutamate + Glutamine | 9.44 | 50.8 |
| Glycine | 1.12 | 6.02 |
| Histidine | 0.92 | 4.95 |
| Isoleucine | 2.28 | 12.3 |
| Leucine | 4.46 | 24.0 |
| Lysine | 4.08 | 21.9 |
| Methionine | 1.04 | 5.59 |
| Phenylalanine | 2.03 | 10.9 |
| Proline | 5.59 | 30.1 |
| Serine | 2.35 | 12.6 |
| Threonine | 2.29 | 12.3 |
| Tryptophan | 0.66 | 3.55 |
| Tyrosine | 1.94 | 10.4 |
| Valine | 2.54 | 13.7 |
| Total | 50.0 | 268.8 |
Adapted from Kim et al. (2004) and Wu et al. (2014).
1The sum of free plus peptide-bound amino acids.
The high abundance of Arg in the tissue proteins of piglets (Wu et al., 1999) and the apparent deficiency of Arg in sow’s milk (Wu and Knabe, 1994; Davis et al., 1994) led to the following important questions: 1) is Arg deficient in sow’s milk? 2) If deficient, what are the nutritional implications? 3) Is there a nutritional significance of the high abundance of glutamine and proline in sow’s milk? Subsequent research to answer these questions yielded discoveries that have fundamentally advanced our knowledge of Arg synthesis and nutrition in pigs over the last 25 yr (Table 2).
Table 2.
NRC-recommended minimal content of arginine in diets for pigs and Wu-recommended optimal content of arginine in diets for pigs (values are % of total diet, unless indicated otherwise)
| Nursery piglets (5 kg BW) |
Weanling piglets (10 kg BW) |
Growing-finishing pigs (20 to 100 kg BW) |
Gestating pigs (140 kg BW at breeding) |
Lactating sows | |
|---|---|---|---|---|---|
| NRC (2012) 1 | 0.75 | 0.68 | 0.62 (20 kg BW)0.38 (100 kg BW) |
0.36 (days 0 to 90) 0.47 (days 90 to 114) |
0.60 (parity 1)2 0.54 (parity 2)2 |
| NRC (1998) 3 | 0.59 | 0.54 | 0.37 (20 kg BW) 0.19 (100 kg BW) |
0.0 | 0.0 |
| Wu (2014) 4 | 1.19 | 1.01 | 0.83 (20 kg BW) 0.64 (100 kg BW) |
1.03 (days 0 to 90) 1.03 (days 90 to 114) |
1.37 |
1Nutrient Requirements of Swine, NRC (2012), minimal content of Arg in diets.
2Standardized ileal digestible value.
3Nutrient Requirements of Swine, NRC (1998), minimal content of Arg in diets.
4Wu-recommended optimal content of true digestible Arg in swine diets. Feed intake for gestating swine is 2 kg/d (days 0 to 90) and 2.3 kg/d (days 90 to 114).
Synthesis of citrulline and arginine from glutamine in pig enterocytes.
Studies involving cannulation of the jejunal artery and jejunal vein of 14- to 58-d-old pigs have shown that the small intestine actively utilizes glutamine from the diet and arterial blood to synthesize citrulline and, to a lesser extent, Arg (Wu et al., 1994a). Of particular interest, glutamine is the only AA in arterial blood that is taken up by the small intestine of pigs in the postabsorptive state (Wu et al., 1994a). To identify the cell type responsible for citrulline and Arg synthesis, Wu et al. (1994b) isolated biochemically viable enterocytes from the small intestine of 1- to 58-d-old pigs. Increasing extracellular concentrations of glutamine from 0.5 to 5 mM dose-dependently increased the synthesis of citrulline and Arg via pyrroline-5-carboxylate (P5C) synthase in porcine enterocytes (Wu et al., 1994b). The de novo synthesis of Arg is consistent with the conversion of [U-14C]glutamine into [14C]Arg in the enterocytes of 0- to 7-d-old pigs (Blachier et al., 1993). All substrates required for this synthetic pathway, including ammonia, HCO3−, glutamate, aspartate, and ATP, are produced from glutamine catabolism (Wu et al., 1994b). Pyrroline-5-carboxylate synthase and N-acetylglutamate synthase are the 2 key regulatory enzymes in the intestinal conversion of glutamine into citrulline (Wu et al., 2004). Other major tissues of preweaning and postweaning pigs (including liver, kidney, heart, pancreas, brain, large intestine, and skeletal muscle) lack P5C synthase for synthesizing citrulline from glutamine or glutamate (Wu et al., 1997; Dillon et al., 1999). These findings establish an essential role for enterocytes in the synthesis of citrulline and Arg from glutamine in pigs.
The reactions for the formation of citrulline from glutamine start in the mitochondria of enterocytes, with the first enzyme being glutaminase for hydrolyzing glutamine into glutamate, and the subsequent conversion of citrulline into Arg takes place in the cytosol (Wu and Morris, 1998). Of interest, in contrast to the ornithine produced in the mitochondria, extracellular ornithine is a very poor substrate for the synthesis of citrulline and Arg in enterocytes (Wu et al., 1994b), which is likely due to the preferential channeling of extracellular ornithine into proline production (Wu et al., 1996b). These results indicate that 1) glutamine plays an important role in the intestinal synthesis of citrulline and Arg and 2) the abundance of glutamine in sow’s milk is of nutritional significance as it compensates for the insufficient provision of dietary Arg required for the optimal growth of piglets (Wu et al., 2004).
Synthesis of citrulline and Arg from proline in pig enterocytes.
The rates of citrulline synthesis from glutamine decrease markedly in 14- to 21-d-old piglets, when compared with 0- to 7-d-old piglets (Wu et al., 1994b, Wu and Knabe, 1995). Thus, we hypothesized that an alternative precursor for intestinal synthesis of citrulline would provide additional Arg for suckling piglets. Intriguingly, when sow-reared piglets were treated with gabaculine (an inhibitor of ornithine aminotransferase, an enzyme for converting P5C into ornithine), concentrations of both glutamine and proline in plasma increased 2-fold (Flynn and Wu, 1996), suggesting that proline may be a substrate for citrulline synthesis. Using radiochemical and chromatographic methodologies, Wu (1997) discovered that proline is extensively catabolized by pig enterocytes to yield P5C, citrulline, and Arg via the proline oxidase pathway. The conversion of proline into citrulline requires ammonia and glutamate, both of which are provided by glutamine degradation (Wu et al., 1994b). Because there is little uptake of proline from arterial blood by the pig’s small intestine (Wu et al., 1994a), enteral provision of large amounts of proline from sow’s milk is crucial in the compensation for the deficiency of Arg in sow’s milk. In support of this view, Brunton et al. (1999) reported that there was little synthesis of Arg from proline in arterial blood in piglets and that the intragastric administration of proline was effective in ameliorating Arg deficiency in neonates.
Synthesis of arginine via the intestinal-renal axis in pigs.
Although Arg is formed in the liver via the urea cycle, there is no net synthesis of Arg by this organ in mammals including pigs (Urschel et al., 2005) due to an exceedingly high activity of cytosolic arginase that rapidly hydrolyzes Arg (Wu and Morris, 1998). The formation of citrulline and Arg from glutamine and proline in enterocytes and the conversion of citrulline into Arg via argininosuccinate synthase and argininosuccinate lyase (ASL) in the kidneys (the primary site for extra-intestinal synthesis of Arg) are known as the intestinal-renal axis for the de novo synthesis of Arg in many mammalian species, including pigs. The absence of intestinal synthesis of citrulline in some mammals (e.g., cats) results in their high requirements for dietary Arg (MacDonald et al. 1984).
At birth, ASL activity is high in enterocytes, but low in the kidneys (Wu and Knabe, 1995). Thus, most of the glutamine- and proline-derived citrulline is utilized locally by the enterocytes of 1- to 7-d-old pigs for Arg synthesis (Wu and Knabe, 1995). The virtual absence of arginase from the enterocytes of 1- to 21-d-old preweaning pigs limits Arg degradation (Wu et al., 1996b), which helps maximize the output of Arg by the neonatal small intestine. Wu and Knabe (1995) estimated that sow’s milk provides, at most, only 40% of Arg required by 7-d-old pigs. Thus, Arg synthesis via the intestinal-renal axis plays a crucial role in maintaining Arg homeostasis in sow-reared piglets. In support of this view, an inhibition of intestinal ornithine aminotransferase for 12 h reduced concentrations of ornithine, citrulline, and Arg in plasma by 59%, 52%, and 76%, respectively, in 4-d-old sow-reared piglets (Flynn and Wu, 1996). Although Wilkinson et al. (2004) reported that gastric feeding of a high-Arg diet to 7-d-old piglets (1.80-g Arg·kg BW−1·d−1) reduced the endogenous synthesis of Arg from proline in comparison with an Arg-deficient diet (0.2-g Arg·kg BW−1·d−1), this finding should not be interpreted to indicate that elevated Arg intake within physiological ranges reduces the intestinal synthesis of citrulline and Arg because the metabolic pathways in piglets fed the high-Arg diet (indicated by a 17% decrease in growth) were likely impaired by the severe imbalance of AA (Wilkinson et al., 2004).
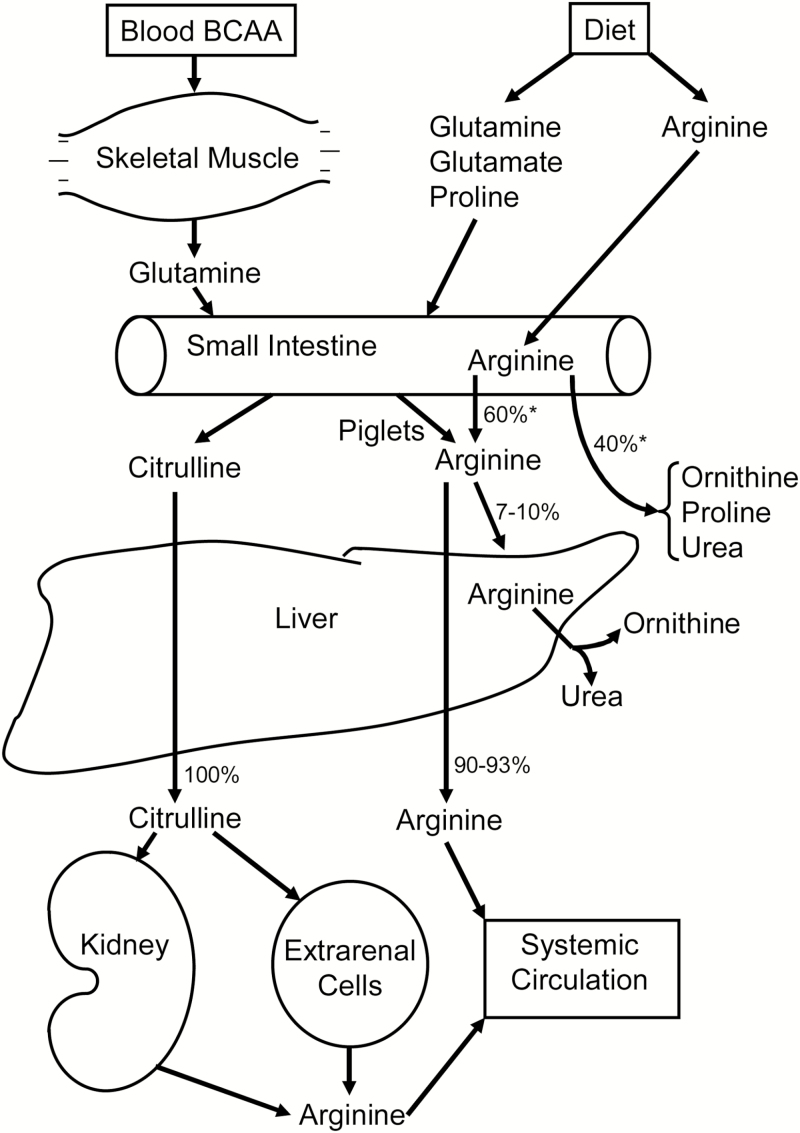
After weaning, a short-term cortisol surge induces the expression of P5C synthase in porcine enterocytes for the enhanced synthesis of citrulline from glutamine (Flynn and Wu, 1997a). In postweaning pigs, ASL activity is high in the kidneys, but low in enterocytes (Wu and Knabe, 1995, Wu et al. 1997). Therefore, the small intestine releases most of the synthesized citrulline into the blood circulation. In both preweaning and postweaning pigs (Wu et al., 2007b), as reported for rats (Dhanakoti et al., 1990), citrulline released by the small intestine is not extracted by the liver, but is utilized for Arg synthesis primarily in the kidneys. Likewise, the uptake of physiological concentrations of Arg by the pig liver is limited (Wu et al., 2007b) due to low activity of the AA transport system y+ (a transporter of basic AA) in hepatocytes (Closs et al., 2004). Therefore, the small intestine is essential for the endogenous synthesis of Arg from both glutamine and proline in pigs (Wu and Morris, 1998; Bertolo et al., 2003), and the citrulline and Arg that are derived from glutamine and proline in the gut are equally effective as a source of Arg for the whole body (Figure 1). Wu et al. (2016) reported that dietary supplementation with 0.5% to 2% Arg did not affect intestinal synthesis of citrulline and Arg from glutamine and proline in postweaning pigs.
Figure 1.
Interorgan metabolism of citrulline and arginine in pigs. There is no net synthesis of Arg in the liver via the urea cycle due to its rapid hydrolysis by arginase. Therefore, the intestinal-renal axis plays an important role in Arg provision in neonatal and postnatal pigs. Branched-chain amino acids (BCAA) in arterial blood are taken up by skeletal muscle for the synthesis of glutamine, which is released into the circulation. Glutamine in arterial blood, as well as dietary glutamine, glutamate, and proline is utilized by enterocytes of the small intestine for the production of citrulline, the immediate precursor of Arg. Virtually all of the intestine-derived citrulline by-pass the liver and are converted into Arg in the kidneys and extra-renal cells (including endothelial cells and macrophages), whereas 7% to 10% of Arg in the portal vein is extracted by the liver in the first pass. The symbol * denotes the flux in postweaning pigs. This figure is reused from Wu et al. (2007b), with permission from Elsevier.
Arginine deficiency limiting the maximal growth of sow-reared piglets.
A plethora of factors, including genetic, nutritional, and stressors (e.g., crowding, disease, ambient temperature, and air pollution), can affect pig growth (Wu, 2018). Data from artificial rearing systems indicate that the biological potential for neonatal pig growth is at least 400 g/d (average from birth to 21 d of age) or more than 74% greater than that for sow-reared piglets (230 g/d) and that suckling piglets start to exhibit submaximal growth from day 8 after birth (Boyd et al., 1995). Interestingly, the submaximal growth of suckling piglets occurs around the time when intestinal synthesis of citrulline and Arg is markedly reduced (Wu and Knabe, 1995) due to the relative deficiency of mitochondrial N-acetylglutamate synthase (Wu et al., 2004). Thus, the intestinal synthesis of citrulline and Arg from glutamine decreased by 70% to 73% in 7-d-old suckling pigs in comparison with newborn pigs and declined further in 14- to 21-d-old pigs (Wu, 1997). Similarly, the rates of citrulline and Arg synthesis from proline were 75% to 88% lower in the enterocytes of 7-d-old pigs, compared with newborn pigs, and remained at reduced levels in 14- to 21-d-old pigs (Wu, 1997). Consequently, the endogenous synthesis of Arg in piglets is reduced remarkably during the suckling period due to the reduced release of citrulline from the small intestine. Accordingly, the concentrations of Arg and its immediate precursors (ornithine and citrulline) in plasma decreased progressively by 20% to 41% from days 3 to 14 of postnatal development (Flynn et al., 2000). In addition, concentrations of ammonia in plasma increased progressively by 18% to 46%, whereas those of nitrite plus nitrate (stable oxidation products of nitric oxide [NO], a metabolite of Arg) decreased by 16% to 29% in 7- to 14-d-old suckling pigs, compared with 1- to 3-d-old pigs (Flynn et al., 2000). These metabolic data showing impaired hepatic ureagenesis and reduced systemic NO synthesis indicate a previously unrecognized deficiency of Arg in 7- to 21-d-old sow-reared pigs.
Arginine Catabolism in Pigs
Synthesis of creatine and homoarginine in pigs.
Based on the urinary excretion of creatinine by 7-d-old pigs, Wu et al. (2004) estimated that these neonates use 70-mg Arg·kg BW−1·d−1 to synthesize creatine, which represents 21% of the Arg catabolized in the body or 17% of the Arg provided from sow’s milk. Similarly, Brosnan et al. (2009) reported that a piglet uses 110-mg Arg/kg BW daily to synthesize creatine between 4 and 11 d of age. In older pigs (e.g., 4 mo of age), 84-mg Arg/kg BW is used daily to produce creatine (Hou et al., 2016). Although homoarginine is formed from Arg and lysine in pigs, this pathway consumes only 0.036-mg Arg·kg BW−1·d−1 and is quantitatively insignificant (Hou et al., 2016).
Metabolism of Arg in the mammary tissue of lactating sows.
The mammary glands of lactating sows take up a large amount of Arg, but its output in milk is much less than its uptake by the lactating glands (Trottier et al., 1997). To understand this observation, O’Quinn et al. (2002) used mammary tissue from lactating sows to conduct metabolic studies, which revealed that this tissue actively degrades Arg to form proline, polyamines, and NO. Two isoforms of arginase (types I and II) are responsible for Arg hydrolysis to produce urea and ornithine, which is subsequently converted into proline via ornithine aminotransferase and P5C reductase. Interestingly, the catabolism of proline is undetectable in porcine mammary tissue due to the absence of proline oxidase (O’Quinn et al., 2002). These findings help us to explain why Arg is remarkably deficient, but proline is highly abundant, in sow’s milk. As noted previously, milk-borne proline is utilized for citrulline and Arg synthesis in the piglet small intestine.
Catabolism of dietary Arg in pigs.
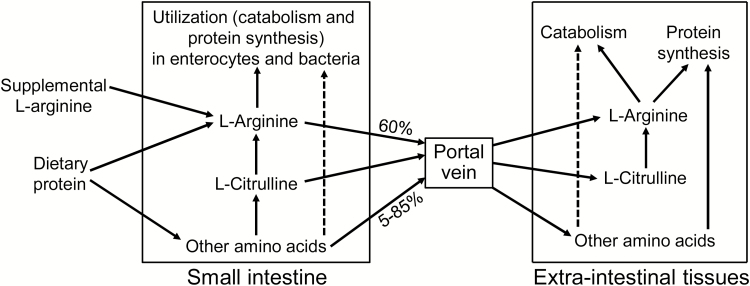
Enterocytes of preweaning pigs express little arginase activity and, therefore, do not synthesize proline from Arg (Wu et al., 1996b). This helps explain why proline is a nutritionally essential AA for young pigs (Ball et al., 1986). During weaning, a cortisol surge induces the expression of intestinal arginase in young pigs (Flynn and Wu, 1997b). Thereafter, arginase activity in enterocytes remains elevated in growing/finishing and adult swine (Wu et al. 2007a; 2016). Enterocytes of postweaning pigs express type-I and type-II arginases (Flynn et al., 1999) to actively degrade Arg into ornithine and urea, with ornithine being further converted into proline by ornithine aminotransferase and P5C reductase (Wu et al., 1996b). Bacteria in the lumen of the pig small intestine also metabolize some dietary Arg to ornithine and possibly a small amount of short-chain fatty acids (Dai et al., 2012). Thus, the gastrointestinal microbiota may affect both Arg availability and host health. In postweaning pigs, intestinal catabolism of Arg reduces the amount of dietary Arg entering the portal vein. Based on the area-under-the curve of Arg in plasma after its oral or intravenous administration, Wu et al. (2007b, 2016) estimated that about 40% of oral Arg is utilized (through catabolism and protein synthesis) by the small intestine in growing and adult pigs, with the remaining portion of Arg entering the portal vein.
In extra-intestinal tissues, the Arg that is not used for protein synthesis enters catabolic pathways initiated by arginase, Arg:glycine amidinotransferase, Arg decarboxylase, and NO synthase (Figure 2). These pathways are used to synthesize ornithine, creatine, agmatine, and NO, respectively. Based on the work of Hu et al. (2015), the small intestine and extra-intestinal tissues can catabolize 1) 153- and 365-mg Arg·kg BW−1·d−1, respectively, in 64-kg pigs fed a basal diet containing 1.35% Arg without Arg supplementation and 2) 405- and 743-mg Arg·kg BW−1·d−1, respectively, in 67-kg pigs fed the basal diet supplemented with 2% Arg. Creatine synthesis is a major pathway for Arg catabolism in growing pigs supplemented with 0-, 315-, and 630-mg Arg·kg BW−1·d−1 (Wu et al., 2016). Based on the production of creatine + homoarginine + agmatine + NO via the nonarginase pathway, the arginase pathway contributed 76%, 82%, and 85% of the Arg catabolized in the extra-intestinal tissues of a 65-kg pig supplemented with 0-, 315-, and 630-mg Arg·kg BW−1·d−1, respectively (Wu et al., 2016). These results indicate that dietary Arg undergoes extensive degradation in postweaning pigs.
Figure 2.
Metabolism of arginine in animals. In the gastrointestinal tract, the hydrolysis of dietary protein by extracellular proteases and peptidases releases Arg, other amino acids, and small peptides into the lumen. Enterocytes take up these products of digestion, degrade Arg, and release citrulline. Catabolism of amino acids also occurs by bacteria in the lumen of the gut. In postweaning pigs and rats, approximately 40% of Arg and 15% (e.g., tryptophan) to 95% (e.g., glutamate) of other AA in the lumen of the small intestine are utilized through catabolism and protein synthesis, during the first pass into the portal vein. Thus, 5% (e.g., glutamate) to 85% (e.g., tryptophan) of AA in the lumen of the small intestine enters the portal vein. In extra-intestinal tissues, the Arg that is not used for protein synthesis enters catabolic pathways initiated by arginase, Arg:glycine amidinotransferase, Arg decarboxylase, and nitric-oxide synthase. This figure is reused from Wu et al. (2016), with permission from Springer Nature.
Arginine metabolites in the pig conceptus.
The discoveries of citrulline and arg synthesis in enterocytes of neonatal pigs (Wu and Knabe, 1995) led to the question of whether this pathway might occur in fetal pigs. In analyzing fetal fluids from pigs at various days of gestation, Wu et al. (1995, 1996a) noted an unusually high abundance of Arg (4 to 6 mM) in porcine allantoic fluid on day 40 of gestation (term = 114 d), when compared with maternal plasma levels (0.1 to 0.14 mM). In addition, there were particularly high concentrations of ornithine (1 to 3 mM) and glutamine (3 to 4 mM) in porcine allantoic fluid on day 40 of gestation, compared with maternal plasma levels (0.05 to 0.1 mM for ornithine and 0.3 to 0.45 mM for glutamine) (Wu et al., 1995; 1996a). Remarkably, the concentrations of Arg, ornithine, and glutamine in porcine allantoic fluid increased by 23-, 18-, and 4-fold, respectively, between days 30 and 40 of gestation, with their nitrogen accounting for 67% of the total free α-AA nitrogen (Wu et al., 1996a). The unusual abundance of members of the Arg family of AA in fetal fluids is associated with maximal placental synthesis of NO and polyamines in the first half of a pregnancy (Wu et al., 2005), when placental growth is most rapid (Wu et al., 2013a). These novel findings provided a basis for the recent studies of the crucial roles for Arg-dependent metabolic pathways in conceptus survival, growth, and development (Wu et al., 2013b).
ARGININE NUTRITION IN PIGS
Results of recent studies indicate that dietary supplementation with Arg to conventional diets may improve the growth, production performance, and health of modern breeds of pigs during gestation, lactation, nursery, weaning, and growing-finishing periods (Wu et al., 2007b; 2010a, 2014). In 2012, the NRC recognized that Arg is a conditionally essential AA for pigs in all phases of their production. Thus, the NRC (2012) has recommended the minimal content of Arg in diets for gestating, lactating, nursing, weanling, and growing-finishing swine (Table 2). Greater values of dietary Arg requirements were suggested by Wu (2014) to maximize the postnatal growth of pigs, milk production by sows, and embryonic/fetal survival in pregnant pigs. This is a paradigm shift in swine AA nutrition, as Arg was not previously considered to be necessary in the diets of gestating or lactating sows (NRC, 1998). Due to dynamic changes in dietary requirements of animals for nutrients (including Arg) depending on physiological and environmental factors (Wu, 2018), NRC-recommended values should be regarded only as a guide in livestock feeding.
Arg Nutrition in Neonatal Pigs
The growth of young pigs is very sensitive to the provision of dietary Arg (Wu et al., 2004), and a severe deficiency of Arg (e.g., Arg concentration in piglet plasma ≤ 26 µM) will rapidly result in hyperammonia and even death in neonates (Brunton et al., 1999). Sow’s milk provides a 7-d-old pig with 0.40-g Arg·kg BW−1·d−1 (Wu et al., 2004). As noted previously, sow’s milk is relatively deficient in Arg. Thus, dietary supplementation with 0.2% and 0.4% Arg to 7- to 21-d-old milk-fed pigs (artificially reared on a liquid-milk feeding system) dose-dependently increased the concentrations of Arg in plasma (30% and 61%), reduced those of ammonia (20% and 35%), and increased the BW gain of piglets (28% and 66%) (Kim et al., 2004). Most recently, Yang et al. (2016) reported that supplementing 0.4% or 0.8% Arg to a milk replacer diet enhanced the weight gain of 4- to 24-d-old piglets by 19% and 22%, respectively, without affecting feed intake. Of interest, supplementation of the preweaning diet with Arg has the carry-over effect of enhancing intestinal growth and development in 25- to 45-d-old pigs (Yang et al., 2016). These metabolic and growth data provide further evidence that adequate Arg in diets is necessary for ensuring the maximal growth of young pigs.
Arg Nutrition in Weanling Pigs
Young pigs can effectively use dietary citrulline to synthesize Arg (Edmonds et al., 1987a; Urschel et al., 2006), but dietary ornithine has little effect on Arg synthesis in the body (Edmonds et al., 1987a), as reported for pig enterocytes (Wu et al., 1994b). Thus, in weanling pigs fed a semipurified diet containing 13.1% CP, 0.89% lysine, and 0.18% Arg, dietary supplementation with 0.18% Arg or an equimolar amount of citrulline increased concentrations of Arg in plasma and growth performance, but adding an equimolar amount of ornithine to the basal diet did not affect either the concentration of Arg in plasma or BW gain, compared with the control group (Edmonds et al., 1987a).
Weanling pigs usually have a reduced rate of feed intake during the first week postweaning, compared with suckling piglets (Wu et al., 2014). Like sow’s milk, typical corn- and soybean meal–based diets (without the addition of fish meal or plasma) for weanling piglets also contain a relatively low content of Arg (Wu et al., 2014). Mertz et al. (1952) demonstrated that the absence of Arg from the diet of weanling piglets greatly limited their growth. Similar results were obtained by Roth et al. (1995). Collectively, these findings indicate insufficient synthesis of Arg by weanling piglets, which results from inadequate intake of dietary AA (e.g., glutamine, glutamate, and proline) and a low activity of mitochondrial N-acetylglutamate synthase (Wu et al., 2004). A quantitative requirement of Arg by weanling piglets was not known until Southern and Baker (1983) reported that the presence of 0.48% bioavailable Arg in a semipurified diet could yield a growth rate comparable to that obtained with a typical corn- and soybean meal–based diet containing 19.1% CP. However, this reference diet may not be optimal for maximal growth of weanling piglets (Wu et al., 2014). For example, supplementing 0.6% Arg to a corn- and soybean meal–based diet enhanced small-intestinal mass and daily weight gain by 22% and 145%, respectively, in 21- to 28-d-old weanling piglets (Wu et al., 2010b).
A diet for weanling pigs should stimulate their feed intake. Balance among AA can be an important factor that affects the feed consumption of animals (Wu, 2014). Southern and Baker (1982) reported that adding 0%, 0.67%, 1.33%, and 2% Arg to a corn- and soybean meal–based diet containing 19% CP, 1.08% lysine, 1.27% Arg, and unknown content of most AA dose-dependently reduced feed intake and weight gain of 5- to 10-kg weanling pigs. Similarly, Hagemeier et al. (1983) showed that supplementing 1.63% Arg to a diet containing 1.03% lysine and an unknown content of protein reduced food intake and daily BW gain of weanling pigs. Likewise, supplementing 0.4%, 0.8%, and 1.6% Arg to a corn- and soybean meal–based diet containing 17.7% CP, 0.89% lysine, and 1.11% Arg dose-dependently reduced feed intake and daily weight gain without affecting the gain:feed ratio (Anderson et al., 1984a). When 0.22% Arg was supplemented to a corn- and soybean meal–based diet containing 14.9% CP, 0.7% lysine, and 0.99% Arg, weanling pigs had normal feed intake and growth performance but exhibited an 11% decrease in the concentration of lysine in plasma, compared with control pigs (Rosell and Zimmerman, 1984). In all of these previous studies, the concentrations of ornithine in the plasma of control pigs were unusually high (2 to 2.7 times the value of 85 to 98 µM in modern breeds of pigs [Wu et al., 1996a; Hu et al., 2015]) and were even much greater than the concentrations of lysine in plasma (e.g., 270 µM ornithine vs. 219 µM lysine, Rosell and Zimmerman, 1984; 204 µM ornithine vs. 134 µM lysine, Hagemeier et al., 1983; 238 µM ornithine vs. 126 µM lysine, Southern and Baker, 1982; and 257 µM ornithine vs. 212 µM lysine, Anderson et al., 1984a). These results raised questions of whether 1) the basal diets contained bacterial arginase to yield ornithine, 2) plasma contained excess arginase to hydrolyze Arg into ornithine possibly because of tissue (e.g., liver) injury, 3) AA in the diets were imbalanced, 4) ornithine metabolism was impaired in the animals, 5) there were differences in ornithine metabolism between different breeds of pigs, or 6) methodological differences in measuring ornithine. Additionally, in all of these previous studies, control diets were not made isonitrogenous to Arg-supplemented diets, and the purity of the crystalline Arg was unknown. Of note, some pharmaceutical grade tryptophan products manufactured before 1990 contained trace amounts of a toxic substance that caused an epidemic of eosinophilia-myalgia syndrome in humans (Mayeno et al., 1990; Trucksess, 1993). It is possible that the Arg product used for the previous studies in the 1980s had an impurity that could negatively affect feed intake and growth performance of pigs. In contrast, recent work could not identify an effect of dietary supplementation with up to 2% Arg on feed intake by weanling pigs, compared with isonitrogenous controls (Wu, 2010b; Hu et al., 2015).
Arg Nutrition in Growing-Finishing Pigs
Because typical corn- and soybean meal–based diets contain a relatively low content of Arg, growing-finishing pigs fed such diets (particularly low-protein diets) do not have sufficient Arg intake to support maximum deposition of protein in the body (Wu et al., 2014). Thus, supplementing Arg to these diets can improve growth and feed efficiency for lean tissue gains in growing-finishing barrows (Tan et al., 2009; Hu et al., 2015). In contrast, a study with growing pigs (27 to 44 kg BW, 28 d) showed that increasing dietary Arg content from 0.31% to 0.68% at the constant dietary content of 0.65% lysine increased feed intake by 4.3% and backfat thickness by 2.4% without affecting BW gain (Anderson et al., 1984b). A further increase in dietary Arg content from 0.68% to 1.01% reduced both feed intake and weight gain without affecting the gain:feed ratio. However, interpretation of these results is confounded because the increase in dietary Arg content from 0.31% to 0.68% was associated with an increase in dietary CP from 10.6% to 14.0% that resulted from feedstuff variation, and because the increase in dietary Arg content from 0.68% to 1.01% was associated with an increase in dietary CP from 14.0% to 16.7% that also resulted from feedstuff variation.
In studying finishing pigs (44 to 97 kg BW, 67 d), Anderson et al. (1984b) showed that increasing dietary Arg content from 0.32% to 0.62% at the constant dietary content of 0.62% lysine increased daily BW gain by 7%, gain:feed ratio by 5.3%, and longissimus muscle area by 10.6%, without affecting feed consumption. A further increase in dietary Arg content from 0.62% to 0.87% reduced BW gain by 4.9%, but did not affect feed intake of the pigs. It is unknown whether these effects of the supplemental Arg could be simply attributed to Arg intake, because the increase in dietary Arg content was associated with an increase in dietary CP from 11.0% to 14.1% that resulted from feedstuff variation, and because the increase in dietary Arg content from 0.62% to 0.87% was associated with an increase in dietary CP from 14.1% to 16.1% that also resulted from feedstuff variation. Nonetheless, this work showed that a diet containing 0.62% to 0.68% Arg and 14.0% to 14.1% CP yielded a better growth response in growing-finishing pigs than a diet containing 0.31% to 0.32% Arg and 10.6% to 11.0% CP. However, because of the differences in the composition of many AA between the 0.62% Arg and 0.87% Arg diets, it is not justified to conclude that an increase in dietary Arg content from 0.62% to 0.87% results in negative effects on feed consumption, growth performance, or carcass quality in growing-finishing pigs.
Easter et al. (1974) reported that nitrogen balance over a 5-d period did not differ between finishing female pigs (120-kg BW) fed a purified basal diet (containing 14.2% glutamic acid plus 4.5% other AA) that was supplemented with 0.0% or 0.38% Arg. These authors concluded that dietary Arg was not required for maintenance and postpubertal growth in the nongravid gilts. However, a lack of change in nitrogen balance does not necessarily mean that maximal lean tissue gain in pigs could be achieved without the provision of dietary Arg, as data from finishing female pigs fed the basal diet containing more than 0.38% Arg were not available. In addition, the basal diet contained an exceedingly large amount (14.2%) of glutamic acid (a precursor for the synthesis of citrulline and Arg in pigs [Wu et al., 1994b]), but the conventional diets of finishing pigs contained less than 1.5% glutamate (Wu et al., 2014).
In growing-finishing pigs, a major concern is that excessive amounts of subcutaneous white adipose tissue (e.g., backfat) are naturally deposited in market-weight pigs fed a conventional finishing diet (NRC, 2012). Notably, supplementing 1% Arg to a corn- and soybean meal–based diet (containing 0.97% Arg) for 110-d-old barrows for 60 d reduced serum triacylglyceride concentrations by 20% and whole-body white fat content by 11%, while increasing whole-body skeletal-muscle content by 5.5%, without affecting BW gain (Tan et al., 2009). Similar results for 30- to 121-d-old pigs were obtained by Hu et al. (2015). This beneficial effect of Arg is achieved through increasing lipolysis and reducing lipogenesis in white adipose tissue, as well as stimulating the oxidation of fatty acids and glucose in skeletal muscle (Wu et al., 2014). Thus, Arg can regulate energy partitioning in the body to favor white-fat reduction and lean-tissue gain. Another important conclusion from the work of Tan et al. (2009) is that a measurement of BW gain alone may not reveal the beneficial effects of Arg supplementation on lean tissue deposition in pigs, as reported for Arg-supplemented rats (Jobgen et al., 2009). Findings from swine may have important implications for treating overweight or obesity in humans (Peeters et al. 2003) and companion animals (Burkholder and Toll 2000; Zoran 2010; Backus and Wara, 2016).
Meat production is the main goal of the swine industry. Of note, dietary Arg also plays a role in meat quality. For example, studies with finishing pigs have shown that dietary Arg supplementation increases glycogen content by 42% and decreases lactate content by 37% in skeletal muscle as a result of reductions in glycogenolysis and glycolysis, leading to a beneficial increase in skeletal muscle pH by a unit of 0.32 at 45-min postmortem and an improvement in water-holding capacity (Tan et al., 2009). Likewise, supplementing 0.5% and 1% Arg to a corn- and soybean meal–based diet (containing 0.95% Arg) for pigs (60- to 110-kg BW) dose-dependently decreased concentrations of cortisol in serum, attenuated drip loss of pork muscle at 48 h postmortem, and enhanced antioxidative capacity in skeletal muscle (Ma et al., 2010).
Arg Nutrition in Gestating Pigs
Easter et al. (1974) investigated whether pregnant gilts required dietary Arg to maintain nitrogen balance over a 5-d period during late gestation. On days 90 to 99 of gestation, gilts (n = 5) which carried 7.2 ± 1.3 live fetuses (mean ± SEM) per gilt were fed an Arg-free purified diet (containing 14.2% glutamic acid plus 4.5% other AA) that was supplemented with no Arg or 0.38% Arg. The lack of Arg in the diet did not affect nitrogen balance in the gestating gilts. Similar results were obtained for pregnant gilts (n = 2 per treatment group) fed the Arg-free basal diet supplemented without Arg or 0.38% Arg between days 30 and 114 of gestation (Easter and Baker, 1976). The absence of Arg from the gestation diet did not affect the number or BW of live-born piglets (a mean value of 8.3 piglets per gilt). The authors suggested that the gravid swine could sufficiently synthesize Arg (Easter et al., 1974; Easter and Baker, 1976). However, no difference in nitrogen balance over a short period does not necessarily mean that maximal fetal growth during late gestation could be achieved without the provision of dietary Arg, as data from gestating gilts fed the basal diet containing >0.38% Arg were not available. In addition, the number of gilts used in the previous study (n = 2/treatment group; Easter and Baker, 1976) was too small to draw a definite conclusion regarding the effects of Arg on pregnancy outcomes. Furthermore, the basal diet used in the experiment contained an exceedingly large amount (14.2%) of glutamic acid (a precursor for the synthesis of citrulline and Arg in the pigs), but the conventional diets for gestating swine contained less than 1.5% glutamate (Wu et al., 2014).
Modern high-prolific sows ovulate 20 to 30 oocytes and deliver 10 to 15 live-born piglets at term (Town et al., 2005). There is a positive relationship between uterine capacity and fetal mortality (Bazer et al., 2014). The greatest restraint on litter size in pigs is placental development and function in early gestation and uterine capacity at all periods of gestation (Bazer et al., 1988). Interestingly, among domestic animals, pigs exhibit the most severe naturally occurring intrauterine growth restriction, and 76% of these compromised piglets do not survive to weaning (Wu et al., 2006). The current restricted feeding program for pregnant swine, which aims at reducing body fat accretion during pregnancy and preventing the associated problems of impaired lactogenesis during lactation (Kim and Wu, 2009), may not be the most desirable for fetal growth and development (Wu et al., 2013a), because dietary provision of Arg alone does not meet requirements during the entire pregnancy (Table 3).
Table 3.
Requirements for arginine and its endogenous synthesis in gestating gilts
| Amounts of arginine (g/d) | ||||
|---|---|---|---|---|
| Days 0 to 30 |
Days 30 to 60 |
Days 60 to 90 |
Days 90 to 114 |
|
| Body weight of sows (kg) | 155 to 160 | 160 to 170 | 170 to 185 | 185 to 200 |
| Arginine requirements for maternal metabolism and conceptus growtha | 15.48 | 16.47 | 17.36 | 19.86 |
| Uptake of portal vein arginine by maternal liver | 0.40 | 0.40 | 0.40 | 0.40 |
| Placental growth | 0.13 | 0.57 | 0.11 | 0.12 |
| Embryonic/fetal growth | 0.005 | 0.17 | 0.85 | 2.60 |
| Allantoic fluid | 0.005 | 0.005 | 0.000 | 0.000 |
| Amniotic fluid | 0.000 | 0.001 | 0.000 | 0.000 |
| Arginine utilization for creatine synthesis | 8.08 | 8.46 | 9.11 | 9.88 |
| Dietary arginine for metabolism via non-arginase and non-NO synthase pathways in extra-intestinal tissues | 5.04 | 5.04 | 5.04 | 5.04 |
| 2 kg diet (0.7% arginine) | 14.0 | 14.0 | 14.0 | 14.0 |
| Indigestible arginine in the diet (15%) | 2.10 | 2.10 | 2.10 | 2.10 |
| Intestinal catabolism (40% of arginine in the lumen) | 4.76 | 4.76 | 4.76 | 4.76 |
| Arginine provision from synthesis in mother plus fetus | ≥ 3.58 | ≥ 4.57 | ≥ 5.46 | ≥ 7.96 |
| (% of daily requirement for maternal metabolism and conceptus growth) | (23) | (28) | (32) | (40) |
Adapted from Wu et al. (2013b). Gilts gestating 12 fetuses are fed daily 2 kg of a typical corn- and soybean meal–based diet (Li et al., 2010).
aThe small intestine of the gestating sow uses 4.76 g of digestible dietary arginine per day. The rates of maternal arginine catabolism via arginase and NO synthase pathways in tissues other than the liver and gut are 2.0 and 0.10 g/d, respectively.
Because both polyamines and NO (products of Arg) play a key role in placental angiogenesis and growth in mammals (Wu et al., 2006), a seminal study was conducted to test the hypothesis that increasing Arg provision may enhance the reproductive performance of gilts (Mateo et al., 2007). The corn- and soybean meal–based diet contained 12% CP, 0.70% Arg, and 0.57% lysine, and the true ileal digestibility of the dietary protein was 85% (Wu et al., 2010). Dietary supplementation with 1.0% Arg-HCl between days 30 and 114 of gestation increased concentrations of Arg, ornithine, and proline in plasma by 77%, 53%, and 30%, respectively (Mateo et al., 2007). The Arg supplementation did not adversely affect the BW or backfat thickness of gilts, but increased the number of live-born piglets by 2 and litter birth-weight by 24% (Mateo et al., 2007). Furthermore, Arg supplementation improved immunity and reduced mortality in sows (Li et al., 2007), as reported for humans and other animals (Alexander and Dorothy, 2014). These novel findings indicate that Arg is a nutritionally essential AA to maximize reproductive performance in swine.
The important role of Arg in embryonic/fetal survival was confirmed by reports from several research groups (Table 4). First, under practical production conditions, there were increases in placental weight (+16%), the number of live-born piglets per litter (+1.1), and litter birth weights of live-born piglets ( + 1.7 kg) for gilts and multiparous sows that received dietary supplementation with 0.83% Arg between days 22 and 114 of gestation (Gao et al., 2012). Second, under practical production conditions, dietary supplementation with 1% Arg to gilts and sows between days 14 and 28 of gestation increased the number of live-born piglets by approximately 1 at birth (Ramaekers et al., 2006). Third, compared with control gilts, dietary supplementation with 0.4% or 0.8% Arg between days 14 and 25 of gestation increased placental growth by 21% to 34% and the number of viable fetuses per litter by approximately 2 (Li et al., 2014). Of note, the rates of embryonic survival did not differ between the 0.4% and 0.8% Arg groups. Fourth, dietary supplementation with 0.9% Arg-HCl between days 14 and 28 of gestation increased the number of fetuses per litter by 3.7 on day 70 of gestation in superovulated gilts (Bérard and Bee, 2010), as well as birth weight, skeletal muscle and organ weights, and myofiber hyperplasia in offspring (Madsen et al. 2017). Fifth, supplementing 1% Arg to the diet of gilts beginning on day 17 of gestation for 16 d increased the number of live-born piglets per litter by 1.2 (De Blasio et al., 2009). Finally, dietary supplementation with 0.83% Arg between days 90 and 114 of gestation increased the average birth weight of live-born piglets by 16% (Wu et al., 2010a). Furthermore, dietary supplementation with 1% Arg on days 25 to 80 of gestation enhanced piglet birth weight by 10% and reduced the percentage of piglets with a birth weight of <0.85 and <1.0 kg by 47% and 33%, respectively, in gilts giving birth to ≤14 piglets per litter (Dallanora et al., 2017). Likewise, dietary supplementation with 0.77% arginine to sows during the last third of pregnancy (days 77 to 114) decreased piglet birth-weight variation by 19% (Quesnel et al. 2014). In addition, dietary supplementation with 1% Arg between days 25 and 53 of gestation increased the size of fiber in the semitendinosus muscle of newborn piglets by 17% (Garbossa et al. 2015). An increase in the number of live-born pigs will markedly increase the profit margin associated with enhanced reproduction and lactation performance of dams (Wu et al., 2013a). Additionally, a reduction in the number of low-birth-weight piglets will greatly improve the management of neonatal pigs and maximize preweaning survival and growth.
Table 4.
Effects of maternal dietary arginine supplementation during pregnancy on litter size and birth weights of piglets in swine
| Authors | Parity of sow | Supple- mental arginine (% of diet or g/sow per day) | Period of arginine supple- mentation | Feed intake per day (kg) | CP content in diet (%) | Energy content in diet (ME; MJ/ kg) | Arginine content in basal diet (%) | Lysine content in basal diet (%) | Placental weight during early- to mid- gestationa or at birthb | Litter size of viable fetuses or live-born piglets | Litter weight of viable fetuses or live-born piglets |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bérard and Bee (2010) | 1 | 0.87% | d 14–28 | 3.0 | 14.3 | 11.5 | 1.07 | 0.88 | No effecta | ↑ by 3.7 per litter |
↑ by 32% per litter |
| 21.7 g | |||||||||||
| Campbell (2009) | 1 and MP |
1% | d 14–28 | ND | ND | ND | ND | ND | ND | ↑ by 1 per litter |
↑ by 6.4% per litter |
| 25 g | |||||||||||
| De Blasio et al. (2009) | 1 | 1% | d 17–33 | 2.5 | ND | ND | ND | ND | ND | ↑ by 1.2 | ND |
| 25 g | |||||||||||
| Gao at al. (2012) | 1 andMP | 0.83%16.6 g | d 22–114 | 2.0 (d 22–90)3.0 (d 90–114) | 13.2 | 13.0 | 0.88 | 0.65 | ↑ by 16%b | ↑ by 1.1 per litter |
↑ by 11% per litter |
| Li (2014) | 1 | 0.40% | d 14–25 | 2.0 | 12.0 | 12.9 | 0.70 | 0.57 | ↑ by 34%b | ↑ by 2.2 per litter |
No effect |
| 8.0 g | |||||||||||
| Li (2014) | 1 | 0.80% | d 14–25 | 2.0 | 12.0 | 12.9 | 0.70 | 0.57 | ↑ by 21%b | ↑ by 1.7 per litter |
No effect |
| 16.0 g | |||||||||||
| Mateo at al. (2007) | 1 | 0.83% | d 30–114 | 2.0 | 12.2 | 13.0 | 0.70 | 0.58 | ND | ↑ by 2.0 per litter |
↑ by 24% per litter |
| 16.6 g | |||||||||||
| Ramaekers (2006) | 1 andMP | 1% | d 14–28 | ND | ND | ND | ND | ND | ND | ↑ by 1 per litter |
ND |
| 25 g | |||||||||||
| Wu at al. (2012) | MP | 0.83% | d 90–114 | 2.0 | 14.7 | 13.5 | 0.80 | 0.78 | ND | No effect | ↑ by 16% per litter |
| 16.6 g |
ME = metabolizable energy; ND = not determined; MP = multiparous; ↑ = increase.
Some studies did not find a beneficial effect of dietary Arg supplementation on pregnancy outcome in swine. For example, Bass et al. (2017) showed that supplementation with 1% Arg-HCl to a corn-, soybean meal-, and distiller’s dried grains with solubles (DDGS)-based diet containing 18.7% CP for pregnant gilts and multiparous sows (consuming 2.72 kg feed/d) between days 93 and 110 of gestation had no effect on the number of pigs born alive, piglet birth weight, or lactation performance. Similar results were obtained when 1% Arg-HCl was supplemented to a corn-, soybean meal-, and wheat middlings-based diet containing 12.05% CP for multiparous sows between days 83 and 116 of gestation (consuming 2.2 kg feed/d on days 0 to 90 and 2.72 kg/d on days 90 to 116; Bass et al. 2017). In a meeting abstract, Greiner et al. (2012) reported that 1) supplementing 1.23% Arg to a corn- and DDGS-based diet (CP content not reported) for gilts and multiparous sows between days 18 and 34 of gestation (consuming 2.27-kg feed/d) did not affect conception rate, farrowing rate, or the total number of piglets born and 2) dietary supplementation with 1.23% Arg to multiparous sows between days 75 and 115 of gestation (consuming 2.27-kg feed/d on days 75 to 100 and 3.28 kg/d on days 100 to 112) reduced individual piglet birth weight by 4% and tended to reduce total litter birth weight. It is possible that the dietary content of CP or AA, feed intake, and the period of gestation influence the effects of dietary Arg supplementation on pregnancy performance in swine.
Arg Nutrition in Lactating Sows
Physiological levels of NO enhance blood flow and milk production by mammary glands of lactating mammals, such as sows (Kim and Wu, 2009), as well as protein synthesis in mammary epithelial cells (Ma et al., 2018). The provision of Arg increases the production of both NO and polyamines in mammary tissue, and, therefore, it promotes milk production (O’Quinn et al., 2002; Kim and Wu, 2009). Supplementing 0.83% Arg (as 1% Arg-HCl) to the diets of primiparous sows enhanced litter weight gain and milk production by 21% in the first week of lactation and by 11% during a 21-d suckling period (Mateo et al., 2008). Notably, the Arg treatment increased the daily BW gain of low-birth-weight piglets (40%) to a greater extent than for normal-birth-weight piglets (15%; Table 5), likely because low-birth-weight piglets have a reduced ability to synthesize Arg due to both reduced mass and dysfunction of the small intestine (Wu et al., 2006). Furthermore, dietary Arg supplementation to sows enhanced the production of lipids in milk (Kirchgessner et al., 1991). Likewise, Laspiur and Trottier (2001) reported that dietary Arg supplementation to lactating sows reduced their BW loss and enhanced their feed efficiency, particularly in hot environments, without affecting their heart and respiration rates or piglet weight gain. These findings have important implications for improving the health of sows, as well as the growth and survival of piglets, especially those that have experienced intrauterine growth restriction.
Table 5.
Growth performance of low- and normal-birth-weight piglets nursed by primiparous sows fed diets supplemented with or without arginine between days 0 and 14 of lactation
| Item | Control-fed sows | Arginine-supplemented sows | ||
|---|---|---|---|---|
| NBW piglets | LBW piglets | NBW piglets | LBW piglets | |
| Body weight of piglets, kg | ||||
| Day 0 | 1.36 ± 0.05 | 0.71 ± 0.03* | 1.38 ± 0.04 | 0.70 ± 0.03* |
| Day 7 | 2.31 ± 0.08 | 1.19 ± 0.05* | 2.70 ± 0.10** | 1.45 ± 0.06*,** |
| Day 14 | 3.75 ± 0.14 | 1.86 ± 0.09* | 4.16 ± 0.17** | 2.31 ± 0.12*,** |
| Daily weight gain of piglets, g/day | ||||
| Days 0 to 7 | 136 ± 5.6 | 69.0 ± 3.3* | 189 ± 8.7** | 106 ± 4.5*,** |
| Days 7 to 14 | 207 ± 9.3 | 95.8 ± 6.1* | 210 ± 9.0 | 123 ± 6.3*,** |
| Days 0 to 14 | 173 ± 7.9 | 82.3 ± 4.6* | 199 ± 8.4** | 115 ± 5.8*,** |
| Milk intake by piglets, mL/kg body weight | ||||
| Day 7 | 302 ± 18 | 294 ± 21 | 326 ± 24 | 312 ± 20 |
| Day 14 | 253 ± 13 | 241 ± 16 | 267 ± 15 | 259 ± 18 |
Adapted from Kim and Wu (2009). Gilts (Yorkshire x Landrace dams and Duroc x Hampshire sires) were bred at ~100 kg BW and fed daily 2.0 kg of a sorghum- and soybean meal–based diet containing 14.0% CP and 0.80% Arg. Between Days 0 and 14 of lactation, primiparous sows had free access to drinking water and the basal diet supplemented with either 1.0% Arg-HCl or 1.7% L-alanine (isonitrogenous control). Arginine or alanine was added to the basal diet at the expense of cornstarch. There were 10 sows in each treatment group. On the day of farrowing, 2 normal-birth-weight (1.3 to 1.5 kg) piglets and 2 low-birth-weight (0.60 to 0.80 kg) littermates were chosen from each of the 10 sows in a treatment group for weekly measurements of body weight and milk consumption. Litter size was equalized to be 9 per sow on day 0. On day 7, milk was obtained for the analysis of AA, and protein concentration was 40.3 ± 0.6 and 43.8 ± 0.8 g/L (P < 0.05), respectively, for control and Arg-supplemented sows. Feed intake was 4.93 ± 0.17 and 5.04 ± 0.19 kg/d during the 14-d period of lactation, respectively, for control and Arg-supplemented sows (P > 0.05). Data are means ± SEM.
*P < 0.01: Different from the corresponding NBW piglets.
**P < 0.01: Different from the corresponding control (alanine-supplemented) group.
LBW = low birth weight; NBW = normal birth weight.
SAFETY OF ARGININE SUPPLEMENTATION IN PIGS
Dietary Arg is extensively catabolized in growing-finishing (Table 6), as well as gestating and lactating (Hou et al., 2016) swine. Hu et al. (2015) assessed the safety of long-term Arg-HCl or Arg supplementation in pigs between 30 and 121 d of age, based on general observations (e.g., behavior, skin health, and hair), feed intake, growth, body composition, as well as hematological and blood chemistry tests (Hu et al., 2015). In that study, male and female pigs were fed a typical corn- and soybean meal–based diet (containing 1.35% Arg) supplemented with either 0%, 1%, 1.5%, or 2% Arg (as either Arg-HCl or Arg) for 91 d. The supplemental doses of 0%, 1%, 1.5%, and 2 % Arg provided pigs with 0-, 315-, 473-, and 630-mg Arg·kg BW−1·d−1, respectively, beyond the amount of digestible Arg in the basal diet (382 mg·kg BW−1·d−1) at the constant feed intake of 31.5 g·kg BW−1·d−1. Hematological and clinical chemistry tests at 1 and 4 h after feeding were performed as follows: 1) the numbers of erythrocytes and white blood cells (including mononuclear and polymorphonuclear cells); 2) blood hemoglobin, pH, and proteins (including enzymes); and 3) total bilirubin, AA, glucose, urea, ammonia, creatinine, lipids, and minerals in plasma. Results of all the measured variables in pigs fed diets supplemented with 0%, 1%, 1.5%, or 2% Arg were within physiological ranges and were not adversely affected by Arg administration (Hu et al., 2015). For example, dietary supplementation with Arg to pigs did not affect concentrations of lysine or histidine in plasma (indicating a lack of antagonism among basic AA), but beneficially increased lean tissue mass and reduced white fat, as well as concentrations of ammonia, free fatty acids, triacylglycerides, and cholesterol in plasma (Go et al., 2012, Hu et al., 2015). These results indicate that dietary supplementation with 315- to 630-mg Arg·kg BW−1·d−1 is safe in pigs fed corn- and soybean meal–based diets supplemented with 1% or 2% Arg for at least 91 d (Wu et al., 2016). Like any other nutrient, excessive intake of Arg may result in adverse effects on the animals. Of note, supplementing 4% Arg (as Arg base) to a corn- and soybean meal–based diet had adverse effects on young pigs, as indicated by AA imbalance and reduced growth performance (Edmonds et al., 1987b). Finally, supplementing up to 1% Arg to the diets for gestating sows (days 14 to 114 of gestation; Mateo et al., 2007, Li et al., 2014) and lactating sows (days 1 to 21 of lactation; Mateo et al., 2008) did not result in any adverse effects on either the mothers or their offspring. Although the general behavior of pigs is not affected by dietary Arg supplementation (Wu et al., 2007a; Li et al., 2010; Hu et al. 2015), animal activity cannot be adequately determined by in-person observation. Rather, 24-h measurements are required with sensor equipment appropriately standardized to specific situations.
Table 6.
Extra-intestinal catabolism of arginine via arginase and nonarginase pathways in pigs supplemented with or without L-arginine for 13 wk
| Supplemental dose of Arg (mg/(kg body weight·d) | Extra-intestinal catabolism of Arg to form its metabolites via nonarginase pathways1 | Extra-intestinal catabolism of Arg via arginase2 |
|||
|---|---|---|---|---|---|
| Homoarginine | Creatine | NOx | Agmatine | ||
| mg Arg·kg BW−1·d−1 | |||||
| 0 | 0.088 ± 0.004c | 83.9 ± 3.7c | 2.86 ± 0.17c | 0.036 ± 0.003c | 278 ± 5.6c |
| 315 | 0.138 ± 0.005b | 96.7 ± 4.3b | 4.35 ± 0.22b | 0.047 ± 0.003b | 453 ± 8.1b |
| 630 | 0.194 ± 0.008a | 109 ± 4.9a | 5.67 ± 0.31a | 0.060 ± 0.004a | 628 ± 11a |
a–cWithin a column for each species, means sharing different superscript letters differ (P < 0.05).
Adapted from Wu et al. (2016). Values are means ± SEM, n = 12 pigs (121 d of age; 6 males and 6 females) per dietary group.
1Homoarginine, creatine, NOx (nitrite plus nitrate), and agmatine were produced from Arg via nonarginase pathways.
2These values are calculated as the rates of Arg catabolism via arginase plus nonarginase pathways minus the rates of Arg catabolism via the nonarginase pathway. The values of extra-intestinal Arg catabolism via the arginase pathway do not include proteins released by extra-intestinal tissues.
An optimal dietary ratio of Arg:lysine likely varies with the age and health status of pigs. When a ratio of Arg:lysine in diets is greater than 3:1, the feed intake and growth performance of postweaning pigs are reduced (Anderson et al., 1984a; Wu et al., 2007b). The underlying mechanisms may involve their competition for 1) absorption from the lumen of the small intestine into enterocytes, 2) transport from blood plasma into cells, and 3) reabsorption into blood from renal tubules, as well as a reduction in intracellular protein synthesis and an acid-base imbalance. Although Edmonds and Baker (1987) reported the failure of excess dietary lysine to antagonize Arg in postweaning pigs, this conclusion is not consistent with their evidence that the addition of 2.3% or 3.45% lysine to the basal diet reduced the daily BW gain of weanling pigs by 10% and 20%, respectively, compared with the lysine-adequate group (the basal diet + 1.15% lysine) (Edmonds and Baker, 1987). Interestingly, neonatal pigs may be particularly sensitive to high Arg intake, as oral administration of Arg twice daily (0.29 g·kg BW−1·d−1) between 1 and 16 d of age reduced the BW of low-birth-weight (0.69 to 0.92 kg) and normal-birth-weight (1.3 to 1.5 kg) piglets by 22% and 13%, respectively, compared with control piglets receiving oral administration of the isonitrogenous amount of alanine (Getty et al., 2015). Caution should be exercised in interpreting this result because the consumption of sow’s milk or dietary intake of AA (including lysine) by all the study piglets was not reported by the authors (Getty et al., 2015). This means that an Arg:lysine ratio in the diet is unknown. In another study, a higher ratio of Arg:lysine (e.g., 1.44:1.00) in the enteral diet providing 1.80-g Arg and 1.25-g lysine·kg BW−1·d−1 had adverse effects of reducing the growth of 7-d-old pigs, compared with the diet providing 0.2-g Arg and 1.25-g lysine·kg BW−1·d−1 (Arg:lysine ratio of 0.16:1.00; Wilkinson et al., 2004). However, because the incomplete basal diet of the young pigs lacked glutamine and aspartate (important AA for intestinal energy supply and function), this finding may not be applicable to piglets fed a complete diet containing all AA.
The timing of Arg supplementation is critical for its safety and beneficial effects in improving pregnancy outcomes in swine (Wu et al., 2010a). This is demonstrated by the adverse effects of Arg supplementation immediately after mating on porcine embryonic survival and growth (Li et al., 2010). Specifically, when compared with the control group of gilts, dietary supplementation with 0.8% Arg between days 0 and 25 of gestation decreased uterine weight (−20%), total number of fetuses (−24%), number of corpora lutea (−17%), total fetal weight (−34%), total volume of allantoic and amniotic fluids (−34% to 42%), concentrations of progesterone in maternal plasma (−33%), as well as total amounts of progesterone (−35%), estrone (−40%), and estrone sulfate (−37%) in allantoic fluid (Li et al., 2010). In the ovary, the follicular development and discharge of mature oocytes with the formation of corpora lutea depend on cell signaling via mitogen activated protein kinases 3 and 1 (also known as extracellular-regulated protein kinases 1 and 2 [ERK1/2]) and liver receptor homolog 1 (Duggavathi et al., 2008). Based on available data, Li et al. (2010) suggest that increased production of NO by Arg supplementation between days 0 and 14 of gestation may impair ERK1/2 signaling and liver receptor homolog 1 function in the porcine ovary, thereby reducing the number of follicles that ovulate and, therefore, the number of corpora lutea, and the concentration of progesterone in the maternal plasma.
In summary, extensive research over the past 25 yr has identified Arg as one of the most abundant AA in the body of pigs and their fetal fluids during pregnancy. About 40% of the dietary Arg is catabolized by the small intestine during the first pass, and endogenous synthesis via interorgan metabolism of AA is crucial for maintaining Arg homeostasis in the whole body. At the cellular level, Arg is physiologically essential for the synthesis of proteins and other nitrogenous substances (including NO, creatine, polyamines, and homoarginine) with key metabolic functions in the body. Thus, this nutrient plays an important role in improving the health, survival, growth, development, lactation, and reproduction of swine. Compelling evidence shows that Arg is a nutritionally-essential AA for weanling pigs to both maintain normal intestinal physiology and enhance efficiency in the utilization of dietary protein for gut maturation and whole-body growth. Additionally, recent findings indicate that adequate amounts of dietary Arg are necessary to support maximum lactation and reproductive performance in pigs. Arginine is truly a functional and conditionally essential AA in swine nutrition. These results also have important implications for improving the nutrition of humans and other animals.
Footnotes
This work was supported by Agriculture and Food Research Initiative Competitive Grants (2014-67015-21770 and 2015-67015-23276) from the USDA National Institute of Food and Agriculture, Texas A&M AgriLife Research (H-8200), Hubei Hundred Talent program, and Natural Science Foundation of Hubei Province (2016CFA070).
We thank our colleagues, students, and technicians for their contribution to this research.
LITERATURE CITED
- Alexander J. W. and Supp D. M.. 2014. Role of arginine and omega-3 fatty acids in wound healing and infection. Adv. Wound Care (New. Rochelle). 3:682–690. doi: 10.1089/wound.2013.0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. C., Lewis A. J., Peo E. R. Jr, and Crenshaw J. D.. 1984a. Effects of excess arginine with and without supplemental lysine on performance, plasma amino acid concentrations and nitrogen balance of young swine. J. Anim. Sci. 58:369–377. [DOI] [PubMed] [Google Scholar]
- Anderson L. C., Lewis A. J., Peo E. R. Jr, and Crenshaw J. D.. 1984b. Effect of various dietary arginine: lysine ratios on performance, carcass composition and plasma amino acid concentrations of growing-finishing swine. J. Anim. Sci. 58:362–368. [DOI] [PubMed] [Google Scholar]
- Backus R. and Wara A.. 2016. Development of obesity: mechanisms and physiology. Vet. Clin. North Am. Small Anim. Pract. 46:773–784. doi: 10.1016/j.cvsm.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Ball R. O., Atkinson J. L., and Bayley H. S.. 1986. Proline as an essential amino acid for the young pig. Br. J. Nutr. 55:659–668. [DOI] [PubMed] [Google Scholar]
- Bass B. E., Bradley C. L., Johnson Z. B., Zier-Rush C. E., Boyd R. D., Usry J. L., Maxwell C. V., and Frank J. W.. 2017. Influence of dietary-arginine supplementation of sows during late pregnancy on piglet birth weight and sow and litter performance during lactation. J. Anim. Sci. 95:248–256. doi: 10.2527/jas.2016.0986 [DOI] [PubMed] [Google Scholar]
- Bazer F. W., Thatcher W. W., Martinat-Botte F., and Terqui M.. 1988. Conceptus development in large white and prolific chinese meishan pigs. J. Reprod. Fertil. 84:37–42. [DOI] [PubMed] [Google Scholar]
- Bazer F. W., Wu G., Johnson G. A., and Wang X.. 2014. Environmental factors affecting pregnancy: endocrine disrupters, nutrients and metabolic pathways. Mol. Cell. Endocrinol. 398:53–68. doi: 10.1016/j.mce.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Bérard J. and Bee G.. 2010. Effects of dietary l-arginine supplementation to gilts during early gestation on foetal survival, growth and myofiber formation. Animal 4:1680–1687. doi: 10.1017/S1751731110000881 [DOI] [PubMed] [Google Scholar]
- Bertolo R. F., Brunton J. A., Pencharz P. B., and Ball R. O.. 2003. Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 284:E915–E922. doi: 10.1152/ajpendo.00269.2002 [DOI] [PubMed] [Google Scholar]
- Blachier F., M’Rabet-Touil H., Posho L., Darcy-Vrillon B., and Duée P. H.. 1993. Intestinal arginine metabolism during development. Evidence for de novo synthesis of L-arginine in newborn pig enterocytes. Eur. J. Biochem. 216:109–117. [DOI] [PubMed] [Google Scholar]
- Boyd R. D., Kensinger R. S., Harrell R. J., and Bauman D. E.. 1995. Nutrient uptake and endocrine regulation of milk synthesis by mammary tissue of lactating sows. J. Anim. Sci. 73 (Suppl. 2):36–56. [Google Scholar]
- Brosnan J. T., Wijekoon E. P., Warford-Woolgar L., Trottier N. L., Brosnan M. E., Brunton J. A., and Bertolo R. F.. 2009. Creatine synthesis is a major metabolic process in neonatal piglets and has important implications for amino acid metabolism and methyl balance. J. Nutr. 139:1292–1297. doi: 10.3945/jn.109.105411 [DOI] [PubMed] [Google Scholar]
- Brunton J. A., Bertolo R. F., Pencharz P. B., and Ball R. O.. 1999. Proline ameliorates arginine deficiency during enteral but not parenteral feeding in neonatal piglets. Am. J. Physiol. 277(2 Pt 1):E223–E231. [DOI] [PubMed] [Google Scholar]
- Burkholder W., and Toll P.. 2000. Obesity. In: Hand, M. S., C. D. Thatcher, R. L. Remillard, P. Roudebush, B. J. Novtony, and L. D. Lews, editors. Small animal clinical nutrition. Mark Morris Institute, Topeka: p. 401–430. [Google Scholar]
- Campbell R. 2009. Pork CRC – NZ Seminar Series: arginine and reproduction http://www/nzpib.co.nz (Accessed 26 April 2013).
- Closs E. I., Simon A., Vékony N., and Rotmann A.. 2004. Plasma membrane transporters for arginine. J. Nutr. 134(10 Suppl):2752S–2759S; discussion 2765S. doi: 10.1093/jn/134.10.2752S [DOI] [PubMed] [Google Scholar]
- Dai Z. L., Li X. L., Xi P. B., Zhang J., Wu G., and Zhu W. Y.. 2012. Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 42:1597–1608. doi: 10.1007/s00726-011-0846-x [DOI] [PubMed] [Google Scholar]
- Dallanora D., Marcon J., Walter M. P., Biondo N., Bernardi M. L., Wentz I., and Bortolozzo F. P.. 2017. Effect of dietary amino acid supplementation during gestation on placental efficiency and litter birth weight in gestating gilts. Livest. Sci. 197:30–35. [Google Scholar]
- Davis T. A., Nguyen H. V., Garcia-Bravo R., Fiorotto M. L., Jackson E. M., Lewis D. S., Lee D. R., and Reeds P. J.. 1994. Amino acid composition of human milk is not unique. J. Nutr. 124:1126–1132. doi: 10.1093/jn/124.7.1126 [DOI] [PubMed] [Google Scholar]
- De Blasio M., Roberts C., and Owens J.. 2009. Effect of dietary arginine supplementation during gestation on litter size of gilts and sows http://www.australianpork.com.au (Accessed 26 April 2013).
- Dhanakoti S. N., Brosnan J. T., Herzberg G. R., and Brosnan M. E.. 1990. Renal arginine synthesis: studies in vitro and in vivo. Am. J. Physiol. 259(3 Pt 1):E437–E442. doi: 10.1152/ajpendo.1990.259.3.E437 [DOI] [PubMed] [Google Scholar]
- Dillon E. L., Knabe D. A., and Wu G.. 1999. Lactate inhibits citrulline and arginine synthesis from proline in pig enterocytes. Am. J. Physiol. 276(5 Pt 1):G1079–G1086. [DOI] [PubMed] [Google Scholar]
- Duggavathi R., Volle D. H., Mataki C., Antal M. C., Messaddeq N., Auwerx J., Murphy B. D., and Schoonjans K.. 2008. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 22:1871–1876. doi: 10.1101/gad.472008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter R. A. and Baker D. H.. 1976. Nitrogen metabolism and reproductive response of gravid swine fed an arginine-free diet during the last 84 days of gestation. J. Nutr. 106:636–641. doi: 10.1093/jn/106.5.636 [DOI] [PubMed] [Google Scholar]
- Easter R. A., Katz R. S., and Baker D. H.. 1974. Arginine: a dispensable amino acid for postpubertal growth and pregnancy of swine. J. Anim. Sci. 39:1123–1128. [DOI] [PubMed] [Google Scholar]
- Edmonds M. S., Gonyou H. W., and Baker D. H.. 1987. Effect of excess levels of methionine, tryptophan, arginine, lysine or threonine on growth and dietary choice in the pig. J. Anim. Sci. 65:179–185. [DOI] [PubMed] [Google Scholar]
- Edmonds M. S. and Baker D. H.. 1987b. Failure of excess dietary lysine to antagonize arginine in young pigs. J. Nutr. 117:1396–1401. doi: 10.1093/jn/117.8.1396 [DOI] [PubMed] [Google Scholar]
- Edmonds M. S., Lowry K. R., and Baker D. H.. 1987a. Urea cycle metabolism: effects of supplemental ornithine or citrulline on performance, tissue amino acid concentrations and enzymatic activity in young pigs fed arginine-deficient diets. J. Anim. Sci. 65:706–716. [DOI] [PubMed] [Google Scholar]
- Flynn N. E., Knabe D. A., Mallick B. K., and Wu G.. 2000. Postnatal changes of plasma amino acids in suckling pigs. J. Anim. Sci. 78:2369–2375. [DOI] [PubMed] [Google Scholar]
- Flynn N. E., Meininger C. J., Kelly K., Ing N. H., Morris S. M. Jr, and Wu G.. 1999. Glucocorticoids mediate the enhanced expression of intestinal type II arginase and argininosuccinate lyase in postweaning pigs. J. Nutr. 129:799–803. doi: 10.1093/jn/129.4.799 [DOI] [PubMed] [Google Scholar]
- Flynn N. E. and Wu G.. 1996. An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Am. J. Physiol. 271(5 Pt 2):R1149–R1155. doi: 10.1152/ajpregu.1996.271.5.R1149 [DOI] [PubMed] [Google Scholar]
- Flynn N. E. and Wu G.. 1997a. Glucocorticoids play an important role in mediating the enhanced metabolism of arginine and glutamine in enterocytes of postweaning pigs. J. Nutr. 127:732–737. doi: 10.1093/jn/127.5.732 [DOI] [PubMed] [Google Scholar]
- Flynn N. E. and Wu G.. 1997b. Enhanced metabolism of arginine and glutamine in enterocytes of cortisol-treated pigs. Am. J. Physiol. 272(3 Pt 1):G474–G480. doi: 10.1152/ajpgi.1997.272.3.G474 [DOI] [PubMed] [Google Scholar]
- Gao K., Jiang Z., Lin Y., Zheng C., Zhou G., Chen F., Yang L., and Wu G.. 2012. Dietary L-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 42:2207–2214. doi: 10.1007/s00726-011-0960-9 [DOI] [PubMed] [Google Scholar]
- Garbossa C. A., Carvalho Júnior F. M., Silveira H., Faria P. B., Schinckel A. P., Abreu M. L., and Cantarelli V. S.. 2015. Effects of ractopamine and arginine dietary supplementation for sows on growth performance and carcass quality of their progenies. J. Anim. Sci. 93:2872–2884. doi: 10.2527/jas.2014-8824 [DOI] [PubMed] [Google Scholar]
- Getty C. M., Almeida F. N., Baratta A. A., and Dilger R. N.. 2015. Plasma metabolomics indicates metabolic perturbations in low birth weight piglets supplemented with arginine. J. Anim. Sci. 93:5754–5763. doi: 10.2527/jas.2015-9293 [DOI] [PubMed] [Google Scholar]
- Go G., Wu G., Silvey D. T., Choi S., Li X., and Smith S. B.. 2012. Lipid metabolism in pigs fed supplemental conjugated linoleic acid and/or dietary arginine. Amino Acids 43:1713–1726. doi: 10.1007/s00726-012-1255-5 [DOI] [PubMed] [Google Scholar]
- Greiner L., Usry J. L., Neill C., Williams N., Connor J., and Allee G.. 2012. The evaluation of supplemental L-arginine during gestation on sow reproductive performance. J. Anim. Sci. 90(Suppl 2):33–34 (abstract). [Google Scholar]
- Hagemeier D. L., Libal G. W., and Wahlstrom R. C.. 1983. Effects of excess arginine on swine growth and plasma amino acid levels. J. Anim. Sci. 57:99–105. [DOI] [PubMed] [Google Scholar]
- Hou Y., Hu S., Jia S., Nawaratna G., Che D., Wang F., Bazer F. W., and Wu G.. 2016. Whole-body synthesis of L-homoarginine in pigs and rats supplemented with L-arginine. Amino Acids 48:993–1001. doi: 10.1007/s00726-015-2145-4 [DOI] [PubMed] [Google Scholar]
- Hu S., Li X., Rezaei R., Meininger C. J., McNeal C. J., and Wu G.. 2015. Safety of long-term dietary supplementation with L-arginine in pigs. Amino Acids 47:925–936. doi: 10.1007/s00726-015-1921-5 [DOI] [PubMed] [Google Scholar]
- Jobgen W., Meininger C. J., Jobgen S. C., Li P., Lee M. J., Smith S. B., Spencer T. E., Fried S. K., and Wu G.. 2009. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J. Nutr. 139:230–237. doi: 10.3945/jn.108.096362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. W., McPherson R. L., and Wu G.. 2004. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J. Nutr. 134:625–630. doi: 10.1093/jn/134.3.625 [DOI] [PubMed] [Google Scholar]
- Kim S. W. and Wu G.. 2009. Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37:89–95. doi: 10.1007/s00726-008-0151-5 [DOI] [PubMed] [Google Scholar]
- Kirchgessner V. M., Rader M. G., and Roth-Maier D. A.. 1991. Influence of an oral arginine supplementation on lactation performance of sows. J. Anim. Physiol. Anim. Nutr. 66:38–44. [Google Scholar]
- Laspiur J. P., and Trottier N. L.. 2001. Effect of dietary arginine supplementation and environmental temperature on sow lactation performance. Livest. Prod. Sci. 70:159–165. [Google Scholar]
- Li X., Bazer F. W., Johnson G. A., Burghardt R. C., Erikson D. W., Frank J. W., Spencer T. E., Shinzato I., and Wu G.. 2010. Dietary supplementation with 0.8% L-arginine between days 0 and 25 of gestation reduces litter size in gilts. J. Nutr. 140:1111–1116. doi: 10.3945/jn.110.121350 [DOI] [PubMed] [Google Scholar]
- Li X., Bazer F. W., Johnson G. A., Burghardt R. C., Frank J. W., Dai Z., Wang J., Wu Z., Shinzato I., and Wu G.. 2014. Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids 46:375–384. doi: 10.1007/s00726-013-1626-6 [DOI] [PubMed] [Google Scholar]
- Li P., Yin Y. L., Li D., Kim S. W., and Wu G.. 2007. Amino acids and immune function. Br. J. Nutr. 98:237–252. doi: 10.1017/S000711450769936X [DOI] [PubMed] [Google Scholar]
- Ma X., Lin Y., Jiang Z., Zheng C., Zhou G., Yu D., Cao T., Wang J., and Chen F.. 2010. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids 38:95–102. doi: 10.1007/s00726-008-0213-8 [DOI] [PubMed] [Google Scholar]
- Ma Q. Q., Hu S. D., Bannai M., and Wu G.. 2018. L-Arginine regulates protein turnover in porcine mammary epithelial cells to enhance milk protein synthesis. Amino Acids 50:621–628. doi: 10.1007/s00726-018-2541-7. [DOI] [PubMed] [Google Scholar]
- MacDonald M. L., Rogers Q. R., and Morris J. G.. 1984. Nutrition of the domestic cat, a mammalian carnivore. Annu. Rev. Nutr. 4:521–562. doi: 10.1146/annurev.nu.04.070184.002513 [DOI] [PubMed] [Google Scholar]
- Madsen J. G., Pardo C., Kreuzer M., and Bee G.. 2017. Impact of dietary l-arginine supply during early gestation on myofiber development in newborn pigs exposed to intra-uterine crowding. J. Anim. Sci. Biotechnol. 8:58. doi: 10.1186/s40104-017-0188-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo R. D., Wu G., Bazer F. W., Park J. C., Shinzato I., and Kim S. W.. 2007. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J. Nutr. 137:652–656. doi: 10.1093/jn/137.3.652 [DOI] [PubMed] [Google Scholar]
- Mateo R. D., Wu G., Moon H. K., Carroll J. A., and Kim S. W.. 2008. Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J. Anim. Sci. 86:827–835. doi: 10.2527/jas.2007-0371 [DOI] [PubMed] [Google Scholar]
- Mayeno A. N., Lin F., Foote C. S., Loegering D. A., Ames M. M., Hedberg C. W., and Gleich G. J.. 1990. Characterization of “peak E,” a novel amino acid associated with eosinophilia-myalgia syndrome. Science 250:1707–1708. [DOI] [PubMed] [Google Scholar]
- Mertz E. T., Beeson W. M., and Jackson H. D.. 1952. Classification of essential amino acids for the weanling pig. Arch. Biochem. Biophys. 38:121–128. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 1998. Nutrient requirements of swine. Natl. Acad. Press, Washington, D.C. [Google Scholar]
- National Research Council (NRC) 2012. Nutrient requirements of swine. Natl. Acad. Press, Washington, D.C. [Google Scholar]
- O’Quinn P. R., Knabe D. A., and Wu G.. 2002. Arginine catabolism in lactating porcine mammary tissue. J. Anim. Sci. 80:467–474. [DOI] [PubMed] [Google Scholar]
- Quesnel H., Quiniou N., Roy H., Lottin A., Boulot S., and Gondret F.. 2014. Supplying dextrose before insemination and L-arginine during the last third of pregnancy in sow diets: effects on within-litter variation of piglet birth weight. J. Anim. Sci. 92:1445–1450. doi: 10.2527/jas.2013-6701 [DOI] [PubMed] [Google Scholar]
- Peeters A., Barendregt J. J., Willekens F., Mackenbach J. P., Al Mamun A., and Bonneux L.; NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group 2003. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann. Intern. Med. 138:24–32. [DOI] [PubMed] [Google Scholar]
- Ramaekers P., Kemp B., and van der Lende T.. 2006. Progenos in sows increases number of piglets born. J. Anim. Sci. 84 (Suppl. 1):394. [Google Scholar]
- Rosell V. L. and Zimmerman D. R.. 1984. Effects of graded levels of lysine and excess arginine and threonine on young pigs fed practical diets. J. Anim. Sci. 59:135–140. [DOI] [PubMed] [Google Scholar]
- Roth F. X., Fickler J., and Kirchgessner M.. 1995. Effect of dietary arginine and glutamic acid supply on the N-balance of piglets. 5. Communication on the importance of nonessential amino acids for protein retention. J. Anim. Physiol. Anim. Nutr. 73:202–212. [Google Scholar]
- Southern L. L. and Baker D. H.. 1982. Performance and concentration of amino acids in plasma and urine of young pigs fed diets with excesses of either arginine or lysine. J. Anim. Sci. 55:857–866. [DOI] [PubMed] [Google Scholar]
- Southern L. L. and Baker D. H.. 1983. Arginine requirement of the young pig. J. Anim. Sci. 57:402–412. [DOI] [PubMed] [Google Scholar]
- Tan B., Yin Y., Liu Z., Li X., Xu H., Kong X., Huang R., Tang W., Shinzato I., Smith S. B., et al. 2009. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 37:169–175. doi: 10.1007/s00726-008-0148-0 [DOI] [PubMed] [Google Scholar]
- Town S. C., Patterson J. L., Pereira C. Z., Gourley G., and Foxcroft G. R.. 2005. Embryonic and fetal development in a commercial dam-line genotype. Anim. Reprod. Sci. 85:301–316. doi: 10.1016/j.anireprosci.2004.05.019 [DOI] [PubMed] [Google Scholar]
- Trottier N. L., Shipley C. F., and Easter R. A.. 1997. Plasma amino acid uptake by the mammary gland of the lactating sow. J. Anim. Sci. 75:1266–1278. [DOI] [PubMed] [Google Scholar]
- Trucksess M. W. 1993. Separation and isolation of trace impurities in L-tryptophan by high-performance liquid chromatography. J. Chromatogr. 630:147–150. [DOI] [PubMed] [Google Scholar]
- Urschel K. L., Shoveller A. K., Pencharz P. B., and Ball R. O.. 2005. Arginine synthesis does not occur during first-pass hepatic metabolism in the neonatal piglet. Am. J. Physiol. Endocrinol. Metab. 288:E1244–E1251. doi: 10.1152/ajpendo.00530.2004 [DOI] [PubMed] [Google Scholar]
- Urschel K. L., Shoveller A. K., Uwiera R. R., Pencharz P. B., and Ball R. O.. 2006. Citrulline is an effective arginine precursor in enterally fed neonatal piglets. J. Nutr. 136:1806–1813. doi: 10.1093/jn/136.7.1806 [DOI] [PubMed] [Google Scholar]
- Wilkinson D. L., Bertolo R. F., Brunton J. A., Shoveller A. K., Pencharz P. B., and Ball R. O.. 2004. Arginine synthesis is regulated by dietary arginine intake in the enterally fed neonatal piglet. Am. J. Physiol. Endocrinol. Metab. 287:E454–E462. doi: 10.1152/ajpendo.00342.2003 [DOI] [PubMed] [Google Scholar]
- Wu G. 1997. Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am. J. Physiol. 272(6 Pt 1):G1382–G1390. doi: 10.1152/ajpgi.1997.272.6.G1382 [DOI] [PubMed] [Google Scholar]
- Wu G. 2014. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J. Anim. Sci. Biotechnol. 5:34. doi: 10.1186/2049-1891-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. 2018. Principles of animal nutrition. CRC Press, Boca Raton, Florida. [Google Scholar]
- Wu G., Bazer F. W., Burghardt R. C., Johnson G. A., Kim S. W., Li X. L., Satterfield M. C., and Spencer T. E.. 2010a. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J. Anim. Sci. 88(13 Suppl):E195–E204. doi: 10.2527/jas.2009-2446 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Cudd T. A., Jobgen W. S., Kim S. W., Lassala A., Li P., Matis J. H., Meininger C. J., and Spencer T. E.. 2007a. Pharmacokinetics and safety of arginine supplementation in animals. J. Nutr. 137(6 Suppl 2):1673S–1680S. doi: 10.1093/jn/137.6.1673S [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Dai Z., Li D., Wang J., and Wu Z.. 2014. Amino acid nutrition in animals: protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2:387–417. doi: 10.1146/annurev-animal-022513-114113 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Davis T. A., Jaeger L. A., Johnson G. A., Kim S. W., Knabe D. A., Meininger C. J., Spencer T. E., and Yin Y. L.. 2007b. Important roles for the arginine family of amino acids in swine nutrition and production. Livest. Sci. 112:8–22. [Google Scholar]
- Wu G., Bazer F. W., Hu J., Johnson G. A., and Spencer T. E.. 2005. Polyamine synthesis from proline in the developing porcine placenta. Biol. Reprod. 72:842–850. doi: 10.1095/biolreprod.104.036293 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Johnson G. A., Burghardt R. C., Li X. L., Dai Z. L., Wang J. J., and Wu Z. L.. 2013a. Maternal and fetal amino acid metabolism in gestating sows. Soc. Reprod. Fertil. Suppl. 68:185–198. [Google Scholar]
- Wu G., Bazer F. W., Satterfield M. C., Li X., Wang X., Johnson G. A., Burghardt R. C., Dai Z., Wang J., and Wu Z.. 2013b. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256. doi: 10.1007/s00726-013-1515-z [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., and Tou W.. 1995. Developmental changes of free amino acid concentrations in fetal fluids of pigs. J. Nutr. 125:2859–2868. doi: 10.1093/jn/125.11.2859 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Tuo W., and Flynn S. P.. 1996a. Unusual abundance of arginine and ornithine in porcine allantoic fluid. Biol. Reprod. 54:1261–1265. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Wallace J. M., and Spencer T. E.. 2006. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J. Anim. Sci. 84:2316–2337. doi: 10.2527/jas.2006-156 [DOI] [PubMed] [Google Scholar]
- Wu G., Borbolla A. G., and Knabe D. A.. 1994a. The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J. Nutr. 124:2437–2444. doi: 10.1093/jn/124.12.437 [DOI] [PubMed] [Google Scholar]
- Wu G., Davis P. K., Flynn N. E., Knabe D. A., and Davidson J. T.. 1997. Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J. Nutr. 127:2342–2349. doi: 10.1093/jn/127.12.2342 [DOI] [PubMed] [Google Scholar]
- Wu Z., Hou Y., Hu S., Bazer F. W., Meininger C. J., McNeal C. J., and Wu G.. 2016. Catabolism and safety of supplemental L-arginine in animals. Amino Acids 48:1541–1552. doi: 10.1007/s00726-016-2245-9 [DOI] [PubMed] [Google Scholar]
- Wu G. and Knabe D. A.. 1994. Free and protein-bound amino acids in sow’s colostrum and milk. J. Nutr. 124:415–424. doi: 10.1093/jn/124.3.415 [DOI] [PubMed] [Google Scholar]
- Wu G. and Knabe D. A.. 1995. Arginine synthesis in enterocytes of neonatal pigs. Am. J. Physiol. 269(3 Pt 2):R621–R629. doi: 10.1152/ajpregu.1995.269.3.R621 [DOI] [PubMed] [Google Scholar]
- Wu G., Knabe D. A., and Flynn N. E.. 1994b. Synthesis of citrulline from glutamine in pig enterocytes. Biochem. J. 299 (Pt 1):115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Knabe D. A., Flynn N. E., Yan W., and Flynn S. P.. 1996b. Arginine degradation in developing porcine enterocytes. Am. J. Physiol. 271(5 Pt 1):G913–G919. doi: 10.1152/ajpgi.1996.271.5.G913 [DOI] [PubMed] [Google Scholar]
- Wu G., Knabe D. A., and Kim S. W.. 2004. Arginine nutrition in neonatal pigs. J. Nutr. 134(10 Suppl):2783S–2790S; discussion 2796S. doi: 10.1093/jn/134.10.2783S [DOI] [PubMed] [Google Scholar]
- Wu G. and Morris S. M. Jr. 1998. Arginine metabolism: nitric oxide and beyond. Biochem. J. 336 (Pt 1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Ott T. L., Knabe D. A., and Bazer F. W.. 1999. Amino acid composition of the fetal pig. J. Nutr. 129:1031–1038. doi: 10.1093/jn/129.5.1031 [DOI] [PubMed] [Google Scholar]
- Wu X., Ruan Z., Gao Y., Yin Y., Zhou X., Wang L., Geng M., Hou Y., and Wu G.. 2010b. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 39:831–839. doi: 10.1007/s00726-010-0538-y [DOI] [PubMed] [Google Scholar]
- Wu X., Yin Y.L., Liu Y. Q., Liua X. D., Liua Z. Q., Li T. J., Huanga R. L., Ruanb Z., Deng Z. Y.. 2012. Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows. Anim. Reprod. Sci. 132:187–192. doi:10.1016/j.anireprosci.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Yang X. F., Jiang Z. Y., Gong Y. L., Zheng C. T., Hu Y. J., Wang L., Huang L., and Ma X. Y.. 2016. Supplementation of pre-weaning diet with L-arginine has carry-over effect to improve intestinal development in young piglets. Can. J. Anim. Sci. 96:52–59. [Google Scholar]
- Zoran D. L. 2010. Obesity in dogs and cats: a metabolic and endocrine disorder. Vet. Clin. North Am. Small Anim. Pract. 40:221–239. doi: 10.1016/j.cvsm.2009.10.009 [DOI] [PubMed] [Google Scholar]