Abstract
The objective of this study was to evaluate the effects of dietary xylanase (X) and a carbohydrase enzyme blend (EB: cellulase, β-glucanase, and xylanase) on nutrient digestibility, intestinal barrier integrity, inflammatory status, and growth performance in weaned piglets fed higher fiber diets. A total of 460 pigs (6.43 ± 0.06 kg BW; F25 × 6.0 Genetiporc) were blocked by initial BW and pens (n = 12 per treatment) were randomly assigned to 1 of 4 dietary treatments. The diets included a higher fiber unsupplemented control diet (CON) and the CON supplemented with 0.01% X, 0.01% EB, or both enzymes, arranged in a 2 × 2 factorial. The diets were based on corn, soybean meal, corn distillers dried grains with solubles (DDGS), and wheat middlings. Pigs had 7 d to adapt to the environment and consumed the same commercial diet. Pigs were fed the experimental diets for 28 d with free access to feed and water. Body weight and feed disappearance were recorded weekly. One pig with BW closest to the pen average from each pen was selected and moved to metabolism crates on day 16 and intragastric gavaged a solution of lactulose and mannitol on day 22 followed by 12-h urine collection. Feces were collected from day 23 to 25. Intestinal tissues and mucosal scrapings were collected on day 28. Data were analyzed using PROC MIXED of SAS (9.4). Xylanase, EB, and their interaction were fixed effects and block was a random effect. The EB, but not X, increased pig BW and improved ADG over 28 d (P < 0.05). Neither carbohydrase impacted ADFI, G:F, or apparent total tract digestibility (ATTD) of DM, GE, or CP. The EB improved ATTD of ADF (32.45 vs. 26.57%; P < 0.01), but had no effect on NDF. Unexpectedly, X reduced ATTD of NDF and ADF (P < 0.01). The EB reduced urinary lactulose:mannitol and increased ileal claudin-3 mRNA abundance (P < 0.05), indicating improved small intestinal barrier integrity. There was a X × EB interaction on ileal secretory immunoglobulin A (sIgA) concentration (P < 0.05); in the absence of X, EB decreased sIgA compared to CON, but this effect disappeared in the presence of X. The EB also reduced ileal IL-22 mRNA abundance (P < 0.05), probably indicating decreased immune activation. In conclusion, EB but not X enhanced growth rate of weaned pigs fed higher fiber diets, which may be partly explained by the improved small intestinal barrier integrity and reduced immune activation, rather than improvement in nutrient digestibility.
Keywords: cellulase, β-glucanase, inflammatory status, intestinal barrier, swine, xylanase
INTRODUCTION
There is a growing interest in including more fibrous coproducts in swine diets due to their lower cost relative to corn and soybean meal as well as potential benefits in enhancing intestinal functions (e.g., improved intestinal morphology, barrier integrity, bacterial population, and microbial metabolites; Chen et al., 2015), thus reducing feed cost and improving animal production. Most coproducts in the United States, such as distillers dried grains with solubles (DDGS) and wheat middlings, originate from corn and wheat processing and contain high levels of insoluble fiber (Gutierrez et al., 2014). A high inclusion rate of these coproducts may decrease nutrient digestibility (Acosta et al., 2017), induce intestinal inflammation (Weber et al., 2008), and depress subsequent growth performance in nursery pigs (Tsai et al., 2017).
Exogenous carbohydrases can be used in nursery diets to mitigate the negative effects caused by high levels of fibrous coproducts and thus improve growth performance (Tsai et al., 2017). Studies have shown that dietary carbohydrase supplementation increased digestibility of nutrients and gross energy in pigs (Patience, 2017; Zeng et al., 2018). Previous research suggested that carbohydrases can degrade dietary fiber (nonstarch polysaccharides) and increase the release of low-molecular-weight oligosaccharides in pigs (Pedersen et al., 2015), which then can enhance gut barrier function and regulate inflammatory responses (Chen et al., 2012). Jiang et al. (2015) also showed that a combination of essential oil and carbohydrases down-regulated the gene abundance of the ileal proinflammatory cytokine IL-1α in weaned piglets. However, limited research has investigated the impact of dietary carbohydrases on intestinal barrier integrity and inflammation in weaning pigs fed higher fiber diets. Intestinal barrier integrity regulates permeability to luminal antigens and pathogens, which in turn modulates the immune status of animals (Hu et al., 2013). This modulation is assumed to regulate the partitioning of energy and nutrients between supporting animal growth and maintaining immune system function (Lochmiller and Deerenberg, 2000; Huntley et al., 2018).
Therefore, the objective of this study was to test our hypothesis that the addition of xylanase (X) and/or a carbohydrase enzyme blend (EB; cellulase, β-glucanase, and xylanase) would improve intestinal barrier integrity, down-regulate markers of inflammation, improve nutrient digestibility, and thereby enhance growth performance of weaned pigs fed higher fiber diets. A better understanding of how xylanase and a carbohydrase enzyme blend modulate gut barrier function and immune status will allow more appropriate and efficient use of these enzymes to improve feed utilization and production of nursery pigs.
MATERIALS AND METHODS
All procedures for this experiment adhered to guidelines for the ethical and humane care of animals used for research and were approved by the Institutional Animal Care and Use Committee at Iowa State University (IACUC #9-15-8097-S).
Animals and Experimental Design
A total of 460 newly weaned pigs (6.43 ± 0.06 kg BW; F25 × 6.0 Genetiporc; PIC Inc., Hendersonville, TN) were blocked by initial body weight and pens were randomly assigned to 1 of 4 dietary treatments. All pigs were vaccinated for porcine reproductive and respiratory syndrome and hemolytic Escherichia coli upon arrival at the Swine Nutrition Farm at Iowa State University. There were 12 blocks and 48 pens, with 12 pens per treatment and 9 or 10 pigs/pen. Each pen (1.2 × 2.4 m2) was equipped with a 4-space stainless steel dry feeder, 2 nipple drinkers, and a fully slatted floor. Sexes were not separated but there were equal number of barrows and gilts per treatment within each block.
Diets and Feeding
The 4 diets included an unsupplemented control diet (CON) and CON supplemented with either 0.01% of X, 0.01% of EB, or both carbohydrases (0.01% X + 0.01% EB) to determine if there would be any effects of X or EB, or of a combination of the two. One gram of X was expected to contain 15,000 EPU of xylanase. One gram of EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase. The enzyme inclusion rate was based on manufacturer’s recommendation (Huvepharma Inc., Peachtree City, GA). For the purposes of this experiment, the Phase 1 and Phase 2 basal diets were formulated to contain slightly elevated levels of fibrous ingredients compared to typical commercial diets; Phase 1, fed from day 0 to 14 of the experiment, contained 5% of each of reduced-oil corn DDGS and wheat middlings and Phase 2, fed from day 15 to 28 of the experiment, contained 10% of the same fibrous ingredients. After arrival at the farm and before the initiation of the experiment, pigs had a 7-d acclimation period to adjust to the environment during which they were fed a common commercial starter diet. Dietary treatments were arranged as a 2 × 2 factorial, with 2 inclusion levels of X (0 or 0.01%) or EB (0 or 0.01%). Diets were formulated to meet or exceed NRC (2012) nutrient requirements (Tables 1 and 2).
Table 1.
Ingredients and chemical composition of the basal diet (as-fed basis)1
| Item | Phase 1 | Phase 2 |
|---|---|---|
| Ingredients, % | ||
| Corn | 48.11 | 49.80 |
| Reduced-oil corn DDGS | 5.00 | 10.00 |
| Wheat middlings | 5.00 | 10.00 |
| Milk whey powder | 7.50 | - |
| Menhaden select fish meal | 5.80 | 2.00 |
| Hamlet protein, HP 3002 | 8.00 | 3.00 |
| Soybean meal, 47.7 | 17.50 | 22.00 |
| Soybean oil | 0.60 | 0.60 |
| Limestone | 0.58 | 1.05 |
| L-Lys HCl | 0.42 | 0.48 |
| DL-Met | 0.14 | 0.08 |
| L-Thr | 0.09 | 0.11 |
| L-Trp | 0.02 | 0.02 |
| Phytase3 | 0.0125 | 0.0125 |
| Vitamin premix4 | 0.25 | 0.25 |
| Trace mineral premix5 | 0.15 | 0.15 |
| Tiamulin6 | 0.18 | - |
| Chlortetracycline 507 | 0.40 | - |
| Carbadox8 | - | 0.20 |
| Salt | 0.25 | 0.25 |
| Calculated nutrient levels, % | ||
| ME, Mcal/kg | 3.35 | 3.29 |
| NE, Mcal/kg | 2.43 | 2.37 |
| CP | 23.86 | 22.49 |
| NDF | 9.58 | 13.33 |
| ADF | 3.73 | 4.99 |
| SID9 Lys | 1.45 | 1.31 |
| SID Met | 0.50 | 0.40 |
| SID Met + Cys | 0.80 | 0.69 |
| SID Thr | 0.85 | 0.77 |
| SID Trp | 0.26 | 0.23 |
| Total Ca | 0.74 | 0.63 |
| Total P | 0.67 | 0.57 |
| STTD P | 0.56 | 0.44 |
DDGS = distillers dried grains with soluble.
1Phase 1 = day 0–14; Phase 2 = day 14–28; 0.01% xylanase or a carbohydrase enzyme blend or both were mixed with premixes and then added to the basal diets; for use in the metabolism trial from day 16 to 25, 0.40% titanium dioxide as an indigestible marker was mixed with 99.60% of each of the Phase 2 diets.
2Enzymatically treated soybean meal, Hamlet Protein Inc., Findlay, OH.
3OptiPhos 4000 G, Huvepharma Inc., Peachtree City, GA; assumed to release 0.15% standardized total tract digestible (STTD) P in the diet based on manufacture’s recommendation.
4Provided 6,614 IU vitamin A, 827 IU vitamin D, 26 IU vitamin E, 2.6 mg vitamin K, 29.8 mg niacin, 16.5 mg pantothenic acid, 5.0 mg riboflavin, and 0.023 mg vitamin B12 per kg of diet.
5Provided 165 mg Zn (zinc sulfate), 165 mg Fe (iron sulfate), 39 mg Mn (manganese sulfate), 17 mg Cu (copper sulfate), 0.3 mg I (calcium iodate), and 0.3 mg Se (sodium selenite) per kg of diet.
6Denagard 10 (tiamulin, 22 g per kg), Elanco Animal Health, Greenfield, IN.
7Aureomycin 50 (chlortetracycline, 110 g per kg), Zoetis Inc., Kalamazoo, MI.
8Mecadox 2.5 (carbadox, 5.5 g per kg), Phibro Animal Health Corp., Ridgefield Park, NJ.
9SID = standardized ileal digestible.
Table 2.
Analyzed nutrient composition of Phase 2 diets1 (day 14–28; as-fed basis)
| Item | Control | X | EB | X+EB |
|---|---|---|---|---|
| DM, % | 90.15 | 90.74 | 90.68 | 90.31 |
| GE, Mcal/kg | 4.07 | 4.09 | 4.08 | 4.06 |
| CP, % | 21.88 | 22.06 | 22.17 | 22.09 |
| aEE2, % | 4.51 | 4.57 | 4.50 | 4.46 |
| NDF, % | 11.04 | 10.74 | 11.09 | 11.16 |
| ADF, % | 2.87 | 2.76 | 3.08 | 2.99 |
| TiO2, % | 0.37 | 0.40 | 0.37 | 0.38 |
1X: Diet with 0.01% xylanase; EB: Diet with 0.01% a carbohydrase enzyme blend; X+EB: Diet with both enzymes at 0.01%; for use in the metabolism trial from day 16–25, 0.40% titanium dioxide as an indigestible marker was mixed with 99.60% of each of the Phase 2 diets.
2Acid-hydrolyzed ether extract (aEE).
Sample Collection
Pigs were individually weighed on day −7, 0, 7, 14, 21, and 28 of the experiment, with day −7 indicating the day of arrival at Swine Nutrition Farm. Feed disappearance was recorded starting on day 0 to allow calculation of ADFI and G:F.
On day 16, 1 barrow close to the average BW within each pen was removed from the pen and transferred to an individual metabolism crate (0.5 × 0.7 m2) in order to collect urine and feces. Pigs had 5 d to adapt to the crates and were fed the same Phase 2 diet with the addition of 0.40% TiO2 as an indigestible marker. Titanium dioxide was mixed with 99.60% of each of the Phase 2 diets in a 200-kg feed mixer. On day 22, after an overnight fast, pigs were orally administrated a solution of lactulose (500 mg/kg BW) and mannitol (50 mg/kg BW) using a gastric tube followed by 12-h total urine collection for the assessment of in vivo small intestinal permeability. One milliliter of 20 g chlorhexidine/L was added to the urine bottles as a preservative. Thereafter, pigs were fed their respective diets and fecal samples were collected via grab sampling from day 23 to 25 and stored at −20 °C until analysis.
On day 28, 1 pig from each pen was euthanized by captive bolt stunning followed by exsanguination. Ileal tissues were collected 15 cm proximal to the ileal-cecal junction. Tissues were rinsed with ice-cold PBS to remove luminal contents, snap-frozen in liquid nitrogen, and kept at −80 °C for later RNA extraction. Mucosal scrapings from the ileum were collected, snap-frozen in liquid N, and stored at −80 °C until later analysis of secretory immunoglobulin A (sIgA).
Analytical Methods
Feces were thawed and homogenized, and then dried at 55 °C to a constant weight. Diets and feces were ground to 1 mm and analyzed in duplicate for DM (method 930.15; AOAC, 2007), acid-hydrolyzed ether extract (aEE; method 2003.06; AOAC, 2007), and nitrogen (N; method 990.03; AOAC, 2007; TruMac; LECO Corp., St. Joseph, MI) with EDTA (9.56% N; Leco Corporation) used as a standard for calibration and was determined to contain 9.56 ± 0.02% of N. Neutral and acid detergent fiber (NDF and ADF) were analyzed in triplicate (Van Soest and Robertson, 1979). Crude protein was calculated as N × 6.25. Gross energy was determined in duplicate using an isoperibolic bomb calorimeter (Parr 6200; Parr Instrument Co., Moline, IL). Benzoic acid (6,318 kcal GE/kg) was used as the standard for calibration and was determined to contain 6,315 ± 3 kcal GE/kg. Titanium dioxide was determined using a spectrophotometer (Synergy 4; BioTek, Winooski, VT) according to the method of Leone (1973).
Concentrations of lactulose and mannitol in urine samples were determined by HPLC following the procedure previously described by Kansagra et al. (2003). The lactulose:mannitol ratio was calculated based on a percent recovery basis and was considered as an index of small intestinal permeability (Wijtten et al., 2011).
Lactulose is a disacchride comprised of galactose and fructose that can be degraded by β-galactosidase, which could be present as a contaminant during commercial enzyme preparation. Thus, it was possible that the EB used in this study may have contained galactosidase, so an in vitro assay was performed to test if the EB could degrade lactulose. Approximately 0.1 g of lactulose standard (≥98.0%; Sigma-Aldrich, St. Louis, MO) was dissoved in 2-mL PBS (pH = 5) either without or with the EB (0.02 g) followed by incubation at 39 °C for 5 h at 150 rpm to mimic the intestinal digestion condition. Lactulose concentration was then determined using a commercially available Lactulose Assay kit (Sigma-Aldrich) according to the manufacturer’s instructions.
Mucosal scrapings (approximately 500 mg) were suspended in 5 mL PBS, homogenized using a homogenizer (PowerGen 700D; Thermo Fisher Scientific, Hanover Park, IL), and centrifuged at 10,000 g for 15 min at 4 °C. The supernatant was then collected for sIgA and total protein content quantification using a porcine-specific IgA ELISA kit (Bethyl Laboratories, Inc., Montgomery, TX) and a Pierce bicinchoninic acid (BCA) Protein Assay kit (Thermo Fisher Scientific, Woltham, MA), respectively. Mucosal sIgA concentration was expressed as milligrams per gram of protein.
RNA Isolation and Quantitative PCR
Ileal tissues (50 to 100 mg) were homogenized in 1 mL of Trizol (Invitrogen, Carlsbad, CA) using the Qiagen Tissuelyser II (Germantown, MD, USA). Total RNA was then isolated according to the manufacturer’s recommendations. The concentration of RNA was quantified using a spectrophotometer (ND-100; NanoDrop Technologies, Inc., Rockland, DE). All samples had 260:280 nm ratios above 1.8. One microgram of isolated RNA was used for cDNA synthesis using the QuantiTect Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. All cDNA samples were diluted 10-fold with nuclease-free water.
Real-time quantitative PCR was performed in 20 µL reactions using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA). The gene-specific primers, shown in Table 3, were diluted to 10 µM with nuclease-free water. Each reaction included 10 µL of SYBR Green Supermix, 1 µL of each forward and reverse primer, 2 µL of cDNA, and 6 µL of nuclease-free water. Each assay plate contained a no-reverse transcriptase negative control and a pooled cDNA reference sample. Each sample was assayed in triplicate. Fluorescence of SYBR Green was quantified with a Real Time PCR Detection System (iQ5; Bio-Rad Laboratories Inc.). The cycling conditions included 5-min initial denaturation at 95 °C followed by 40 PCR cycles (95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s) and a dissociation curve to verify the amplification of a single PCR product. Optical detection was performed at 55 °C. Analyses of amplification plots were performed with an Optical System Software version 2.0 (iQ5; Bio-Rad Laboratories Inc.) and cycle threshold (Ct) values for each reaction was obtained. Ribosomal protein - L19 (RPL19) was included as an endogenous reference gene. The mRNA abundance for each sample was normalized to RPL19 and the pooled sample, and fold change was calculated using the 2−ΔΔCTmethod (Livak and Schmittgen, 2001).
Table 3.
Primers used for real-time quantitative PCR
| Gene | Primer sequence, 5→3′ | Product size, base pair | Accession no. | |
|---|---|---|---|---|
| Forward | Reverse | |||
| IL-1β | TTGAATTCGAGTCTGCCCTGT | CCCAGGAAGACGGGCTTT | 76 | NM_214055 |
| IL-6 | GGCTGTGCAGATTAGTACC | CTGTGACTGCAGCTTATCC | 124 | AF518322 |
| IL-10 | TGGGTTGCCAAGCCTTGT | GCCTTCGGCATTACGTCTTC | 61 | L20001 |
| IL-17 | CCAGACGGCCCTCAGATTAC | CACTTGGCCTCCCAGATCAC | 103 | AB102693 |
| IL-22 | AAGCAGGTCCTGAACTTCAC | CACCCTTAATACGGCATTGG | 133 | AY937228 |
| Occludin | TCGTCCAACGGGAAAGTGAA | ATCAGTGGAAGTTCCTGAACCA | 95 | NM_001163647 |
| Claudin-3 | TTGCATCCGAGACCAGTCC | AGCTGGGGAGGGTGACA | 85 | NM_001160075 |
| ZO-11 | CTCTTGGCTTGCTATTCG | AGTCTTCCCTGCTCTTGC | 197 | XM_003353439 |
| RPL192 | AACTCCCGTCAGCAGATCC | AGTACCCTTCCGCTTACCG | 147 | AF435591 |
1ZO-1 = zonula occluden-1.
2RPL19 = ribosomal protein - L19.
Statistical Analysis
All data were analyzed in SAS 9.4 (SAS Institute Inc., Cary, NC). Data were analyzed as a 2 × 2 factorial in a randomized complete block design using PROC MIXED. The UNIVARIATE procedure was used to check normality and equal variance of residuals, and to identify statistical outliers (defined as values greater than 3 SD away from the mean). Xylanase, EB, and their interaction were fixed effects. Initial BW was used as a covariate. Block was considered a random effect. Pen was the experimental unit for performance data and individual pig was the experimental unit for other parameters. Performance data were analyzed as repeated measurements with week as the repeated effect and a variance structure of auto-regressive 1 (AR1) was applied. Means of the main effect within each week were separated using the least square means statement and the slice = week option. The interactions between X and EB within each week were calculated using the lsmestimate statement. Differences were considered significant if P ≤ 0.05 and a tendency if 0.05 < P ≤ 0.10.
RESULTS
There were no X × EB interactions on any performance parameters (Tables 4 and 5); therefore, all results are presented as main effects. No treatment × week interactions were observed. Xylanase had no impact on BW or ADG. Xylanase increased ADFI during day 22 to 28 and improved G:F during day 0 to 7 (P < 0.05). The addition of EB tended (P = 0.063) to improve BW on day 7 and improved (P < 0.05) BW on day 14 and day 28 compared to diets without EB. Consequently, pigs consumed diets with EB tended (P = 0.066) to have greater ADG during day 8 to 14 and had greater (P < 0.05) ADG during day 14 to 21. Over the 28-d experimental period, EB increased ADG (P < 0.05). Neither carbohydrase had an impact on ADFI or G:F across 4 wk.
Table 4.
Effect of xylanase (X) and a carbohydrase enzyme blend (EB) on BW of weaned pigs, kg1
| Day | X2 | EB3 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| - | + | - | + | X | EB | X × EB | ||
| 0 | 6.50 | 6.47 | 6.44 | 6.53 | 0.21 | 0.888 | 0.645 | 0.890 |
| 7 | 7.95 | 7.97 | 7.83 | 8.09 | 0.20 | 0.892 | 0.209 | 0.581 |
| 14 | 11.60 | 11.65 | 11.43 | 11.82 | 0.20 | 0.774 | 0.063 | 0.448 |
| 21 | 15.47 | 15.51 | 15.26 | 15.72 | 0.21 | 0.861 | 0.027 | 0.350 |
| 28 | 19.88 | 19.95 | 19.59 | 20.24 | 0.21 | 0.749 | 0.002 | 0.404 |
1 n = 24 pens per treatment (main effects); data were analyzed using repeated measurements; week was significant (P < 0.05), and no X × EB × Week interaction (P > 0.10).
2Xylanase activity was expected to be 15,000 EPU/g and inclusion rate was 0.01% according to the manufacturer’s recommendation.
3One gram EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of EB was 0.01% according to the manufacturer’s recommendation.
Table 5.
Effect of xylanase (X) and a carbohydrase enzyme blend (EB) on growth performance of weaned pigs1
| Item | X2 | EB3 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| - | + | - | + | X | EB | X × EB | ||
| ADG, kg | ||||||||
| Day 0–7 | 0.21 | 0.21 | 0.20 | 0.22 | 0.01 | 0.922 | 0.168 | 0.676 |
| Day 8–14 | 0.51 | 0.52 | 0.50 | 0.52 | 0.01 | 0.425 | 0.066 | 0.762 |
| Day 15–21 | 0.53 | 0.55 | 0.53 | 0.55 | 0.01 | 0.170 | 0.049 | 0.177 |
| Day 22–28 | 0.64 | 0.65 | 0.64 | 0.64 | 0.01 | 0.679 | 0.942 | 0.315 |
| Average | 0.47 | 0.48 | 0.47 | 0.48 | 0.01 | 0.237 | 0.026 | 0.926 |
| ADFI, kg | ||||||||
| Day 0–7 | 0.27 | 0.26 | 0.26 | 0.27 | 0.002 | 0.799 | 0.518 | 0.792 |
| Day 8–14 | 0.57 | 0.57 | 0.56 | 0.58 | 0.002 | 0.990 | 0.483 | 0.606 |
| Day 15–21 | 0.78 | 0.79 | 0.78 | 0.80 | 0.002 | 0.647 | 0.433 | 0.671 |
| Day 22–28 | 0.99 | 1.04 | 1.03 | 1.01 | 0.002 | 0.026 | 0.453 | 0.826 |
| Average | 0.65 | 0.67 | 0.66 | 0.66 | 0.001 | 0.388 | 0.647 | 0.741 |
| G:F | ||||||||
| Day 0–7 | 0.77 | 0.80 | 0.77 | 0.80 | 0.01 | 0.047 | 0.201 | 0.753 |
| Day 8–14 | 0.90 | 0.91 | 0.91 | 0.90 | 0.01 | 0.628 | 0.802 | 0.605 |
| Day 15–21 | 0.69 | 0.69 | 0.68 | 0.69 | 0.01 | 0.704 | 0.540 | 0.342 |
| Day 22–28 | 0.64 | 0.62 | 0.63 | 0.64 | 0.01 | 0.295 | 0.486 | 0.653 |
| Average | 0.75 | 0.76 | 0.75 | 0.76 | 0.01 | 0.317 | 0.184 | 0.698 |
1 n = 24 pens per treatment (main effect) for weekly data and 96 for 28-d average data; data were analyzed using repeated measurements of weeks; week was significant (P < 0.05), and no X × EB × Week interaction (P > 0.10).
2Xylanase activity was expected to be 15,000 EPU/g and the inclusion rate of X was 0.01% according to the manufacturer’s recommendation.
3One gram EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of EB was 0.01% according to the manufacturer’s recommendation.
No X × EB interactive effects were observed for apparent total tract digestibility (ATTD) of nutrients or energy (Table 6); therefore, all results are presented as main effects. Neither enzyme impacted ATTD of DM, GE, or CP. There was a trend for decreased aEE digestibility in X-supplemented diets (P = 0.073). Supplementation of EB increased the ATTD of ADF by 22% (P < 0.05), but did not impact NDF or hemicellulose. Unexpectedly, X addition decreased the ATTD of NDF, ADF, and hemicellulose (P < 0.05).
Table 6.
Effect of xylanase (X) and a carbohydrase enzyme blend (EB) on apparent total tract digestibility of dietary constituents, %1,2
| Item | X3 | EB4 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| - | + | - | + | X | EB | X × EB | ||
| DM | 83.79 | 83.59 | 83.45 | 83.93 | 0.28 | 0.600 | 0.225 | 0.966 |
| GE | 82.30 | 82.11 | 82.04 | 82.37 | 0.33 | 0.700 | 0.488 | 0.965 |
| CP | 83.66 | 83.94 | 83.21 | 84.39 | 0.56 | 0.726 | 0.151 | 0.705 |
| aEE5 | 62.82 | 61.05 | 61.78 | 62.10 | 0.68 | 0.073 | 0.737 | 0.285 |
| NDF | 48.95 | 46.10 | 47.11 | 47.94 | 0.65 | 0.004 | 0.375 | 0.535 |
| ADF | 31.71 | 27.30 | 26.57 | 32.45 | 1.09 | 0.008 | 0.001 | 0.824 |
| Hemicellulose | 55.23 | 52.77 | 54.26 | 53.74 | 0.53 | 0.003 | 0.494 | 0.273 |
1Fecal samples were collected on day 23–25 and stored at −20 °C until analysis; pigs were housed in metabolism crates and had free access to feed and water.
2 n = 24 pigs per treatment (main effects).
3Xylanase activity was expected to be 15,000 EPU/g and the inclusion rate of X was 0.01% according to the manufacturer’s recommendation.
4One gram EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of EB was 0.01% according to the manufacturer’s recommendation.
5Acid-hydrolyzed ether extract (aEE).
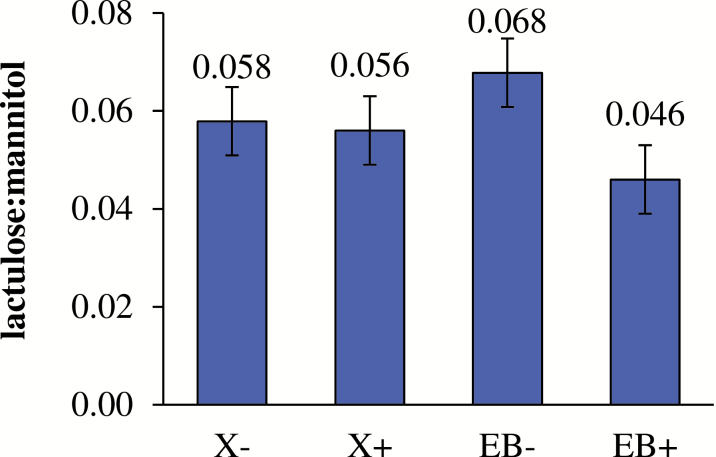
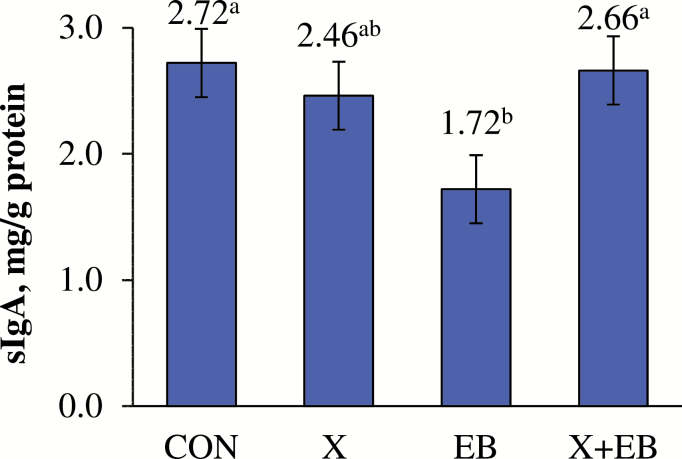
The lactulose concentration in lactulose standard samples without or with EB was not different (312.8 vs. 320.2 pmol/μL, respectively). The addition of EB decreased urinary lactulose:mannitol on a percent recovery basis (P < 0.01; Fig. 1). No effects of X or an interaction of X × EB were observed for lactulose:mannitol. There was an interaction of X × EB on ileal sIgA concentration (P < 0.05; Fig. 2); in the absence of X, EB decreased sIgA compared to the control, but this effect disappeared in the presence of X.
Figure 1.
Main effect of xylanase (X) and a carbohydrase enzyme blend (EB) on urinary lactulose:mannitol based on percent recovery basis; X: P = 0.788; EB: P = 0.003; X × EB: P = 0.191; n = 24 per treatment (main effects).
Figure 2.
Interaction effect of xylanase (X) and a carbohydrase enzyme blend (EB) on ileal mucosal secretory immunoglobulin A (sIgA) concentration; X+EB: diets with both X and EB; a,bMeans without a common superscript differ (P < 0.05); n = 12 per treatment.
There were no effects of X × EB interaction or X addition on ileal transcript abundance of cytokine and tight junction protein genes (Table 7). Similarly, EB did not alter the mRNA abundance of IL-1β, IL-6, IL-10, IL-17, or occludin. However, EB increased mRNA abundance of claudin-3 and decreased mRNA abundance of IL-22 compared with no EB treatment (P < 0.05). Pigs fed EB-supplemented diets tended to have greater mRNA abundance of zonula occluden-1 (ZO-1) than that fed diets without EB (P = 0.067).
Table 7.
Effect of xylanase (X) and a carbohydrase enzyme blend (EB) on mRNA abundance of cytokines and tight junction proteins in the ileum1,2
| Gene | X3 | EB4 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| - | + | - | + | X | EB | X × EB | ||
| IL-1ß | 1.00 | 0.96 | 1.09 | 0.87 | 0.12 | 0.141 | 0.527 | 0.453 |
| IL-6 | 0.48 | 0.58 | 0.63 | 0.44 | 0.11 | 0.230 | 0.116 | 0.605 |
| IL-10 | 0.79 | 0.79 | 0.78 | 0.79 | 0.13 | 0.628 | 0.882 | 0.388 |
| IL-17 | 0.76 | 0.83 | 0.91 | 0.68 | 0.16 | 0.720 | 0.176 | 0.723 |
| IL-22 | 0.73 | 0.57 | 0.84 | 0.46 | 0.13 | 0.363 | 0.037 | 0.742 |
| Occludin | 1.50 | 1.21 | 1.21 | 1.50 | 0.27 | 0.684 | 0.226 | 0.225 |
| Claudin-3 | 1.39 | 1.16 | 1.05 | 1.50 | 0.22 | 0.573 | 0.007 | 0.260 |
| ZO-15 | 0.25 | 0.24 | 0.21 | 0.29 | 0.05 | 0.281 | 0.067 | 0.803 |
1Ileal tissue samples were collected on day 28.
2 n = 24 per treatment.
3Xylanase activity was expected to be 15,000 EPU/g and the inclusion rate of X was 0.01% according to the manufacturer’s recommendation.
4One gram EB was expected to contain 7,000 CU of cellulase, 5,000 U of β-glucanase, and 1,000 EPU of xylanase; the inclusion rate of EB was 0.01% according to the manufacturer’s recommendation.
5ZO-1 = zonula occluden-1.
DISCUSSION
Due to the growing interest by the swine industry in feeding fibrous coproducts, the use of exogenous carbohydrases has received increasing interest. The objective of most of these studies has been to improve the nutritional value of higher fiber diets for pork production (Nortey et al., 2008; Passos et al., 2015). Because older pigs generally have greater digestive capacity to utilize dietary fiber than young piglets, most research has focused on evaluating the effects of carbohydrases in growing or finishing pigs fed diets high in coproducts (Jacela et al., 2010; Kiarie et al., 2012; Ndou et al., 2015).
This experiment evaluated the impact of dietary xylanase and/or a carbohydrase enzyme blend in weaned pigs fed higher fiber diets; however, the fiber levels were not extreme. All diets contained 500 phytase units (FTU) per kg diet and 0.15% standardized total tract digestible (STTD) P was assumed to be released by the phytase according to the manufacture’s recommendation. The results showed that neither X nor EB impacted ADFI and G:F over 28-d period, which was in agreement with Olukosi et al. (2007), Jang et al. (2017), and Tsai et al. (2017). The addition of EB had no impact ADG during day 0 to 7 and a tendency to improve ADG during day 8 to 14, and significantly increased ADG during day 15 to 21 and over the 28-d trial, indicating EB-supplemented diets need to be fed for at least 3 wk to show positive effects on growth rate in nusery pigs. But X had no impact on any BW or ADG. It appears that a combination of multiple enzymes (e.g., EB containing cellulase, β-glucanase, and xylanase) is more effective than individual enzymes (e.g., X) to improve growth performance of nursery pigs. This is supported by Tsai et al. (2017), who reported that adding both xylanase and β-glucanase into nursery diets with 30% corn DDGS improved ADG from day 7 to 35, but not when the enzyme was individually added.
However, it is worth noting that improved growth rate by adding exogenous carbohydrases in pigs has been inconsistent. For example, Jones et al. (2010) did not detect beneficial effects of an enzyme blend on growth performance in nursery pigs fed diets containing 30% DDGS. Interestingly, Bloxham et al. (2018) recently reported that a combination of xylanase and β-glucanase resulted in poorer growth performance in nursery pigs, despite improvements in nutrient digestibility. Multiple factors could result in the inconsistent impact on growth performance, such as health status and age of pigs, sources and inclusion levels of fibrous ingredients, efficacy of supplemented enzymes, feed processing, and the housing environment.
In this experiment, neither X nor EB improved the ATTD of DM, GE, CP, or aEE, indicating the improved growth rate from adding EB was not likely due to improvement in nutrient digestibility. However, because the apparent ileal digestibility (AID) of nutrients was not measured, it is unknown whether the lack of difference in ATTD of nutrients was a result of limited carbohydrase efficacy in breaking down the cell wall components or increased bacterial fermentation of undigested nutrients in the large intestine of pigs fed a diet without supplemented carbohydrase. Zeng et al. (2018) recently reported that addition of an enzyme blend (galactanase, xylanase, mannanase, α-amylase, and cellulase) improved AID, but not ATTD, of energy, NDF, and nonstarch polysaccharides (NSP) constituents in wheat bran containing diets. This indicates that enzyme supplementation may shift fiber degradation from the hindgut to the small intestine, which is supported by Ndou et al. (2015) and Moran et al. (2016). Energy absorbed in the small intestine will be used by the pig with greater efficiency than if it is released by fermentation in the lower gut (Patience, 2017). Another possibility could be that pigs adapt to fibrous diets with time and increase their capacity to digest or ferment fiber (Tsai et al., 2017; Bloxham et al., 2018). Unexpectedly, xylanase addition reduced the ATTD of NDF and ADF, which contradicted the unchanged or improved fiber or NSP digestibility reported in most other studies. A decrease in AID of fiber in pigs fed xylanase-supplemented diets was also reported by Weiland (2017). The reason for this observation is unclear, but may likely be attributed to the contamination of endogenous materials in the feces, such as mucin and bacteria, in the fiber analysis process, leading to underestimated fiber digestibility (Montoya et al., 2016).
Because EB had no impact on the ATTD of nutrients except for ADF, it is possible that it improved BW gain through other mechanisms such as improving intestinal barrier function. The intestinal barrier is composed primarily of epithelial cells and a mucous layer covering the epithelium (France and Turner, 2017). Tight junctions “seal” the paracellular space between neighboring epithelial cells; thus, they are a major regulator of paracellular permeability (Günzel and Yu, 2013). Impaired barrier integrity could result in increased permeability to luminal antigens and pathogens, which in turn induces immune activation (Hu et al., 2013). As such, energy and nutrients will be diverted away from growth to support immune defense processes, leading to reduced growth performance (Elsasser et al., 2008; Huntley et al., 2018).
The use of lactulose and mannitol as marker probes to assess small intestinal barrier integrity is considered a reliable and noninvasive in vivo method (Barboza Jr et al., 1999). To our knowledge, there is no previously published information about the impact of carbohydrases on in vivo small intestinal permeability using lactulose and mannitol. It is known that lactulose can only traverse the intestinal wall by paracellular pathways, whereas mannitol passes by both paracellular and transcellular routes (Wijtten et al., 2011). The EB did not degrade lactulose in vitro; therefore, a lower urinary lactulose:mannitol ratio in pigs fed diets with EB in the current study indicates reduced paracellular permeability and improved barrier integrity. Decreased urinary lactulose:mannitol was likely associated with the increased mRNA abundance of claudin-3 in the ileum, an important tight junction protein. The observed association between increased expression of tight junction proteins and reduced intestinal permeability (decreased urinary lactulose:mannitol) in weaning pig was also reported in a dietary Zn supplementation study (Zhang and Guo, 2009). The reason for enhanced barrier integrity in EB-supplemented diets is unclear. It may be due to modulation of intestinal microbiota and microbial metabolites (e.g., VFA) by low-molecular-weight oligosaccharides released from fiber degradation in the small intestine (Van Craeyveld et al., 2008; Pedersen et al., 2015). The exact mechanisms need to be explored in future studies.
To further assess ileal function and immune defense, sIgA was measured. Secretory IgA, along with mucus, is considered to be a first line of defense against adhesion and invasion of microorganisms in the ileal mucosa (Pabst, 2012). Lower levels of ileal sIgA were observed in pigs fed diets with EB alone, but not in combination with X, compared to the control. This may be related with the improved gut barrier function in the small intestine, which likely resulted in reduced access of microbiota to ileal lamina propria, and hence reduced stimulation of IgA production (Lewis et al., 2013). In piglets, Xiong et al. (2015) reported that supplementing a low dosage of chito-oligosaccharide led to an increase in intestinal mucosal IL-10 and sIgA, suggesting immune activation resulted in modulation of cytokines and antibodies. In the same study, compromised intestinal barrier integrity was also observed. Thus, decreased sIgA levels in the current study are suggestive of decreased immune activation and association with increased barrier integrity.
An activated immune system has higher priority over growth production functions and is expensive in terms of energy and nutrients (Lochmiller and Deerenberg, 2000; Huntley et al., 2018). While it has been shown that dietary fiber can modulate immune status of pigs likely through intestinal microbes and microbial metabolites (Weber et al., 2008; Chen et al., 2013), whether supplementing carbohydrases can alter the immune modulation indirectly triggered by dietary fiber is less known. Therefore, investigating the effects of dietary carbohydrases on intestinal immune status is of importance in understanding the mechanisms whereby carbohydrases impact growth performance in nursery pigs as it relates to immune activation. In this study, the lack of carbohydrase effects on intestinal mRNA abundance of most cytokine genes evaluated was probably attributed to the fact that pigs were not clinically affected by pathogenic or immunogenic agents; thus, the intestinal immune system was not overly activated. However, these data suggest that changes from dietary EB were not overtly inflammatory. Interlukin-22 is a cytokine produced mainly by T-helper 17 (Th17) cells, γδ T cells, as well as type 3 innate lymphoid cells (ILC), and plays an important role in orchestrating antibacterial immunity and host defense in the intestine (Parks et al., 2016). Its primary function is to promote epithelial barrier functions and restore intestinal homeostasis after infection; however, not all the roles of IL-22 in intestinal homeostasis are completely understood (reviewed in Parks et al., 2016). The decrease in ileal IL-22 mRNA levels in EB-supplemented group may suggest a reduction in activated host defense mechanisms that was associated with reduced intestinal permeability (i.e., improved barrier integrity). It is unclear if the change in IL-22 expression was due to differences in abundance of immune cell populations (e.g., ILC; Sonnenberg et al., 2012) or changes in bacterial recognition due to microbiota changes or decreased intestinal permeability. Collectively, enhanced barrier integrity was associated with markers of reduced immune activation (sIgA and IL-22) and this may be an additional mechanism through which EB spares energy for growth in weaned pigs, contributing to improved BW gain in the current study.
In conclusion, the addition of EB, but not X, increased weaned pig 28 d growth rate, suggesting a combination of multiple carbohydrases is more effective than individual carbohydrase. This improvement was not due to increased feed intake, feed efficiency, or improved nutrient digestibility. This EB improved markers of small intestinal integrity (i.e., decreased urinary lactulose:mannitol and up-regulated claudin-3 mRNA abundance). The supplementation of EB individually, but not in combination with X, decreased ileal sIgA compared to the control. Moreover, pigs fed diets with the EB had lower mRNA abundance of IL-22 compared to diets without EB. The improved intestinal barrier integrity and reduced markers of immune activation may contribute to the improved growth rate in weaned pigs fed higher fiber diets supplemented with the EB. This study provides evidence of one possible mode of action of a carbohydrase enzyme blend in improving performance in weaned pigs. Further studies need to explore the mechanisms by which EB improves intestinal barrier function and modulates the immune status of pigs.
Conflict of interest statement. None declared.
Footnotes
The authors would like to thank Huvepharma Inc. for financial support of this research. Appreciation is also expressed to Ajinomoto Heartland, DSM Nutritional Products, and Hamlet Protein for in-kind support.
LITERATURE CITED
- Acosta J. A., Boyd R. D., and Patience J. F.. 2017. Digestion and nitrogen balance using swine diets containing increasing proportions of coproduct ingredients and formulated using the net energy system. J. Anim. Sci. 95:1243–1252. doi: 10.2527/jas.2016.1161 [DOI] [PubMed] [Google Scholar]
- AOAC. 2007. Official methods of analysis. 18th rev. ed. AOAC Int, Gaithersburg, MD. [Google Scholar]
- Barboza M. S. Jr, Silva T. M., Guerrant R. L., and Lima A. A.. 1999. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz. J. Med. Biol. Res. 32:1499–1504. doi: 10.1590/S0100-879X1999001200008 [DOI] [PubMed] [Google Scholar]
- Bloxham D., Dove C., and Azain M.. 2018. Effect of wheat as a feedstuff in starter diets on nursery pig growth performance and digestibility. Livest. Sci. 207:98–104. doi: 10.1016/j.livsci.2017.11.016 [DOI] [Google Scholar]
- Chen H. H., Chen Y. K., Chang H. C., and Lin S. Y.. 2012. Immunomodulatory effects of xylooligosaccharides. Food Sci. Technol. Res. 18:195–199. doi: 10.3136/fstr.18.195 [DOI] [Google Scholar]
- Chen H., Mao X., He J., Yu B., Huang Z., Yu J., Zheng P., and Chen D.. 2013. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 110:1837–1848. doi: 10.1017/S0007114513001293 [DOI] [PubMed] [Google Scholar]
- Chen H., Wang W., Degroote J., Possemiers S., Chen D., De Smet S., and Michiels J.. 2015. Arabinoxylan in wheat is more responsible than cellulose for promoting intestinal barrier function in weaned male piglets. J. Nutr. 145:51–58. doi: 10.3945/jn.114.201772 [DOI] [PubMed] [Google Scholar]
- Elsasser T. H., Caperna T. J., Li C. J., Kahl S., and Sartin J. L.. 2008. Critical control points in the impact of the proinflammatory immune response on growth and metabolism. J. Anim. Sci. 86(14 Suppl):E105–E125. doi: 10.2527/jas.2007-0634 [DOI] [PubMed] [Google Scholar]
- France M. M. and Turner J. R.. 2017. The mucosal barrier at a glance. J. Cell Sci. 130:307–314. doi: 10.1242/jcs.193482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzel D. and Yu A. S.. 2013. Claudins and the modulation of tight junction permeability. Physiol. Rev. 93:525–569. doi: 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez N., Serão N., Kerr B., Zijlstra R., and Patience J.. 2014. Relationships among dietary fiber components and the digestibility of energy, dietary fiber, and amino acids and energy content of nine corn coproducts fed to growing pigs. J. Anim. Sci. 92:4505–4517. doi: 10.2527/jas.2013-7265 [DOI] [PubMed] [Google Scholar]
- Hu C. H., Xiao K., Luan Z. S., and Song J.. 2013. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91:1094–1101. doi: 10.2527/jas.2012-5796 [DOI] [PubMed] [Google Scholar]
- Huntley N. F., Nyachoti C. M., and Patience J. F.. 2018. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 9:47. doi: 10.1186/s40104-018-0264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacela J., Dritz S., DeRouchey J., Tokach M., Goodband R., and Nelssen J.. 2010. Effects of supplemental enzymes in diets containing distillers dried grains with solubles on finishing pig growth performance. Prof. Anim. Sci. 26:412–424. doi: 10.15232/S1080-7446(15)30623-9 [DOI] [Google Scholar]
- Jang Y., Wilcock P., Boyd R., and Lindemann M.. 2017. Effect of combined xylanase and phytase on growth performance, apparent total tract digestibility, and carcass characteristics in growing pigs fed corn-based diets containing high-fiber coproducts. J. Anim. Sci. 95:4005–4017. doi: 10.2527/jas2017.1781 [DOI] [PubMed] [Google Scholar]
- Jiang X. R., Awati A., Agazzi A., Vitari F., Ferrari A., Bento H., Crestani M., Domeneghini C., and Bontempo V.. 2015. Effects of a blend of essential oils and an enzyme combination on nutrient digestibility, ileum histology and expression of inflammatory mediators in weaned piglets. Animal 9:417–426. doi: 10.1017/S1751731114002444 [DOI] [PubMed] [Google Scholar]
- Jones C., Bergstrom J., Tokach M., DeRouchey J., Goodband R., Nelssen J., and Dritz S.. 2010. Efficacy of commercial enzymes in diets containing various concentrations and sources of dried distillers grains with solubles for nursery pigs. J. Anim. Sci. 88:2084–2091. doi: 10.2527/jas.2009-2109 [DOI] [PubMed] [Google Scholar]
- Kansagra K., Stoll B., Rognerud C., Niinikoski H., Ou C. N., Harvey R., and Burrin D.. 2003. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G1162–G1170. doi: 10.1152/ajpgi.00243.2003 [DOI] [PubMed] [Google Scholar]
- Kiarie E., Owusu-Asiedu A., Peron A., Simmins P., and Nyachoti C.. 2012. Efficacy of xylanase and β-glucanase blend in mixed grains and grain co-products-based diets for fattening pigs. Livest. Sci. 148:129–133. doi: 10.1016/j.livsci.2012.05.020 [DOI] [Google Scholar]
- Leone J. L. 1973. Collaborative study of the quantitative determination of titanium dioxide in cheese. J. Assoc. Off. Anal. Chem. 56:535–537. [PubMed] [Google Scholar]
- Lewis M. C., Patel D. V., Fowler J., Duncker S., Zuercher A. W., Mercenier A., and Bailey M.. 2013. Dietary supplementation with Bifidobacterium lactis NCC2818 from weaning reduces local immunoglobulin production in lymphoid-associated tissues but increases systemic antibodies in healthy neonates. Br. J. Nutr. 110:1243–1252. doi: 10.1017/S0007114513000251 [DOI] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lochmiller R. L., and Deerenberg C.. 2000. Trade‐offs in evolutionary immunology: Just what is the cost of immunity?Oikos. 88:87–98. doi: 10.1034/j.1600-0706.2000.880110.x [DOI] [Google Scholar]
- Montoya C. A., Henare S. J., Rutherfurd S. M., and Moughan P. J.. 2016. Potential misinterpretation of the nutritional value of dietary fiber: Correcting fiber digestibility values for nondietary gut-interfering material. Nutr. Rev. 74:517–533. doi: 10.1093/nutrit/nuw014 [DOI] [PubMed] [Google Scholar]
- Moran K., de Lange C. F., Ferket P., Fellner V., Wilcock P., and van Heugten E.. 2016. Enzyme supplementation to improve the nutritional value of fibrous feed ingredients in swine diets fed in dry or liquid form. J. Anim. Sci. 94:1031–1040. doi: 10.2527/jas.2015-9855 [DOI] [PubMed] [Google Scholar]
- Ndou S., Kiarie E., Agyekum A., Heo J., Romero L., Arent S., Lorentsen R., and Nyachoti C.. 2015. Comparative efficacy of xylanases on growth performance and digestibility in growing pigs fed wheat and wheat bran-or corn and corn DDGS-based diets supplemented with phytase. Anim. Feed Sci. Technol. 209:230–239. doi: 10.1016/j.anifeedsci.2015.08.011 [DOI] [Google Scholar]
- Nortey T. N., Patience J. F., Sands J. S., Trottier N. L., and Zijlstra R. T.. 2008. Effects of xylanase supplementation on the apparent digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs. J. Anim. Sci. 86:3450–3464. doi: 10.2527/jas.2007-0472 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Olukosi O. A., Sands J. S., and Adeola O.. 2007. Supplementation of carbohydrases or phytase individually or in combination to diets for weanling and growing-finishing pigs. J. Anim. Sci. 85:1702–1711. doi: 10.2527/jas.2006-709 [DOI] [PubMed] [Google Scholar]
- Pabst O. 2012. New concepts in the generation and functions of iga. Nat. Rev. Immunol. 12:821–832. doi: 10.1038/nri3322 [DOI] [PubMed] [Google Scholar]
- Parks O. B., Pociask D. A., Hodzic Z., Kolls J. K., and Good M.. 2016. Interleukin-22 signaling in the regulation of intestinal health and disease. Front. Cell Dev. Biol. 3:85. doi: 10.3389/fcell.2015.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos A. A., Park I., Ferket P., von Heimendahl E., and Kim S. W.. 2015. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim. Nutr. 1:19–23. doi: 10.1016/j.aninu.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patience J. F. 2017. Meeting energy requirements in pig nutrition. In: Wiseman J. editor, Achieving sustainable production of pig meat. Burleigh Dodds Science Publ, Cambridge, UK: p. 127–143. doi: 10.19103/AS,2017.0013.07 [DOI] [Google Scholar]
- Pedersen M. B., Yu S., Arent S., Dalsgaard S., Bach Knudsen K. E., and Lærke H. N.. 2015. Xylanase increased the ileal digestibility of nonstarch polysaccharides and concentration of low molecular weight nondigestible carbohydrates in pigs fed high levels of wheat distillers dried grains with solubles. J. Anim. Sci. 93:2885–2893. doi: 10.2527/jas.2014-8829 [DOI] [PubMed] [Google Scholar]
- Sonnenberg G. F., Monticelli L. A., Alenghat T., Fung T. C., Hutnick N. A., Kunisawa J., Shibata N., Grunberg S., Sinha R., Zahm A. M., et al. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336:1321–1325. doi: 10.1126/science.1222551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T., Dove C., Cline P., Owusu-Asiedu A., Walsh M., and Azain M.. 2017. The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livest. Sci. 197:46–52. doi: 10.1016/j.livsci.2017.01.008 [DOI] [Google Scholar]
- Van Craeyveld V., Swennen K., Dornez E., Van de Wiele T., Marzorati M., Verstraete W., Delaedt Y., Onagbesan O., Decuypere E., Buyse J., et al. 2008. Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J. Nutr. 138:2348–2355. doi: 10.3945/jn.108.094367 [DOI] [PubMed] [Google Scholar]
- Van Soest P., and Robertson J.. 1979. Systems of analysis for evaluating fibrous feeds Standardization of analytical methodology for feeds: Proceedings. IDRC, Ottawa, ON, Canada. [Google Scholar]
- Weber T. E., Ziemer C. J., and Kerr B. J.. 2008. Effects of adding fibrous feedstuffs to the diet of young pigs on growth performance, intestinal cytokines, and circulating acute-phase proteins. J. Anim. Sci. 86:871–881. doi: 10.2527/jas.2007-0330 [DOI] [PubMed] [Google Scholar]
- Weiland S. A. 2017. The impact of xylanase and body weight, and their interaction, on the utilization of dietary components in swine. Master Thesis, Iowa State University, Ames, IA. [Google Scholar]
- Wijtten P. J., van der Meulen J., and Verstegen M. W.. 2011. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 105:967–981. doi: 10.1017/S0007114510005660 [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H. S., Wang X. C., Hu Q., Liu C. X., Wu X., Deng D., Hou Y. Q., Nyachoti C. M., Xiao D. F., et al. 2015. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 93:1089–1097. doi: 10.2527/jas.2014-7851 [DOI] [PubMed] [Google Scholar]
- Zeng Z., Li Q., Tian Q., Xu Y., and Piao X.. 2018. The combination of carbohydrases and phytase to improve nutritional value and non-starch polysaccharides degradation for growing pigs fed diets with or without wheat bran. Anim. Feed Sci. Technol. 235:138–146. doi: 10.1016/j.anifeedsci.2017.11.009 [DOI] [Google Scholar]
- Zhang B. and Guo Y.. 2009. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 102:687–693. doi: 10.1017/S0007114509289033 [DOI] [PubMed] [Google Scholar]