Abstract
Fermentation has attracted increasing attention in pig industry, because of low costs and numerous benefits on pig growth and health as well as environmental improvement, although the mechanisms remain largely unknown. In the present study, fermented corn-soybean meal significantly improved average daily gain and gain:food ratio (P < 0.05). Fermented feed (FF) significantly increased insulin-like growth factor 1 (IGF1) transcription in liver (P < 0.05). Meanwhile, fermented meal significantly enhanced the binding of CCAAT/enhancer-binding protein beta (C/EBPβ) to IGF1 promoter and C/EBPβ expression in liver (both P < 0.05). FF tended to increase IGF1 proteins in liver and serum too (both 0.05 < P < 0.10). Meanwhile, FF slightly but significantly increased hepatic and circulating triglyceride and total cholesterol levels, as well as serum ratio of high-density to low-density cholesterol (all P < 0.05). Our data indicated that FF could significantly augment the binding of C/EBPβ to IGF1 promoter and promote hepatic IGF1 expression and production, thus boost pig growth.
Keywords: fermented corn-soybean meal, insulin-like growth factor 1, liver, pig
INTRODUCTION
Recently, solid-state fermented corn-soybean meal is increasingly used in pig farms for its numerous benefits. Fermentation dramatically degrades the antinutritional factors in soybean meal (Mukherjee et al., 2016) and increases amino acid and phosphorus digestibility (Shi et al., 2017); thus, nitrogen and phosphorus emissions were accordingly decreased (Cheng et al., 2017). More importantly, fermented soybean meal has been reported to improve the growth performance of nursery (Cheng et al., 2017) and weaned piglets (Zhu et al., 2017). Furthermore, supplementing fermented corn-soybean feed in sow diets was reported to enhance the growth performance of their progeny (Wang et al., 2018a). So fermented feed (FF) might be a novel and attractive alternative to antibiotic growth promoters in pig industry. People have noticed the beneficial effects of FF on swine gut microbiota, probably due to the beneficial effects of both pre- and probiotics (reviewed in Wang et al. (2018b)); however, more and detailed mechanism how FF boosts pig growth still remains largely unknown.
Insulin-like growth factor 1 (IGF1) is a well-known endocrine growth factor. IGF1 is primarily produced in the liver, as a critical mediator of the growth hormone (GH) pulse which is originally derived from anterior pituitary gland. IGF1 acts on almost every cell type in the body during the processes controlling tissue growth and reconstruction as well as carbohydrate and lipid metabolism (Álvarez-Nava and Lanes, 2017). The canonical GH-IGF1 axis is highly conserved in mammals (Berryman et al., 2008). Meanwhile, expression of IGF1 in liver is also sensitive to dietary nutrients (Frieten et al., 2018), toxins (Li et al., 2017), and even maternal stimuli (Meyer et al., 2017; Tuersunjiang et al., 2017). The current study was conducted to investigate whether IGF1 mediates the growth-promoting effects of fermented meal in the grower-finisher pigs.
MATERIALS AND METHODS
Ethical Approval
The experiment was approved by the Institutional Animal Ethical Committee, College of Animal Sciences & Technologies, Northwest A&F University.
Animals and Diets
This experiment was performed at Yuanheng Ecological Agriculture Co., Ltd (Tongchuan, Shaanxi, China). A total of 48 crossbred castrated boars with similar body weights were randomly allotted into 2 groups (6 pigs per pen with 4 pens per group). Pens had a drybox feeder and a nipple drinker. Body weights and food intake were recorded weekly. Pigs had free access to feed and clean water throughout the whole experiment. Pigs in Ctrl group were fed with commercial corn-soybean meal (Beijing Great North Agricultural Technology Group Co. Ltd., Beijing, China), and the others in FF group were fed with the same meal fermented with EM probiotics (a mixture containing 60% Lactobacillus, 20% Clostridium, 8% Bifidobacteria, and others, purchased from Nongfukang Co., Ltd., Zhengzhou, China). Briefly, 1 kg of EM probiotics was diluted 1:40 (wt/vol) with sterile water. The commercial corn-soybean meal was mixed with probiotics (moisture content was about 35%) and incubated at 30 to 35 °C for 48 h as per the manufacturer’s instructions. Unfermented and fermented feeds were analyzed for dry matter (DM), gross energy (GE), crude protein (CP), crude fat (CF), and amino acids profile using the AOAC International guidelines (2005). The contents of total polyphenol were measured by Folin-Ciocalteu assay, and total flavone was determined by spectrophotometric colorimetry (Rutin as the standard control) (Table 1).
Table 1.
Ingredients and chemical composition of experimental diets
| Items | Normal meal | Fermented meal |
|---|---|---|
| Ingredients, % | ||
| Corn | 67.0 | 67.0 |
| Soybean meal | 21.0 | 21.0 |
| Wheat bran | 8 | 8 |
| Mineral/vitamin premix1 | 4.0 | 4.0 |
| Analyzed chemical composition | ||
| DM, % | 88.04 | 63.02 |
| Gross energy, kcal∙kg−1 DM | 12.48 | 12.43 |
| Crude protein, %DM | 16.14 | 16.12 |
| Crude fat, %DM | 2.31 | 2.85 |
| Polyphenols, g∙kg−1 DM | 22.57 | 31.85 |
| Flavonoids, g∙kg−1 DM | 0.57 | 1.22 |
| Profile of amino acids | ||
| Total amino acid, %DM | 14.94 | 16.44 |
| Val, %DM | 0.66 | 0.76 |
| Met, %DM | 0.17 | 0.19 |
| Ile, %DM | 0.55 | 0.60 |
| Leu, %DM | 1.40 | 1.57 |
| Thr, %DM | 0.67 | 0.63 |
| Phe, %DM | 0.69 | 0.72 |
| Lys, %DM | 1.08 | 0.83 |
| His, %DM | 0.49 | 0.58 |
| Arg, %DM | 0.77 | 0.87 |
| Asp, %DM | 1.29 | 1.36 |
| Tyr, %DM | 0.50 | 0.62 |
| Ser, %DM | 0.69 | 0.78 |
| Glu, %DM | 2.55 | 2.80 |
| Pro, %DM | 1.86 | 2.32 |
| Gly, %DM | 0.55 | 0.62 |
| Ala, %DM | 0.87 | 0.96 |
| Cys, %DM | 0.14 | 0.21 |
1Premix supplied per kilogram of diet: vitamin A, 6,480 IU; vitamin D3, 2800 IU; vitamin E, 26 mg; vitamin K, 2 mg; vitamin B1, 50 mg; vitamin B2, 4 mg; vitamin B6, 3 mg; vitamin B12, 0.03 mg; nicotinic acid, 20 mg; pantothenic acid, 9 mg; folic acid, 1.2 mg; biotin, 0.2 mg; choline chloride, 300 mg; Fe, 200 mg; Cu, 95 mg; Zn 100 mg; Mn, 30 mg; I, 0.35 mg; Se, 0.36 mg; Ca, 0.9 %; P 0.1%; NaCl, 0.5%; and Lysine, 0.1%.
When pigs reached the slaughter weights (around 110 kg), 6 hogs closest to the average body weight were selected from each group and fasted for 12 h (but free access to water) before sampling. Blood (no anticoagulant) were collected via the anterior vena cava 1 h before slaughter. Blood samples were centrifuged at 3000 rpm for 10 min at 4 °C to separate the serum. The serum was collected and kept at −20 °C until use. Pigs were slaughtered using electrical shock followed by exsanguination. Tissues of liver, longissimus dorsi (LD) muscle, were quickly isolated and stored in liquid nitrogen until further use.
RNA Isolation and Real-Time PCR
Around 50 mg of frozen tissues were grinded in liquid nitrogen and then transferred into 1 ml of Trizol regent (Takara, Japan) to purify total RNA as the manufacturer suggested. RNA quality was validated by the ratio of A260/A280. Total RNA was reverse transcripted with mRNA reverse transcription kit (Takara, Japan).
Real-time PCR were performed using a SYBR green kit (Vazyme, China) with a Bio-Rad iQ5 (Bio-Rad, USA). Relative expression of genes of interest was calculated using 2−∆∆Ct method, with β-actin or PPIA (peptidylprolyl isomerase A) as the internal control. Primers targeting β-actin and PPIA (Li et al., 2016), IGF1 and IGF1 promoter (Tang et al., 2015), and IGFBP3 (Wang et al., 2011) were got from previous studies. Primers for IGFBP5 were designed with the aid of NCBI Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). All primers were synthesized by Sangon Biotech (Shanghai, China), and the sequences are listed in Table 2.
Table 2.
Primer sequences
| Sequences (5′ → 3′) | Product length | |
|---|---|---|
| IGF1 | F: art tot tea agg taa aga tgc a R: cag ccc cac aga ggg tct ca |
117 bp |
| IGFBP3 | F: gac acg ctg aac cac ctc a R: cgt act tat cca cgc acc ag |
151 bp |
| IGFBP5 | F: caa ctg tga ccg caa ggg att R: cga agc tgt ggc act gga ag |
149 bp |
| β-actin | F: gga ctt cga gca gga gat gg R: agg aag gag ggc tgg aag ag |
138 bp |
| IGF1 promoter | F: aag tta atc aga gga cag cat cag t R: ttg ggc atg gtg aca aat aac atc |
138 bp |
| PPIA1 | F: gac tga gtg gtt gga tgg R: tga tct tct tgc tgg tct t |
116 bp |
1PPIA = peptidylprolyl isomerase A.
Western Blot
To detect the protein levels of CCAAT/enhancer-binding protein beta (C/EBPβ) in liver, about 200 mg of frozen livers were homogenized in RIPA buffer with protein inhibitors and then centrifuged at 5000 rpm at 4 °C for 10 min to aspirate the supernatant. Protein concentration was determined with BCA assay, and equal amount of proteins were subjected to SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes. Then the membranes were blocked with a blocking solution for 2 h and incubated overnight at 4 °C with anti-C/EBPβ (sc-150, 1:200, Santa Cruz Biotechnology, Santa Cruz, CA) or anti-β-Actin (sc-47778, 1:200, Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were rinsed with TBST for 3 times and then incubated with the secondary antibody coupled with horseradish peroxidase. Blots were detected by ECL chemiluminescence (Pierce, Rockford, IL), and the densities were quantified using Image J (National Institutes of Health, USA).
ELISA Assay
Porcine IGF1 protein levels in serum and livers were analyzed with commercial kits (Cat #SEA050Po, Cloud-Clone Corp., TX) according to the manufacturer’s suggestion. As for the tissue samples, 100 mg of frozen liver were homogenized in cold PBS, and the supernatant was collected for further assay.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) assay was done with a commercial kit (#9003, Cell Signaling Technology, Danvers, MA) according to manufacturers’ instrument. Briefly, 25 mg of frozen samples were grinded in liquid nitrogen and cross-linked in 1.5% formaldehyde. The cross-linking reaction was stopped using 2.5M glycine. The pellets were washed using cold PBS and rinsed with lysis buffer (0.1% sodium dodecylsulfate, 0.5% Triton X-100, 150 mM NaCl, and 20 mM Tris-HCl, pH 8.1). The samples were sonicated on ice for 3 min with 10 s on/off intervals (#JY92-II, Xinzhi Biotechnology Co., Ltd., Ningbo, China) to produce DNA fragments around 500 bp. Protein-DNA complex was diluted in ChIP dilution buffer and incubated with anti-C/EBPβ (sc-150, 1:50, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C. And a rabbit IgG antibody was used as a control for each sample. The immunoprecipitated chromatin complexes were captured by protein G agarose beads. After sequentially washing, the antibody/protein/DNA complexes were eluted from protein G agarose beads. Finally, DNA fragments were released from the immunoprecipitated complex by incubating at 65 °C for 5 h and purified using the QIAquick Spin kit (Qiagen, CA) for real-time PCR analysis.
Biochemical Assay
The contents of total cholesterol, low-density cholesterol (LDL-c), high-density cholesterol (HDL-c), triglyceride as well as malondialdehyde (MDA), lipid peroxide (LPO), the activities of glutathione reductase (GSH-Px), super oxide dismutase (SOD), catalase (CAT), and total antioxidative activity (T-AOC) were tested by respective commercial kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) according to the manufacturer’s introduction. As regarding the tissue samples, 100 mg of frozen tissues of liver or LD were homogenized in cold PBS, and the supernatant was collected for further biochemical and antioxidative assays. The OD values were monitored by BioTek Epoch microplate reader (BioTek, VT).
Statistic Analysis
The data from the experiment were analyzed using independent t-tests using IBM SPSS 19.0 (International Business Machines Corporation, USA). Data were presented as mean ± SEM, and differences were considered significant if P < 0.05.
RESULTS
Table 3 shows the growth performance of the grower-finisher pigs in the experiment. In our study, pigs with similar initial body weights (57.47 ± 4.71 vs. 58.23 ± 2.61 kg) were arranged to be fed with either normal meal (Ctrl group) or fermented feed (FF group), slaughter around 110-kg body weights (114.67 ± 9.61 vs. 116.55 ± 5.48 kg). Fermented meal significantly increased average daily gain (0.753 ± 0.064 vs. 1.041 ± 0.052 kg∙d−1, P < 0.05), and there were no significant differences regarding the average food intake (2.198 ± 0.071 vs. 2.299 ± 0.068 kg∙d−1), so gain:food ratio (0.343 ± 0.029 vs. 0.453 ± 0.021, P < 0.05) was significantly higher in pigs fed with fermented meal compared with that fed with normal feed (P < 0.05). FF shortened the fattening period by 20 d (76 vs. 56 d).
Table 3.
Effects of fermented meal on growth performance of grower-finisher pigs
| Items | Normal meal | Fermented feed |
|---|---|---|
| Initial body weight (kg) | 57.47 (4.71) | 58.23 (2.61) |
| Final body weight (kg) | 114.67 (9.61) | 116.55 (5.48) |
| Average daily gain (kg∙d−1) | 0.753 (0.064) | 1.041 (0.052)* |
| Average daily intake (kg∙d−1) | 2.198 (0.071) | 2.299 (0.068) |
| Gain:food ratio | 0.343 (0.029) | 0.453 (0.021)* |
| Feeding period | 76 | 56 |
1Results are presented as mean (SE; n = 4 pens per group).
*A significant difference between the pigs fed with normal and fermented meal, P < 0.05.
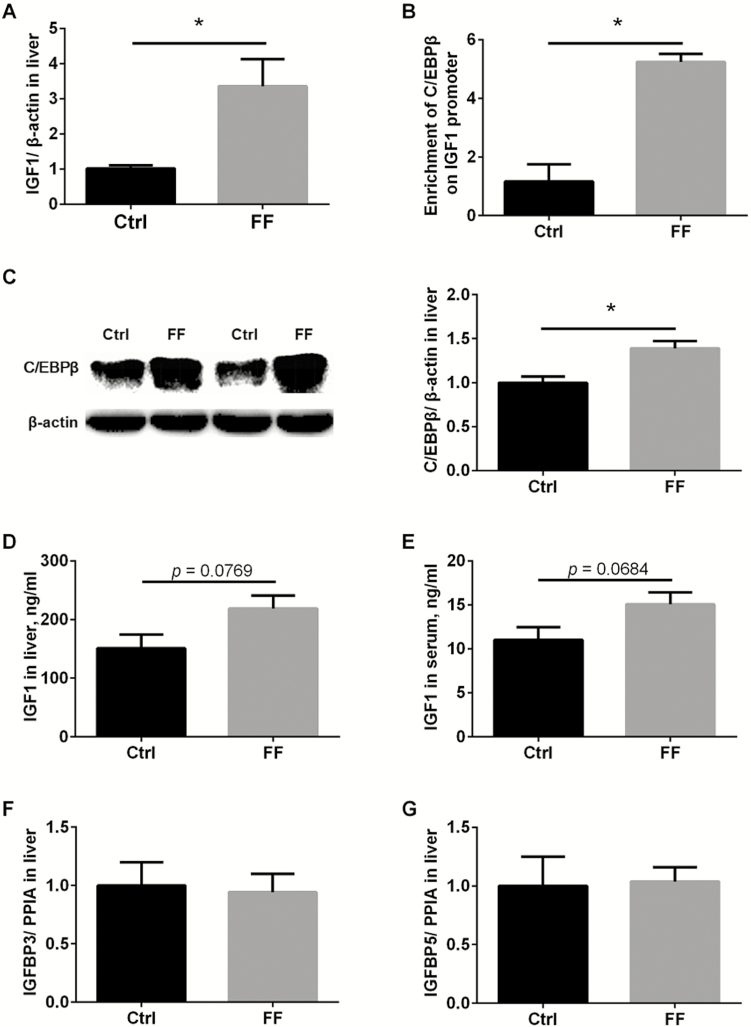
To explore the effects of FF on IGF1 expression and production in liver, fresh liver samples were collected immediately after slaughter. Real-time PCR show that FF significantly upregulated IGF1 transcripts in liver (Figure 1A, P < 0.05). C/EBPβ was previously reported to activate IGF1 transcription in porcine livers (Tang et al., 2015), and ChIP-PCR show that significantly much more C/EBPβ proteins were enriched in the promoter of IGF1 gene (Figure 1B, p < 0.05). Corresponding to ChIP-PCR assay, results of western blot show that C/EBPβ proteins were upregulated in liver fed with FF (Figure 1C, P < 0.05). ELISA was further employed to investigate the changes of IGF1 at protein levels. In line with that at transcriptional levels, an increasing tendency of IGF1 protein in liver and serum was observed in pigs with FF (Figure 1D and E, 0.05 < P < 0.10), but the changes did not reach statistical differences due to large variation. We moved on to test the expression of IGF1-related factors, insulin-like growth factor-binding proteins IGFBP3, IGFBP5, although their transcripts were comparable in livers fed with FF and normal meal (Figure 1F and G, P > 0.05).
Figure 1.
Fermented meal enhanced hepatic IGF1 synthesis. Hepatic IGF1 mRNAs were analyzed using qPCR (A). C/EBPβ enrichment within IGF1 promoter was detected with ChIP-PCR (B). C/EBPβ (C) and IGF1(D) proteins in liver were analyzed by western blot. Serum IGF1 levels were analyzed with ELISA (E). IGFBP3 (F) and IGFBP5 (G) mRNAs were analyzed using qPCR. Ctrl: control group with normal commercial feed; FF: pigs fed with fermented meal; n = 6 per group; *P < 0.05.
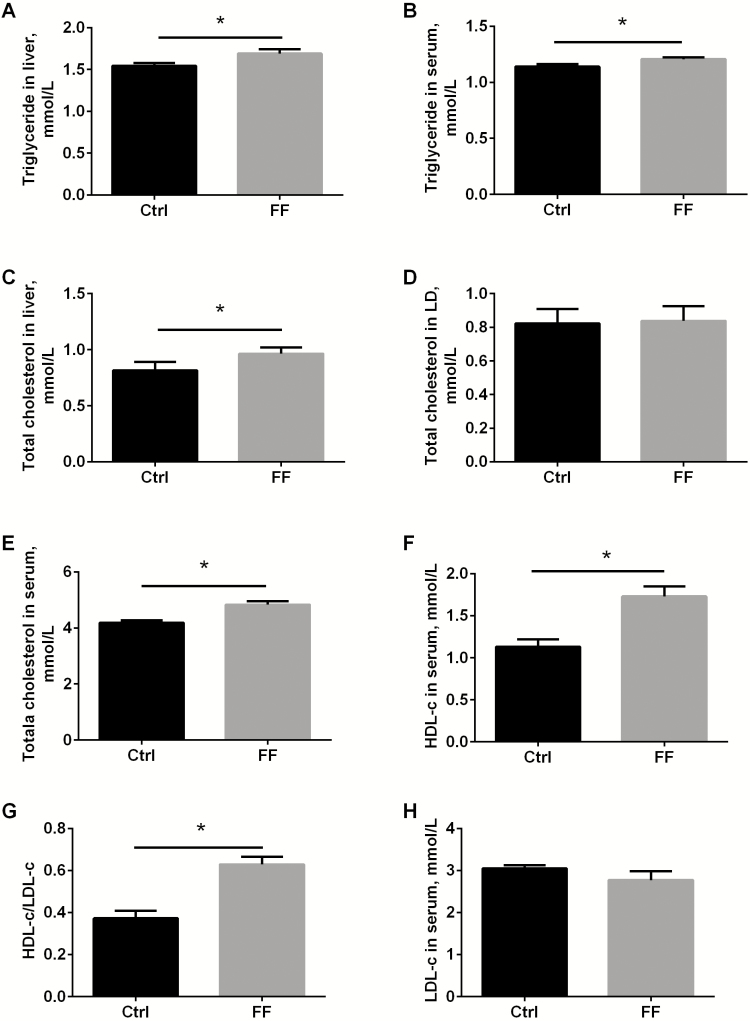
Given the fact that C/EBPβ is a predominant factor for adipogenesis (Guo et al., 2015), and overexpression (Schroeder-Gloeckler et al., 2007) or activation(Zhao et al., 2017) of C/EBPβ is reported to increase triglyceride accumulation in liver, lipid profiles were detected here. Fermented meal indeed significantly raised up the levels of triglyceride in both liver and serum (Figure 2A and B, both P < 0.05). FF significantly increased the total cholesterol content in liver (Figure 2C, P < 0.05), but the total cholesterol in LD muscle was unchanged yet (Figure 2D, P > 0.05). Fermented meal also significantly increased total cholesterol (Figure 2E, P < 0.05), HDL-c (Figure 2F, P < 0.05), the HDL-c/LDL-c ratio in serum (Figure 2G, P < 0.05), and the circulating LDL remained comparable between fermented and unfermented feed (Figure 2H, P > 0.05). To rule out the healthy concerns about the elevated triglyceride and cholesterol, oxidative stress markers were analyzed. Results show that the activities of antioxidative enzymes CAT, GSH-Px, SOD, and T-AOC in liver were not dramatically affected by fermented meal (Supplementary Figure S1A–D, all P > 0.05). Accordingly, oxidative products, MDA and LPO were not changed either (Supplementary Figure S1E and F, all P > 0.05).
Figure 2.
Fermented meal altered cholesterol and triglyceride levels in serum and liver. Triglyceride in liver (A) and serum (B), total cholesterol in liver (C), LD muscle (D) and serum (E) were determined by commercial kits. HDL-c (F), ratio of HDL-c/LDL-c (G), and LDL-c (H) were analyzed. Ctrl: control group with normal commercial feed; FF: pigs fed with fermented meal; LD means longissimus dorsi muscle; n = 6 per group; *P < 0.05.
DISCUSSION
In our study, one-stage solid-state fermentation in our study greatly increased the content of both essential and nonessential amino acids in corn-soybean meals (especially Val by 14.65%, Arg by 12.89%, and Cys by 9.93%), supporting the results reported previously (Frias et al., 2008; Song et al., 2008). Moreover, fermented corn-soybean meal in our study improved daily gain by 40%, and shortened the time to market by 20 d, indicating fermented meal dramatically promoted pig growth. Based on the well-documented effects of Val (Zhang et al., 2018) or Arg (Hu et al., 2017) on pig growth, we speculate that the increased supply of functional amino acids might contribute to the growth-promoting effects of FF.
IGF1 is a predominant growth factor during postnatal development. IGF1 is produced primarily by the liver, and hepatic IGF1 transcription is regulated by GHR-JAK2 (Janus kinase 2)-STAT5B (signal transducer and activator of transcription 5B) axis (Sawada et al., 2017) as a response to pituitary GH plus. A pioneer’s work revealed that GH and amino acid supply could interact synergistically to regulate IGF1 expression and production in cultured bovine hepatocytes (Wheelhouse et al., 1999). Another study reported that 1.0% Arg or 0.5% Arg + 0.5% Gln could significantly increase the circulating GH and IGF1 in pigs (Wu et al., 2013). Therefore, we next examined whether IGF1 expression in liver might be altered by the FF. Our results show that IGF1 transcripts were significantly increased by FF, and IGF1 protein levels in liver and serum demonstrated the same tendency. Thereby, our data provide a solid evidence that FF could enhance IGF1 expression in liver.
C/EBPβ, a liver-enriched transcription factor, was previously unveiled to be involved in the interaction of GH and amino acid on hepatic IGF1 expression in vitro (Wheelhouse et al., 1999). Latter, C/EBPβ was confirmed to modulate prenatal and postnatal hepatic IGF1 transcription through directly binding to the promoter, and overexpression of C/EBPβ could enhance IGF1 expression in porcine primary hepatocytes (Tang et al., 2015). Here, increased C/EBPβ expression was found in livers with fermented meal, and meanwhile, more C/EBPβ was enriched in IGF1 promotor. Together with previous data, these findings suggest that fermented meal augmented hepatic IGF1 expression is at least partially through enhancing the binding of C/EBPβ to IGF1 promotor, thus facilitating IGF1 transcription.
Intriguingly, hepatic as well as circulating triglyceride and total cholesterol contents were slightly but significantly increased in pigs fed with fermented meal, which might be due to the increased crude fat in the diet and/ or enhanced C/EBPβ expression, for C/EBPβ activates the expression of SREBP-1c (Sterol Regulatory Element-Binding Protein-1c) transcription (Tian et al., 2016), and SREBP-1c further activates genes encoding enzymes required for fat synthesis. C/EBPβ interference decreases triglyceride content in the liver (Schroeder-Gloeckler et al., 2007). However, in human, serum IGF1 levels are negatively related to liver fat content (Nguyen et al., 2018). As the core factor for both triglyceride accumulation and IGF1 synthesis in liver, how C/EBPβ coordinates both cellular processes are worthy of further study.
Taken together, our study shows that fermented corn-soybean meal significantly strengthens IGF1 transcription in liver through enhancing the binding of C/EBPβ to its promoter, thus increases circulating IGF1 levels to promote pig growth.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Animal Science online.
Footnotes
This work was supported by the Technical Innovation Guidance Special (Foundation) Project of Shaanxi Province (2017ZKC07-114), National Natural Science Foundation (31501925), and Major Projects for Genetically Modified Organisms Breeding (2014ZX0800947B).
LITERATURE CITED
- Álvarez-Nava F., and Lanes R.. 2017. GH/IGF-1 signaling and current knowledge of epigenetics: a review and considerations on possible therapeutic options. Int. J. Mol. Sci. 18:1624. doi: 10.3390/ijms18101624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman D. E., J. S. Christiansen G. Johannsson M. O. Thorner, and Kopchick J. J.. 2008. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm. IGF Res. 18:455–471. doi: 10.1016/j.ghir.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. S., Y. Li S. J. Geng L. S. Hu X. F. Fu, and Han X. Y.. 2017. Effects of dietary fresh fermented soybean meal on growth performance, ammonia and particulate matter emissions, and nitrogen excretion in nursery piglets. J. Zhejiang Univ. Sci. B 18:1083–1092. doi: 10.1631/jzus.B1700180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias J., Y. S. Song C. Martínez-Villaluenga E. González de Mejia, and Vidal-Valverde C.. 2008. Immunoreactivity and amino acid content of fermented soybean products. J. Agric. Food Chem. 56:99–105. doi: 10.1021/jf072177j [DOI] [PubMed] [Google Scholar]
- Frieten D., C. Gerbert C. Koch G. Dusel K. Eder A. Hoeflich B. Mielenz, and Hammon H. M.. 2018. Influence of ad libitum milk replacer feeding and butyrate supplementation on the systemic and hepatic insulin-like growth factor I and its binding proteins in holstein calves. J. Dairy Sci. 101:1661–1672. doi: 10.3168/jds.2017-13603 [DOI] [PubMed] [Google Scholar]
- Guo L., X. Li, and Tang Q. Q.. 2015. Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β. J. Biol. Chem. 290:755–761. doi: 10.1074/jbc.R114.619957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. J., Q. Y. Jiang T. Zhang Y. L. Yin F. N. Li J. Y. Su G. Y. Wu, and Kong X. F.. 2017. Dietary supplementation with arginine and glutamic acid enhances key lipogenic gene expression in growing pigs. J. Anim. Sci. 95:5507–5515. doi: 10.2527/jas2017.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., F. He H. Wen J. Li Y. Si M. Liu Y. Huang, and Meng L.. 2017. Low salinity affects cellularity, DNA methylation, and mrna expression of igf1 in the liver of half smooth tongue sole (cynoglossus semilaevis). Fish Physiol. Biochem. 43:1587–1602. doi: 10.1007/s10695-017-0395-7 [DOI] [PubMed] [Google Scholar]
- Li X., K. Huang F. Chen W. Li S. Sun X. E. Shi, and Yang G.. 2016. Verification of suitable and reliable reference genes for quantitative real-time PCR during adipogenic differentiation in porcine intramuscular stromal-vascular cells. Animal 10:947–952. doi: 10.1017/S1751731115002748 [DOI] [PubMed] [Google Scholar]
- Meyer K. F., R. N. Verkaik-Schakel W. Timens L. Kobzik T. Plösch, and Hylkema M. N.. 2017. The fetal programming effect of prenatal smoking on igf1r and igf1 methylation is organ- and sex-specific. Epigenetics 12:1076–1091. doi: 10.1080/15592294.2017.1403691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R., R. Chakraborty, and Dutta A.. 2016. Role of fermentation in improving nutritional quality of soybean meal – A review. Asian-Australas. J. Anim. Sci. 29:1523–1529. doi: 10.5713/ajas.15.0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A., F., Ricolfi B., Lemogne S., Aho S., Lemaire B., Bouillet L., Duvillard D., Denimal C., Fourmont R., Loffroy, et al. 2018. Liver fat content in people with pituitary diseases: influence of serum IGF1 levels. Horm. Metab. Res. 50:303–307. doi: 10.1055/s-0043-120673 [DOI] [PubMed] [Google Scholar]
- Sawada T., D. Arai X. Jing M. Miyajima S. J. Frank, and Sakaguchi K.. 2017. Molecular interactions of epha4, growth hormone receptor, janus kinase 2, and signal transducer and activator of transcription 5B. Plos One. 12:e0180785. doi: 10.1371/journal.pone.0180785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder-Gloeckler J. M., S. M., Rahman R. C., Janssen L., Qiao J., Shao M., Roper S. J., Fischer E., Lowe D. J., Orlicky J. L., McManaman, et al. 2007. CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in lepr(db/db) mice. J. Biol. Chem. 282:15717–15729. doi: 10.1074/jbc.M701329200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Y. Zhang Y. Yin C. Wang Z. Lu F. Wang J. Feng, and Wang Y.. 2017. Amino acid and phosphorus digestibility of fermented corn-soybean meal mixed feed with bacillus subtilis and enterococcus faecium fed to pigs. J. Anim. Sci. 95:3996–4004. doi: 10.2527/jas2017.1516 [DOI] [PubMed] [Google Scholar]
- Song Y. S., J. Frias C. Martinez-Villaluenga C. Vidal-Valdeverde, and de Mejia E. G.. 2008. Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem. 108:571–581. doi: 10.1016/j.foodchem.2007.11.013 [DOI] [PubMed] [Google Scholar]
- Tang Y., K. Xiong M. Shen Y. Mu K. Li, and Liu H.. 2015. CCAAT-enhancer binding protein (C/EBP) β regulates insulin-like growth factor (IGF) 1 expression in porcine liver during prenatal and postnatal development. Mol. Cell. Biochem. 401:209–218. doi: 10.1007/s11010-014-2308-8 [DOI] [PubMed] [Google Scholar]
- Tian J., Goldstein J. L., and Brown M. S.. 2016. Insulin induction of SREBP-1c in rodent liver requires LXRα-C/EBPβ complex. Proc. Natl. Acad. Sci. USA. 113:8182–8187. doi: 10.1073/pnas.1608987113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuersunjiang N., J. F. Odhiambo D. R. Shasa A. M. Smith P. W. Nathanielsz, and Ford S. P.. 2017. Maternal obesity programs reduced leptin signaling in the pituitary and altered GH/IGF1 axis function leading to increased adiposity in adult sheep offspring. PLoS ONE 12:e0181795. doi: 10.1371/journal.pone.0181795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., C. Lin W. Su Y. Zhang F. Wang Y. Wang C. Shi, and Lu Z.. 2018a. Effects of supplementing sow diets with fermented corn and soybean meal mixed feed during lactation on the performance of sows and progeny. J. Anim. Sci. 96:206–214. doi: 10.1093/jas/skx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., C. Shi Y. Zhang D. Song Z. Lu, and Wang Y.. 2018b. Microbiota in fermented feed and swine gut. Appl. Microbiol. Biotechnol. 102:2941–2948. doi: 10.1007/s00253-018-8829-4 [DOI] [PubMed] [Google Scholar]
- Wang L., G. Zhang F. Lin B. Jiang F. Dong, and Liu H.. 2011. Expression of the insulin-like growth factor system in skeletal muscle during embryonic and postnatal development in the first filial generation pigs from erhualian and yorkshire reciprocal crosses. Gen. Comp. Endocrinol. 173:56–62. doi: 10.1016/j.ygcen.2011.04.025 [DOI] [PubMed] [Google Scholar]
- Wheelhouse N. M., A. K. Stubbs M. A. Lomax J. C. MacRae, and Hazlerigg D. G.. 1999. Growth hormone and amino acid supply interact synergistically to control insulin-like growth factor-I production and gene expression in cultured ovine hepatocytes. J. Endocrinol. 163:353–361. [DOI] [PubMed] [Google Scholar]
- Wu L., W., Wang K., Yao T., Zhou J., Yin T., Li L., Yang L., He X., Yang H., Zhang, et al. 2013. Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS ONE 8:e69502. doi: 10.1371/journal.pone.0069502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., X. Liu H. Jia P. He X. Mao S. Qiao, and Zeng X.. 2018. Valine supplementation in a reduced protein diet regulates growth performance partially through modulation of plasma amino acids profile, metabolic responses, endocrine, and neural factors in piglets. J. Agric. Food Chem. 66:3161–3168. doi: 10.1021/acs.jafc.8b01113 [DOI] [PubMed] [Google Scholar]
- Zhao N. Q., X. Y. Li L. Wang Z. L. Feng X. F. Li Y. F. Wen, and Han J. X.. 2017. Palmitate induces fat accumulation by activating C/ebpβ-mediated G0S2 expression in hepg2 cells. World J. Gastroenterol. 23:7705–7715. doi: 10.3748/wjg.v23.i43.7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., M., Gao R., Zhang Z., Sun C., Wang F., Yang T., Huang S., Qu L., Zhao Y., Li, et al. 2017. Effects of soybean meal fermented by L. Plantarum, B. Subtilis and S. Cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell Fact. 16:191. doi: 10.1186/s12934-017-0809-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.