Abstract
Heat stress is a major environmental factor contributing to lower production of poultry. The objective of present study was to evaluate the influence of constant or intermittent high temperature on the production performance and redox status of plasma and hypothalamus in laying ducks. A total of 288 weight- and laying-matched laying ducks were randomly assigned to 1 of 4 treatments (each with 6 replicates of 12 birds): control, pair-fed, constant high temperature (24 h, 34 ± 1°C), and intermittent high temperature (10 h, 34 ± 1°C). Blood and hypothalamic tissue samples were collected on days 1, 21, and 55 to determine redox status. Average daily feed intake and egg weight was reduced (P < 0.001) during imposition of both high-temperature treatments but was not different (P > 0.05) among the treatments during the recovery period. Lower (P < 0.05) egg mass was observed in pair-fed and intermittent high-temperature treatment during high-temperature period and in constant high temperature during the recovery period. Haugh units from high temperature–treated ducks were significantly lower than those from control or pair-fed ducks (P < 0.05) during the high-temperature period. Both models of heat exposure decreased plasma concentrations of glutathione (GSH) at day 1, and constant high temperature decreased plasma activity of GSH peroxidase (GSH-PX) at day 21 (P < 0.05). Hypothalamic expression of antioxidant genes GSH reductase (GR) and mitochondrial NADH dehydrogenase subunit (Complex Ι) were decreased by both high-temperature treatments at day 1. Hypothalamic expression of genes for pro-oxidant enzymes cyclooxygenase-2 (COX-2), 5-lipoxygenase (5-LOX), and cytochrome P450 7A1 (CYP7A1) were decreased (P < 0.05) by both models of high temperature but transcripts of cyclooxygenase-1 (COX-1) of ducks that were pair-fed or were exposed to constant high temperature were increased at day 21. The transcripts of NADPH oxidase 1 (NOX-1) were decreased at day 1 by both high-temperature treatments (P < 0.05) but increased during the recovery period. These results indicate that, for laying ducks, intermittent high temperature caused much greater negative production performance effects than constant high temperature during high-temperature period, but laying ducks exposed to constant high temperature tend to take longer to recover their production performance. High-temperature stress, either constant or intermittent, altered hypothalamic expression of antioxidation and pro-oxidation genes.
Keywords: anti, and pro-oxidant enzymes, duck, egg production, heat stress
INTRODUCTION
Heat stress is a condition that results when an animal experiences an imbalance between thermogenesis, heat gain, and net energy released into the surrounding environment (Ajakaiye et al., 2011). Feeding efficiency, growth rate, reproductive efficiency, egg quantity, and efficiency of the immune response decline with increasing environmental temperature, and these cause an enormous economic loss in the poultry industry (Zeng et al., 2014; Mignon-Grasteau et al., 2015). As the world warms and highly centralized animal production develops, heat stress is a major environmental factor contributing to lower production of poultry. The negative effects of heat stress will become increasingly apparent in the future (Kamineni, 2015).
During heat stress, the balance between the oxidative and antioxidant defense system can be disturbed by excessive levels of reactive oxygen species (ROS), which can cause lipid peroxidation and oxidative damage to proteins and DNA (Lin et al., 2006; Feng et al., 2008). In previous studies, liver (Del Vesco and Gasparino, 2013), muscle (Zhang et al., 2015), and heart (Zeng et al., 2014) were studied extensively for explaining the disordered redox system after high temperature, but redox status in the hypothalamus during heat stress remains unclear. The hypothalamus plays a major role in thermoregulation of birds and initiates heat stress responses (Kamineni, 2015; Lin et al., 2006). Many reports have focused on the alteration of the high-temperature period, regardless of the duration of recovery (Morera et al., 2012; Song et al., 2012; Sun et al., 2015). It remains unknown how long it takes for birds to recover after exposure to high ambient temperature. The objective of the present study, therefore, was to investigate the impact of exposing laying ducks to constant or intermittent high temperature on production performance, and redox status of plasma and hypothalamus during the period of high temperature and subsequent recovery.
MATERIALS AND METHODS
Treatments, Diets, and Animals
Animal care procedures outlined by the guidelines of the Animal Care and use Committee of the Guangdong Academy of Agricultural Sciences were complied with for all aspects of management, housing, and slaughter. A total of 288 weight- and laying-matched laying ducks (Shanma ducks, Anas platyrhynchos) were randomly assigned to 1 of 4 groups, each with 6 replicates with a total of 12 birds. Birds were caged individually in humidity- and temperature-controlled rooms (cage size, 30 cm width × 35 cm depth × 35 cm height). During the high-temperature period, the treatments imposed a 30-d period of high temperature (34 ± 1°C), either continuously (24 h/d) or intermittently (10 h/d, from 09:00 to 19:00); for pair-fed and control ducks, the room temperature was held at 25°C for 24 h/d. Feed was provided ad libitum for ducks from control, intermittent, and constant high temperature. The pair-fed ducks were fed the same amount of feed that birds exposed to intermittent high temperature consumed the day before. After the high-temperature period, all ducks were held at 25°C for the subsequent 25-d recovery period when all ducks had free access to feed. The relative humidity for all treatments varied from 76% to 84% during the high-temperature period, and from 74% to 78% during the recovery period. Feed was provided twice daily (09:00 and 16:00). The composition of the diet is shown in Table 1. Water was provided ad libitum to ducks and a 17-h daily photoperiod was provided throughout. Residual feed was measured at 08:00 and used to calculate the previous day’s feed intake and ADFI. The number and total egg weight from each replicate was recorded daily, the daily egg production, egg weight, egg mass, and feed conversion ratio (FCR) were calculated accordingly.
Table 1.
Ingredients and calculated composition of basal diet (air-dry basis, %)
| Ingredients | % | Calculated composition | |
|---|---|---|---|
| Corn | 54.51 | ME (Mcal/Kg) | 2.50 |
| Wheat bran | 11.52 | CP, % | 17.00 |
| Soybean meal | 22.70 | Lys, % | 0.86 |
| DL-Met | 0.15 | Met, % | 0.40 |
| Limestone | 8.45 | Met + Cys, % | 0.68 |
| CaHPO4 | 1.37 | Arg, % | 1.10 |
| NaCl | 0.30 | Ca, % | 3.60 |
| Premix1 | 1.00 | Total P, % | 0.63 |
| Total | 100 | Available P, % | 0.35 |
1Vitamin-trace mineral premix provided the following minerals in milligrams per kilogram of diet: Fe, 52; Cu, 10.4; Mn, 91; Se, 0.20; I, 0.52; Co, 0.26; and the following vitamins per kg of diet: thiamine, 3.0 mg; riboflavin, 9.6 mg; niacinamide, 114 mg; D-pantothenic acid, 28.5 mg; choline chloride, 500 mg; cobalamin, 30 µg; menadione, 0.96 mg; DL-α-tocopheryl acetate, 6 IU; vitamin A, 12 000 IU; cholecalciferol D3, 1 800 IU.
Sample Collection
At the end of period of high temperature (day 30) and recovery (day 55), 4 eggs within each replicate were collected to determine egg quality. On days 1, 21 (high-temperature period) and 55 (recovery period), 2 ducks from each replicate were randomly selected for blood sampling; 5 mL of blood was collected from the brachial vein using heparinized tubes (BD vacutainer Systems, Franklin Lakes, NJ), centrifuged (1500 g, 10 min, 4°C) and aliquots of plasma were stored at −80°C until analyses. After blood sampling, ducks were killed by cervical dislocation. The hypothalamic tissue was quickly excised from each duck, snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Egg Quality Determination
After storing eggs overnight at 4°C, the shape index was measured from the length and width, then shell-breaking strength was measured by an Egg Force Reader (ORKA Food Technology Ltd., Ramat Hasharon, Israel). Eggs were broken onto a level surface. Haugh units and yolk color were measured with an egg analyzer (model EA-01, ORKA), after which yolk was separated and weighed; albumen weight was calculated by subtraction. Shells were washed under running water, dried and weighed. The shell thickness without membrane at three locations (air cell, equator, and sharp end) was measured using a digital micrometer (model IT-014UT, Mitutoyo, Kawasaki, Japan) and shell thickness was the mean of the 3 values.
Determination of Reproductive Organ Variables
After ducks were killed (days 21 and 55), the ovary and oviduct were excised and the weight of whole ovary and oviduct length and weight were recorded. Dominant follicles (diameter >1 cm) were dissected from the ovary and number and weight were measured and recorded.
Determination of Plasma Antioxidant Enzymes, Glutathione, and Malondialdehyde
Plasma activities of superoxide dismutase (SOD) and glutathione (GSH) peroxidase (GSH-PX) and levels of malondialdehyde (MDA) and reduced GSH were measured with colorimetric assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Real-Time PCR
Total RNA was isolated from hypothalamus using Trizol (Invitrogen, Carlsbad, CA). RNA concentration and purity were determined with a spectrophotometer (NanoDrop Technologies, Wilmington, MA). The integrity of RNA was examined using 1.2% agarose gels containing 0.1% ethidium bromide. One microgram of total RNA was reverse transcribed using PrimeScript RT reagent kit with gDNA eraser (TaKaRa, Shiga, Japan). Real-time PCR (qPCR) was performed in triplicate on 2 µL of the cDNA template in a total volume of 20 µL containing 10 µL of SYBR Green Supermix (BioRad, Hercules, CA) and 0.8 µM forward/reverse primers (Table 2) using CFX-96 (BioRad). The gene expression levels were normalized using β-actin mRNA as an internal control. PCR products were examined by 2% agarose/Tris-borate-EDTA gel electrophoresis to confirm amplification specificity and amplicon size. The specificity of the reaction was confirmed by determining the product-melting curve. The following protocol was used for qPCR: initial denaturation for 30 s at 95°C, followed by 40 cycles of 20 s at 95°C, 30 s at 60°C, and 20 s at 72°C. The relative expression levels of the genes tested were calculated using the 2 −ΔΔCt method, as described previously (Livak and Schmittgen, 2001).
Table 2.
Gene primer sequences used for quantitative RT-PCR in laying ducks
| Gene1 | Primer sequence (5′–3′) | Product size (bp) | Accession number |
|---|---|---|---|
| COX-1 | Forward: GCAGTGAACCCGTGTTGCTATTAC | 252 | XM_021278679.1 |
| Reverse: GAGCCTCATCAGCGTGTCCCT | |||
| COX-2 | Forward: AAACAGGAGCATCCAGAGTG | 216 | XM_005015351.3 |
| Reverse: GTGCCAGTGGTACAGGGTAT | |||
| NOX-1 | Forward: TCGCCTCCATCCTGAAGTCC | 256 | XM_021270354.1 |
| Reverse: GTGTCCGTGGCAGTGTCGTG | |||
| NOX-4 | Forward: AGCCACCCATTCACCCTTAC | 249 | XM_005029579.3 |
| Reverse: AATACCACCAGCCACGCAGA | |||
| 5-LOX | Forward: AATACCAAAGCCCGAGAACA | 133 | XM_005025227.3 |
| Reverse: GGAAGCAGAGTGAGCTGTAAGTC | |||
| CYP7A1 | Forward: ATCAGACTTTCATTAGAACCCTTCA | 169 | NM_001310351.1 |
| Reverse: GACTCAAACATCACCTGGCAAC | |||
| GR | Forward: CCCATCTGGACTACAGCAACA | 182 | XM_005025125.3 |
| Reverse: CTTCATCACGCACTTCACCTTC | |||
| MnSOD | Forward: CACCACAGCAAGCACCACG | 174 | XM_005015908.2 |
| Reverse: AGGCGAAAGATTTGTCCAGAA | |||
| CAT | Forward: AGATATGGTATGGGACTTTTGGAG | 180 | XM_013091878.2 |
| Reverse: TCAGTCTTCACATGAAACTTGCA | |||
| Complex Ι | Forward: GTCACCACCCTGGATAACGG | 250 | XM_005023916.3 |
| Reverse: GTCACCACCCTGGATAACGG | |||
| CoQ10 | Forward: TGTTTCACTGTTAGGCATTGTATTG | 146 | XM_021274031.1 |
| Reverse: GACAACTCATTCCCTCCTTTCA | |||
| β-actin | Forward: GCTATGTCGCCCTGGATTT | 174 | EF_667345.1 |
| Reverse: GGATGCCACAGGACTCCATAC |
1 COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; NOX-1, NADPH oxidase-1; NOX-4, NADPH oxidase-4; 5-LOX, 5-lipoxygenase; CYP7A1, cytochrome P450 7A1; GR, glutathione reductase; Complex I, NADH dehydrogenase subunit Ι; CoQ10, Coenzyme Q10.
Statistical Analysis
All data from the high-temperature and recovery periods were tested for normal distribution then analyzed using the general linear models procedure of SAS (SAS Inst. Inc., Cary, NC) in a completely randomized design. The following statistical model (Li et al.,2018) was used: Yij = μ + Ti + eij where Y is the analyzed variable, μ is the overall mean, T is the effect of treatment (i = 1 … 4), and e is the residual error (i = 1 … 4, j = 1 … 6). Replicate was taken as the experimental unit. Duncan’s multiple-range tests were used to compare the group means when the F test in the analysis of variance table was significant. Differences were considered to be significant when P values were less than 0.05. The data are presented as means ± SEM.
RESULTS
Production Performance and Egg Quality
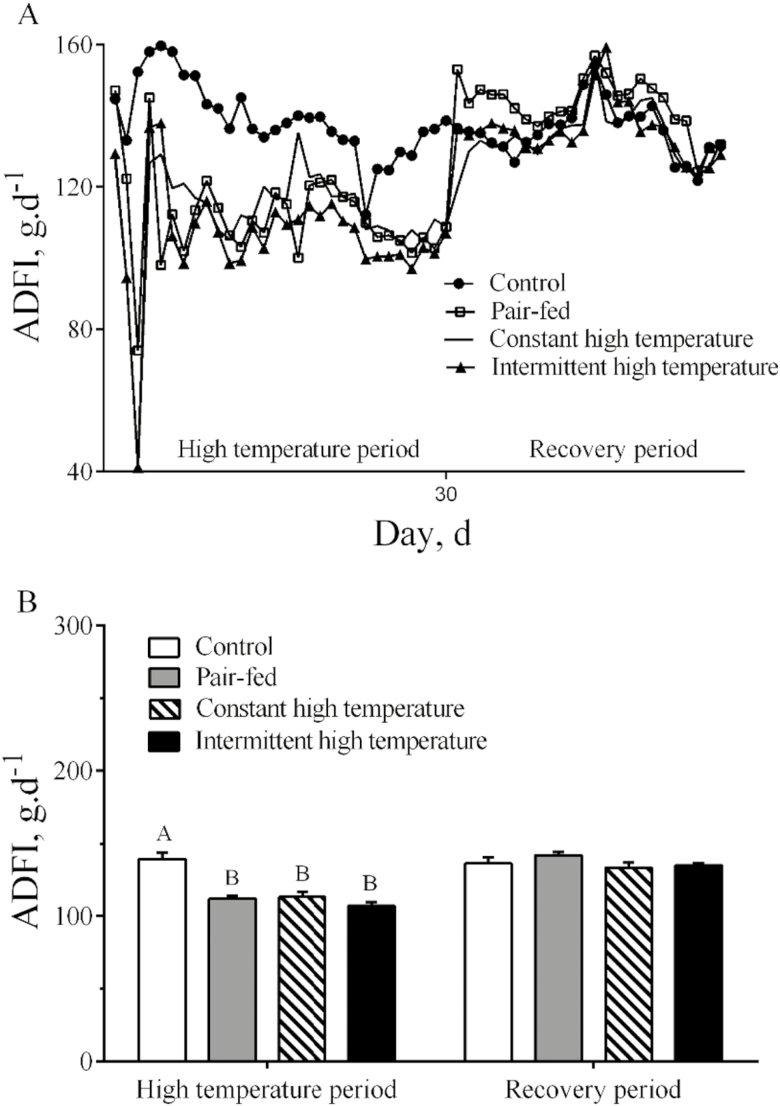
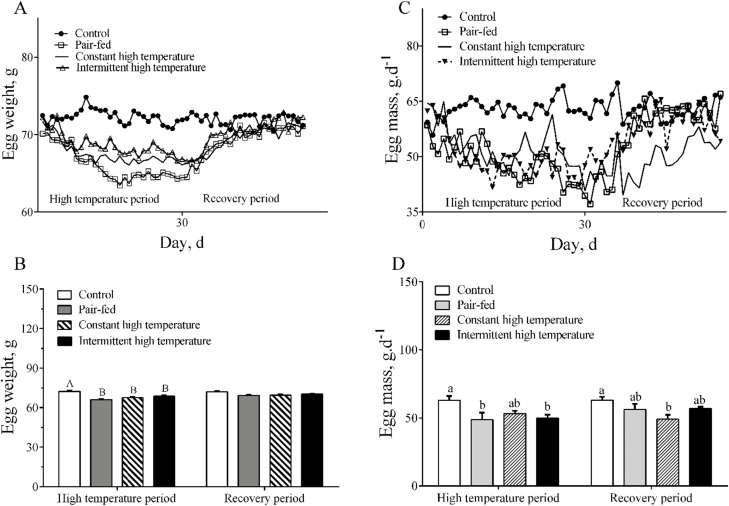
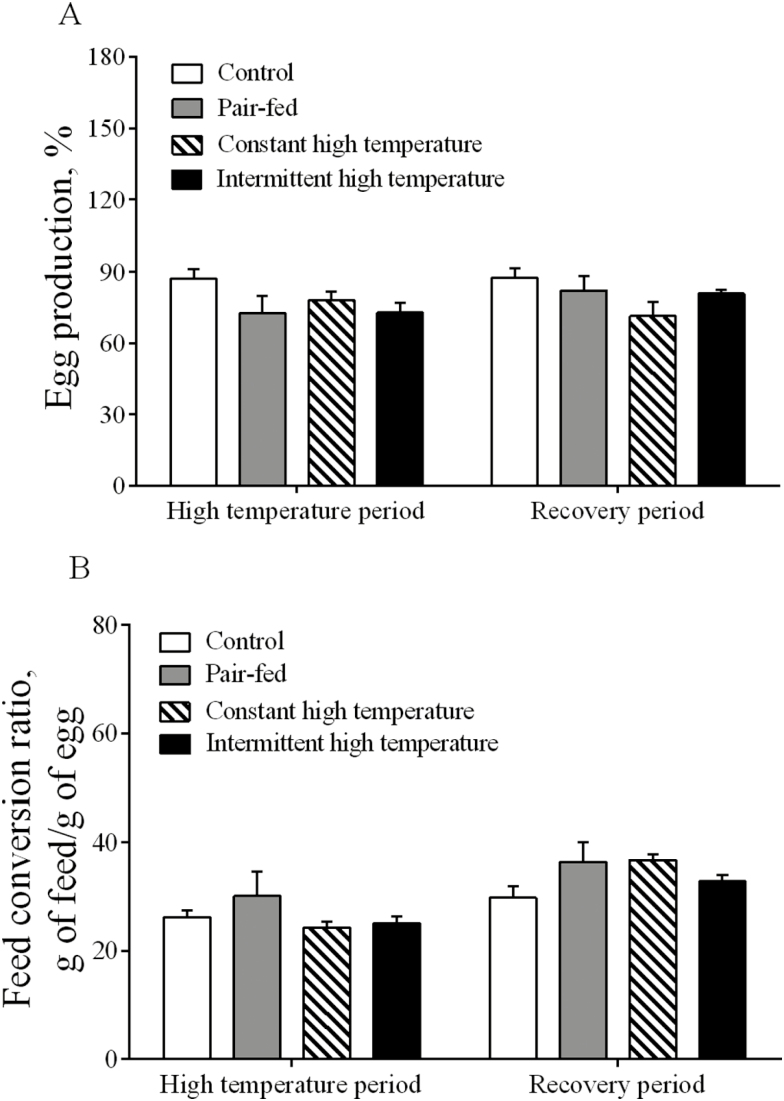
During the high-temperature period, overall ADFI of ducks in the 2 high-temperature treatments was lower (P < 0.001) than in the controls housed at 25°C (Fig. 1A and B). During the recovery period, ADFI returned quickly to normal levels and there were no differences (P > 0.05) between the 4 treatments (Fig. 1A and B). Egg weight was reduced (P < 0.001) during imposition of both high-temperature treatments (Fig. 2A and B) but was not different (P > 0.05) among the treatments during the recovery period. Lower (P < 0.05) egg mass was observed in pair-fed and intermittent high-temperature treatment (Fig. 2C) and egg mass was lower (P < 0.05) during the recovery period only after exposure to constant high temperature (Fig. 2D). There were no statistical differences (P > 0.05) in egg production or FCR among the 4 treatments (Fig. 3A and B). There were no differences (P > 0.05) in most egg quality characteristics (Table 3), including shape index, shell-breaking strength, shell proportion, and average shell thickness. In contrast, both the egg albumen height and Haugh units from high temperature–treated ducks were significantly lower than those from control or pair-fed ducks (P < 0.05) during the high-temperature period. Yolk color scores were lower in pair-fed or constant high temperature than in controls (P < 0.05, Table 3). Yolk proportion of the pair-fed ducks was higher than other groups during the recovery period (P < 0.05, Table 3).
Figure 1.
Feed intake of laying ducks during the period of high temperature and recovery. (A) Daily feed intake changes; (B) statistical results of feed intake among treatments. Data are means ± SEM, n = 6 for each treatment. Different uppercase letters indicate significant difference at the 0.001 probability level, different lowercase letters indicate significant difference at the 0.05 probability level.
Figure 2.
Egg weight and egg mass of laying ducks during the period of high temperature and recovery. (A) Changes in egg weight; (B) statistical results of egg weight among treatments; (C) changes in egg mass; (D) statistical results of egg mass among treatments. Data are means ± SEM, n = 6 for each treatment. Different letters indicate significant difference at the 0.001 probability level.
Figure 3.
Egg production (A) and feed conversion ratio (B) in laying ducks during the period of high temperature and recovery. Data are means ± SEM. n = 6 for each treatment. Different uppercase letters indicate significant difference at the 0.001 probability level, different lowercase letters indicate significant difference at the 0.05 probability level.
Table 3.
Egg quality traits of laying ducks during the high-temperature and recovery periods1
| Trait | Treatment | |||||
|---|---|---|---|---|---|---|
| Control | Pair-fed | Constant high temperature | Intermittent high temperature | SEM | P value | |
| High-temperature period | ||||||
| Shape index | 1.30 | 1.33 | 1.34 | 1.33 | 0.01 | 0.34 |
| Haugh units | 81.74a | 83.71a | 74.99b | 74.85b | 2.10 | 0.01 |
| Albumen height, mm | 7.28ab | 7.33a | 6.13b | 6.20b | 0.28 | < 0.01 |
| Yolk color score | 6.42a | 5.33c | 5.58bc | 6.17ab | 0.27 | 0.03 |
| Yolk proportion, % | 31.85 | 32.00 | 30.97 | 31.62 | 0.69 | 0.73 |
| Albumen proportion, % | 58.93 | 58.73 | 59.77 | 58.03 | 0.86 | 0.56 |
| Shell proportion, % | 9.22 | 9.27 | 9.26 | 9.27 | 0.92 | 0.81 |
| Shell-breaking strength, kg.cm-2 | 4.02 | 4.04 | 4.00 | 3.68 | 0.24 | 0.68 |
| Average shell thickness, mm | 0.33 | 0.32 | 0.32 | 0.33 | 0.01 | 0.60 |
| Recovery period | ||||||
| Shape index | 1.31 | 1.34 | 1.32 | 1.32 | 0.01 | 0.37 |
| Haugh units | 79.29 | 79.90 | 73.93 | 80.15 | 2.42 | 0.25 |
| Albumen height, mm | 6.93 | 6.75 | 6.10 | 6.81 | 0.33 | 0.32 |
| Yolk color score | 7.08 | 6.17 | 6.75 | 6.83 | 0.25 | 0.11 |
| Yolk proportion, % | 31.83ab | 33.05a | 31.15b | 30.93b | 0.51 | 0.04 |
| Albumen proportion, % | 59.20a | 57.60b | 59.68a | 59.96a | 0.46 | 0.01 |
| Shell proportion, % | 8.97 | 9.35 | 9.17 | 9.10 | 0.16 | 0.37 |
| Shell-breaking strength, kg.cm-2 | 3.42 | 3.82 | 3.63 | 3.59 | 2.27 | 0.69 |
| Average shell thickness, mm | 0.31 | 0.31 | 0.31 | 0.31 | 0.01 | 0.84 |
a, bValues within a row with different superscripts differ significantly at P < 0.05.
1 n = 6 for each treatment.
Reproductive Organ Indices
Oviduct length was greater (P < 0.05) in both of the high-temperature treatments at day 21 (Table 4). There were no differences among the treatments in oviduct mass, ovary weights, dominant follicle number, or weight during either the high-temperature or recovery period.
Table 4.
Reproductive organ variables of laying ducks at the end of high-temperature and recovery periods.1
| Trait | Treatment | |||||
|---|---|---|---|---|---|---|
| Control | Pair-fed | Constant high temperature | Intermittent high temperature | SEM | P value | |
| Day 21 | ||||||
| Oviduct length, cm | 38.60c | 41.88bc | 46.93ab | 48.50a | 2.13 | 0.02 |
| Oviduct mass, g | 53.40 | 47.42 | 45.17 | 49.204 | 3.72 | 0.54 |
| Ovary mass, g | 57.21 | 54.85 | 53.91 | 54.86 | 4.51 | 0.97 |
| Dominant follicle number | 5.20 | 4.75 | 5.29 | 4.83 | 0.37 | 0.64 |
| Dominant follicle mass, g | 47.53 | 47.06 | 45.79 | 45.01 | 3.98 | 0.97 |
| Day 55 | ||||||
| Oviduct length, cm | 45.86 | 43.57 | 49.36 | 48.29 | 1.78 | 0.12 |
| Oviduct mass, g | 50.34 | 42.23 | 51.05 | 49.76 | 3.43 | 0.26 |
| Ovary mass, g | 46.99 | 45.68 | 51.90 | 45.15 | 4.78 | 0.73 |
| Dominant follicle number | 4.86 | 4.86 | 5.43 | 5.00 | 0.42 | 0.74 |
| Dominant follicle mass, g | 37.35 | 38.65 | 43.25 | 38.09 | 4.62 | 0.53 |
a, bValues within a row with different superscripts differ significantly at P < 0.05.
1 n = 6 for each treatment.
Redox Metabolites and Antioxidant Enzyme Activities in Plasma
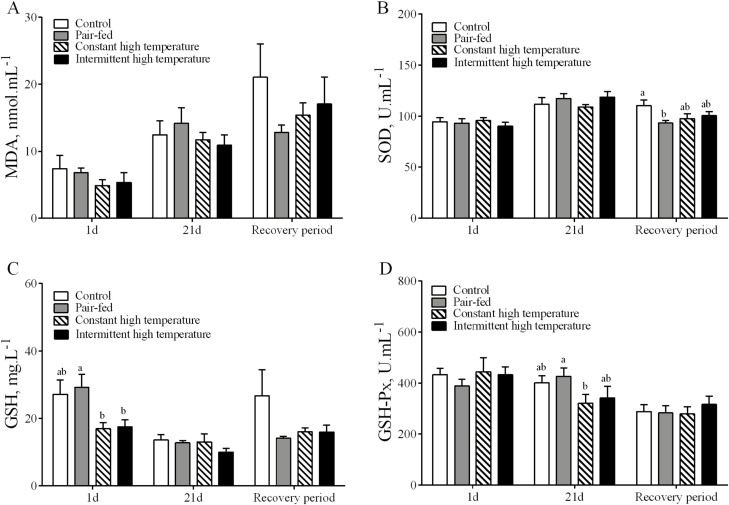
There were no differences (P > 0.05) between treatments and controls in plasma concentrations of MDA or in activities of SOD at any sampling times (Fig. 4A and B). The plasma concentrations of GSH were decreased at day 1 in ducks exposed to high temperature (P < 0.05, Fig. 3C), but there were no differences between the 4 treatments at day 21 or after the recovery period at day 25. The plasma GSH-PX activities from ducks exposed to constant high temperature, but not those exposed to intermittent high temperature, at day 21 were lower than in pair-fed ducks (P < 0.05, Fig. 3D), but there were no differences at day 1 of high temperature or after the recovery period (day 55).
Figure 4.
Redox metabolites and antioxidant enzyme activities in plasma of laying ducks during the period of high temperature (days 1 and 21) and recovery (day 55). (A) The leve of malondialdehyde (MDA); (B) The activity of superoxide dismutase (SOD); (C) The level of glutathione (GSH); (D) The activity of GSH peroxidase (GSH-Px). Data are means ± SEM, n = 6 for each treatment at each time points. Different letters indicate significant difference at the 0.05 probability level.
Hypothalamic Expression of Genes for Pro-oxidant and Antioxidant Enzymes
As shown in Table 5, the hypothalamic expression of genes for the antioxidant enzymes GSH reductase (GR) and mitochondrial NADH dehydrogenase subunit (Complex Ι) were reduced at day 1 by both models of high temperature. Compared with the control ducks, the hypothalamic expression of genes for pro-oxidant enzymes cyclooxygenase-2 (COX-2), 5-lipoxygenase (5-LOX), and cytochrome P450 7A1 (CYP7A1) were reduced (P < 0.05) by both high-temperature treatments (P < 0.05). The transcripts of cyclooxygenase-1 (COX-1) in ducks exposed to constant high temperature were increased at day 21. The transcripts of NADPH oxidase 1 (NOX-1) were increased (P < 0.05) by both high-temperature treatments at day 55, but were decreased at day 1. There were no effects of high temperature (P > 0.05) on the relative abundance of gene transcripts related to antioxidation and pro-oxidation enzymes, and mitochondria electron transport including manganese SOD (MnSOD), NADPH oxidase 4 (NOX-4), and Coenzyme Q10 (CoQ10) at any sampling time.
Table 5.
Relative abundance of gene transcripts of antioxidation and pro-oxidase enzymes in hypothalamus of laying ducks during the periods of high temperature and recovery1
| Transcript2 | Treatment | |||||
|---|---|---|---|---|---|---|
| Control | Pair-fed | Constant high temperature | Intermittent high temperature | SEM | P value | |
| Day 1 | ||||||
| NOX-1 | 1.15a | 0.92b | 0.83b | 0.80b | 0.08 | 0.02 |
| NOX-4 | 1.01 | 0.90 | 1.02 | 1.00 | 0.06 | 0.40 |
| COX-1 | 1.11 | 0.94 | 0.99 | 0.98 | 0.11 | 0.75 |
| COX-2 | 1.01 | 0.88 | 0.94 | 0.81 | 0.08 | 0.32 |
| 5-LOX | 1.12 | 0.87 | 1.12 | 0.91 | 0.09 | 0.11 |
| CYP7A1 | 1.05a | 0.74ab | 1.06a | 0.46b | 0.14 | 0.02 |
| Complex Ι | 1.01ab | 1.16a | 0.77bc | 0.73c | 0.08 | <0.01 |
| CoQ10 | 1.10 | 0.98 | 1.10 | 0.82 | 0.08 | 0.07 |
| GR | 1.05a | 1.05a | 0.68b | 0.59b | 0.12 | 0.02 |
| MnSOD | 1.02 | 1.15 | 1.03 | 0.97 | 0.09 | 0.84 |
| CAT | 1.01a | 0.74b | 1.15a | 1.05a | 0.07 | <0.01 |
| Day 21 | ||||||
| NOX-1 | 1.06 | 1.21 | 1.04 | 0.97 | 0.12 | 0.56 |
| NOX-4 | 1.01 | 1.19 | 1.30 | 1.29 | 0.10 | 0.14 |
| COX-1 | 1.42b | 3.40a | 2.67a | 1.29c | 0.50 | <0.01 |
| COX-2 | 1.03a | 0.96ab | 0.78b | 0.75b | 0.08 | 0.05 |
| 5-LOX | 1.27a | 0.47b | 0.57b | 0.43b | 0.21 | 0.02 |
| CYP7A1 | 1.12b | 1.87a | 0.56c | 0.55c | 0.16 | <0.01 |
| Complex Ι | 1.01 | 1.37 | 1.07 | 1.04 | 0.14 | 0.27 |
| CoQ10 | 1.65 | 2.40 | 2.00 | 2.15 | 0.34 | 0.53 |
| GR | 1.48 | 1.92 | 1.58 | 1.82 | 0.27 | 0.64 |
| MnSOD | 1.01 | 1.25 | 1.27 | 1.32 | 0.10 | 0.13 |
| CAT | 1.01 | 1.15 | 1.16 | 1.10 | 0.09 | 0.66 |
| Day 55 | ||||||
| NOX-1 | 0.89b | 0.86b | 1.12ab | 1.26a | 0.09 | 0.02 |
| NOX-4 | 1.01 | 0.94 | 0.98 | 1.02 | 0.05 | 0.85 |
| COX-1 | 1.06 | 0.84 | 1.25 | 1.22 | 0.12 | 0.09 |
| COX-2 | 1.09 | 1.13 | 1.27 | 1.26 | 0.11 | 0.59 |
| 5-LOX | 1.01 | 1.14 | 0.90 | 1.19 | 0.21 | 0.08 |
| CYP7A1 | 1.04 | 0.86 | 0.82 | 0.91 | 0.11 | 0.52 |
| Complex Ι | 1.02 | 0.91 | 1.23 | 1.09 | 0.09 | 0.14 |
| CoQ10 | 1.07 | 1.07 | 1.00 | 1.04 | 0.22 | 0.98 |
| GR | 1.06 | 1.14 | 1.00 | 0.91 | 0.12 | 0.59 |
| MnSOD | 1.01 | 1.13 | 1.21 | 1.13 | 0.21 | 0.36 |
| CAT | 1.01 | 0.95 | 1.03 | 1.10 | 0.07 | 0.48 |
a, bValues within a row with different superscripts differ significantly at P < 0.05.
1 n = 6 for each treatment.
2 COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; NOX-1, NADPH oxidase-1; NOX-4, NADPH oxidase-4; 5-LOX, 5-lipoxygenase; CYP7A1, cytochrome P450 7A1; GR, glutathione reductase; Complex I, NADH dehydrogenase subunit Ι; CoQ10, Coenzyme Q10.
DISCUSSION
Birds are compromised when they are exposed to high, potentially life threatening temperatures. The most debilitating environmental factors that affect layer chickens in production is heat stress, because of their feather covering and lack of sweat glands, making thermolysis difficult (Ajakaiye et al., 2011). Like chickens, ducks are sensitive to high ambient temperature (Zeng et al., 2013; Ma et al., 2014). It was found here that egg weight, egg mass, and feed intake of laying ducks were reduced by high-temperature treatments and in pair-fed controls at thermoneutrality, but these variables gradually returned to normal levels during the subsequent 25-d recovery period at normal temperature. It appeared that intermittent high temperature caused much greater negative effects on production performance than did constant high temperature. This is probably because the laying ducks are susceptible to the variation of ambient temperature within a day, as reduced production is observed commonly in practice in the summer, especially when environmental weather changes. Daily feed intake, egg mass, and egg production in laying hens were significantly reduced by chronic heat stress (Mignon-Grasteau et al., 2015). The increased temperature decreases energy requirement and feed intake but requirements for protein, minerals, and vitamins do not decrease, therefore the biological mechanism by which heat stress impacts production performance is partly explained by reduced feed intake (Das et al., 2011). The results of the present study provide initial evidence of the biological mechanisms through which the reduced production performance is probably due to the reduced feed intake when laying ducks were exposed to high ambient temperature. Thus, the heat stress during summer high temperatures may be partly offset by feeding diets with increased nutrient density (De Andrade et al., 1977; Usayran et al., 2001). Haugh units are indicative of egg quality, especially as quality related to albumen (Eisen et al., 1962). In the present study, the Haugh units of duck eggs were decreased by intermittent or constant high temperature, consistent with previous work from this laboratory (Ma et al., 2014). The important albumen proteins, including ovalbumin, ovotransferrin, ovomucoid, ovo mucin, lysozyme, and globulins, are largely secreted by the tubular gland cells of the magnum (Toussant et al., 1995; Sah and Mishra, 2018). The secretion of these protein may be affected by high temperature, because there was an abnormal increase in oviduct length from laying ducks exposed to high temperature and the previous study (Ma et al., 2014) showed that exposure to constant high temperature (34°C) for 28 days impaired the morphology of the magnum in laying ducks, thereby negatively affecting albumen secretion. The present finding of decreased albumen height with high temperature differed from Durmus and Kamanli (2015), who found no effects of high temperature on albumen height. On the contrary, Melesse et al. (2010) reported a decline of albumen and yolk quality as the environmental temperature increased. The difference between these studies might be due to the duration, nature, and imposed temperature; the 30-d duration of high temperature (34°C) used here account for the negative effects. Previous studies rarely evaluated production and egg quality during recovery after a period of high temperature. As found here, 25 d is sufficient for laying ducks to recover to normal performance after high temperature exposure, based on feed intake and laying performance returning to the levels of the controls, although laying ducks exposed to constant high temperature tend to take longer to recover their production performance.
Although ROS are a part of normal physiology and have biological roles, including those in cell signaling and protection against environmental insults. Serious damage to proteins, DNA, and lipids, however, can be caused by ROS (Dostalek et al., 2008). Production of ROS and their elimination is in dynamic equilibrium but is readily disturbed when the organism suffers environmental insults, such as imposed heat stress. The antioxidant enzyme systems including SOD and GSH-Px serve as the first line of antioxidant defense. The balance between the production of ROS and the antioxidant system was altered by modification of the activity of these enzymes (Zeng et al., 2014). Compared to the controls and pair-fed ducks, there were no changes observed in plasma SOD activity, indicating that it was unaffected by either form of high temperature. Similarly, there were no effects on MDA concentrations in plasma. These results are in accordance with a study in broiler chickens (Lin et al., 2006), noting that antioxidant enzyme activity was not effected by heat stress. The present findings of a substantial decrease in GSH content with high temperature at day 1, coupled with the smaller decrease in GSH-Px activity at day 21, indicated that the GSH-mediated-antioxidant system was greatly affected by the constant high temperature but not by the intermittent high temperature.
Chowdhury et al. (2014) have indicated the potential relationship between heat stress and the antioxidant defense system. Several enzyme systems have been considered in the production of ROS, including NADPH oxidase, xanthine oxidase, (uncoupled) mitochondrial electron transport, and cytochrome P450 (Dostalek et al., 2008; Peter, 2015). The present study attempted to explore the relationship between the antioxidant defense system and heat stress by quantifying expression of oxidase and antioxidase genes in the hypothalamus, including COX-1, COX-2, NOX-1, NOX-4, 5-LOX, CYP7A1, GR, MnSOD, and CAT. The abundance of COX-1 transcripts in pair-fed ducks and those exposed to constant high temperature increased at day 21, but transcripts in ducks stressed with intermittent high temperature decreased. COX-2 is a central proinflammatory mediator and plays a role in ROS production (Zheng et al., 2012). Hypothalamic COX-2 gene expression was reduced in both high-temperature treatments as compared to the controls. High temperature can activate NADPH oxidase through NOX-1 upregulation and NOX-1 knockdown significantly inhibited ROS production (Moon et al., 2010). Here, the expression of NOX-1 was downregulated in laying duck hypothalamus after exposure to high temperature for 1 d, but there were no differences at day 21 of the high-temperature period. 5-LOX was a reasonable candidate based on its known proinflammatory properties (Mehrabian, 2002). The abundance of 5-LOX transcripts in both high-temperature treatments and pair-fed ducks at day 21 was strikingly lower than in controls, showing that 5-LOX in duck hypothalamus was affected by high temperature and may play a role in suppressing feed intake. Expression of the CYP7A1 gene in hypothalamus of ducks exposed to intermittent high temperature was markedly decreased at day 1, and this transcript on day 21 was lower in both heat treatments than in controls and pair-fed ducks. These data suggest that there is a possible protective mechanism in ducks through downregulating expression of pro-oxidant enzymes COX-1, COX-2, and CYP7A1 during long-term heat stress. The modulation of antioxidant enzymes affects the thermal sensitivity of the cell (Slimen et al., 2014). Compared to the controls and pair-fed ducks, the expression of GR, MnSOD, CAT, Complex Ι, and CoQ10 genes did not change with 21 d of high-temperature treatment, but expression of Complex Ι and GR genes in heat-treated ducks were decreased at day 1. The findings indicate that GR and mitochondrial NADH dehydrogenase subunit (Complex Ι) in hypothalamus of laying ducks were most obviously affected by early stage of high temperature but not by late stage of high temperature.
In conclusion, for laying ducks, intermittent high temperature caused more severe negative production performance than did constant high temperature during a 30-d treatment period, but laying ducks exposed to constant high temperature tend to take longer to recover their production performance. In the early response to high temperature of laying ducks, hypothalamic expression of Complex Ι and GR genes was downregulated and plasma GSH decreased while after 21 d of high temperature resulted in downregulated hypothalamic COX-1, COX-2, and CYP7A1 expression and reduced plasma GSH-Px. These results showed that high temperature of laying ducks disrupted the balance between the oxidative-antioxidative system in the hypothalamus, and then suppressed appetite.
Footnotes
We sincerely thank Dr. W. Bruce Currie (Emeritus Professor, Cornell University) for his help in the presentation of this manuscript. This work was supported by the Fund for China Agricultural Research System (CARS–42–13), National Natural Science Foundation of China (Grant No. 31301995), The Science and Technology Program for Pearl River Nova of Guangzhou City (201710010159), the Science and Technology Program of Guangdong Province (2016A020210043), China National Key Research and Development Program (Grant No. 2018YFD0501504).
LITERATURE CITED
- Ajakaiye J. J., Perezbello A., and Mollinedatrujillo A.. 2011. Impact of heat stress on egg quality in layer hens supplemented with l-ascorbic acid and dl-tocopherol acetate. Vet. Arh. 81:119–132. [Google Scholar]
- Chowdhury V. S., S. Tomonaga T. Ikegami E. Erwan K. Ito J. F. Cockrem, and Furuse M.. 2014. Oxidative damage and brain concentrations of free amino acid in chicks exposed to high ambient temperature. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 169:70–76. doi: 10.1016/j.cbpa.2013.12.020 [DOI] [PubMed] [Google Scholar]
- Das S., Palai T. K., Mishra S. R., Das D., and Jena B.. 2011. Nutrition in relation to diseases and heat stress in poultry. Vet. World. 4:429–432. doi:10.5455/vetworld.2011.429-432 [Google Scholar]
- De Andrade A. N., Rogler J. C., Feathersto W. R., and Alliston C. W.. 1977. Interrelationships between diet and elevated temperatures (cyclic and constant) on egg production and shell quality. Poult. Sci. 56:1178–1977. doi:10.3382/ps.0561178 [Google Scholar]
- Del Vesco A. P. and Gasparino E.. 2013. Production of reactive oxygen species, gene expression, and enzymatic activity in quail subjected to acute heat stress. J. Anim. Sci. 91:582–587. doi: 10.2527/jas.2012-5498 [DOI] [PubMed] [Google Scholar]
- Dostalek M., K. D., Hardy G. L., Milne J. D., Morrow C., Chen F. J., Gonzalez J., Gu X., Ding D. A., Johnson J. A., Johnson, et al. 2008. Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems. J. Biol. Chem. 283:17147–17157. doi: 10.1074/jbc.M802447200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmus I., and Kamanli S.. 2015. Effects of cold and heat stress on egg quality traits of a newly developed native hybrid layer. Turkish JAF Sci. Tech. 3:444–447. doi:10.24925/turjaf.v3i6.44-447.344 [Google Scholar]
- Eisen, E. J., B. B. Bohren, and H. E. Mckean. 1962. The haugh unit as a measure of egg albumen quality. Poult. Sci. 41(5): 1461–1468. doi:10.3382/ps.0411461 [Google Scholar]
- Feng J., M. Zhang S. Zheng P. Xie, and Ma A.. 2008. Effects of high temperature on multiple parameters of broilers in vitro and in vivo. Poult. Sci. 87:2133–2139. doi: 10.3382/ps.2007-00358 [DOI] [PubMed] [Google Scholar]
- Kamineni L. P. 2015. Transcriptomic analysis of hypothalamic responses to heat stress in modern and legacy chicken lines. PhD Diss. Univ. of Delaware, Newark, NJ. [Google Scholar]
- Li S., J., Zheng K., Deng L., Chen X. L., Zhao X., Jiang Z., Fang L., Che S., Xu B., Feng, et al. 2018. Supplementation with organic acids showing different effects on growth performance, gut morphology, and microbiota of weaned pigs fed with highly or less digestible diets. J. Anim. Sci. 96:3302–3318. doi: 10.1093/jas/sky197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., E. Decuypere, and Buyse J.. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 144:11–17. doi: 10.1016/j.cbpa.2006.01.032 [DOI] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Ma X., Y. Lin H. Zhang W. Chen S. Wang D. Ruan, and Jiang Z.. 2014. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim. Reprod. Sci. 145:182–190. doi: 10.1016/j.anireprosci.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Mehrabian M., H. Allayee J. Wong W. Shi X. P. Wang Z. Shaposhnik C. D. Funk A. J. Lusis, and Shih W.. 2002. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ. Res. 91:120–126. doi:10.1161/01.RES.0000028008.99774.7F [DOI] [PubMed] [Google Scholar]
- Melesse A., Maak S., and Von Lengerken G.. 2010. Effect of long-term heat stress on egg quality traits of Ethiopian naked neck chickens and their F1 crosses with Lohmann White and New Hampshire chicken breeds. Livest. Res. Rural Dev. 22 (4) :071. http://www.lrrd.org/lrrd22/4/mele22071.htm [Google Scholar]
- Mignon-Grasteau S., U. Moreri A. Narcy X. Rousseau T. B. Rodenburg M. Tixier-Boichard, and Zerjal T.. 2015. Robustness to chronic heat stress in laying hens: a meta-analysis. Poult. Sci. 94:586–600. doi: 10.3382/ps/pev028 [DOI] [PubMed] [Google Scholar]
- Moon E. J., P. Sonveaux P. E. Porporato P. Danhier B. Gallez I. Batinic-Haberle Y. C. Nien T. Schroeder, and Dewhirst M. W.. 2010. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. U. S. A. 107:20477–20482. doi: 10.1073/pnas.1006646107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morera P., L. Basiricò K. Hosoda, and Bernabucci U.. 2012. Chronic heat stress up-regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. J. Mol. Endocrinol. 48:129–138. doi: 10.1530/JME-11-0054 [DOI] [PubMed] [Google Scholar]
- Peter S. F. 2015. Antioxidant systems in poultry biology: superoxide dismutase. J. Anim. Res. Nutr. 1:8. doi:10.21767/2572-5459.100008 [Google Scholar]
- Sah N., and Mishra B.. 2018. Regulation of egg formation in the oviduct of laying hen. Worlds Poult. Sci. J. 74:1–13. [Google Scholar]
- Slimen I. B., T. Najar A. Ghram H. Dabbebi M. Ben Mrad, and Abdrabbah M.. 2014. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperthermia. 30:513–523. doi: 10.3109/02656736.2014.971446 [DOI] [PubMed] [Google Scholar]
- Song Z., L. Liu A. Sheikhahmadi H. Jiao, and Lin H.. 2012. Effect of heat exposure on gene expression of feed intake regulatory peptides in laying hens. J. Biomed. Biotechnol. 2012:484869. doi: 10.1155/2012/484869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., R. Jiang S. Xu Z. Zhang G. Xu J. Zheng, and Qu L.. 2015. Transcriptome responses to heat stress in hypothalamus of a meat-type chicken. J. Anim. Sci. Biotechnol. 6:6. doi: 10.1186/s40104-015-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussant M. J., D. E. Swayne, and Latshaw J. D.. 1995. Morphologic characteristics of oviducts from hens producing eggs of different Haugh units caused by genetics and by feeding vanadium as determined with computer software-integrated digitizing technology. Poult. Sci. 74:1671–1676. doi: 10.3382/ps.0741671 [DOI] [PubMed] [Google Scholar]
- Usayran N., M. T. Farran H. H. Awadallah I. R. Al-Hawi R. J. Asmar, and Ashkarian V. M.. 2001. Effects of added dietary fat and phosphorus on the performance and egg quality of laying hens subjected to a constant high environmental temperature. Poult. Sci. 80:1695–1701. doi: 10.1093/ps/80.12.1695 [DOI] [PubMed] [Google Scholar]
- Wu F., X. J. Dong H. Q. Zhang L. Li Q. L. Xu Z. F. Liu Z. T. Gu, and Su L.. 2016. Role of MnSOD in propofol protection of human umbilical vein endothelial cells injured by heat stress. J. Anesth. 30:410–419. doi: 10.1007/s00540-015-2129-2 [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Z. P. Hu C. H. Lu M. X. Yang L. L. Zhang, and Wang T.. 2015. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 93:1656–1665. doi: 10.2527/jas.2014-8244 [DOI] [PubMed] [Google Scholar]
- Zeng T., X. Jiang J. Li D. Wang G. Li L. Lu, and Wang G.. 2013. Comparative proteomic analysis of the hepatic response to heat stress in Muscovy and Pekin ducks: insight into thermal tolerance related to energy metabolism. Plos One. 8:e76917. doi: 10.1371/journal.pone.0076917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Y. M. Kang W. Liu W. J. Zang C. Y. Bao, and Qin D. N.. 2012. Inhibition of cyclooxygenase-2 reduces hypothalamic excitation in rats with adriamycin-induced heart failure. Plos One. 7:e48771. doi: 10.1371/journal.pone.0048771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T., J. J. Li D. Q. Wang G. Q. Li G. L. Wang, and Lu L. Z.. 2014. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell Stress Chaperones. 19:895–901. doi: 10.1007/s12192-014-0514-7 [DOI] [PMC free article] [PubMed] [Google Scholar]