Abstract
The main objective of this study was to determine how feeding different dietary calcium (Ca) concentrations in combination with a negative dietary cation–anion difference (DCAD) would affect the cow’s response to induced hypocalcemia. We conducted an experiment with multiparous, nonlactating, nonpregnant Holstein cows fed a negative DCAD (average −18.2 across all diets) for 21 d with low (LC; 0.45% Ca; n = 5), medium (MC; 1.13% Ca; n = 6), or high (HC; 2.02% Ca; n = 6) concentrations of dietary Ca. Urine and blood samples were collected and urine pH measured daily during the 21-d feeding period prior to hypocalcemia challenge. Cows were then subjected to a controlled induction of hypocalcemia to determine how dietary Ca intake affected the response to a hypocalcemia challenge. On days 22, 23, and 24, hypocalcemia was induced with an intravenous infusion of 5% EGTA in 2 different cows from each treatment daily. During infusion, blood samples were collected every 15 min until 60% of prechallenge ionized calcium (iCa) concentrations were achieved. Samples were collected postinfusion at 0, 2.5, 5, 10, 15, 30, and every 30 min thereafter until 90% of prechallenge iCa was reached. Blood pH, hematocrit, and serum total Ca (tCa), sodium (Na), potassium (K), phosphorous (P), magnesium (Mg), and serotonin did not differ (P > 0.05) among treatments during the feeding period. Blood iCa (P = 0.04) and glucose (P = 0.03) were significantly elevated in HC compared with LC and MC cows during the feeding period. Urine pH was less than 6.0 in all cows, but was lowest in LC (P = 0.02) compared with MC and HC cows during the feeding period. Urine Ca, P, Mg, and deoxypyridinoline did not differ among treatments (P > 0.05). Cows fed HC maintained higher concentrations of iCa (P = 0.03) during the challenge period than MC (P = 0.04), and LC (P = 0.004), and required a longer time to reach 60% of whole blood iCa, and required more EGTA to reach 60% iCa than MC or LC cows (P = 0.01). Serum tCa decreased in all cows during infusion (P < 0.0001) but did not differ among treatments. Serotonin concentrations were elevated in MC cows compared with HC and LC cows during EGTA infusion (P = 0.05), suggesting an interdependent relationship between iCa and serotonin. Cows fed HC had a slower rate of decrease in iCa, but not tCa, when induced with hypocalcemia, indicating potential metabolic benefits of feeding higher dietary Ca in combination with a negative DCAD.
Keywords: calcium, dairy cows, dietary cation–anion difference, EGTA, serotonin
INTRODUCTION
Calcium (Ca) homeostasis in dairy cows is critical for maternal function, as well for milk synthesis. The dairy cow has approximately 3 g of Ca in the blood, 8 to 9 g of Ca in the extracellular fluids aside from bone, and 6 to 15 g in the fluid within the bone canaliculi (Goff, 2004). Maintenance of Ca homeostasis in mammalian species is controlled by an endocrine feedback loop that involves parathyroid hormone (PTH), calcitonin, and 1,25-dihydroxyvitamin D (Goff, 2004). These 3 hormones regulate the increased or decreased absorption of Ca in the intestine, increased or decreased resorption of Ca in the kidney, and finally regulate resorption of bone to release Ca, depending on the calcemic state of the cow. Modulation of normocalcemia in the dairy cow is of particular interest, as the physiological state of lactation creates an enormous stress on Ca homeostasis.
The practice of feeding a diet with a negative dietary cation–anion difference (DCAD) during the prepartum period is a common preventative method used on-farm to reduce cases of subclinical and clinical hypocalcemia in dairy cows (Block, 1984; Goff, 2008). The premise behind the use of acidogenic salts is to activate Ca metabolism in response to an induced compensated metabolic acidosis. It was hypothesized that the acidogenic properties of anions would increase concentrations of blood total (tCa) and ionized (iCa) calcium during times of Ca stress due to an increase in intestinal Ca absorption and mobilization of Ca from bone (Block, 1984; Goff, 2008). Furthermore, research has shown that the compensated metabolic acidosis created by diets with negative DCAD in combination with adequate magnesium (Mg) concentrations in the diet allowed for improved interaction of PTH with its receptor on bone tissue to stimulate Ca resorption (Goff, 2008; Goff et al., 2014).
An additional dietary method that has been used to improve Ca concentrations in dairy cows is the feeding of low dietary Ca. Low Ca diets initially cause a decrease in plasma Ca concentrations, which then result in the stimulation of PTH, resulting in improved Ca absorption in the gut, reduction of urinary Ca loss, and also increased bone mobilization of Ca (Goings et al., 1974; Green et al., 1981; Kichura et al., 1982). To stimulate PTH with a low Ca diet, the diet has to provide less than 20 g available Ca/d, which is difficult given the forages available to formulate these diets (Goff, 2004). Two meta-analyses revealed the possibility that high Ca diets may also reduce the risk of hypocalcemia (Oetzel, 1991; Lean et al., 2006). Therefore, it seems plausible to explore how high Ca may influence the physiology controlling Ca homeostasis.
Very few targeted studies have focused on the use of high dietary Ca, particularly in combination with a negative DCAD diet and how the diets may affect Ca metabolism in the dairy cow. One study in a periparturient model indicated that the use of acidogenic salts improved postpartum tCa and iCa concentrations, but dietary Ca (0.6% vs. 1.2% of the diet) had no effect on these blood parameters (Oetzel et al., 1988). An additional experiment in older Jersey cows (greater than fourth lactation) revealed no effect of dietary Ca on blood Ca concentrations in which the amount of Ca in the diet was 0.5% vs. 1.5%, and also focused on the amount of potassium in the diets (Goff and Horst, 1997). Another experiment conducted in periparturient Holstein cows (12 primiparous and 9 multiparous) with 0.99% and 1.5% Ca in the diet in combination with a negative DCAD also observed no differences in postpartum tCa concentrations (Chan et al., 2006). There are no recent studies that have explored the level of dietary Ca necessary in combination with a negative DCAD diet. Therefore, further controlled experiments investigating the levels of DCAD and dietary Ca are of importance.
The Ca and Mg chelator EDTA has been used since the 1970s to induce hypocalcemia in dairy cows (Muir et al., 1972; Belyea et al., 1976). A previous study using EDTA in a nonlactating, nonpregnant dairy cow model revealed that the optimum dietary Ca concentration was dependent on the DCAD value when examining the recovery of iCa concentrations after the hypocalcemia challenge (Oba et al., 2011). A more recent study examined the effects of an induced subclinical hypocalcemia (SCH) using EGTA, which is a selective Ca chelator, to examine the impacts of SCH on immune function (Martinez et al., 2014).
Therefore, in this experiment, we tested the hypothesis that high levels of dietary Ca in combination with a negative DCAD diet will improve the resistance against a hypocalcemia challenge using the Ca-specific chelator, EGTA. The objectives of the experiment were to examine the response of iCa and tCa concentrations to the hypocalcemia challenge, as well as the recovery from the hypocalcemia challenge, after feeding a negative DCAD diet with varying levels of dietary Ca using a nonpregnant, nonlactating dairy cow model.
MATERIALS AND METHODS
The College of Agriculture and Life Sciences Animal Care and Use Committee at the University of Wisconsin-Madison approved all experimental procedures. Care and use protocol guidelines (A005295) were strictly followed for this experiment.
Animals and Experimental Design
Eighteen multiparous, nonlactating, nonpregnant Holstein cows (average lactation number 2.4 ± 0.25) at the University of Wisconsin Dairy Cattle Research Center were enrolled in the experiment in May of 2016. Cows were housed in tie-stalls throughout the entire experiment, and weekly body weights were collected. All cows were fitted with rumination collars (SCR by Allflex North America, Madison, WI), and randomly assigned and balanced by lactation number to 1 of 3 dietary treatments for 21 d (day 1 of the experiment) prior to the hypocalcemia challenge: 1) 0.4% dietary Ca (n = 6; LC); 2) 1.0% dietary Ca (n = 6; MC); and 3) 1.60% dietary Ca (n = 6; HC). All diets were formulated using Animate (Phibro Animal Health Corporation, Teaneck, NJ) in total mixed rations (TMR) to reduce DCAD to the target level of −15.2/100 g of DM (Leno et al., 2017b). Animate is a patented anionic mineral supplement designed for transition dairy cow rations that contains chloride (Cl), sulfur (S), Mg, and 32% crude protein, and the millequivalents of macrominerals are −7064 mEq/kg. The following equation was used to calculate DCAD: DCAD mEq/100 g of DM = (Na+ + K+) − (Cl− + S2−).
Diet Formulation, Composition, and DMI
Compositions of formulated diets are found in Table 1. Diets were formulated using the Cornell Net Carbohydrate and Protein System (v. 6.55, Cornell University, Ithaca, NY). The basal diets consisted of corn silage, wheat straw, and a supplement providing protein, minerals, and vitamins, and were designed to supply 1.47 Mcal/kg NEL of DM of energy, 1,300 g of metabolizable protein/cow/d, 47 g of P, 65 g of Mg, 166 g of K, 14 g of Na, 127 g of Cl, and 51 g of S. The target DCAD was −15.2 mEq/100 g of DM. Samples of each diet were collected using the TMR sampling protocol (Robinson and Meyer, 2010; University of California, Agriculture and Natural Resources) daily for wet chemical analysis (SureTech Laboratories, Indianapolis, IN) during the entire feeding period (n = 28 per treatment group). Analyzed composition of dietary treatments are shown in Table 2. All cows were limit-fed 12.7 kg DM of their respective diets once daily and no orts were present during the feeding period. Cows on days 22, 23, and 24 that were not assigned to the hypocalcemia challenge on that particular day were fed identical diets, with no orts, as they had received during the feeding period. After completing the challenge (day 24 of the experiment), cows no longer were on the study. The final analyzed composition of the LC, MC, and HC diets provided 57.2 g (0.45%), 143.5 g (1.1%), and 256.6 g (2.0%) of Ca per d, respectively.
Table 1.
Formulated ingredient composition of the dietary treatments (% DM)
| Ingredient (% of DM) | Dietary treatments1 | ||
|---|---|---|---|
| Low calcium | Medium calcium | High calcium | |
| Corn Silage | 45.44 | 45.41 | 45.12 |
| Wheat Straw | 17.82 | 17.81 | 17.52 |
| Soy Plus2 | 11.05 | 11.04 | 11.04 |
| Distillers Grain | 8.37 | 8.37 | 8.37 |
| Soybean Hulls | 7.80 | 7.80 | 7.80 |
| Animate3 | 4.70 | 4.63 | 4.56 |
| Oat Hulls | 2.15 | 1.07 | 0.00 |
| AB-204 | 1.08 | 0.54 | 0.00 |
| Calcium Carbonate | 0.07 | 1.60 | 3.70 |
| Molasses | 0.71 | 0.71 | 0.71 |
| Salt | 0.14 | 0.14 | 0.18 |
| Mg Oxide | 0.16 | 0.19 | 0.21 |
| VTM Premix5 | 0.44 | 0.44 | 0.43 |
| Mono-dicalcium Phosphate | 0.07 | 0.25 | 0.36 |
1Dietary treatments: All diets were formulated to supply 1.47 Mcal/kg NEL of DM of energy, 1,300 g of metabolizable protein/cow/d, 47 g of P, 65 g of Mg, 166 g of K, 14 g of Na, 127 g of Cl, and 51 g of S. The target dietary cation–anion difference was −15.2 mEq/100 g of DM.
2Heat-treated soybean meal, a source of rumen bypass protein (Dairy Nutrition Plus, Landus Cooperative, Ames, IA).
3Composed of 38.0% CP, 3.2% ether extract, 3.1% sugar, 5.8% starch, 15.9% NDF, 1.4% Ca, 0.4% P, 4.8% Mg, 5.4% S, 13.9% Cl (Phibro Animal Health Corp., Teaneck, NJ).
4Advanced anticaking agent and pelleting aid for feed and feed ingredients (Phibro Animal Health Corp., Teaneck, NJ).
5Composed of 1.3 (min)–1.8% (max) Ca, 68.4 ppm Se, 1,771,234 IU/kg vitamin A, 552,098 IU/kg vitamin D3, 25,259 IU vitamin E/kg, 3.37 g/kg monensin (Elanco Animal Health, Greenfield, IN) fed at a rate of 202 mg/hd/d (VitaPlus Corporation, Madison, WI).
Table 2.
Analyzed chemical composition of the experimental diets1,2,3
| Item | Dietary treatments | ||
|---|---|---|---|
| Low calcium | Medium calcium | High calcium | |
| DM (%) | 52.93 (1.16)3 | 53.11 (1.24) | 53.19 (1.16) |
| CP (% DM) | 15.58 (1.63) | 15.72 (1.07) | 15.85 (1.17) |
| ADF (%DM) | 25.85 (2.59) | 25.60 (2.02) | 24.91 (3.00) |
| NDF (%DM) | 40.99 (2.89) | 40.59 (2.18) | 39.44 (2.44) |
| Fat (%DM) | 2.98 (0.29) | 3.01 (0.38) | 3.11 (0.36) |
| Ca (% DM) | 0.45 (0.02) | 1.13 (0.16) | 2.02 (0.43) |
| P (% DM) | 0.38 (0.02) | 0.41 (0.03) | 0.43 (0.03) |
| Mg (% DM) | 0.61 (0.02) | 0.66 (0.04) | 0.63 (0.02) |
| K (% DM) | 1.54 (0.05) | 1.53 (0.04) | 1.51 (0.05) |
| S (% DM) | 0.49 (0.02) | 0.50 (0.02) | 0.49 (0.02) |
| Cl (% DM) | 1.09 (0.03) | 1.18 (0.04) | 1.12 (0.04) |
| Na (% DM) | 0.13 (0.01) | 0.11 (0.01) | 0.14 (0.02) |
| DCAD (mEq/100g DM) | −16.70 (2.61) | −20.78 (2.13) | −17.26 (1.93) |
| Predicted composition | |||
| NEL (Mcal/kg DM)4 | 1.47 (0.01) | 1.47 (0.01) | 1.48 (0.36) |
| MP (g/kg DM)5 | 102.4 | 101.3 | 100.3 |
1Chemical composition of the diet was determined on composites of daily samples (N = 28) from each diet (SureTech Laboratories, Indianapolis, IN) during the 21-d feeding period.
2All diets were formulated to supply 1.47 Mcal/kg NEL of DM of energy, 1300 g of metabolizable protein/cow/d, 47 g of P, 65 g of Mg, 166 g of K, 14 g of Na, 127 g of Cl, and 51 g of S. The target dietary cation–anion difference (DCAD) was −15.2 mEq/100 g of DM.
3Cows were limit fed 12.7 kg DM of their respective diets, ORTS were not present.
4% of DM of component of the diet provided with the standard deviation (SD) calculated on the 28 samples taken for each diet as described.
5Calculated from chemical composition (NRC, 2001).
6Predicted by Cornell Net Carbohydrate and Protein System (v 6.5, Cornell University, Ithaca, NY) based on composite forage analysis, average ingredient composition, and average DMI. Metabolizable protein (MP) is a calculated value, so we are unable to report a standard deviation for this variable. The high Ca diet supplied 1,274 g of MP in 12.7 kg of DM, the medium Ca diet 1,287 g of MP in 12.7 kg of DM, and the low Ca diet supplied 1300 g of MP in 12.7 kg of DM.
Solution Preparation for Induction of Hypocalcemia
Solutions were prepared as described by Martinez et al., (2014). Briefly, 50 g of EGTA (# E3889, Sigma Aldrich, St. Louis, MO) were added to 900 mL of sterile, endotoxin-free 0.9% NaCl (Hospira Inc., Lake Forest, IL) and brought to a pH of 7.4 by addition of 5 M NaOH (Martinez et al., 2014). The solution was mixed until salts were fully dissolved and then sterile filtered using a 0.22-μM filter in a laminar flow hood. Solutions were prepared fresh daily.
Hypocalcemia Challenge and Recovery Periods
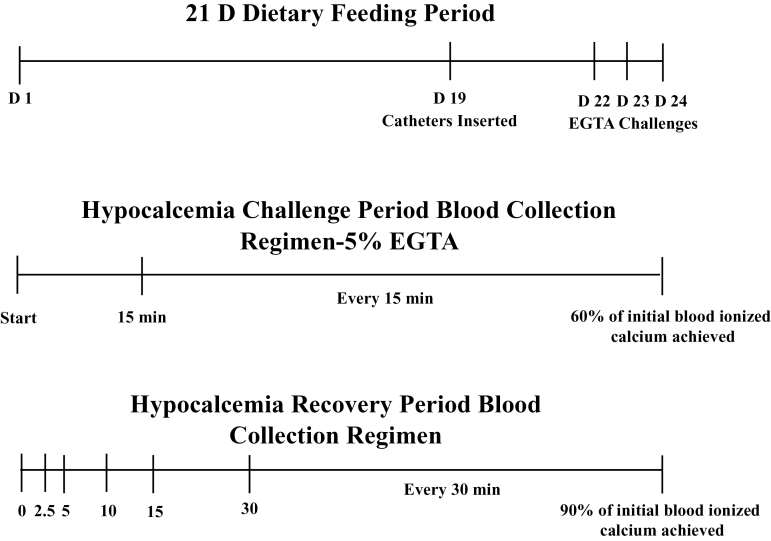
A timeline for the experimental periods is provided in Figure 1. Cows were fitted with 40-cm long indwelling jugular catheters, made from gas sterilized Tygon microbore tubing (ID = 0.040, OD = 0.070; Cole Parmer Scientific, Vernon Hills, IL), in both jugular veins on day 19. Catheters were threaded into the jugular veins with 12-gauge × 2ʺ hypodermic needles (#JO174EE; Jorgensen Laboratories, Loveland, CO). Catheters were then sutured to the neck of the cow and placed into an Elasticon (4ʺ width, Johnson and Johnson, New Brunswick, NJ) pouch, which was secured by wrapping Elasticon around the neck of the cow. Catheters were flushed with 10 units/mL of heparinized 0.9% saline to maintain patency and prevent clotting. On days 22 to 24 of the experiment, 6 cows per d (randomly selected, 2 cows from each treatment) were administered 5% EGTA at a continuous rate of 500 mL/h (Martinez et al., 2014), until blood iCa concentrations were 60% of prehypocalcemia challenge iCa concentrations (Heron et al., 2009; Oba et al., 2011). The time required for blood iCa concentrations to reach 60% of initial concentrations was recorded. Continuous infusion was performed using a controlled infusion pump (Heska Vet IV, Loveland, CO). Once 60% of initial iCa concentrations were achieved, EGTA infusions were terminated and cows were allowed to recover until they achieved 90% of their initial iCa concentrations which were measured prior to the initiation of the EGTA challenge. The time required for blood iCa concentrations to reach 90% of pre-EGTA challenge concentrations was measured. Whole blood samples were collected every 15 min during the EGTA infusions and at 0, 2.5, 5, 10, 15, 30 min, and every 30 min thereafter during the recovery period until 90% of pre-EGTA iCa concentrations were reached in each individual cow.
Figure 1.
Experimental design and sampling timelines for the 21-d feeding period, 5% EGTA challenge, and recovery periods.
Blood Analyses
One cow from the LC treatment was removed from all analyses as her iCa concentrations fell well below the 60% iCa concentrations within 15 min of EGTA treatment, resulting in an n = 5 for the LC treatment.
During the treatment period, whole blood was collected daily from the coccygeal vein. During the challenge and recovery periods, whole blood was collected from the jugular catheter opposite to the infusion site. Whole blood iCa concentrations, pH, and glucose concentrations were measured using a handheld cow-side biochemical analyzer using CG8+ cartridges (VetScan iSTAT System, Abbott Laboratories, Princeton, NJ). Serum was isolated using 10-mL BD Vacutainer Serum Plus Tubes (367820, BD, Franklin Lakes, NJ) to measure serotonin, tCa, total protein, phosphorus (P), and Mg concentrations. Following collection, blood was allowed to clot in serum tubes for 30 min at room temperature and then centrifuged for 3,000 × g for 20 min at 4 °C to collect the serum fraction. Serum was then stored at −80 °C in triplicates until analysis.
During the hypocalcemia challenge and recovery periods, iCa was also measured using a commercially available Horiba LAQUAtwin Compact Calcium Ion Meter (#B-751; Horiba Scientific, Irvine, CA) in order to compare values obtained to the iSTAT System. The Horiba LAQUAtwin Compact Calcium Ion Meter was calibrated per the manufacturer’s instructions prior to every measurement.
For all assays performed, a quality control reference sample was analyzed on each plate to assure the integrity of the assay. Serum serotonin concentrations were analyzed using a Serotonin Enzyme Immunoassay (EIA) Kit (IM1749, Immunotech, Beckman Coulter, Marseille, France) as described previously (Laporta et al., 2015; Moore et al., 2015; Weaver et al., 2016). The interassay and intra-assay CVs for the serotonin assay were 15% and 18%, respectively. Serum tCa was analyzed using a colorimetric calcium assay kit (# 700550; Cayman Chemical; Ann Arbor, MI) as described previously (Laporta et al., 2015; Moore et al., 2015; Weaver et al., 2016), and interassay and intra-assay CVs for tCa were less than 10% and 9.24%, respectively. To measure total P concentrations, an aliquot of serum was precipitated with tricarboxylic acid (10% wt/vol), and the P concentration in the supernatant was determined using a molybdovanadate, colorimetric procedure in a cuvette. Absorbance was measured at 660 nm using a spectrophotometer (Gilford Spectrophotometer 260, Gilford Instrument Laboratories, Inc.). Serum Mg was measured using a colorimetric magnesium assay kit (#MG531, Randox Laboratories, United Kingdom) adapted for a 96-well plate. Briefly, 3 μL of sample and standards were loaded in duplicates into the plate and 300 μL of Randox detection solution was then added to the samples and standards. The plate was incubated at room temperature for 15 to 30 min and subsequently read at 520 nm. The interassay and intra-assay CVs were 5% and 9.6%, respectively.
Urine Sample Analysis
Urine was obtained by gently stimulating the area between the udder and the vulva. Urine samples were collected each day during the 21-d feeding period. Urine pH was analyzed from mid-stream urine samples immediately upon collection using a Horiba LAQUAtwin Compact pH Meter and samples were also stored at −20 °C to analyze tCa, Mg, P, creatinine, and deoxypyridinoline (DPD) concentrations. During the hypocalcemia challenge, a urine sample was collected immediately prior to the start of the EGTA infusion and 30 min later. Urine pH was measured mid-stream as previously described, and samples were collected and stored at −20 °C to analyze Ca, Mg, P, creatinine, and DPD concentrations.
Urine tCa, Mg, and P concentrations were analyzed by the University of Wisconsin-Madison, Veterinary Diagnostic Laboratory (Madison, WI) by inductively coupled plasma/mass spectrophotometry. Urine DPD concentrations were measured using an EIA (DPD MicroVue Bone Health). Samples were diluted 1:10 to fit within the standard curve as previously described by Weaver et al. (2016). The interassay and intra-assay CVs for the DPD assay were 10% and 10.1%, respectively. The urine Ca, Mg, P, and DPD concentrations were corrected against creatinine concentrations to account for varying urine volumes. Creatinine concentrations were determined using a colorimetric assay as previously described by Weaver et al. (2016) (DICT-500, QuantiChrom Creatinine Assay Kit, BioAssay Systems, 3191 Corporate Pl, Hayward, CA 94545). The interassay and intra-assay CVs for DPD were 4.7% and 6.3%, respectively.
Statistical Analysis
Data were analyzed using the mixed model procedure of SAS (Statistical Analysis Systems, Cary, NC). Statistical significance was set at P < 0.05. The experimental unit for statistical analysis was each individual cow. During the feeding period, all blood and urine variables were analyzed using a repeated measures analysis. The repeated measures model contained dietary treatment, day, and the interaction of dietary treatment*day as fixed effects and a random effect of cow (treatment). Two cows from each treatment group were randomly selected to balance out a day effect during the challenge period. To account for correlated errors due to repeated measurements on the same experimental unit (cow), an auto-regressive error term of order one was implemented, as seen during the feeding period and the hypocalcemia infusion period. The spatial power error structure was implemented when the times were unevenly spaced, as seen during the recovery period after the challenge.
To compare slopes for iCa during the infusion period, an analysis of covariance (ANCOVA) was used. The ANCOVA model included the fixed effects of diet (categorical), time (continuous), and the diet*time interaction. To account for correlated errors due to repeated measurements on the same experimental unit (cow), an auto-regressive error term of order one was implemented. This analysis included data up to 360 min, due to cows dropping out during the EGTA challenge.
Comparison of the Horiba LAQUAtwin iCa meter and the Abbaxis VetScan iSTAT meter was conducted by performing Pearson correlations on the residuals. Additionally, a log-likelihood ratio test was implemented in order to determine whether the variances of the residuals differed between the 2 meters.
RESULTS
21-D Feeding Period
Circulating serotonin, tCa, and total protein, blood pH, P, Mg, Na, K, and hematocrit were unaffected by dietary treatments (P > 0.05; Table 3). However, there was an effect due to day (P < 0.05) for the above-mentioned variables. Blood iCa (P = 0.04) and glucose (P = 0.03) concentrations were significantly increased in HC cows compared with their LC and MC cohorts (Table 3). Urine DPD, Ca, Mg, and P were not significantly different (P > 0.05) during the feeding period. Urine pH was significantly decreased (P = 0.02; Table 3) in the LC group compared with the MC and HC treatment groups during the feeding period. Time spent ruminating as measured by the SCR collars was not significantly different (P > 0.05) among treatments and no orts were reported for any cows in any treatment group. All cows were weighed weekly throughout the experimental period and cows randomly allocated to the MC diet were the heaviest (P = 0.0003) compared with cows on the LC and the HC diets, with no day (P > 0.05) or treatment*day interaction (P > 0.05) effects.
Table 3.
Urine, blood, and feeding variables measured during the 21-d feeding period
| Mean (SEM)1 | Dietary treatments | P value | ||
|---|---|---|---|---|
| LC | MC | HC | ||
| Urine variables | ||||
| Ca:Creatinine (mg/L:mM) | 75.6 (19.6) | 56.5 (17.9) | 81.9 (17.9) | 0.95 |
| Mg:Creatinine (mg/L:mM) | 49.1 (11.9) | 56.7 (10.8) | 69.4 (10.8) | 0.45 |
| P:Creatinine (mg/L:mM) | 217.5 (89.5) | 193.1 (89.4) | 110.3 (97.9) | 0.71 |
| DPD:Creatinine (mg/L:mM) | 8.78 (1.6) | 8.31 (1.4) | 9.24 (1.4) | 0.90 |
| pH | 5.42 (0.10)a | 5.84 (0.10)b | 5.81 (0.11)b | 0.02 |
| Blood variables | ||||
| pH | 7.411 (0.006) | 7.397 (0.006) | 7.397 (0.006) | 0.22 |
| Ionized Ca (mM) | 1.185 (0.010)a | 1.208 (0.010)a | 1.228 (0.011)b | 0.04 |
| Glucose (mg/dL) | 60.5 (2.40)a | 60.9 (2.40)a | 69.1 (2.43)b | 0.03 |
| Hematocrit (%PCV fraction) | 25.1 (0.73) | 25.3 (0.73) | 27.0 (0.74) | 0.15 |
| Total Ca (mM) | 2.35 (0.08) | 2.41 (0.08) | 2.37 (0.08) | 0.86 |
| Total P (mg/dL) | 7.24 (0.35) | 7.05 (0.35) | 6.94 (0.35) | 0.83 |
| Total Mg (mg/dL) | 1.94 (0.13) | 2.14 (0.13) | 1.84 (0.13) | 0.30 |
| Serotonin (ng/mL) | 5070 (1364) | 7441 (1246) | 7716 (1264) | 0.33 |
| Feeding variables | ||||
| Rumination time (min/24 h) | 380.2 (29.1) | 364.1 (26.6) | 314.1 (26.7) | 0.24 |
| Average weekly body weight (kg) | 722.0 (7.74)a | 796.9 (5.09)b | 727.6 (7.37)a | 0.0003 |
a,bResults with different superscripts significantly differ (P < 0.05).
1Multiparous, nonlactating, nonpregnant Holstein cows were fed a negative dietary cation–anion difference (average −18.2 across all diets) for 21 d with low (LC; 0.45% Ca; n = 5), medium (MC; 1.13% Ca; n = 6) or high (HC; 2.02% Ca; n = 6) concentrations of dietary Ca. On days 22, 23, and 24, hypocalcemia was induced with an intravenous infusion of 5% EGTA in 2 different cows from each treatment daily. Urine and blood samples were collected and urine pH measured daily during the 21-d feeding period prior to hypocalcemia challenge. Data presented as the mean value (SEM).
Hypocalcemia Challenge Period
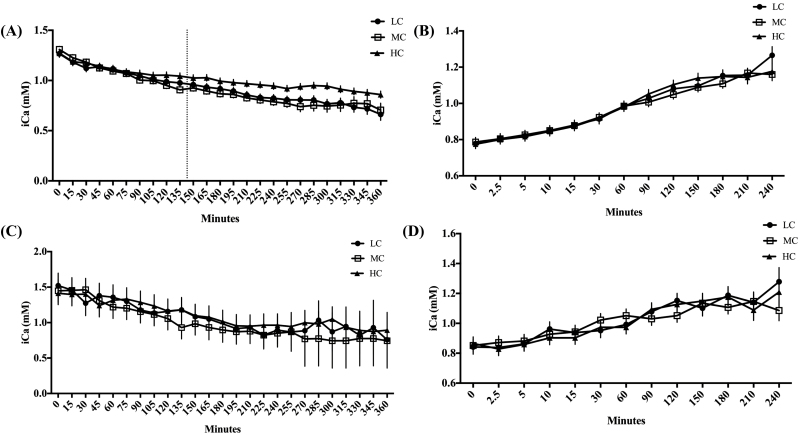
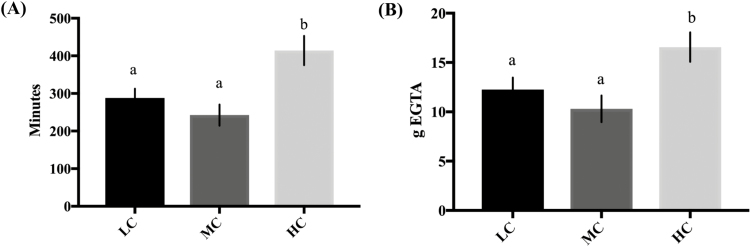
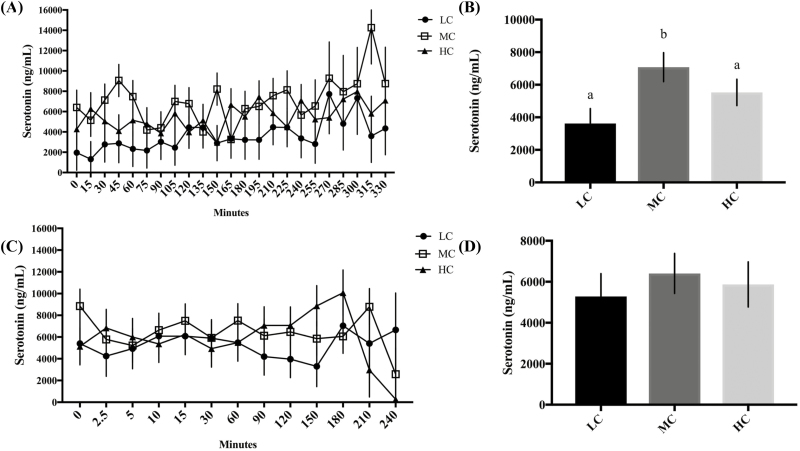
Cows fed the HC diet had the highest average iCa concentrations (P = 0.03) compared with MC, and LC, throughout the entire hypocalcemia challenge period (Figure 2A). The rate at which iCa concentrations decreased during the first 145 min of the challenge was slower (P = 0.003) in HC cows than for either MC or LC fed cows (Figure 2A). Cows fed the MC diet had the fastest rate of decline (P = 0.003) compared with the LC and HC groups through 145 min (Figure 2A). Between 145 and 360 min of EGTA infusion, the rate of decline was similar among groups (P > 0.05; Figure 2A), but the HC group had increased iCa concentrations compared with the MC (P = 0.001) and LC groups (P = 0.001; Figure 2A). We analyzed the overall average time it took each dietary treatment to reach 60% of iCa compared with where their initial Ca was and total grams of EGTA used based on cow body weight, using a one-way ANOVA. Cows fed HC took significantly more time than the MC (P = 0.004) and LC (P = 0.04) groups to reach 60% of initial blood iCa (Figure 3A). Total grams of EGTA based on cow body weight (Figure 3B) required to reach 60% of initial iCa were increased (P = 0.01) in HC cows compared with LC and MC cows. Serum serotonin concentrations (Figure 4A and B) were significantly increased (P = 0.05) in MC group compared with HC and LC fed cows. Cows fed HC tended (P = 0.09) to have elevated concentrations of serotonin relative to the LC fed cows. Serum tCa concentrations decreased in response to induction (P < 0.0001), but was unaffected by treatment (P > 0.05; Figure 5A). Serum total Mg concentrations increased in response to induction (P = 0.01); however, they were unaffected by treatment (P > 0.05; data not shown). There were no treatment*time interactions for serotonin, iCa, or Mg during the induction period (P > 0.05). Blood pH, Na, K, glucose, and hematocrit (data not shown) were unaffected by diet during the induction period (P > 0.05). Urine pH, DPD, Ca, Mg, and P were also unaffected by treatment and time during the hypocalcemia challenge (P > 0.05; data not shown).
Figure 2.
Ionized calcium (iCa) concentrations in response to challenge and recovery from an EGTA/hypocalcemia challenge in multiparous, nonlactating, nonpregnant Holstein cows fed a negative dietary cation–anion difference (average −18.2 across all diets) for 21 d with low (LC; 0.45% Ca; n = 5), medium (MC; 1.13% Ca; n = 6) or high (HC; 2.02% Ca; n = 6) concentrations of dietary Ca. On days 22, 23, and 24, hypocalcemia was induced with an intravenous infusion of 5% EGTA in 2 different cows from each treatment daily. The LC treatment groups is represented by the solid circles, the MC treatment group by the open squares, and the HC treatment group by the solid triangles. (A) Ionized calcium (iCa) concentrations as measured by the Abaxis iSTAT in response to EGTA challenge. Line break at 145 min is representative of where of the slopes of the lines change. Prior to 145 min the slopes of the lines are different (P < 0.0001) and after 145 min the slopes of all the lines are equal (P > 0.05). (B) Ionized calcium (iCa) concentrations as measured by the Abaxis iSTAT during the recovery to EGTA challenge. (C) Ionized calcium (iCa) concentrations as measured by the Horiba LAQUAtwin Compact Calcium Ion meter in response to EGTA challenge. (D) Ionized calcium (iCa) concentrations as measured by the Horiba LAQUAtwin Compact Calcium Ion meter during the recovery from the EGTA challenge. All data are represented as the least square means ± standard error of the mean.
Figure 3.
Average time and quantity of EGTA required to reach 60% of initial ionized calcium (iCa) concentrations during induction of hypocalcemia in multiparous, non-lactating, nonpregnant Holstein cows fed a negative dietary cation–anion difference (average −18.2 across all diets) for 21 d with low (LC; 0.45% Ca; n = 5), medium (MC; 1.13% Ca; n = 6), or high (HC; 2.02% Ca; n = 6) concentrations of dietary Ca. On days 22, 23, and 24, hypocalcemia was induced with an intravenous infusion of 5% EGTA in 2 different cows from each treatment daily. (A) Amount of time required to reaching 60% of initial ionized calcium (iCa) concentrations in response to EGTA. a,bResults with different superscripts significantly differ (P<0.05). (B) Amount of EGTA, corrected for body weight, required to reaching 60% of initial ionized calcium (iCa) concentrations in response to EGTA. a,bResults with different superscripts significantly differ (P<0.05). All data are represented as the least square means ± standard error of the mean.
Figure 4.
Serum serotonin concentrations in response to induction and recovery from a hypocalcemia challenge in multiparous, nonlactating, nonpregnant Holstein cows fed a negative dietary cation–anion difference (average -18.2 across all diets) for 21 d with low (LC; 0.45% Ca; n = 5), medium (MC; 1.13% Ca; n = 6), or high (HC; 2.02% Ca; n = 6) concentrations of dietary Ca. On days 22, 23, and 24, hypocalcemia was induced with an intravenous infusion of 5% EGTA in 2 different cows from each treatment daily. The LC treatment groups is represented by the solid circles, the MC treatment group by the open squares, and the HC treatment group by the solid triangles. (A) Serum serotonin concentrations in response to EGTA. (B) Average serum serotonin concentrations of all time points during the EGTA challenge. a,bResults with different superscripts significantly differ (P<0.05). (C) Serum serotonin concentrations during the recovery period from the EGTA challenge. (D) Average serum serotonin concentrations of all time points during the recovery period from the EGTA challenge. All data are represented as the least square means ± standard error of the mean.
Figure 5.
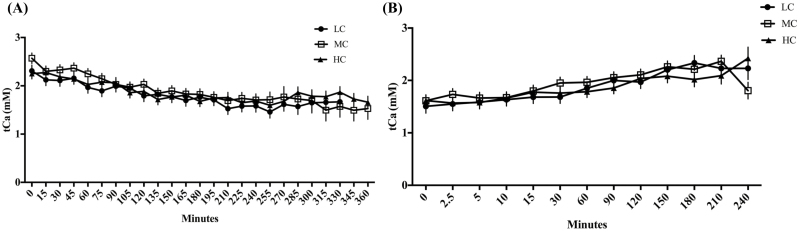
Total calcium (tCa) concentrations in response to induction and recovery from a hypocalcemia challenge in multiparous, nonlactating, nonpregnant Holstein cows fed a negative dietary cation–anion difference (average −18.2 across all diets) for 21 d with low (LC; 0.45% Ca; n = 5), medium (MC; 1.13% Ca; n = 6), or high (HC; 2.02% Ca; n = 6) concentrations of dietary Ca. The LC treatment group is represented by the solid circles, the MC treatment group by the open squares, and the HC treatment group by the solid triangles. (A) Total calcium (tCa) concentrations in response to EGTA challenge. (B) Total calcium (tCa) concentrations during the recovery from the EGTA. All data are represented as the least square means ± standard error of the mean.
Hypocalcemia (EGTA) Recovery Period
During the recovery period, only iCa (Figure 2B) and tCa (Figure 5B) were affected by time. Ionized and tCa concentrations increased (P < 0.0001) with time. No treatment effects (P > 0.05) or an interaction between treatment and time (P > 0.05) were detected. All other variables measured were not affected by time (P > 0.05), treatment (P > 0.05), or the time by treatment interaction (P > 0.05) during the recovery period.
iSTAT vs. Horiba Meter
We assessed the use of the Horiba LAQUAtwin ionized calcium meter as a quick and inexpensive cow-side tool to measure iCa concentrations (Figure 2C and D). The Horiba meter was calibrated at every measurement and used at every time point during the hypocalcemia challenge and recovery periods in tandem with the VetScan iSTAT. Variance between the 2 meters was evaluated by a likelihood ratio to determine equality. The variance for the VetScan iSTAT was 0.02745, and the variance for the Horiba meter was 0.14447 for iCa measurements during the hypocalcemia (EGTA) challenge and recovery periods. The chi-square value for these variances was significant (P < 0.0001). These results indicate that the Horiba meter is more variable when measuring blood iCa concentration and produces values that are significantly different and higher (P < 0.001) than values obtained from the VetScan iSTAT. Additionally, because the values obtained from the Horiba meter were significantly increased, it is probable that using this meter would result in an under diagnosis of SCH and more false negative cases when compared with the VetScan iSTAT.
DISCUSSION
The practice of feeding dry cows a diet containing acidogenic salts to manipulate Ca homeostasis has been implemented and evaluated in North America for over 30 years. Based on 2 meta-analyses that involved over 2,500 cows, researchers determined that not only feeding a higher DCAD diet prepartum resulted in a greater risk for hypocalcemia, but that dietary Ca supplementation during the prepartum period also had a quadratic effect for determining the pathogenesis of milk fever (Oetzel, 1991; Lean et al., 2006). Furthermore, they demonstrated an interdependence between levels of DCAD and dietary Ca supplementation, with pathogenesis of milk fever (Oetzel, 1991; Lean et al., 2006). However, there are studies that were performed in which feeding diets with a range of higher dietary Ca concentrations in combination with a negative DCAD did not result in improve postpartum Ca homeostasis, as measured by plasma tCa (Oetzel et al., 1988; Goff and Horst, 1997). It should be noted that these studies did not use a dietary Ca level of 2.02%, which is what was used in our study. Moore et al. (2000) performed an experiment where varying levels of dietary Ca were included in the diets; however, the DCAD level was not held constant. The results of this study demonstrated that feeding a diet consisting of −15 DCAD and 1.50% dietary Ca to mature cows resulted in elevated Ca concentrations postpartum compared with the other dietary treatment groups (Moore et al., 2000). These findings also suggest that elevated dietary Ca may be critical for improving postpartum Ca concentrations; however, the effect of a negative DCAD diet in combination with varying levels of dietary Ca was not explored. Many research studies have been completed to reduce hypocalcemia by focusing on feeding a low Ca diet, in order to stimulate the classical negative feedback pathway between Ca, gastrointestinal absorption of Ca, and bone resorption (Jorgensen, 1974; Barton et al., 1981). Furthermore, prepartum diets have been routinely formulated to feed a range of dietary Ca in combination with a negative DCAD to reduce hypocalcemia (Oetzel et al., 1988; Goff and Horst, 1997; Leno et al., 2017b). The premise of the experiment described herein was to determine the ability of varying dietary Ca levels, in combination with a negative DCAD, to improve the response to an induced hypocalcemic challenge.
21-D Feeding Period
Cows fed the HC diet had increased blood iCa and glucose concentrations during the feeding period. Previous research has shown that periparturient cows with SCH have increased concentrations of both nonesterified fatty acids and β-hydroxybutyrate concentrations, when compared with normocalcemic cows (Martinez et al., 2012). Interestingly, in a hypocalcemia induction model performed in nonlactating, nonpregnant dairy cows, it was observed that blood glucose concentrations were elevated during the induction of hypocalcemia (Martinez et al., 2014). It is possible that the increased blood glucose concentrations are a result of increased iCa; however, this has not been previously demonstrated, as previous research has shown that this was due to suppression of insulin secretion in hypocalcemic cows (Littledike et al., 1968; Witzel and Littledike, 1973). However, it could be important to explore other endocrine parameters to interpret this finding, given that previous work demonstrated that inducing hypocalcemia increased blood glucose concentrations (Martinez et al., 2014). The relationship of energy parameters and endocrine hormones to Ca was not an objective of these experiments; however, it could be important in future experiments. Particularly, the observed increase in blood glucose concentrations in cows in the Martinez et al. (2014) experiment was fed a negative DCAD of 160 mEq/kg, and 0.24% Ca, which is substantially different from the diets in our experiment. A recent study demonstrated that cows with less than or equal to 2.4 mM tCa concentrations prepartum were at an increased risk for developing SCH postpartum (Neves et al., 2017). Therefore, the increased iCa concentrations found during our study could be a possible explanation for the resistance to the hypocalcemia challenge in the HC group that was evident; however, this should be explored more thoroughly to determine whether this is truly the mechanism of action. However, these cows were dry and nonpregnant, which is a physiologically different state compared with the cows in the Neves et al. (2017) experiment, in which the cows were dry and pregnant. Pregnancy and lactation both alter Ca homeostasis when compared with animals that are nonpregnant and nonlactating (Wysolmerski, 2010). Additionally, other than a lower urine pH in the LC treatment group, we saw no differences in excretion of minerals or DPD in the urine. These data suggest that during the feeding period, the LC cows did not increase their bone turnover to compensate for the low dietary Ca, compared with the HC and MC groups. This is not surprising, given that these cows did not have the Ca demand of a developing fetus or colostrum/milk synthesis. We would not expect to see large changes in the status of mineral reserves within the cows until colostrum and milk formation begins, such as during late pregnancy and lactation (Benzie et al., 1955; El-Samad et al., 2002).
Hypocalcemia Challenge and Recovery Periods
These data demonstrated that feeding 2.02% DM dietary Ca, in combination with a negative DCAD diet, resulted in a slower reduction in blood iCa in response to an induced hypocalcemia challenge when compared with cows fed the LC or MC diets. Cows fed the LC diet (0.45% dietary Ca) had a faster decrease in iCa during the hypocalcemia challenge during the first 145 min of the challenge compared with cows fed the HC diet. However, during the hypocalcemia challenge period, cows fed the MC diet had the fastest rate of decline in iCa concentrations, whereas cows fed the HC diet had the slowest rate of decline. Cow fed the LC treatment had a moderate rate of decline in iCa concentrations compared with the HC and MC fed cows. These results were not surprising, given the predictions related to clinical milk fever based on the meta-analyses by Oetzel (1991) and Lean et al. (2006). It may be of interest to extrapolate their findings to tCa and iCa concentrations in the future to determine whether the analysis would still apply. Both data sets indicated that using DCAD = (Na+ + K+) − (Cl− + 0.6S2−) was the best model for predicting milk fever incidence, and that low or high dietary Ca percentage fed prepartum reduced milk fever incidence. Surprisingly, very little effort has been focused on the use of high Ca diets to prevent milk fever, particularly in combination with negative DCAD diets. Previous research on “high” Ca diets used a dietary Ca concentration that was closer to our MC dietary treatment and improvements in hypocalcemia were not observed when feeding these diets (Kichura et al., 1982; van de Braak et al., 1982; Chan et al., 2006; Liesegang et al., 2007). Other studies that fed 1.6% dietary Ca prepartum did not examine the effects of a low Ca diet in combination with feeding acidogenic salts (Tucker et al., 1992). In a study where cows were fed an acidogenic diet with 1.5% dietary Ca, they did not see a benefit on incidence of milk fever; however, our study used 2.02% dietary Ca (Goff and Horst, 1997). Our data indicate that feeding 2.02% dietary Ca, along with a negative DCAD, resulted in the slowest rate of decline in blood iCa concentrations during the first 145 min of the challenge. This may simulate what could happen in a peripartum cow fed high dietary Ca. However, this experiment must be repeated in periparturient cows to determine whether we would observe the same response given the difference in physiological state. Cows that exhibit more rapid and severe declines in blood Ca at calving are more likely to succumb to hypocalcemia (Kichura et al., 1982). Unexpectedly, cows fed the LC diet were not as resistant to the hypocalcemia challenge as the HC treatment group. However, given the dietary forages fed, the LC treatment group received 57 g/d of Ca rather than the <20-g/d level determined to be the maximum level for activation of the negative feedback loop for Ca homeostasis through bone resorption (Goff and Horst, 1997; Thilsing-Hansen et al., 2002). Additionally, we determined that the use of an inexpensive, cow-side meter, the Horiba LAQUAtwin iCa meter was not adequate for measuring iCa concentrations compared with the Abbott VetScan iSTAT. However, there is a recently published study demonstrating the use of an updated prototype of the Horiba LAQUAtwin iCa meter that is more sensitive and specific for iCa than the model utilized in this experiment (Neves et al., 2018). The use of this prototype for the analysis of iCa should be further investigated.
Surprisingly, although there was evidence for time-dependent changes in iCa in response to the hypocalcemia challenge, we saw no treatment effects on tCa concentrations. Although tCa is the most common method for clinical assessment of hypocalcemia, iCa is the biologically active form of the mineral (Schenck and Chew, 2008; Baird 2011). Calcium status as determined by tCa is based on the assumption that tCa and iCa are proportional and that the proportional relationship is static. Previous research in both humans and dogs have demonstrated that both tCa concentrations and tCa concentrations that have been adjusted for variables such as serum albumin are unacceptable for predicting iCa status (Thode et al., 1989; Schenck and Chew, 2005). This is 1 possible explanation for why we were unable to detect treatment differences in tCa concentrations in this experiment. Albumin is the primary protein in blood that provides short-term maintenance of a stable iCa concentration by acting as a buffer and it is known that the correlation between iCa and tCa concentrations significantly decreases when there are alterations in serum albumin concentrations (Baird, 2011). Previous data in periparturient dairy cows have shown that plasma protein and albumin concentrations were decreased when cows were fed acidogenic diets. However, this was not examined in the context of varying levels of dietary Ca (Grunberg et al., 2011). Furthermore, our study utilized nonpregnant, nonlactating dairy cows with presumably stable Ca balance. One study conducted in early postpartum dairy cows demonstrated that due to the variation between iCa and tCa, they cannot be used interchangeably during the early postpartum period (Leno et al., 2017a). This study determined that the assumed relationship between tCa and iCa do not reliably identify the same population of cows during the early postpartum period. These findings support our observations that iCa was the variable most effected by the EGTA challenge. More research is needed to understand the relationship between circulating tCa, iCa, and protein concentrations in dairy cows.
Serum serotonin concentrations were also significantly different between dietary treatment groups during the EGTA challenge period. The MC cows, which would be at the highest risk for developing hypocalcemia based on the models created by Oetzel (1991) and Lean et al. (2006), had the highest concentrations of serotonin during the EGTA challenge period. Furthermore, the LC group has the lowest and the HC group had intermediate serotonin concentrations. These data suggest that Ca and serotonin are working in a feedback loop to regulate Ca homeostasis. Previous research in rodents has demonstrated that administration of a precursor to serotonin increases mRNA and protein expression of several mammary Ca pumps and transporters, as well as increases in milk Ca concentrations (Laporta et al., 2013, 2014). Furthermore, when administering a serotonin precursor to late lactation dairy cows, milk Ca concentrations were increased (Laporta et al., 2015). Finally, in a recent study in periparturient dairy cows, providing a serotonin precursor prepartum increased tCa concentrations postpartum, independent of parathyroid hormone (Hernandez-Castellano et al., 2017). It is possible that serotonin and Ca are working together, independent of the classic Ca homeostasis pathways, to maintain circulating iCa concentrations. However, this hypothesis requires further investigation.
We did not see treatment effects on any parameters measured during the recovery period. Upon cessation of the EGTA infusion, iCa and tCa concentrations recovered in all treatment groups at equal, rapid rates. In a similar study where EDTA was used as the Ca chelator, nonlactating, nonpregnant cows fed a negative DCAD with high Ca diets experienced a shorter recovery time after hypocalcemia challenge than cows fed a negative DCAD with low Ca diets (Oba et al., 2011). Although we did not observe this in our experiment, EDTA is not a selective Ca chelator. Furthermore, this finding supports the fact that blood Ca concentrations are very tightly controlled, particularly in the nonpregnant, nonlactating dairy cow (Baird, 2011). Additionally, these data suggest that when hypocalcemia occurs, the resistance to the hypocalcemia challenge may be the critical measurement, not necessarily the recovery from the challenge.
Overall Conclusions
Multiparous, dry, nonpregnant cows fed 2.02% dietary Ca in combination with a negative DCAD diet had a slower rate of decline in iCa concentrations in response to the induction of hypocalcemia using a 5% EGTA challenge compared with cows fed 0.45% or 1.13% dietary Ca. The HC cows also maintained higher iCa concentrations throughout the entire hypocalcemia challenge period compared with the other 2 dietary groups. The MC cows had the fastest rate of decline in response to the EGTA challenge compared with the HC and LC fed groups. More EGTA and time were required for HC cows to reach 60% of their initial iCa concentration during the EGTA challenge compared with MC and LC fed cows. Cows fed the MC diet also had increased serotonin concentrations during the hypocalcemia challenge compared with the HC and LC fed groups, during the first 145 min of the EGTA challenge period. Cows fed the HC diet also had increased blood iCa and glucose concentrations during the 21-d feeding period prior to initiation of the hypocalcemia challenge. These data suggest that the role of feeding negative DCAD diets and the amount of dietary Ca on the resistance to hypocalcemia should be further investigated.
LITERATURE CITED
- Baird G. S. 2011. Ionized calcium. Clin. Chim. Acta. 412:696–701. doi: 10.1016/j.cca.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Barton B. A., Horst R. L., Jorgensen N. A., and DeLuca H. F.. 1981. Concentration of calcium, phosphorus, and 1,25-dihydroxyvitamin D in plasma of dairy cows during the lactation cycle. J. Dairy Sci. 64:850–852. doi: 10.3168/jds.S0022-0302(81)82657-4 [DOI] [PubMed] [Google Scholar]
- Belyea R. L., Coppock C. E., and Lake G. B.. 1976. Effects of a low calcium diet on feed intake, milk production, and response to blood calcium challenge in lactating Holstein cows. J. Dairy Sci. 59:1068–1077. doi: 10.3168/jds.S0022-0302(76)84325-1 [DOI] [PubMed] [Google Scholar]
- Benzie D., Boyne A. W., Dalgarno A. C., Duckworth J., Hill R., and Walker D. M.. 1955. Studies of the skeleton of the sheep: the effect of different levels of dietary calcium during pregnancy and lactation on individual bones. J. Agri. Sci. 46:425–444. [Google Scholar]
- Block E. 1984. Manipulating dietary anions and cations for prepartum dairy cows to reduce incidence of milk fever. J. Dairy Sci. 67:2939–2948. doi: 10.3168/jds.S0022-0302(84)81657-4 [DOI] [PubMed] [Google Scholar]
- van de Braak A. E., van’t Klooster E. T., Malestein A., and Faber J. A. J.. 1986. Effects of low and high calcium intake prepartum on calcium mobilization rate around parturition in dairy cows. Vet. Q. 8:12–23. doi:10.1080/01652176.1986.9694013 [DOI] [PubMed] [Google Scholar]
- Chan P. S., West J. W., and Bernard J. K.. 2006. Effect of prepartum dietary calcium on intake and serum and urinary mineral concentrations of cows. J. Dairy Sci. 89:704–713. doi: 10.3168/jds.S0022-0302(06)72133-6 [DOI] [PubMed] [Google Scholar]
- El-Samad H., Goff J. P., and Khammash M.. 2002. Calcium homeostasis and parturient hypocalcemia: an integral feedback perspective. J. Theor. Biol. 214:17–29. doi: 10.1006/jtbi.2001.2422 [DOI] [PubMed] [Google Scholar]
- Goff J. P. 2004. Macromineral disorders of the transition cow. Vet. Clin. North Am. Food Anim. Pract. 20:471–94, v. doi: 10.1016/j.cvfa.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Goff J. P. 2008. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 176:50–57. doi: 10.1016/j.tvjl.2007.12.020 [DOI] [PubMed] [Google Scholar]
- Goff J. P. 2014. Calcium and magnesium disorders. Vet. Clin. North Am. Food Anim. Pract. 30:359–81, vi. doi: 10.1016/j.cvfa.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Goff J. P., and Horst R. L.. 1997. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 80:1260–1268. doi: 10.3168/jds.S0022-0302(97)76055-7 [DOI] [PubMed] [Google Scholar]
- Goff J. P., Liesegang A., and Horst R. L.. 2014. Diet-induced pseudohypoparathyroidism: a hypocalcemia and milk fever risk factor. J. Dairy Sci. 97:1520–1528. doi: 10.3168/jds.2013-7467 [DOI] [PubMed] [Google Scholar]
- Goings R. L., Jacobson N. L., Beitz D. C., Littledike E. T., and Wiggers K. D.. 1974. Prevention of parturient paresis by a prepartum, calcium-deficient diet. J. Dairy Sci. 57:1184–1188. doi: 10.3168/jds.S0022-0302(74)85034-4 [DOI] [PubMed] [Google Scholar]
- Green H. B., Horst R. L., Beitz D. C., and Littledike E. T.. 1981. Vitamin D metabolites in plasma of cows fed a prepartum low-calcium diet for prevention of parturient hypocalcemia. J. Dairy Sci. 64:217–226. doi: 10.3168/jds.S0022-0302(81)82557-X [DOI] [PubMed] [Google Scholar]
- Grunberg W., Donkin S. S., and Constable P. D.. 2011. Periparturient effects of feeding a low dietary cation-anion difference diet on acid-base, calcium, and phosphorus homeostasis and on intravenous glucose tolerance test in high-producing dairy cows. J. Dairy Sci. 94:727–745. doi: 10.3168/jds.2010-3230 [DOI] [PubMed] [Google Scholar]
- Hernandez-Castellano L. E., Hernandez L. L., Sauerwein H., and Bruckmaier R. M.. 2017. Endocrine and metabolic changes in transition dairy cows are affected by prepartum infusions of a serotonin precursor. J. Dairy Sci. 100:5050–5057. doi: 10.3168/jds.2016-12441 [DOI] [PubMed] [Google Scholar]
- Heron V. S., Tremblay G. F., and Oba M.. 2009. Timothy hays differing in dietary cation-anion difference affect the capability of dairy cows to maintain their calcium homeostasis. J. Dairy Sci. 92:238–246. doi: 10.3168/jds.2008-1357 [DOI] [PubMed] [Google Scholar]
- Jorgensen N. A. 1974. Combating milk fever. J. Dairy Sci. 57:933–944. doi: 10.3168/jds.S0022-0302(74)84989-1 [DOI] [PubMed] [Google Scholar]
- Kichura T. S., Horst R. L., Beitz D. C., and Littledike E. T.. 1982. Relationships between prepartal dietary calcium and phosphorus, vitamin D metabolism, and parturient paresis in dairy cows. J. Nutr. 112:480–487. doi: 10.1093/jn/112.3.480 [DOI] [PubMed] [Google Scholar]
- Laporta J., Keil K. P., Vezina C. M., and Hernandez L. L.. 2014. Peripheral serotonin regulates maternal calcium trafficking in mammary epithelial cells during lactation in mice. PLoS One 9:e110190. doi: 10.1371/journal.pone.0110190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporta J., Moore S. A., Weaver S. R., Cronick C. M., Olsen M., Prichard A. P., Schnell B. P., Crenshaw T. D., Peñagaricano F., Bruckmaier R. M.,. et al. 2015. Increasing serotonin concentrations alter calcium and energy metabolism in dairy cows. J. Endocrinol. 226:43–55. doi: 10.1530/JOE-14-0693 [DOI] [PubMed] [Google Scholar]
- Laporta J., Peters T. L., Weaver S. R., Merriman K. E., and Hernandez L. L.. 2013. Feeding 5-hydroxy-l-tryptophan during the transition from pregnancy to lactation increases calcium mobilization from bone in rats. Domest. Anim. Endocrinol. 44:176–184. doi: 10.1016/j.domaniend.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Lean I. J., DeGaris P. J., McNeil D. M., and Block E.. 2006. Hypocalcemia in dairy cows: meta-analysis and dietary cation anion difference theory revisited. J. Dairy Sci. 89:669–684. doi: 10.3168/jds.S0022-0302(06)72130-0 [DOI] [PubMed] [Google Scholar]
- Leno B. M., Martens E. M., Felippe M. J. B., Zanzalari K. P., Lawrence J. C., and Overton T. R.. 2017a. Short communication: relationship between methods for measurement of serum electrolytes and the relationship between ionized and total calcium and neutrophil oxidative burst activity in early postpartum dairy cows. J. Dairy Sci. 100:9285–9293. doi: 10.3168/jds.2017-12971 [DOI] [PubMed] [Google Scholar]
- Leno B. M., Ryan C. M., Stokol T., Kirk D., Zanzalari K. P., Chapman J. D., and Overton T. R.. 2017b. Effects of prepartum dietary cation-anion difference on aspects of peripartum mineral and energy metabolism and performance of multiparous Holstein cows. J. Dairy Sci. 100:4604–4622. doi: 10.3168/jds.2016-12221 [DOI] [PubMed] [Google Scholar]
- Liesegang A., Chiappi C., Risteli J., Kessler J., and Hess H. D.. 2007. Influence of different calcium contents in diets supplemented with anionic salts on bone metabolism in periparturient dairy cows. J. Anim. Physiol. Anim. Nutr. (Berl). 91:120–129. doi: 10.1111/j.1439-0396.2006.00651.x [DOI] [PubMed] [Google Scholar]
- Littledike E. T., Witzel D. A., and Whipp S. C.. 1968. Insulin: evidence for inhibition of release in spontaneous hypocalcemia. Proc. Soc. Exp. Biol. Med. 129:135–139. PMID: 5693728. [DOI] [PubMed] [Google Scholar]
- Martinez N., Risco C. A., Lima F. S., Bisinotto R. S., Greco L. F., Ribeiro E. S., Maunsell F., Galvão K., and Santos J. E.. 2012. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J. Dairy Sci. 95:7158–7172. doi: 10.3168/jds.2012-5812 [DOI] [PubMed] [Google Scholar]
- Martinez N., Sinedino L. D., Bisinotto R. S., Ribeiro E. S., Gomes G. C., Lima F. S., Greco L. F., Risco C. A., Galvão K. N., Taylor-Rodriguez D.,. et al. 2014. Effect of induced subclinical hypocalcemia on physiological responses and neutrophil function in dairy cows. J. Dairy Sci. 97:874–887. doi: 10.3168/jds.2013-7408 [DOI] [PubMed] [Google Scholar]
- Moore S. A., Laporta J., Crenshaw T. D., and Hernandez L. L.. 2015. Patterns of circulating serotonin and related metabolites in multiparous dairy cows in the peripartum period. J. Dairy Sci. 98:3754–3765. doi: 10.3168/jds.2014-8841 [DOI] [PubMed] [Google Scholar]
- Moore S. J., VandeHaar M. J., Sharma B. K., Pilbeam T. E., Beede D. K., Bucholtz H. F., Liesman J. S., Horst R. L., and Goff J. P.. 2000. Effects of altering dietary cation-anion difference on calcium and energy metabolism in peripartum cows. J. Dairy Sci. 83:2095–2104. doi: 10.3168/jds.S0022-0302(00)75091-0 [DOI] [PubMed] [Google Scholar]
- Muir L. A., Hibbs J. W., Conrad H. R., and Smith K. L.. 1972. Effect of estrogen and progesterone on feed intake and hydroxyproline excretion following induced hypocalcemia in dairy cows. J. Dairy Sci. 55:1613–1620. doi: 10.3168/jds.S0022-0302(72)85729-1 [DOI] [PubMed] [Google Scholar]
- Neves R. C., Leno B. M., Stokol T., Overton T. R., and McArt J. A. A.. 2017. Risk factors associated with postpartum subclinical hypocalcemia in dairy cows. J. Dairy Sci. 100:3796–3804. doi: 10.3168/jds.2016-11970 [DOI] [PubMed] [Google Scholar]
- Neves R. C., Stokol T., Bach K. D., and McArt J. A. A.. 2018. Method comparison and validation of a prototype device for measurement of ionized calcium concentrations cow-side against a point-of-care instrument and a benchtop blood-gas analyzer reference method. J. Dairy Sci. 101:1334–1343. doi: 10.3168/jds.2017-13779 [DOI] [PubMed] [Google Scholar]
- NRC 2001. Nutrient requirements of dairy cattle. 7th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Oba M., Oakley A. E., and Tremblay G. F.. 2011. Dietary Ca concentration to minimize the risk of hypocalcemia in dairy cows affected by the dietary cation-anion differences. Anim. Feed Sci. Technol. 164:147–153. doi:10.1016/j.anifeedsci.2011.01.002 [Google Scholar]
- Oetzel G. R. 1991. Meta-analysis of nutritional risk factors for milk fever in dairy cattle. J. Dairy Sci. 74:3900–3912. doi: 10.3168/jds.S0022-0302(91)78583-4 [DOI] [PubMed] [Google Scholar]
- Oetzel G. R., Olson J. D., Curtis C. R., and Fettman M. J.. 1988. Ammonium chloride and ammonium sulfate for prevention of parturient paresis in dairy cows. J. Dairy Sci. 71:3302–3309. doi: 10.3168/jds.S0022-0302(88)79935-X [DOI] [PubMed] [Google Scholar]
- Robinson P. H., and Meyer D.. 2010. Total mixed ration (TMR) sampling protocol University of California, Agricultural and Natural Resources, Richmond, CA. ANR Publication; 8413. http://anrcatalog.ucdavis.edu [Google Scholar]
- Schenck P. A., and Chew D. J.. 2005. Prediction of serum ionized calcium concentration by use of serum total calcium concentration in dogs. Am. J. Vet. Res. 66:1330–1336. [DOI] [PubMed] [Google Scholar]
- Schenck P. A., and Chew D. J.. 2008. Calcium: total or ionized?Vet. Clin. North Am. Small Anim. Pract. 38:497–502, ix. doi: 10.1016/j.cvsm.2008.01.010 [DOI] [PubMed] [Google Scholar]
- Thilsing-Hansen T., Jørgensen R. J., and Østergaard S.. 2002. Milk fever control principles: a review. Acta Vet. Scand. 43:1–19. doi:10.1186/1751-0147-43-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thode J., Juul-Jørgensen B., Bhatia H. M., Kjaerulf-Nielsen M., Bartels P. D., Fogh-Andersen N., and Siggaard-Andersen O.. 1989. Comparison of serum total calcium, albumin-corrected total calcium, and ionized calcium in 1213 patients with suspected calcium disorders. Scand. J. Clin. Lab. Invest. 49:217–223. PMID: 2662382. [PubMed] [Google Scholar]
- Tucker W. B., Hogue J. F., Adams G. D., Aslam M., Shin I. S., and Morgan G.. 1992. Influence of dietary cation-anion balance during the dry period on the occurrence of parturient paresis in cows fed excess calcium. J. Anim. Sci. 70:1238–1250. PMID: 1582954. [DOI] [PubMed] [Google Scholar]
- Weaver S. R., Prichard A. P., Endres E. L., Newhouse S. A., Peters T. L., Crump P. M., Akins M. S., Crenshaw T. D., Bruckmaier R. M., and Hernandez L. L.. 2016. Elevation of circulating serotonin improves calcium dynamics in the peripartum dairy cow. J. Endocrinol. 230:105–123. doi: 10.1530/JOE-16-0038 [DOI] [PubMed] [Google Scholar]
- Witzel D. A., and Littledike E. T.. 1973. Suppression of insulin secretion during induced hypocalcemia. Endocrinology 93:761–766. doi: 10.1210/endo-93-4-761 [DOI] [PubMed] [Google Scholar]
- Wysolmerski J. J. 2010. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann. N. Y. Acad. Sci. 1192:161–169. doi: 10.1111/j.1749-6632.2009.05249.x [DOI] [PMC free article] [PubMed] [Google Scholar]