Abstract
Mycotoxins are toxic secondary metabolites produced by various fungi and are known to contaminate animal feed ingredients especially cereals. One of the most common mycotoxins in swine diets is deoxynivalenol (DON) which is known to decrease growth performance. The objective of the present study was to evaluate the effects of single or repeated short-term DON challenges on growth performance, and feeding behavior in finishing pigs. A total of 160 pigs were distributed to four experimental groups in two successive replicates with each pig individually measured for live BW and individually fed using an electronic feeding station. The pigs in control group CC were fed with a standard finisher diet during the whole duration of the experimental period. Groups DC, CD, and DD were given the DON-contaminated diet (3.02 mg DON/kg feed) for 7 d at 113 d, at 134 d, and at 113 and 134 d of age, respectively. The DON-contaminated diet was formulated with a naturally contaminated corn. During challenge periods, ADFI was decreased by 26% to 32% (P < 0.05) and ADG by 40% to 60% (P < 0.05). The drop in ADFI during DON challenges was associated with changes in the feeding behavior: when compared to the nonchallenged pigs, pigs fed with DON-contaminated diet had lower number of meals per day (9.6 versus 8.2 meals per day on average; P < 0.05) and slower feeding rate (42.0 g/min versus 39.9 g/min on average; P < 0.05). For the whole trial period, pigs submitted to the DON challenge at the end of the experiment (i.e., first time for CD group and second time for DD group) had a lower (P < 0.05) ADFI (2.67 and 2.59 kg/d, respectively) when compared to the control CC group of pigs (2.87 kg/d). An intermediate value was reported for the DC groups (2.79 kg/d). All challenged groups, i.e., DC, CD, and DD pigs, had lower (P < 0.05) overall ADG (970, 940, and 900 g/day, respectively) than CC (1,050 g/day) for the whole trial period. Pigs challenged early in the trial, i.e., DC and DD groups, had a higher (P < 0.05) FCR than CC group (3.00 and 3.06 versus 2.80, respectively) while group CD showed intermediate results (2.92). This study demonstrates that the severity of DON toxicity in pig performance can be related to the age of exposure (113 or 134 d) and the number of exposures to the toxin (one or two). Exposure to DON also resulted to long-term effects because challenged pigs showed limited ability to recover after the DON-induced reduction of feed intake.
Keywords: deoxynivalenol, feeding behavior, growth, mycotoxin, pig
INTRODUCTION
Trichothecene mycotoxins are closely related compounds produced by several Fusarium species mostly in cereals and grains. Among these, deoxynivalenol (DON) is often associated to a reduced performance in livestock animals with symptoms including feed refusal, reduced growth, and digestive disorders. Pigs are the most sensitive among farm animals because they have limited metabolic ability to transform DON into less toxic products (Wu et al., 2010). The susceptibility of pigs to DON is also related to their cereal-rich diet which increases their exposure probability (Pinton and Oswald, 2014).
In swine, as in other animal species toxic effects of DON include protein synthesis inhibition (Dänicke et al., 2006), neuroendocrine changes (Bonnet et al., 2012), inflammatory effect (Alassane-Kpembi et al., 2017a), damages to intestinal epithelial cells leading to loss of barrier functions (Pinton et al., 2010), and vomiting among others (Payros et al., 2016). Reported effects in gilts include ovarian lesions and a dose-dependent decreased linear trend in fetal weight and length (Friend et al., 1983; Gerez et al., 2017). In terms of growth, the most highlighted effect of DON is its negative impact on feed intake with subsequent effects on growth rate. However, there is high variability within reports about its impact severity because the toxic effects are highly dependent on dose and source of the toxin, animal age, duration of exposure, and interaction with other compounds, e.g., other mycotoxin (EFSA, 2017).
Although there are many studies about DON-contaminated diets in growing pigs (Forsyth et al., 1977; Friend et al., 1986; Trenholm et al., 1994; Rotter et al., 1995; House et al., 2002; Dänicke et al., 2004; Pinton et al., 2012; Alizadeh et al., 2015; Gerez et al., 2015), little is known about the short- and long-term effects of an acute DON challenge. There are also few studies on the ability of the pigs to adapt to DON exposure. Thus, the objective of this work is to study the short- and long-term effects of DON challenges on the pig performance and feeding behavior. It also aims to investigate the effect of age of the pig and/or previous exposures on these responses to the DON challenge.
MATERIALS AND METHODS
The experiment was conducted in accordance with the French legislation on animal experimentation and approved by the Regional Ethical Committee (authorization: 2016022415253973).
Animals and Treatments
A total of 160 Pietrain × (Large White × Landrace) pigs were used in the experiment in two replicates, with each pig individually measured for live BW and individually fed using an electronic feeding station. The two replicates of the experiment were conducted in the INRA experimental facilities at the Unité Expérimentale Porcs de Rennes (UEPR) located in Saint Gilles, France. The first replicate was conducted from April to June 2017 and the second one from August to October 2017. The average climatic parameters during replicates 1 and 2 are as follows: temperature at 22.9 and at 23.1 °C, respectively, relative humidity at 68.2% and 75.1%, respectively, and dew point at 16.7 and 18.4 °C, respectively. Each replicate had an equal number of castrated males and females. Within each replicate, pigs were blocked according to sex and litter origin, and were allotted to four experimental treatments in a randomized complete block design. From 91 to 98 d of age, the pigs started the transition from a commercial grower feed to the control finisher feed. The experiment started and ended at 99 and 154 d of age, respectively, and all pigs were slaughtered at 161 d of age.
The two diets used were based on corn and soybean meal and were formulated to contain the same amount of standardized ileal digestible lysine per MJ of NE (0.8 g/MJ NE) and to meet the ideal protein profile for essential amino acids (Table 1). The control diet was based on a corn with a very low DON concentration. The DON-contaminated diet was obtained by using a naturally contaminated corn containing an initial amount of 4.8 mg DON/kg. The analyzed DON content in the control and DON-contaminated diets were 0.14 and 3.02 mg DON/kg feed, respectively (Table 2).
Table 1.
Composition of experimental diets1
| Diet | Control | DON contaminated |
|---|---|---|
| Ingredients, % as-fed | ||
| Corn | 75.00 | — |
| Corn, DON contaminated | — | 75.00 |
| Wheat bran | 1.54 | 1.64 |
| Soybean meal | 18.10 | 18.00 |
| Molasses | 2.00 | 2.00 |
| Lysine HCL | 0.21 | 0.21 |
| dl-Methionine | 0.08 | 0.08 |
| l-Threonine | 0.06 | 0.06 |
| l-Tryptophan | 0.01 | 0.01 |
| Dicalcium phosphate | 1.00 | 1.00 |
| Calcium carbonate | 1.00 | 1.00 |
| Salt | 0.50 | 0.50 |
| Vitamin–mineral premix2 | 0.50 | 0.50 |
| Calculated composition3 | ||
| Crude protein, % | 14.3 | 14.4 |
| NE, MJ/kg | 10.3 | 10.3 |
| SID Lys, g/kg | 7.7 | 7.7 |
| SID Lys/NE, g/MJ | 0.8 | 0.8 |
| SID SAA, g/kg | 0.6 | 0.6 |
| SID Thr, g/kg | 0.7 | 0.7 |
| SID Trp, g/kg | 0.2 | 0.2 |
| Ca, g/kg | 7.8 | 6.5 |
| Dig P, g/kg | 2.5 | 2.1 |
| Dig P/NE, g/MJ | 0.3 | 0.3 |
| Ca/P dig | 3.1 | 3.1 |
| Analyzed composition4 | ||
| Dry matter, % | 87.0 | 87.0 |
| Organic matter, % | 79.5 | 77.9 |
| Crude protein, % | 14.2 | 14.1 |
| Crude fat, % | 3.1 | 2.3 |
| Crude fiber, % | 1.5 | 1.7 |
| NDF, % | 6.3 | 7.5 |
| ADF, % | 1.8 | 2.0 |
| ADL, % | 0.3 | 0.2 |
| Starch, % | 50.6 | 50.7 |
| GE, MJ/kg | 15.8 | 15.7 |
| NE, MJ/kg5 | 10.7 | 10.7 |
1Diet fed in pellet form.
2Provided per kilogram of complete diet: vitamin A, 1,000,000 IU; vitamin D, 3, 200,000 IU; vitamin E, 4,000 mg; vitamin B1, 400 mg; vitamin B2, 800 mg; calcium pantothenate, 2,170 mg; niacin, 3,000 mg; vitamin B12, 4 mg; vitamin B6, 200 mg; vitamin K3, 400 mg; folic acid, 200 mg; biotin, 40 mg; choline chloride, 100,000 mg; iron (sulfate), 11,200 mg; iron (carbonate), 4,800 mg; copper (sulfate), 2,000 mg; zinc (oxide), 20,000 mg; manganese (oxide), 8,000 mg; iodine (iodate), 40 mg; cobalt (carbonate), 20 mg; and selenium (selenite), 30 mg.
3As-fed basis. SID = standardized ileal digestible.
4As-fed basis. Values are calculated for the same dry matter content (87.0%).
5As-fed basis. Values are calculated based on equation set by Noblet et al. (1994, eq. 11) for calculating NE in growing pigs.
Table 2.
Analyzed mycotoxin composition of the experimental diets1,2
| Mycotoxin concentration, mg/kg | Control | DON contaminated |
|---|---|---|
| Deoxynivalenol | 0.14 | 3.02 |
| Nivalenol | 0.02 | 0.62 |
| Zearalenone | 0.10 | 0.76 |
| Fumonisin B1 | 0.45 | 0.06 |
| Fumonisin B2 | 0.10 | 0.01 |
| Aflatoxin3 | <0.004 | <0.004 |
1Dietary mycotoxin concentrations were analyzed by a commercial laboratory (GIP Labocea, 22440 Ploufragan, FR).
2All values are expressed as-fed basis and are calculated based on the same dry matter content of 87%.
3Sum of Aflatoxins B1, B2, G1, and G2.
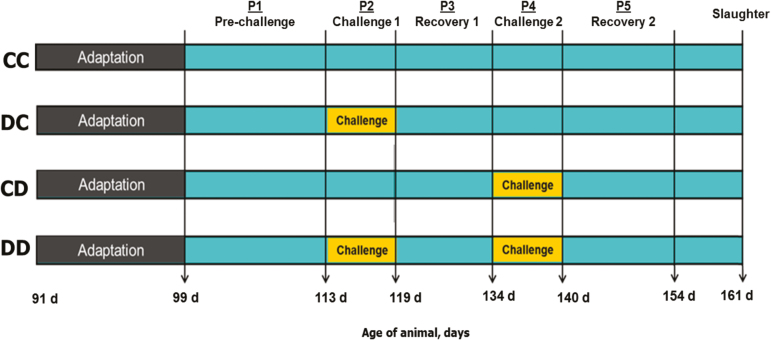
The description of the experimental study is presented in Figure 1. During the experimental period, the pigs from the experimental group CC received the control diet throughout the finishing period (99 to 154 d of age). The pigs of the experimental group DC were given the DON-contaminated diet for 7 d starting at 113 d of age (i.e., 113 to 119 d of age). The pigs of the experimental group CD were given the DON-contaminated diet also for 7 d starting at 134 d of age (i.e., 134 to 140 d of age). The pigs of the experimental group DD were challenged with the DON-contaminated diet for 7 d starting at 113 d of age and for another 7 d starting at 134 d of age. After each challenge, a 14-d recovery period was observed. During the pre- and the postchallenge periods, pigs were fed with the control diet.
Figure 1.
Description of the experimental design of study. The pigs were given two diets during the 56-d experiment: control and DON contaminated. Pigs from the experimental groups CC (n = 39) were fed the control diet all over the experimental period. DC (n = 39), CD (n = 38), and DD (n = 39) experimental groups of pigs were challenged on period 2, period 4, or on periods 2 and 4, respectively, with the DON-contaminated diet. Pigs were slaughtered 7 d after the end of the experimental period.
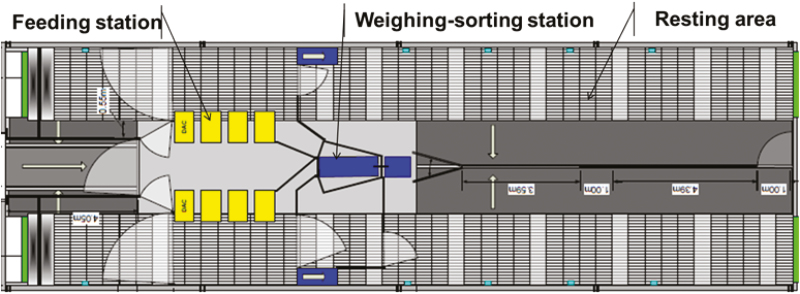
Before moving to the experimental room, pigs were tagged in the right ear with a serial number and an RFID chip for identification in the sorter (which also served as the weighing machine) and in the automatic feeders. The layout of the experimental room is shown in Figure 2 and a more detailed description of the automatic and intelligent precision feeders (AIPF) used in the experiment is given by Pomar et al. (2011). To sum up, the experimental room had two feeding zones (zones A and B) which were accessed by the pigs through an automatic sorter. Each feeding zone was equipped with four automatic feeders. During nonchallenge periods, the sorter was programmed in random order so pigs can access either zone. During challenge periods, zone A feeders were filled with the DON-contaminated feeds and zone B feeders with the control. During these periods, animals of the challenged groups fed with the DON-contaminated diet only had access to zone A (via the sorter) while control animals fed with the control diet only had access to zone B (without going through the sorter). During the challenge period, the resting areas of the control and DON-challenged groups were also separated to avoid cross-contaminations by feces. Feed and water were provided ad libitum. After the end of the trial period (or end of P5), all pigs remained in the experimental room for another 7 d and were provided with the control diet before they were slaughtered at 161 d of age in both replicates.
Figure 2.
Experimental room layout.
Measurements and Calculations
The trial was divided into five sub-periods which are as follows: prechallenge (14 d; 99–112 d of age; P1), challenge 1 (7 d; 113 to 119 d of age; P2), recovery 1 (14 d; 120–133 d of age; P3), challenge 2 (7 d; 134–140 d of age; P4), and recovery 2 period (14 d; 141–154 d of age; P5) (Figure 1). Five pigs were removed from the final calculation, and statistical analysis because of poor performance, leg injury, and/or death with causes unrelated to the treatments.
Live BW was measured automatically when the pigs pass through the automatic sorter. The average daily BW was calculated to be the average of all BW recordings each day. Daily feed intake was calculated based on the recordings of the AIPF on the number of feed servings the pig requested (in theory, one serving = 25 g) and calibration factor (CF). Calibration measurements were done weekly on all feeders to correct for the actual amount of feed delivered per serving. From these measurements, the CF was calculated to be the ratio of the actual amount delivered to the theoretical value. In order to evaluate adaptive ability of the pigs during challenge periods, the daily marginal change in daily feed intake (DFI) of the challenged pigs (or the feed intake retrieval rate) was calculated as feed intake difference between days 1 and 7 of challenge divided by 6 d of the challenge.
All pigs were fasted 24 h before slaughter and BW at slaughter was measured by passing the pigs through the automatic sorter before they were transported to the slaughterhouse. Ultrasound backfat thickness (BFT) measurements were taken behind the last rib (at the boundary of thoracic and lumbar vertebrae), 3 cm off the midline (P2 points) at the beginning of the experiment, at the end of periods P2, P4, and P5. Dressing yield and lean meat percentage were also measured in a commercial slaughterhouse.
For the feeding behavior parameters, data obtained in the first day of the experiment (99 d of age) and from the other days of BFT measurements were removed from the whole database. Number of feeder visits and meals per day were recorded by the AIPF. One visit is recorded each time a pig is detected by the AIPF. The duration of each visit was calculated based on the recorded time the pig entered and was detected by the feeder and the time the pig left. Two consecutive visits separated by a time interval not longer than a given meal criterion are considered to belong to one meal. If time at feeder exceeded 5 min, the meal criterion duration no longer affected the number of meals. From this result, the adopted meal criterion for the present study was 5 min and this value was chosen for further calculation of daily components of feeding behavior criteria. These components were the meal frequency (meals per day), DFI (g/d), and average rate of feed intake (total feed intake/total consumption time, g/min).
Statistical Analyses
Data were analyzed through an ANOVA using the MIXED procedure (SAS Inst. Inc., Cary, NC) considering the pig as the experimental unit. Effects of the experimental group (n = 4), sex (n = 2), replication (n = 2), period (n = 5), and their interactions were tested and considered as fixed effects on the growth and slaughter performance, BFT, and feeding behavior parameters. Aside from the slaughter parameters, all other parameters were all subjected to a repeated measurement ANOVA based on the periods mentioned. Initial BW was also added as a covariable to the described model for growth parameters in which it was found to be significant, i.e., BW, ADG, and ADFI, and on the feeding behavior parameters. Initial BFT was also added as a covariable on the analysis of the BFT data. For analyzing the kinetics of DFI and daily rate of feed intake (DRFI) of the different experimental groups, the whole database of DFI and of DRFI were first analyzed using SAS (Proc MIXED) with the pig as the experimental unit. The model included experimental group (n = 4), sex (n = 2), replication (n = 2), and age in days (n = 55) as fixed effects and with initial BW as a covariable. The least square means of each experimental group were compared at each given age (in days) using the Tukey test. In the first challenge, CC was not different to CD, and DC was not different to DD. Based on these results, statistical contrasts were analyzed between groups (challenged versus unchallenged) depending on the challenge period. The feed intake retrieval rate was also subjected to contrast analysis among the challenged groups. In all parameters, differences were considered statistically significant at P < 0.05.
RESULTS
Growth and Slaughter Parameters
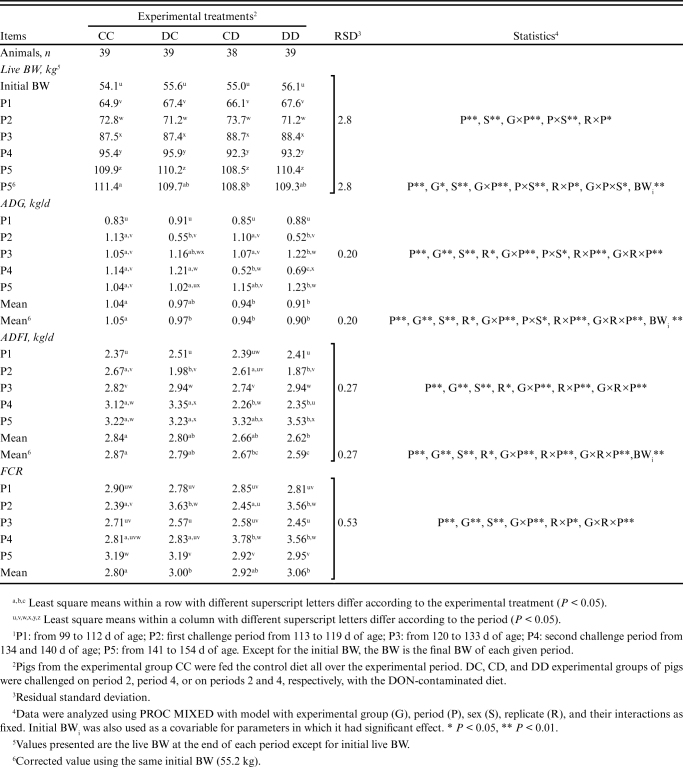
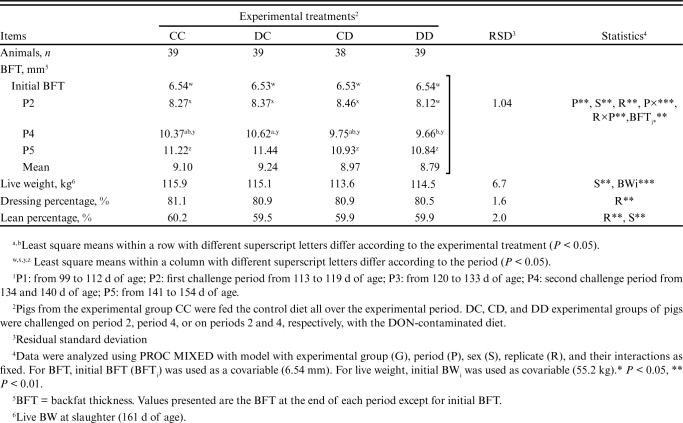
Even though the average initial BW of pigs did not differ among the experimental groups (P = 0.672), it was found to have a significant effect as a covariable on the final BW, ADG, and ADFI during the whole experimental period (Table 3). Looking at the overall performance during the whole experimental period, the overall ADG of the challenged groups was similar (P > 0.05) and were significantly lower than that measured in the CC group (0.94 kg/d on average versus 1.05 kg/d; P < 0.05). Groups challenged in P4 (CD and DD) had lower overall ADFI than CC (2.63 on average versus 2.87 kg/d; P < 0.05). An intermediate value was reported for the DC pigs (2.79 kg/d). Groups challenged in P2 (DC and DD) had higher FCR than the control group CC (3.00 and 3.06 versus 2.80; P < 0.05) while an intermediate value was found for the CD group (2.92). The slaughter parameters are shown in Table 4. The experimental group did not influence the BFT at the end of the experimental period (11.11 mm on average; P > 0.05) and the BW at slaughter (114.8 kg; P > 0.05). Lean meat percentage and dressing yield were also similar between the experimental groups (60.0% and 80.8%, respectively; P > 0.05).
Table 3.
Effects of DON mycotoxin challenge on the growth performance of the finishing pigs.1
Table 4.
Effects of DON mycotoxin challenge on slaughter characteristics of the finishing pigs.1
Feeding Behavior
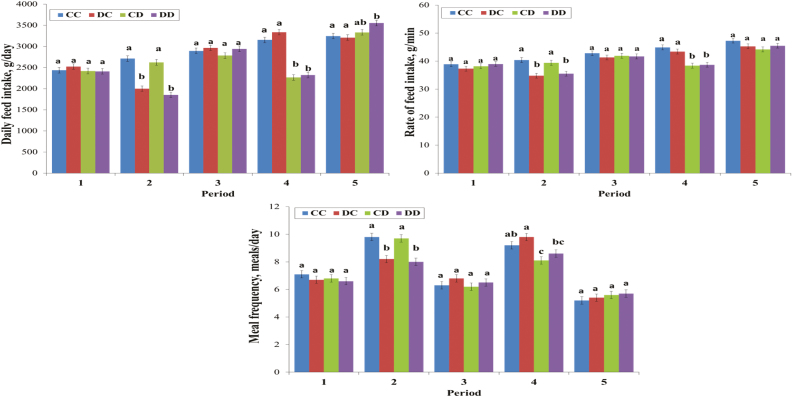
Looking at the parameters per period, the feeding behavior parameters were not significantly influenced by the dietary treatment before P2: on average, pigs had 6.8 meals per day, the feeding rate was 38.4 g/min, and the DFI was 2,448 g/day (Figure 3). During challenges, the reduced feed intake in DON-fed pigs was mainly related to a reduction of the meal frequency (8.1 versus 9.7 meals/day in P2, and 8.3 versus 9.5 meals/day in P4 on average; P < 0.05), whereas the average meal size, albeit different between periods, remained constant within the period (282 and 348 g/meal on the average for P2 and P4, respectively; P > 0.05). The average rate of feed intake per period of DON-fed pigs was significantly reduced only in P2 and P4 (35.1 versus 39.9 g/min on average, and 38.6 versus 44.2 g/min on average, respectively; P < 0.05).
Figure 3.
Effect of the experimental group and period on the feeding behavior in finishing pigs (LSmeans ± SEM). Diets: Control and DON contaminated. Pigs from the experimental groups CC (n = 39) were fed the control diet all over the experimental period. DC (n = 39), CD (n = 38), and DD (n = 39) experimental groups of pigs were challenged on period 2, period 4, and on periods 2 and 4, respectively, with the DON-contaminated diet. a,b,cLSmeans with different superscript letters differ according to the experimental treatment (P < 0.05).
Compared to the nonchallenged pigs, pigs fed with the DON-contaminated diet experienced a significant drop in ADG and ADFI during the 7-d challenged periods (−51% and −28% on average, respectively; P < 0.001). Compared to the CC group, ADFI during the 7 d of challenge was reduced in P2 by 0.73 and 0.86 kg/day for the DC and the DD groups, respectively, and in P4 by 0.89 and 0.83 kg/day for the CD and DD groups, respectively. Between the challenged groups in P4, ADG of CD pigs was lower than DD (0.52 versus 0.69 kg/day; P = 0.011), whereas ADFI was similar in both groups (P = 0.915). In recovery periods following a specific challenge, ADG and ADFI of DON-fed pigs were higher than the control pigs by 0.13 and 0.07 kg/day during P3 and by 0.14 and 0.16 kg/day during P5. After being subjected to two challenges, ADG and ADFI of DD during its second recovery period (P5) was higher than CC and DC (P < 0.003 and P < 0.020, respectively) but was not different to CD (P = 0.563 and 0.159, respectively). Groups fed with DON-contaminated diet had higher FCR than the control-fed groups during challenged periods (3.59 versus 2.43 on the average during P2, and 3.66 versus 2.82 during P4; P < 0.001). There was no significant difference in the FCR among the groups during nonchallenge periods.
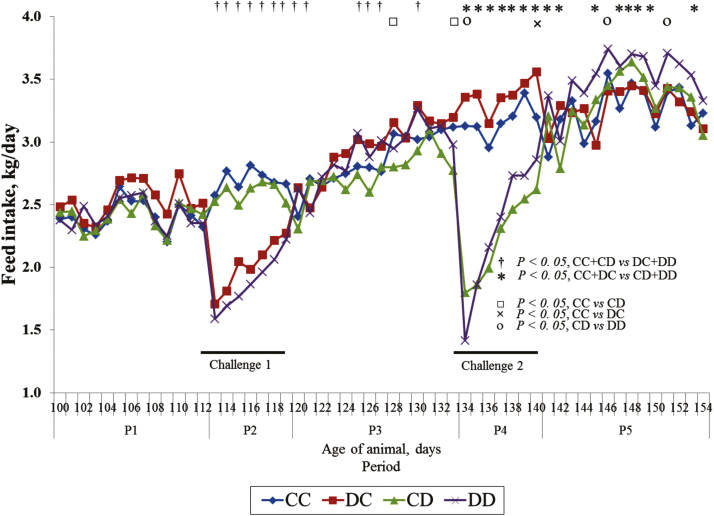
Finally, looking at the daily feeding behavior parameter measured during the trial (Figure 4), the ADFI of the challenged groups significantly dropped on day 1 of exposure and showed gradual feed intake retrieval thereafter during the following 6 d. From the generation of contrast between adjacent DFI from the day prior the challenge to the 7 d of challenge, the challenged groups in P2 (DC and DD) had similar drops in feed intake on the first day (−0.77 and −0.75 kg/d, respectively; P = 0.483) and had similar feed intake retrieval rate (0.09 and 0.11 kg∙d−2; P = 0.387). In the groups challenge in P4 (CD and DD), the group DD which was previously challenged in P2 had a bigger feed intake drop on the first day than group CD (1.56 versus 1.00 kg, respectively; P = 0.003) but it had a faster feed intake retrieval rate than the group CD (0.24 and 0.14 kg∙d−2; P = 0.007). Whatever the challenge period, ADFI of the challenged groups remained significantly lower (P < 0.001) than that of the unchallenged groups until the end of each challenge. In the recovery periods (P3 and P5), there seemed to be a lag time of around 3 days before the challenged groups started their feed intake compensation. After the lag times, feed intake of the challenged groups increased and was continuously higher than the unchallenged groups for the rest of both recovery periods. Compared to unchallenged groups, feed intake was significantly higher in DD and DC during P3 on days 125 to 127 and day 130, and in DD and CD during P5 on days 145, 147 to 150, and day 153. Between the two challenged groups during P5 (CD and DD groups), DD was constantly higher than CD for the whole recovery period though it was only significant on days 146 and 151.
Figure 4.
Effect of the experimental treatment on the daily feed intake pattern in finishing pigs. Diets: Control and DON contaminated. Pigs from the experimental groups CC (n = 39) were fed the control diet all over the experimental period. DC (n = 39), CD (n = 38), and DD (n = 39) experimental groups of pigs were challenged on period 2, period 4, and on periods 2 and 4, respectively, with the DON-contaminated diet.
DISCUSSION
Among livestock farm animals, pigs are the most exposed to DON because of their cereal-rich-based diet. In contrast to ruminant and poultry, pigs are also more sensitive because they have a low ability to detoxify DON and other mycotoxins to less toxic products (Pinton and Oswald, 2014). A reduction in feed intake is the most common effect of dietary exposure to DON. In the present study and whatever the challenge period, ADFI was reduced on average by 26% to 32% when compared to the control groups. This is similar to the average value of 26% obtained in a meta-analysis by Andretta et al. (2012). One cause of the feed intake reduction could be related to the decreased palatability due to mold presence in naturally contaminated feed (Higgins and Brinkhaus, 1999). However, it could also be caused by other mechanisms since in this study, the decreased feed intake was also related to a reduction in the rate of feed intake and the meal frequency, the average meal size remaining constant. The DON effect of slower rate of feed intake is comparable to findings by Goyarts et al. (2005) in which pigs fed with DON took longer to finish the same amount of ration than control-fed pigs. Moreover, the decreased meal frequency in the current study was similar to the study of Girardet et al. (2011b) in mice for which they reported reduction in both meal frequency and meal size. In the aforementioned study, the satiety and satiation effect of DON can be related to the altered anorexigenic balance in the brain (Girardet et al., 2011b).This appetite-reducing effect of DON could be a result of its effect in the brain such as an increased serotonin turnover in the brain (Prelusky, 1993). Bonnet et al. (2012) also suggested activation of proopiomelanocortin neurons which induces decreased food intake and nesfatinergic neurons which are associated with meal termination mechanisms. Some studies reported that DON can induce the release of satiety hormones from endocrine cells found in the gut (Zhou and Pestka, 2015). According to Alassane-Kpembi et al. (2017a), the pro-inflammatory cytokine produced upon exposure to DON can also participate to the observed anorexia.
There is a large variability of results regarding the degree DON toxicity in pig performance (Andretta et al., 2012). First, this variability could be attributed to diet-related factors such as source of contamination, level of DON contamination, or combined contamination with others mycotoxins among others (Mirocha et al., 1976; Foster et al., 1986; Trenholm et al., 1994; Andretta et al., 2016; Guerre, 2016; Alassane-Kpembi et al., 2017b). In the current experiment, the analyzed DON and zearalenone (ZEN) content in the control diet was 0.14 and 0.10 mg/kg feed which is lower than the guideline levels based on EU Commission Recommendation 2006/576/EC (2006) of 0.90 and 0.25 mg/kg feed, respectively. Meanwhile, the DON and ZEN content in the DON-contaminated diet (i.e., 3.02 and 0.76 mg/kg feed, respectively) exceeded the guideline levels. Concentration of nivalenol, a closely related molecule to DON, was also higher in the DON-contaminated diet than the control (0.62 versus 0.02 mg/kg feed). Both nivalenol and ZEN are known to co-occur with DON in naturally contaminated feed and feed ingredients (Mirocha et al., 1976; Yoshizawa and Hosokawa, 1983; Zinedine et al., 2007; Gerez et al., 2015; Calori-Domingues et al., 2016; Smith et al., 2016; Bryła et al., 2018) and were found to have a synergistic toxic effect with DON (Gerez et al., 2015; Ren et al., 2016; Alassane-Kpembi et al., 2017b). The concentration of DON contamination also affects the severity of the impact in which multiple studies have already reported its dose-dependent effect particularly in feed intake (Rotter et al., 1994; Dänicke et al., 2006; Guerre, 2016). In the present study, a level of 3.02 mg DON/kg feed was sufficient to decrease the feed intake of the finishing pigs.
The toxicity of DON and mycotoxins in general is also influenced by animal-related factors such as the age or physiological state of the animal (Pier et al., 1980; Broom, 2015). The present study also suggests this age-related effects indicated by the greater decrease in ADFI expressed as kilograms per day in pigs challenged late in the trial (P4) than those challenged early (P2). However, when it was expressed in percentage relative to the control, the feed intake reduction was comparable in both periods (−30% and −27% in P2 and P4, respectively).
According to Dänicke et al. (2004), ADFI was reduced by about 9% in finishing pigs fed with a contaminated diet containing 3.7 mg DON/kg for 10 weeks from 68 to 104 kg BW. This reduced ADFI was much lower than the value reported in the present study for a 7-d challenge period, suggesting that the duration of the challenge can also modulate the negative effect of DON in pig appetite. In the present study, the drop in the feed intake in the first day of DON exposure could be associated to the palatability or appetite-reducing effect previously discussed. After this initial drop in feed intake, the gradual increase in ADFI (feed intake retrieval) clearly indicated that pigs have the ability to adapt to the presence of DON in the diet. This result is in agreement with previous studies (Foster et al., 1986; Prelusky, 1997). Although the adaptive mechanism of pigs to DON has not been fully understood, Prelusky (1997) suggests that this adaptation is stronger when DON is fed orally than when given intravenously. One reason could be related to the effect on DON in the gut and especially its ability to alter the intestinal microbiota composition (Tenk et al., 1982; Mayer et al., 2017). The role of gut microbiota in the biotransformation of DON into less toxic products through de-epoxidation has been reported in many studies on pigs, chickens, mice, and humans (Swanson et al., 1988; Young et al., 2007; Pierron et al., 2016; Gratz et al., 2018). In pigs, Waché et al. (2009) reported that microbiota composition was significantly affected when the host was fed with a DON-contaminated diet for 4 weeks. It is assumed that this variation of microbiota composition would help the animal to cope with a DON challenge. In our experimental conditions, a 7-d challenge length was too short to allow the pig to fully adapt to DON and retrieve their normal feeding level. Similarly, Prelusky et al. (1994) reported that 7-week-old pigs fed with 3.26 mg DON/kg feed also adapted to DON but were not able to fully retrieve a feed intake similar to the control even until the end of a 4-weekchallenge. Meanwhile, Cote et al. (1985) reported that pigs subjected to 3.1 to 5.8 mg DON/kg feed took 5 weeks to adapt to DON and have the same feed intake as the control. All these results suggest that more than 4 weeks are needed for a total adaptation to a DON-contaminated diet and explain why shorter challenges lead to a more severe impact of the toxin.
In the present study, a previous DON exposure did not make the pigs resistant to a second interrupted DON challenge as indicated by the greater drop in feed intake of the DD pigs in the first day of P4. However, based on the faster retrieval rate in feed intake, this previous experience seemed to improve pig response to the repeated DON challenge. These results were similar to those reported by Flannery et al. (2011) in mice subjected to an intermittent repeated DON challenge. According to this study, the adaptation to DON exposure was dose dependent: multiple exposures at a level of 5 mg/kg can improve tolerance to the toxin but not at levels below 2.5 mg/kg. In the present study, this improved tolerance seemed to be mediated by changes in the feeding behavior: in the second DON challenge, meal frequency was affected only in the CD groups but not for the DD pigs. This improved resilience to the toxin could be related to the previously mentioned possibility of a modified pig microbiota that is more able to detoxify DON due to previous exposure. It could also be due to the impact of DON on immune system. Cano et al. (2013) indicated that DON exposure can induce inflammatory response leading to activation of T helper 17 cells. These cells have important functions in the adaptive immunity and in vaccine-induced memory immune responses against pathogens (Ouyang et al., 2008; Vautier et al., 2010). The pigs subjected to a previous DON challenge could have had increased their tolerance to a repeated exposure possibly because of a better immune response. However, there are currently no studies on the development of acquired immunity to mycotoxins so this remains to be purely hypothetical.
In this study, the negative impact of DON in feed intake was only seen during the challenge period. During the recovery periods, challenged pigs had higher feed intake but this compensation was observed only after 2 to 3 d postchallenge. In rodents, DON was found to disrupt motor complexes in the intestine which slows down intestinal transit and inhibits gastric emptying (Fioramonti et al., 1993). This effect of DON on the gut could possibly have carryover effect during the few days postchallenge which limits the stomach or gut capacity of pigs, thus inhibiting them to increase their feed intake immediately after the challenge.
In agreement with previous studies (Bergsjø et al., 1992; Rotter et al., 1994; Dänicke et al., 2006), the negative effect of DON on feed intake resulted in a decreased amount of nutrient available for growth with a subsequent negative impact on growth performance. The challenged pigs in this study showed overall growth retardation when compared to the control pigs. Some studies also reported that pigs fed with DON-contaminated diets took longer to reach market weight (Friend et al., 1986; House et al., 2002). In the present study, the slaughter BW was numerically lower in challenged pigs, especially in DON-fed pigs late in the trial (CD and DD pigs), but the difference was not statistically significant with the control group. Compensatory growth mechanisms during recovery period could explain this attenuated effect of DON challenge on the market weight since pigs were slaughtered 21 d after the last day of P4.
Based on the FCR, feed efficiency was significantly lower during the challenge periods. This reduction in feed efficiency could be related to the increased maintenance requirement. It has been reported that DON induces inflammatory and sickness-like responses with associated changes in locomotor and thermoregulatory activities (Girardet et al., 2011a), all of which contribute to increased maintenance requirements in pigs (Whittemore et al., 2001). Bonnet et al. (2012) also reported that DON can activate neurons involved in increased energy expenditure. This effect could also be directly related to the lower feeding level which automatically increases the proportion of energy intake used for maintenance at the expense of growth requirements. However, looking at the overall performance, the lower feeding level did not seem to be the only limiting factor. The pigs challenged only once and only early in the trial (DC pigs) still had lower overall weight gain despite similar feed intake with the control in the postchallenge periods and in the overall trial period which resulted to an overall lower feed efficiency. This decreased feed efficiency, then, could be also partially explained by a decreased ability of the pigs to digest and utilize feed. Some studies reported toxic effects of DON such as decreased stomach integrity, inflammation in the small intestine, decrease villi length in the jejunum, and altered intestinal epithelium in general (Kolf-Clauw et al., 2009; Pinton et al., 2012; Lucioli et al., 2013; Pinton and Oswald, 2014). These results suggest long-term DON effects in which the gut integrity of early-challenged pigs could have been altered early on thus preventing them to fully recover from the toxin.
In conclusion, the study confirms previous reports of the negative impact of DON in feed intake, feeding behavior, and growth performance of finishing pigs. This study also demonstrates that the severity of DON toxicity in pig performance can be attributed to the duration, the age of exposure, and the number of toxin challenges. The effect of DON seemed to be long-lasting and to continue after the challenge has ended; this resulted in a delayed compensatory effect and an inability to fully compensate the lost productive days.
ACKNOWLEDGMENTS
The authors thank I. Oswald for reviewing this paper, B. Duteil, P. Roger, N. Muller, J. Delamarre, H. Demay from the UEPR, INRA, 35590 Saint-Gilles, France for animal care and sample collection, C. Perrier, G. Robin, and A. Marchais for lab analyses.
Footnotes
This study was funded by the Feed-a-Gene Project which has received funding from the European Union’s H2020 Program under grant agreement no. 633531.
LITERATURE CITED
- Alassane-Kpembi I., Puel O., Pinton P., Cossalter A. M., Chou T. C., and Oswald I. P.. 2017a. Co-exposure to low doses of the food contaminants deoxynivalenol and nivalenol has a synergistic inflammatory effect on intestinal explants. Arch. Toxicol. 91:2677–2687. doi: 10.1007/s00204-016-1902-9 [DOI] [PubMed] [Google Scholar]
- Alassane-Kpembi I., Schatzmayr G., Taranu I., Marin D., Puel O., and Oswald I. P.. 2017b. Mycotoxins co-contamination: methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 57: 3489–3507. doi: 10.1080/10408398.2016.1140632 [DOI] [PubMed] [Google Scholar]
- Alizadeh A., Braber S., Akbari P., Garssen J., and Fink-Gremmels J.. 2015. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins (Basel). 7:2071–2095. doi: 10.3390/toxins7062071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretta I., Kipper M., Hauschild L., Lehnen C. R., Remus A., and Melchior R.. 2016. Meta-analysis of individual and combined effects of mycotoxins on growing pigs. Sci. Agric. 73:328–331. doi: 10.1590/0103-9016-2015-0132 [DOI] [Google Scholar]
- Andretta I., Kipper M., Lehnen C. R., Hauschild L., Vale M. M., and Lovatto P. A.. 2012. Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs. Animal 6:1476–1482. doi: 10.1017/S1751731111002278 [DOI] [PubMed] [Google Scholar]
- Bergsjø B., Matre T., and Nafstad I.. 1992. Effects of diets with graded levels of deoxynivalenol on performance in growing pigs. Zentralbl. Veterinarmed. A. 39:752–758. doi: 10.1111/j.1439-0442.1992.tb00240.x [DOI] [PubMed] [Google Scholar]
- Bonnet M. S., Roux J., Mounien L., Dallaporta M., and Troadec J. D.. 2012. Advances in deoxynivalenol toxicity mechanisms: the brain as a target. Toxins (Basel). 4:1120–1138. doi: 10.3390/toxins4111120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L. 2015. Mycotoxins and the intestine. Anim. Nutr. 1:262–265. doi: 10.1016/j.aninu.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryła M., Ksieniewicz-Woźniak E., Waśkiewicz A., Szymczyk K., and Jędrzejczak R.. 2018. Natural occurrence of nivalenol, deoxynivalenol, and deoxynivalenol-3-glucoside in Polish winter wheat. Toxins (Basel). 10: 81. doi: 10.3390/toxins10020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calori-Domingues M. A., Bernardi C. M., Nardin M. S., de Souza G. V., Dos Santos F. G., Stein M. D. E. A., Gloria E. M., Dias C. T., and de Camargo A. C.. 2016. Co-occurrence and distribution of deoxynivalenol, nivalenol and zearalenone in wheat from Brazil. Food Addit. Contam. Part B. Surveill. 9:142–151. doi: 10.1080/19393210.2016.1152598 [DOI] [PubMed] [Google Scholar]
- Cano P. M., Seeboth J., Meurens F., Cognie J., Abrami R., Oswald I. P., and Guzylack-Piriou L.. 2013. Deoxynivalenol as a new factor in the persistence of intestinal inflammatory diseases: an emerging hypothesis through possible modulation of th17-mediated response. PLoS One. 8:e53647. doi: 10.1371/journal.pone.0053647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote L. M., Beasley V. R., Bratich P. M., Swanson S. P., Shivaprasad H. L., and Buck W. B.. 1985. Sex-related reduced weight gains in growing swine fed diets containing deoxynivalenol. J. Anim. Sci. 61:942–950. doi: 10.2527/jas1985.614942x [DOI] [PubMed] [Google Scholar]
- Dänicke S., Goyarts T., Döll S., Grove N., Spolders M., and Flachowsky G.. 2006. Effects of the fusarium toxin deoxynivalenol on tissue protein synthesis in pigs. Toxicol. Lett. 165:297–311. doi: 10.1016/j.toxlet.2006.05.006 [DOI] [PubMed] [Google Scholar]
- Dänicke S., Goyarts T., Valenta H., Razzazi E., and Böhm J.. 2004. On the effects of deoxynivalenol (DON) in pig feed on growth performance, nutrients utilization and DON metabolism. J. Anim. Feed Sci. 13: 539–556. doi: 10.22358/jafs/67624/2004 [DOI] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) , Knutsen K. H., Alexander J., Barregard L., Bignami M., Bruschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Grasl-Kraupp B.,. et al. 2017. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 15: 4718. doi: 10.2903/j.efsa.2017.4718 [DOI] [Google Scholar]
- EU Commission Recommendation 2006/576/EC 2006. EU Commission Recommendation 2006/576/EC on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (17 August 2006). https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32006H0576 [Google Scholar]
- Fioramonti J., Dupuy C., Dupuy J., and Bueno L.. 1993. The mycotoxin, deoxynivalenol, delays gastric emptying through serotonin-3 receptors in rodents. J. Pharmacol. Exp. Ther. 266:1255–1260. http://jpet.aspetjournals.org/content/266/3/1255 [PubMed] [Google Scholar]
- Flannery B. M., Wu W., and Pestka J. J.. 2011. Characterization of deoxynivalenol-induced anorexia using mouse bioassay. Food Chem. Toxicol. 49:1863–1869. doi: 10.1016/j.fct.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth D. M., Yoshizawa T., Morooka N., and Tuite J.. 1977. Emetic and refusal activity of deoxynivalenol to swine. Appl. Environ. Microbiol. 34:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster B., Trenholm H., Friend D., Hartin K., and Thompson B.. 1986. Evaluation of different sources of deoxynivalenol (vomitoxin) fed to swine. Can. J. Anim. Sci. 66: 1149–1154. doi: 10.4141/cjas86-128 [DOI] [Google Scholar]
- Friend D., Trenholm H., Fiser P., Hartin K., and Thompson B.. 1983. Effect on dam performance and fetal development of deoxynivalenol (vomitoxin) contaminated wheat in the diet of pregnant gilts. Can. J. Anim. Sci. 63: 689–698. doi: 10.4141/cjas83-078 [DOI] [Google Scholar]
- Friend D., Trenholm H., Prelusky D., Hartin K., and Thompson B.. 1986. Effect of deoxynivalenol (DON)-contaminated diet fed to growing-finishing pigs on their performance at market weight, nitrogen retention and DON excretion. Can. J. Anim. Sci. 66: 1075–1085. doi: 10.4141/cjas86-118 [DOI] [Google Scholar]
- Gerez J. R., Desto S. S., and Bracarense A. P. F. R. L.. 2017. Deoxynivalenol induces toxic effects in the ovaries of pigs: an ex vivo approach. Theriogenology 90:94–100. doi: 10.1016/j.theriogenology.2016.10.023 [DOI] [PubMed] [Google Scholar]
- Gerez J. R., Pinton P., Callu P., Grosjean F., Oswald I. P., and Bracarense A. P.. 2015. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 67:89–98. doi: 10.1016/j.etp.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Girardet C., Bonnet M. S., Jdir R., Sadoud M., Thirion S., Tardivel C., Roux J., Lebrun B., Mounien L., Trouslard J.,. et al. 2011a. Central inflammation and sickness-like behavior induced by the food contaminant deoxynivalenol: a PGE2-independent mechanism. Toxicol. Sci. 124:179–191. doi: 10.1093/toxsci/kfr219 [DOI] [PubMed] [Google Scholar]
- Girardet C., Bonnet M. S., Jdir R., Sadoud M., Thirion S., Tardivel C., Roux J., Lebrun B., Wanaverbecq N., Mounien L.,. et al. 2011b. The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS One 6:e26134. doi: 10.1371/journal.pone.0026134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyarts T., Dänicke S., Rothkötter H. J., Spilke J., Tiemann U., and Schollenberger M.. 2005. On the effects of a chronic deoxynivalenol intoxication on performance, haematological and serum parameters of pigs when diets are offered either for ad libitum consumption or fed restrictively. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52:305–314. doi: 10.1111/j.1439-0442.2005.00734.x [DOI] [PubMed] [Google Scholar]
- Gratz S., Currie V., Richardson A., Duncan G., Holtrop G., Farquharson F., Louis P., Pinton P., and Oswald I.. 2018. Porcine small and large intestinal microbiota rapidly hydrolyze the masked mycotoxin deoxynivalenol-3-glucoside and release deoxynivalenol in spiked batch cultures in vitro. Appl. Environ. Microbiol. 84: 1–9. doi: 10.1128/AEM.02106-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerre P. 2016. Worldwide mycotoxins exposure in pig and poultry feed formulations. Toxins (Basel). 8: 350. doi: 10.3390/toxins8120350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C., and Brinkhaus F.. 1999. Efficacy of several organic acids against molds. J. Appl. Poult. Res. 8: 480–487. doi: 10.1093/japr/8.4.480 [DOI] [Google Scholar]
- House J., Abramson D., Crow G., and Nyachoti C.. 2002. Feed intake, growth and carcass parameters of swine consuming diets containing low levels of deoxynivalenol from naturally contaminated barley. Can. J. Anim. Sci. 82: 559–565. doi: 10.4141/A02-033 [DOI] [Google Scholar]
- Kolf-Clauw M., Castellote J., Joly B., Bourges-Abella N., Raymond-Letron I., Pinton P., and Oswald I. P.. 2009. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: histopathological analysis. Toxicol. In Vitro 23:1580–1584. doi: 10.1016/j.tiv.2009.07.015 [DOI] [PubMed] [Google Scholar]
- Lucioli J., Pinton P., Callu P., Laffitte J., Grosjean F., Kolf-Clauw M., Oswald I. P., and Bracarense A. P.. 2013. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: interest of ex vivo models as an alternative to in vivo experiments. Toxicon 66:31–36. doi: 10.1016/j.toxicon.2013.01.024 [DOI] [PubMed] [Google Scholar]
- Mayer E., Novak B., Springler A., Schwartz-Zimmermann H. E., Nagl V., Reisinger N., Hessenberger S., and Schatzmayr G.. 2017. Effects of deoxynivalenol (DON) and its microbial biotransformation product deepoxy-deoxynivalenol (DOM-1) on a trout, pig, mouse, and human cell line. Mycotoxin Res. 33:297–308. doi: 10.1007/s12550-017-0289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirocha C. J., Pathre S. V., Schauerhamer B., and Christensen C. M.. 1976. Natural occurrence of fusarium toxins in feedstuff. Appl. Environ. Microbiol. 32:553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noblet J., Fortune H., Shi X. S., and Dubois S.. 1994. Prediction of net energy value of feeds for growing pigs. J. Anim. Sci. 72:344–354. doi: 10.2527/1994.722344x [DOI] [PubMed] [Google Scholar]
- Ouyang W., Kolls J. K., and Zheng Y.. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28:454–467. doi: 10.1016/j.immuni.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payros D., Alassane-Kpembi I., Pierron A., Loiseau N., Pinton P., and Oswald I. P.. 2016. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 90:2931–2957. doi: 10.1007/s00204-016-1826-4 [DOI] [PubMed] [Google Scholar]
- Pier A. C., Richard J. L., and Cysewski S. J.. 1980. Implications of mycotoxins in animal disease. J. Am. Vet. Med. Assoc. 176:719–724. [PubMed] [Google Scholar]
- Pierron A., Mimoun S., Murate L. S., Loiseau N., Lippi Y., Bracarense A. P., Schatzmayr G., He J. W., Zhou T., Moll W. D.,. et al. 2016. Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci. Rep. 6:29105. doi: 10.1038/srep29105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Braicu C., Nougayrede J. P., Laffitte J., Taranu I., and Oswald I. P.. 2010. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 140:1956–1962. doi: 10.3945/jn.110.123919 [DOI] [PubMed] [Google Scholar]
- Pinton P., and Oswald I. P.. 2014. Effect of deoxynivalenol and other type B trichothecenes on the intestine: a review. Toxins (Basel). 6:1615–1643. doi: 10.3390/toxins6051615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Tsybulskyy D., Lucioli J., Laffitte J., Callu P., Lyazhri F., Grosjean F., Bracarense A. P., Kolf-Clauw M., and Oswald I. P.. 2012. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 130:180–190. doi: 10.1093/toxsci/kfs239 [DOI] [PubMed] [Google Scholar]
- Pomar J., López V., and Pomar C.. 2011. Agent-based simulation framework for virtual prototyping of advanced livestock precision feeding systems. Comput. Electron. Agric. 78: 88–97. doi: 10.1016/j.compag.2011.06.004 [DOI] [Google Scholar]
- Prelusky D. B. 1993. The effect of low-level deoxynivalenol on neurotransmitter levels measured in pig cerebral spinal fluid. J. Environ. Sci. Health B. 28:731–761. doi: 10.1080/03601239309372851 [DOI] [PubMed] [Google Scholar]
- Prelusky D. B. 1997. Effect of intraperitoneal infusion of deoxynivalenol on feed consumption and weight gain in the pig. Nat. Toxins 5:121–125. doi: [DOI] [PubMed] [Google Scholar]
- Prelusky D. B., Gerdes R. G., Underhill K. L., Rotter B. A., Jui P. Y., and Trenholm H. L.. 1994. Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat. Toxins 2:97–104.doi: 10.1002/nt.2620020302 [DOI] [PubMed] [Google Scholar]
- Ren Z., Deng H., Deng Y., Deng J., Zuo Z., Yu S., Shen L., Cui H., Xu Z., and Hu Y.. 2016. Effect of the Fusarium toxins, zearalenone and deoxynivalenol, on the mouse brain. Environ. Toxicol. Pharmacol. 4662–70. doi: 10.1016/j.etap.2016.06.028 [DOI] [PubMed] [Google Scholar]
- Rotter B., Thompson B., and Lessard M.. 1995. Effects of deoxynivalenol-contaminated diet on performance and blood parameters in growing swine. Can. J. Anim. Sci. 75: 297–302. doi: 10.4141/cjas95-046 [DOI] [Google Scholar]
- Rotter B. A., Thompson B. K., Lessard M., Trenholm H. L., and Tryphonas H.. 1994. Influence of low-level exposure to fusarium mycotoxins on selected immunological and hematological parameters in young swine. Fundam. Appl. Toxicol. 23:117–124. doi: 10.1093/toxsci/23.1.117 [DOI] [PubMed] [Google Scholar]
- Smith M. C., Madec S., Coton E., and Hymery N.. 2016. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins (Basel). 8:94. doi: 10.3390/toxins8040094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson S. P., Helaszek C., Buck W. B., Rood H. D. Jr, and Haschek W. M.. 1988. The role of intestinal microflora in the metabolism of trichothecene mycotoxins. Food Chem. Toxicol. 26:823–829. doi: 10.1016/0278-6915(88)90021-X [DOI] [PubMed] [Google Scholar]
- Tenk I., Fodor E., and Szathmáry C.. 1982. The effect of pure fusarium toxins (T-2, F-2, DAS) on the microflora of the gut and on plasma glucocorticoid levels in rat and swine. Zentralbl. Bakteriol. Mikrobiol. Hyg. A. 252:384–393. doi: 10.1016/S0174-3031(82)80013-9 [DOI] [PubMed] [Google Scholar]
- Trenholm H., Thompson B., Foster B., Charmley L., Hartin K., Coppock R., and Albassam M.. 1994. Effects of feeding diets containing Fusarium (naturally) contaminated wheat or pure deoxynivalenol (DON) in growing pigs. Can. J. Anim. Sci. 74: 361–369. doi: 10.4141/cjas94-049 [DOI] [Google Scholar]
- Vautier S., Sousa M. d. a. G., and Brown G. D.. 2010. C-type lectins, fungi and th17 responses. Cytokine Growth Factor Rev. 21:405–412. doi: 10.1016/j.cytogfr.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waché Y. J., Valat C., Postollec G., Bougeard S., Burel C., Oswald I. P., and Fravalo P.. 2009. Impact of deoxynivalenol on the intestinal microflora of pigs. Int. J. Mol. Sci. 10:1–17. doi: 10.3390/ijms10010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore C., Knap P., and Green D.. 2001. Technical review of the energy and protein requirements of growing pigs: energy. Anim. Sci. 73: 199–215. doi: 10.1017/S1357729800058185 [DOI] [Google Scholar]
- Wu Q., Dohnal V., Huang L., Kuca K., and Yuan Z.. 2010. Metabolic pathways of trichothecenes. Drug Metab. Rev. 42:250–267. doi: 10.1080/03602530903125807 [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., and Hosokawa H.. 1983. Natural cooccurrence of deoxynivalenol and nivalenol, trichothecene mycotoxins, in commercial foods. Food Hyg. Safe Sci. 24: 413–415. doi: 10.3358/shokueishi.24.413 [DOI] [Google Scholar]
- Young J. C., Zhou T., Yu H., Zhu H., and Gong J.. 2007. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 45:136–143. doi: 10.1016/j.fct.2006.07.028 [DOI] [PubMed] [Google Scholar]
- Zhou H. R., and Pestka J. J.. 2015. Deoxynivalenol (vomitoxin)-induced cholecystokinin and glucagon-like peptide-1 release in the STC-1 enteroendocrine cell model is mediated by calcium-sensing receptor and transient receptor potential ankyrin-1 channel. Toxicol. Sci. 145:407–417. doi: 10.1093/toxsci/kfv061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinedine A., Soriano J. M., Moltó J. C., and Mañes J.. 2007. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem. Toxicol. 45:1–18. doi: 10.1016/j.fct.2006.07.030 [DOI] [PubMed] [Google Scholar]