Abstract
Intestinal epithelial cells undergo rapid renewal along the crypt-villus axis (CVA), which ensures intestinal functions. Weaning stress differentially effects intestinal epithelial cell metabolism and physiological states along the CVA. Sulfur amino acids (SAA) play a key role in intestinal epithelial cell functioning. This study evaluated the effects of SAA dietary supplementation on weaning pig jejunal epithelial cells along the CVA. Sixteen Duroc × Landrace × Yorkshire piglets (6.16 ± 0.22 kg BW) were weaned at 21 d of age and were blocked by BW and gender and the randomly assigned to 1 of 2 groups fed diets consisting of low (0.53%) or high (0.85%) levels of SAA for a 7-d period. All piglets were euthanized for tissue sampling on day 7 postweaning. Jejunal epithelial cells were isolated along the CVA to yield 3 “cell fractions” (upper villus, middle villus, and crypt cells). The number of proliferating cells per crypt of piglets fed the high SAA diet was lower (P < 0.05) than that for low SAA diet. High SAA diet piglets tended to have decreased (P = 0.059) sucrase activities compared low SAA diet piglets. A high SAA diet increased (P < 0.05) total antioxidant capacity, catalase, and superoxide dismutase activities compared with a low SAA diet. mRNA expression levels of claudin-1, Slc5a1, and Slc7a9 in high SAA diet piglets were lower (P < 0.05) than for low SAA diet piglets. There were no interactions between dietary SAA and cell sections along the CVA for enzyme activities and mRNA expression in any of the weaned piglets. Protein amounts and phosphorylation levels related to Wnt/β-catenin and mechanistic targeting of rapamycin (mTOR) signaling pathways were affected by SAA in weaning piglets. These findings indicate that dietary SAA affects jejunal cell proliferation and functions in weaning piglets. There appears to be no interactions between dietary SAA and cell sections along the CVA. The effects of SAA may be partly through affecting antioxidant capacity, and Wnt/β-catenin and mTOR signaling pathway.
Keywords: crypt-villus axis, intestine, piglet, sulfur amino acids, weaning
INTRODUCTION
Intestinal epithelial cells perform the primary functions of digestion and absorption of nutrients, while defending against luminal pathogens and toxins (Pinto and Clevers, 2005). Intestinal epithelial cells undergo rapid renewal via highly coordinated cellular proliferation, differentiation, and apoptosis processes along the crypt-villus axis (CVA). This ensures intestinal function and recovery from tissue damage (Fan et al., 2001; Yang et al., 2013a; Yang et al., 2016a). Intestinal epithelial cell renewal along the CVA is regulated by mechanistic targeting of rapamycin (mTOR), Wnt/β-catenin signaling pathways, and antioxidant capacity (Yang et al., 2013, 2016b). Weaning stress in piglets usually disrupts intestinal epithelial cell renewal along the CVA and related signaling pathways and can result in morphological intestinal atrophy and dysfunction (Jiang et al., 2000; Yang et al., 2013a).
The sulfur amino acids (SAA), methionine and cysteine, have been suggested as the main factors affecting animal growth and intestinal health (Kim et al., 2006; Bauchart-Thevret et al., 2009). SAA deficiency reportedly upregulates intestinal methionine cycle activity while suppressing epithelial growth in neonatal pigs (Bauchart-Thevret et al. 2009). Our previous study showed that weaning stress affected intestinal functions by differentially affecting metabolism and signaling pathways in intestinal epithelial cells along the CVA (Xiong et al., 2015; Yang et al., 2016a, c). This study hypothesizes that dietary SAA may affect intestinal epithelial cell renewal and cell function along the CVA in weaning piglets. This study was conducted to explore the effects of SAA on enzyme activities, tight-junction protein expression, amino acid transport, Wnt/β-catenin-related protein synthesis, and mTOR signaling pathways along the CVA in weaning piglets.
MATERIALS AND METHODS
The experimental design and procedures in this study were reviewed and approved by the Animal Care and Use Committee of Hunan Normal University, Changsha City, Hunan, China.
Animals and Experimental Treatments
Sixteen piglets (Duroc × Landrace × Yorkshire; 8 males and 8 females; 6.16 ± 0.22 kg BW) were weaned at 21 d of age. The piglets were grouped by BW and gender and randomly assigned to 1 of 2 treatment groups fed a diet of either low (0.53%) or high (0.85%) SAA levels for a 7-d period. These doses were based on the results of prior studies (Ettle et al., 2010; Zong et al., 2018). Experimental diets were formulated to meet NRC (2012) nutrient requirements (Table 1) except for SAA levels. There were 8 pens (1 piglet/pen) per treatment. All piglets were housed individually caged and had ad libitum access to drinking water and the food throughout the experimental period. Feed intake was determined by daily weighing of feed amounts. At the end of study, individual piglets were weighed. ADG, ADFI, and G:F were then calculated.
Table 1.
Ingredient and chemical composition of experimental piglet diets (as-fed basis)
| Item | Dietary SAA, % | |
|---|---|---|
| 0.53 | 0.85 | |
| Corn | 53.23 | 53.12 |
| Soybean meal | 7.00 | 6.70 |
| Extruded soybean | 15.00 | 15.00 |
| Whey powder | 10.00 | 10.00 |
| Extruded blood meal | 8.00 | 8.00 |
| Dicalcium phosphate | 1.00 | 1.00 |
| Limestone | 1.10 | 1.10 |
| Salt | 0.40 | 0.40 |
| Choline chloride | 0.10 | 0.10 |
| Zinc Oxide | 0.30 | 0.30 |
| Lys | 0.26 | 0.27 |
| Met | 0.00 | 0.39 |
| Thr | 0.08 | 0.09 |
| Try | 0.03 | 0.03 |
| Soybean oil | 2.50 | 2.50 |
| Vitamin and mineral premix1 | 1.00 | 1.00 |
| Calculated composition | ||
| CP, % | 20.82 | 20.87 |
| DE, MJ/kg | 14.60 | 14.60 |
| Ca, % | 0.81 | 0.81 |
| Total P, % | 0.55 | 0.55 |
| Available P, % | 0.37 | 0.37 |
| Lys,2 % | 1.35 | 1.35 |
| Met,2 % | 0.27 | 0.59 |
| SAA,2,3 % | 0.53 | 0.85 |
| Thr,2 % | 0.79 | 0.79 |
| Trp,2 % | 0.21 | 0.21 |
| Met,% | 0.33 | 0.71 |
| SAA,% | 0.65 | 0.94 |
1Vitamin-mineral premix supplied per kilogram of feed: 10,000 IU of Vitamin A, 1,000 IU of Vitamin D3, 80 IU of Vitamin E, 2.0 mg of Vitamin K3, 0.03 mg of Vitamin B12, 12 mg of riboflavin, 40 mg of niacin, 25 mg of d-pantothenic acid, 0.25 mg of biotin, 1.6 mg of folic acid, 3.0 mg of thiamine, 2.25 mg of pyridoxine, 300 mg of choline chloride, 150 mg of Fe (FeSO4), 100 mg of Zn (ZnSO4), 30 mg of Mn (MnSO4), 25 mg of Cu (CuSO4), 0.5 mg of I (KIO3), 0.3 mg of Co (CoSO4), 0.3 mg of Se (Na2SeO3), and 4.0 mg of ethoxyquin.
2Standardized ileal-digestible.
3SAA = Met +Cys.
Sample Collection
At day 28 of age, each piglet was administered a general anesthesia and euthanized via an intravenous (jugular vein) injection of 4% sodium pentobarbital solution (40 mg/kg BW). Intestinal tissues from the jejunum middle section (approximately 2 cm long) were isolated using sterilized instruments and flushed with a phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, PH = 7.4). These sections were then fixed with a 4% formaldehyde-phosphate buffer and kept at 4 °C for Ki-67 immunohistochemistry. Five piglets were randomly selected from each treatment and intestinal epithelial cells were collected. A sequential isolation of these cells along CVA was performed based on Fan’s (Fan et al., 2001). Specfically, divided mid-jejunum segments were thoroughly cleaned with an ice-cold physiological saline solution and then warmed at 37 °C for 30 min with oxygenated PBS.
Jejunum segments were then filled with 15 to 30 mL of isolation buffer. The buffer consisted of 5 mM Na2EDTA; 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES); 0.5 mM DTT; 0.25% bovine serum albumin (BSA); and 2.5 mM D-glucose, 2.5 mM L-glutamine, and 0.5 mM DL-hydroxybutyrate sodium salt, oxygenated with an O2/CO2 mixture (19:1 vol/vol)] for sequential isolation of three epithelial cell fractions (upper villus: F1; middle villus: F2; crypt cells: F3) from the villus tip to the crypt bottom. The collected cells were washed twice with an oxygenated cell—suspension buffer (155 mM KCl at pH 7.4). The cells were retained via centrifugation at 400 × g and 4 °C for 10 min. The washed cells were immediately frozen using liquid nitrogen and the stored at −80 °C for further enzyme activity, RNA isolation, and western blot analysis testing (Tan et al., 2011).
Ki67 Immunohistochemistry
Serial slides were processed according to standard immunohistochemistry protocols with minor modifications. After slide dewaxing and rehydration, endogenous peroxidases were inhibited by incubation with freshly prepared 3% hydrogen peroxide for 10 min in the dark. Antigen retrieval was performed by twice boiling in 10 mM sodium citrate buffer (pH = 6.0). A 5% bovine serum albumin (BSA; Boster Biological Technology Co. Ltd, Wuhan, China) was used in 1:10 dilution for 30 min incubation at 37 °C to block nonspecific binding. Following overnight incubation with a Ki67 antibody (Abcam, 1:300 dilution), sections were treated with a goat antirabbit IgG secondary antibody (ZSGB-BIO, Beijing, China) for 45 min at 37 °C. Except for blocking, each step was followed by three 5-min PBS washes. Positive cells were visualized with diaminobenzidine (DAB) (ZSGB-BIO, Beijing, China). Sections were counterstained with hematoxylin, then dehydrated, and mounted. Negative control sections were processed using the same protocols except that the primary antibody was replaced with PBS. Ki67 scores (number of positive cells per field under 200× magnification) were evaluated using Image-Pro Plus 6.0 software (Media Cybernetics; San Diego, CA).
Enzyme Activity Anaylsis
Alkaline phosphatase (ALP), lactase, and sucrase in isolated cell fractions activities were analyzed using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to manufacturer instructions. The antioxidant capacities, including total antioxidant capacity (T-AOC), catalase (CAT), malondialdehyde (MDA), superoxide dismutase (SOD), and CuZn-SOD, were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following manufacturer protocols.
Quantitative Real-Time PCR Analysis
RNA isolation and RT-PCR analysis of isolated cells were performed according to Yang’s methods (Yang et al., 2013b). Total RNA was isolated from isolated cells using a TRIZOL reagent (Invitrogen, Carlsbad, CA). RNA quality and quantity was determined by ultraviolet spectroscopy using a spectrophotometer (NanoDrop ND-1000; Thermo Fisher Scientific, DE). Selected gene primer sequences appear in Table 2. Real-time quantitative PCR (RT-PCR) analyses were performed using an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Real-time PCR was duplicated for each cDNA sample, using SYBR Green I as PCR core reagents in a final volume of 10 μL. After a predenaturation process (10 s at 95 °C), 40 amplification cycles (each cycle consisted of 95 °C for 5 s and 60 °C for 20 s) were performed. This was followed by a melting curve program (from 60 to 99 °C with a heating rate of 0.1 °C/s) and the fluorescence was collected. The housekeeping gene β-actin was used in each sample as an internal control to normalize target gene expression. mRNA expression abundance (A) of target genes was calculated according to this formula: A = 2−ΔΔCt(treat − control), where −ΔΔCt(treat − control) = (Ct gene of interest − Ct β-actin)treat − (Ct gene of interest − Ct β-actin)control.
Table 2.
Primers used for real-time PCR analysis
| Genes | Primers | Sequences (5′–3′) | Size (bp) | GenBank accession No. |
|---|---|---|---|---|
| Claudin-1 | Forward | CTAGTGATGAGGCAGATGAA | 250 | XM_005670262.3 |
| Reverse | AGATAGGTCCGAAGCAGAT | |||
| Occludin | Forward | GAGTGATTCGGATTCTGTCT | 181 | XM_005672525.3 |
| Reverse | TAGCCATAACCATAGCCATAG | |||
| ZO-1 | Forward | TTGATAGTGGCGTTGACA | 126 | XM_021098896.1 |
| Reverse | CCTCATCTTCATCATCTTCTAC | |||
| Slc15a1 | Forward | CGGCTGGAATGACAATCT | 134 | NM_214347.1 |
| Reverse | CGATGGACAACGACACAA | |||
| Slc5a1 | Forward | ATCTCTGTCATCGTCATCTAC | 121 | NM_001164021.1 |
| Reverse | GCCACCACACCATACTTC | |||
| Slc6a19 | Forward | CACAACAACTGCGAGAAG | 152 | XM_003359855.3 |
| Reverse | TTGATAAGCGTCAGGATGT | |||
| Slc7a9 | Forward | GAAGAAGCCTCCTAGAAGTG | 268 | XM_013988544.1 |
| Reverse | CCAGTGTCGCAAGAATCC | |||
| Slc1a1 | Forward | GCTGTGCTGAAGAGAAGAA | 181 | NM_001164649.1 |
| Reverse | GTGGCGGTGATACTGATAG | |||
| β-actin | Forward | AGTTGAAGGTGGTCTCGTGG | 216 | XM_003357928.4 |
| Reverse | TGCGGGACATCAAGGAGAAG |
Western Blotting Analysis
Ice-cold RIPA lyses buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl at pH 7.4) from Beyotime Biotechnology, Shanghai, China, which contained 0.1mM phenylmethylsulfonyl fluoride (PMSF) was used to extract total protein fractions from isolated cells. The sample was then centrifuged at 10,000 × g and 4 °C for 10 min. The protein concentration in the supernatant fluid was determined using a Bicinchoninic Acid assay (Beyotime Biotechnology, China). All samples were adjusted to an equal protein concentration and then diluted with 5 × loading buffer (Beyotime Biotechnology, China) to a final volume of 1 mL and heated in boiling water for 5 min.
Soluble proteins were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA), blocked with 5% nonfat milk in TBS-0.05% Tween-20 for 1 h, and incubated overnight with primary antibodies followed by horseradish peroxidase-linked secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA). The bound antibodies were using enhanced chemiluminescence (Applygen Technologies Inc., Beijing, China) for detection (Yang et al., 2013b). Antibodies for mTOR(7C10), phospho-mTOR (Ser2448), β-Catenin (D10A8), non-phosphor-β-Catenin (Ser45), and Bmil (D42B3) western blot analysis were purchased from Cell Signaling Technology (Cedarlane, ON, Canada). The antibodies for β-actin western blot analysis were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Target protein abundance was normalized via β-actin. AlphaImager 2200 software (Alpha Innotech Corporation, CA) was used to quantify the bands of each protein per sample.
Statistical Analysis
All data were expressed as the mean ± SEM, and differences in Ki67 immunohistochemistry, amounts of mTOR, P-mTOR, β-Catenin, non-phosphor-β-Catenin, and Bmil were analyzed using t test. Enzyme activities data and mRNA expression were analyzed as a 2 × 2 factorial using SPSS software (SPSS 20.0). P values of <0.05 were used to indicate statistical significance.
RESULTS
Growth Performance, Ki67 Immunohistochemistry, and Enzyme Activities
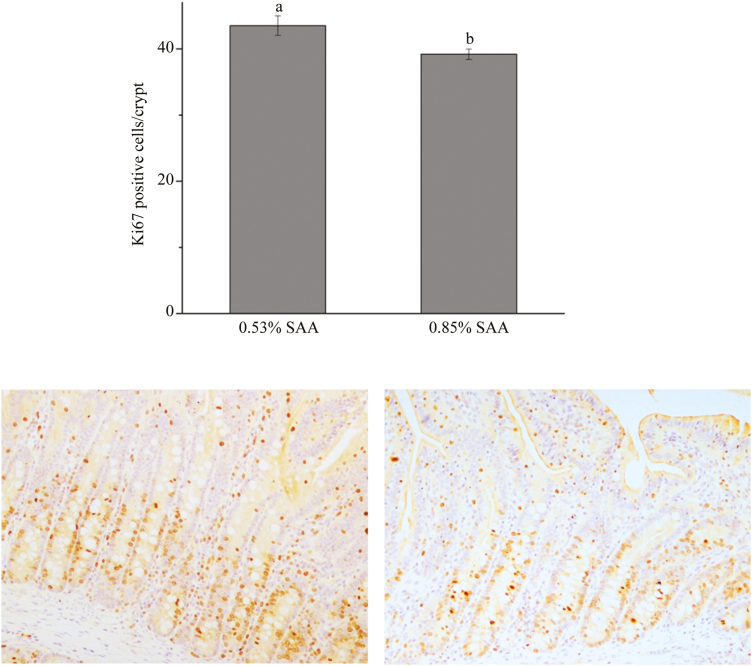
No significant differences in ADG (78.57 ± 8.95 vs. 55.10 ± 10.69 g), ADFI (276.90 ± 26.61 vs. 244.40 ± 25.78 g), or G:F (0.28 ± 0.06 vs. 0.23 ± 0.11) were detected between low- and high-SAA diet piglets. Intestinal cell proliferation in intestine of animals was evaluated by immunohistochemistry for Ki67. Ki67 positive cells per crypt in the jejunum were counted. Piglets fed the high-SAA diet had a lower number of proliferating cells per crypt (P < 0.05) than those fed a low-SAA diet (Figure 1). High-SAA diet piglets tended to decrease (P = 0.059) the activities of sucrase compared with low-SAA diet piglets. The upper villus cells had greater (P < 0.05) ALP activities than middle villus and crypt cells (Table 3). There were no significant lactase activitiy differences in the three fractions of the 2 groups. A high-SAA diet increased (P < 0.05) T-AOC, CAT, and SOD activities. The SOD activity in middle villus and crypt cells was higher (P < 0.05) than in upper villus cells (Table 4). The high SAA diet tended to increase (P = 0.088) CuZn-SOD activities compared with the low-SAA diet. No differences in MDA contents were observed between the 2 groups. There were no observed interactions between dietary SAA and cell sections along the CVA for any of the weaned piglets’ enzyme activities.
Figure 1.
Immunohistochemistry for Ki67. a,bWithin a variable, values with different superscripts differ (P < 0.05).
Table 3.
Effects of dietary supplementation with sulfur amino acids (SAA) on activity of digestive enzymes in intestinal epithelial cells along the crypt villus axis in weaning piglets
| Item | 0.53% SAA | 0.85% SAA | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | T | S | T × S | |
| ALP, U/g of protein | 196.55 | 124.46 | 135.12 | 246.70 | 100.09 | 90.58 | 0.847 | 0.014 | 0.456 |
| Lactase, U/mg of protein | 14.07 | 13.44 | 11.43 | 17.94 | 6.19 | 10.16 | 0.529 | 0.103 | 0.195 |
| Sucrase, U/mg of protein | 7.91 | 15.80 | 7.89 | 7.74 | 3.95 | 3.12 | 0.059 | 0.461 | 0.225 |
T = effects of dietary treatment; S = effects of crypt villus axis section; T × S = effect of interaction between dietary treatment and crypt villus axis section.
ALP = alkaline phosphatase; F1 = upper villus; F2 = middle villus; F3 = crypt cells.
Table 4.
Effects of dietary supplementation with sulfur amino acids (SAA) on activity of antioxidant enzymes in intestinal epithelial cells along the crypt villus axis in weaning piglets
| Item | 0.53% SAA | 0.85% SAA | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | T | S | T × S | |
| CAT, U/mg of protein | 231.79 | 294.34 | 305.05 | 542.70 | 528.38 | 549.60 | 0.000 | 0.881 | 0.874 |
| MDA, nmol/mg of protein | 0.59 | 0.55 | 0.30 | 0.69 | 0.39 | 0.55 | 0.520 | 0.137 | 0.169 |
| T-AOC, mmol/mg of protein | 122.21 | 124.61 | 107.67 | 119.77 | 145.13 | 139.44 | 0.016 | 0.190 | 0.105 |
| SOD, U/mg of protein | 29.17 | 33.06 | 29.17 | 29.08 | 36.78 | 37.65 | 0.035 | 0.039 | 0.172 |
| CuZn-SOD, U/mg of protein | 29.60 | 32.15 | 28.30 | 28.08 | 36.16 | 36.17 | 0.088 | 0.116 | 0.164 |
T = effects of dietary treatment; S = effects of crypt villus axis section; T × S = effect of interaction between dietary treatment and crypt villus axis section.
T-AOC = total antioxidant capacity; CAT = catalase; MDA = malondialdehyde; SOD = superoxidedismutase; F1 = upper villus; F2 = middle villus; F3 = crypt cells.
mRNA Expression of Tight Junction Protein and AA Transporter
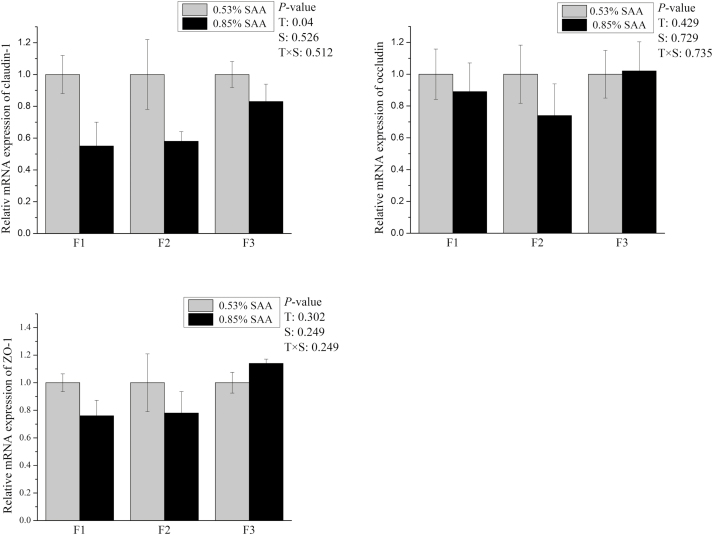
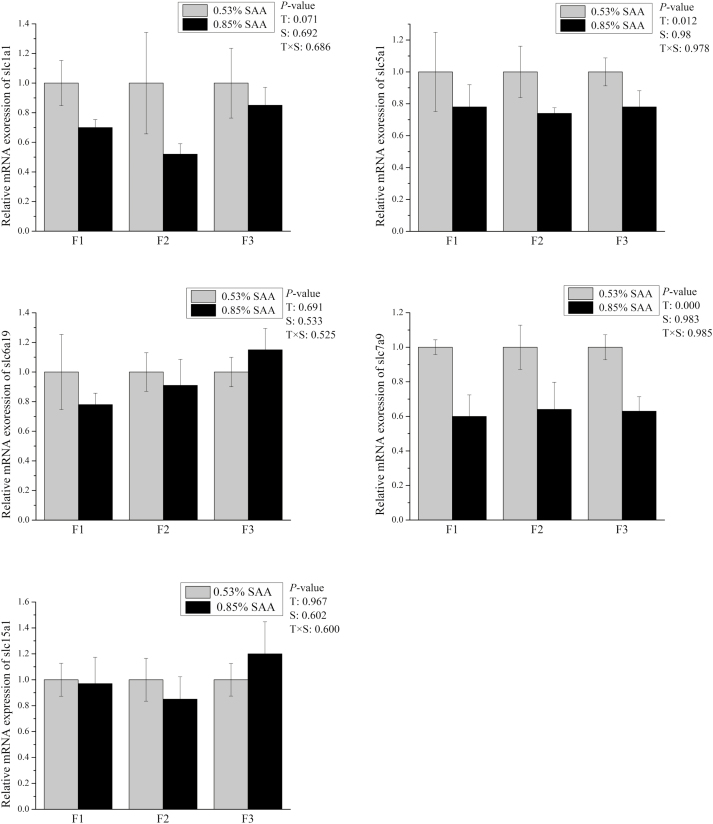
The high-SAA diet decreased (P < 0.05) mRNA expression levels of claudin-1, Slc5a1, and Slc7a9 in weaned piglets villus-crypt axis (Figures 2 and 3). The mRNA expression of Slc1a1 (P = 0.071) in the villus-crypt axis of high-SAA diet piglets tended to be less compared with low-SAA diet piglets (Figure 3). No treatment effect was observed in the mRNA expression of Occludin, ZO-1, Slc15a1, or Slc6a19 between low- and high-SAA groups. There were no significant differences in the mRNA expression of ZO-1, Occludin, Slc15a1, Slc6a19, or Slc5a1 among the 3 fractions. There were no interactions observed between dietary SAA and cell sections along the CVA for mRNA expression level in weaned piglets.
Figure 2.
Effects of dietary supplementation with sulfur amino acids (SAA) on mRNA expression of tight junction of F1 to F3 cell fraction in weaning piglets. The mRNA expression abundances of Claudin-1, Occludin, and ZO-1 were normalized using β-actin as an internal control. P values of <0.05 were used to indicate statistical significance. Data are expressed as means ± SEM; n = 5. T = effects of dietary treatment; S = effects of crypt villus axis section; T × S = effect of interaction between dietary treatment and crypt villus axis section.
Figure 3.
Effects of dietary supplementation with sulfur amino acids (SAA) on mRNA expression of peptide transporters of F1 to F3 cell fraction in weaning piglets. The mRNA expression abundances of Slc15a1, Slc1a1, Slc5a1, Slc6a19, and Slc7a9 were normalized using β-actin as an internal control. P values of <0.05 were used to indicate statistical significance. Data are expressed as means ± SEM; n = 5. T = effects of dietary treatment; S = effects of crypt villus axis section; T × S = effect of interaction between dietary treatment and crypt villus axis section.
Wnt/β-Catenin, mTOR Pathway Analysis
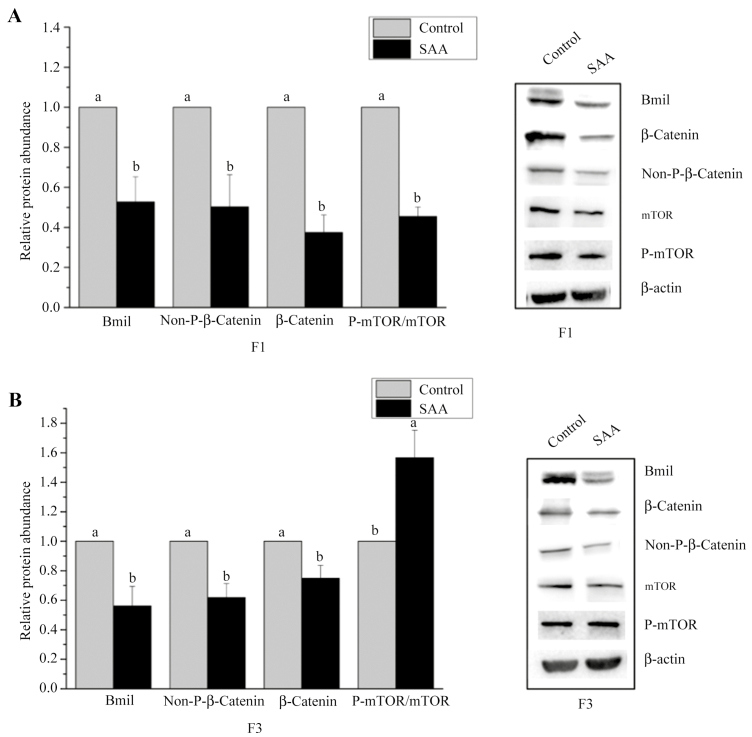
The high-SAA diet reduced (P < 0.05) relative expressions of Bmil and non-phosphor-β-catenin in the upper villus and crypt cells of weaning piglets (Figure 4). High-SAA diet piglets had decreased (P < 0.05) relative expression of β-catenin in crypt cells and significantly reduced (P < 0.01) β-catenin and p-mTOR/mTOR amounts in the upper villus (Figure 4) compared with the low-SAA diet group. p-mTOR/mTOR amounts in crypt cells increased (P < 0.05) due to dietary SAA (Figure 4).
Figure 4.
Effects of dietary supplementation with sulfur amino acids (SAA) on protein abundance of mammalian target of rapamycin signaling pathway (mTOR) and Wnt/β-catenin signaling pathway of upper villus or crypt cell fraction in weaning piglets. (A) the relative abundance of proteins in mTOR and Wnt/β-catenin signaling pathway in F1. (B) The relative abundance of proteins in mTOR and Wnt/β-catenin signaling pathway in F3. β-Actin was used as an internal control to normalize abundance. Data are means ± SEM, n = 5.
DISCUSSION
The small intestine plays key roles in the digestion and absorption of nutrients and affects the function of the entire organism (Yang et al., 2016d). The intestinal lumen is lined with epithelial cells (or mucosa) that play critical roles in well-known digestive and absorptive functions and maintaining a barrier against noxious antigens and bacteria (Pacha, 2000). Cells positive for Ki67 in the epithelium were mostly present in crypts and cell proliferation of the gastrointestinal tract epithelium and were evaluated by counting Ki67 positive cells (De Conto et al., 2010). Bauchart-Thevret et al. (2009) reported that dietary SAA deficiencies suppressed epithelial growth in neonatal pigs. Similarly, the present study also showed that the number of proliferating cells per crypt in high-SAA diet piglets was lower than that of low-SAA diet piglets. This suggests that a high-SAA diet suppressed epithelial growth in weaned piglets.
ALP is a marker of enterocyte differentiation and is regarded as a key marker enzyme for changes in small intestine digestive and absorptive functions (Hodin et al., 1995). Early weaning has been reported to decrease piglet intestinal ALP digestive capacity (Lackeyram et al., 2010). In this study, no differences in ALP activities were observed between the 2 groups. Results did indicate that ALP activities in the villus were higher than in the crypt. This result is in line with Fan et al. (2001), who reported that ALP activities in small intestine epithelial cells increased along the CVA in piglets. mRNA expression levels of AA transporters (Slc5a1 and Slc7a9) were reduced in a high SAA-diet piglets. Amino acids and peptides are mainly absorbed by the enterocytes of the small intestine with the core process for absorption of dietary AA or peptides mainly mediated by specific transporters (Bröer, 2008; Yang et al., 2013b). This study’s results suggest that digestion and absorption capacities in low-SAA diet are more effective than that in high-SAA diets. A possible explanation is that SAA effects on epithelial growth in weaned piglets associate with the lower small intestine digestion and absorption capacities.
Tight junctions are the principal determinants of epithelial, endothelial, and paracellular barrier functions (Shen et al., 2011; Ren et al., 2014). Tight junction proteins consist of a complex of integral membrane proteins tethered to cytoplasm plaque proteins which play key roles in maintaining intestinal epithelial tight junctions (Xiong et al., 2015). Prior studies showed that early weaning induced sustained impairment in the intestinal barrier by decreasing tight-junction protein expression (Hu et al., 2013). Chen et al. (2014) showed that dietary supplementation with L-Met improved jejunal epithelial barrier function in postweaning piglets. In the present study, a high-SSA diet decreased mRNA expression levels of claudin-1, suggesting that a high-SAA diet may compromise piglet intestinal epithelium integrity.
Oxidative damage is a strong indicator of health status and the well-being of animals in terms of stress, nutritional status, and disease (Xie et al., 2016). Antioxidant defenses can be enzymatic or nonenzymatic. Enzymatic defenses include SOD, CAT, and GSH-Px, which are major aspects of the as antioxidant system (Yin et al., 2013). Recent studies show that weaning may damage the intestine by contributing to oxidative stress and, might, eventually led to enterocyte apoptosis and cell cycle arrest in weaned piglet small intestines (Chen et al., 2018b).
SAA has been reported to play an important role in antioxidant function in neonatal pigs (Bauchart-Thevret et al., 2009). Previous study showed that increasing maternal consumption of methionine promoted intestinal antioxidant ability of neonatal pigs (Zhong et al., 2016). It is widely recognized that CAT and SOD, 2 important endogenous antioxidant enzymes, play crucial roles in preventing oxidative damage (Chen et al., 2018a). In the present study, high dietary SAA increased the activities of T-AOC, CAT and tended to increase the activities of CuZn-SOD. These results suggest that high dietary SAA contents enhanced the antioxidative activity of weaned piglets.
The mTOR signaling pathway is a serine/threonine protein kinase belonging to the phosphoinositide-3-kinase (PI3K)-related kinase family (Weichhart. 2012). The mTOR signaling pathway affects cell proliferation and growth by affecting protein biosynthesis (Wullschleger et al., 2006; Makky et al., 2007). The Wnt signaling pathway is an evolutionarily conserved, cysteine-rich, secreted glycoproteins system which plays an important role in cell polarity, proliferation, differentiation, and migration (Klaus and Birchmeier, 2008). In mTOR and Wnt/β-catenin pathways, expression and phosphorylation levels of the relevant functional proteins are important parameters in measuring cell proliferation (Fan et al., 2017).
In this study, the high-SAA diet reduced the relative expressions of Bmil and nonphosphor-β-catenin in the upper villus and crypt cells of weaning piglets. High-SAA diet piglets had decreased relative expression of β-catenin in crypt cells and decreased amounts of β-catenin and p-mTOR/mTOR in the upper villus. Proliferating cells usually have high activity of the mTOR signaling pathway because mTOR signaling pathways play key roles in integrating signals from nutrients and growth factors to regulate cell cycle progression and cell growth coordinately (Fingar et al., 2004). A high-SAA diet increased the amount of p-mTOR/mTOR in crypt cells. Our previous study showed that mTOR signaling pathway activity in intestinal epithelial cells of piglets decreased from crypt to villus tip (Yang et al., 2016b). This indicates that a high-SAA diet decreased the amount of p-mTOR/mTOR in crypt cells and may be the result of mTOR signaling pathway activity in the crypt being higher than in the villi.
In summary, the results of the present study showed that dietary SAA affects jejunal cell proliferation and functions in weaning piglets and this affect may be partly via its antioxidant capacity, and Wnt/β-catenin and mTOR signaling pathway.
LITERATURE CITED
- Bauchart-Thevret C., B. Stoll S. Chacko, and Burrin D. G.. 2009. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 296:E1239–E1250. doi: 10.1152/ajpendo.91021.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S. 2008. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 88:249–286. doi: 10.1152/physrev.00018.2006 [DOI] [PubMed] [Google Scholar]
- Chen Y., D. Li Z. Dai X. Piao Z. Wu B. Wang Y. Zhu, and Zeng Z.. 2014. L-methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 46:1131–1142. doi: 10.1007/s00726-014-1675-5 [DOI] [PubMed] [Google Scholar]
- Chen J., Li Y., Yu B., Chen D., Mao X., Luo J., and He J.. 2018a. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 3:1108–1118. doi: 10.1093/jas/skx078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Xie H., Chen D., Yu B., Mao X., Zheng P., Yu J., Luo Y., Luo J., and He J.. 2018b. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs. ACS Omega 3:2211–2219. doi: 10.1021/acsomega.7b01971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Conto C., A. Oevermann I. A. Burgener M. G. Doherr, and Blum J. W.. 2010. Gastrointestinal tract mucosal histomorphometry and epithelial cell proliferation and apoptosis in neonatal and adult dogs. J. Anim. Sci. 88:2255–2264. doi: 10.2527/jas.2009-2511 [DOI] [PubMed] [Google Scholar]
- Ettle T., Rademacher M., Htoo J. K., and Roth F. X.. 2010. Dietary preference for methionine sources in weaned pigs. Anim. Feed. Sci. Tech. 155:201–205. doi: 10.1016/j.anifeedsci.2009.11.001 [DOI] [Google Scholar]
- Fan M. Z., B. Stoll R. Jiang, and Burrin D. G.. 2001. Enterocyte digestive enzyme activity along the crypt-villus and longitudinal axes in the neonatal pig small intestine. J. Anim. Sci. 79:371–381. [DOI] [PubMed] [Google Scholar]
- Fan H. B., Zhai Z. Y., Li X. G., Gao C. Q., Yan H. C., Chen Z. S., and Wang X. Q.. 2017. CDX2 stimulates the proliferation of porcine intestinal epithelial cells by activating the mTORC1 and Wnt/β-Catenin signaling pathways. Int. J. Mol. Sci.18:2447. doi: 10.3390/ijms18112447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar D. C. and Blenis J.. 2004. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23:3151–3171. doi: 10.1038/sj.onc.1207542 [DOI] [PubMed] [Google Scholar]
- Hodin R. A., S. M. Chamberlain, and Meng S.. 1995. Pattern of rat intestinal brush-border enzyme gene expression changes with epithelial growth state. Am. J. Physiol. 269(2 Pt 1):C385–C391. doi: 10.1152/ajpcell.1995.269.2.C385 [DOI] [PubMed] [Google Scholar]
- Hu C. H., K. Xiao Z. S. Luan, and Song J.. 2013. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91:1094–1101. doi: 10.2527/jas.2012-5796 [DOI] [PubMed] [Google Scholar]
- Jiang R., Chang X., Stoll B., Fan M. Z., Arthington J., Weaver E., Campbell J., Burrin D.G.. 2000. Dietary plasma protein reduces small intestinal growth and lamina propria cell density in early weaned pigs. J. Nutr. 130:21–26. doi:org/10.1093/jn/130.1.21 [DOI] [PubMed] [Google Scholar]
- Kim B. G., M. D. Lindemann M. Rademacher J. J. Brennan, and Cromwell G. L.. 2006. Efficacy of DL-methionine hydroxy analog free acid and DL-methionine as methionine sources for pigs. J. Anim. Sci. 84:104–111. doi:10.2527/2006.841104x [DOI] [PubMed] [Google Scholar]
- Klaus A., and Birchmeier W.. 2008. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer. 5:387–398. doi: 10.1038/nrc2389 [DOI] [PubMed] [Google Scholar]
- Lackeyram D., C. Yang T. Archbold K. C. Swanson, and Fan M. Z.. 2010. Early weaning reduces small intestinal alkaline phosphatase expression in pigs. J. Nutr. 140:461–468. doi: 10.3945/jn.109.117267 [DOI] [PubMed] [Google Scholar]
- Makky K., J. Tekiela, and Mayer A. N.. 2007. Target of rapamycin (TOR) signaling controls epithelial morphogenesis in the vertebrate intestine. Dev. Biol. 303:501–513. doi: 10.1016/j.ydbio.2006.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pácha J. 2000. Development of intestinal transport function in mammals. Physiol. Rev. 80:1633–1667. doi: 10.1152/physrev.2000.80.4.1633 [DOI] [PubMed] [Google Scholar]
- Pinto D. and Clevers H.. 2005. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp. Cell Res. 306:357–363. doi: 10.1016/j.yexcr.2005.02.022 [DOI] [PubMed] [Google Scholar]
- Ren W. K., Yin J., Wu M. M., Liu G., Yang G., Xion Y., Su D., Wu L., Li T. J., Chen S., Duan J. L.,. et al. 2014. Serum amino acids profile and the beneficial effects of L-Arginine or L-Glutamine supplementation in dextran sulfate sodium colitis. PLOS ONE. 9:e88335–88340. doi: 10.1371/journal.pone.0088335.eCollection2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., C. R. Weber D. R. Raleigh D. Yu, and Turner J. R.. 2011. Tight junction pore and leak pathways: a dynamic duo. Annu. Rev. Physiol. 73:283–309. doi: 10.1146/annurev-physiol-012110-142150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B. E., Yin Y. L., Liu Z. Q., Tang W. J., Xu H. J., Kong X. F., Li X. G., Yao K., Gu W. T., Smith S. B.,. et al. 2011. Dietary L-arginine supplementation differentially regulates expression of fat-metabolic genes in porcine adipose tissue and skeletal muscle. J. Nutr. Biochem. 22:441–445. doi: 10.1016/j.jnutbio.2010.03.012 [DOI] [PubMed] [Google Scholar]
- Weichhart T. 2012. Mammalian target of rapamycin: a signaling kinase for every aspect of cellular life. Methods Mol. Biol. 821:1–14. doi: 10.1007/978-1-61779-430-8_1 [DOI] [PubMed] [Google Scholar]
- Wullschleger S., R. Loewith, and Hall M. N.. 2006. TOR signaling in growth and metabolism. Cell 124:471–484. doi: 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Xie C., X. Wu C. Long Q. Wang Z. Fan S. Li, and Yin Y.. 2016. Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. BMC Vet. Res. 12:243. doi: 10.1186/s12917-016-0872-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., H. S., Yang X. C., Wang Q., Hu C. X., Liu X., Wu D., Deng Y. Q., Hou C. M., Nyachoti D. F., Xiao, et al. 2015. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 93:1089–1097. doi: 10.2527/jas.2014-7851 [DOI] [PubMed] [Google Scholar]
- Yang H. S., D. Z. Fu X. F. Kong W. C. Wang X. J. Yang C. M. Nyachoti, and Yin Y. L.. 2013b. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned huanjiang mini-pig piglets. J. Anim. Sci. 91:2740–2748. doi: 10.2527/jas.2012-5795 [DOI] [PubMed] [Google Scholar]
- Yang H. S., Wang X. C., Xiong X., Li T. J., and Yin Y. L.. 2016c. Effects of weaning on intestinal crypt epithelial cells in piglets. Sci. Rep. 6:36939. doi: 10.1038/srep36939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. S., Wang X. C., Xiong X., and Yin Y. L.. 2016d. Energy metabolism in intestinal epithelial cells during maturation along the crypt-villus axis. Sci. Rep. 6:31917. doi: 10.1038/srep31917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. S., Xiong X., Wang X. C., Tan B., Li T. J., and Yin Y. L.. 2016a. Effects of weaning on intestinal upper villus epithelial cells of piglets. PLOS ONE. 11: e0150216. doi: 10.1371/journal.pone.0150216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., X. Xiong X. Wang, and Yin Y.. 2016b. Mammalian target of rapamycin signaling pathway changes with intestinal epithelial cells renewal along crypt-villus axis. Cell. Physiol. Biochem. 39:751–759. doi: 10.1159/000445665 [DOI] [PubMed] [Google Scholar]
- Yang H., Xiong X., and Yin Y.. 2013a. Development and renewal of intestinal villi in pigs. In: Blachier F., Wu G., and Y. Yin, editors, Nutritional and physiological functions of amino acids in pigs. Springer, New York: p. 29–47. [Google Scholar]
- Yin J., W. Ren G. Liu J. Duan G. Yang L. Wu T. Li, and Yin Y.. 2013. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic. Res. 47:1027–1035. doi: 10.3109/10715762.2013.848277 [DOI] [PubMed] [Google Scholar]
- Zhong H. J., Li H., Liu G. M., Wan H. F., Mercier Y., Zhang X. L., Lin Y., Che L. Q., Xu S. Y., Tang L.,. et al. 2016. Increased maternal consumption of methionine as its hydroxyl analog promoted neonatal intestinal growth without compromising maternal energy homeostasis. J. Anim. Sci. Biotechnol. 7:46. doi: 10.1186/s40104-016-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong E., P. Huang W. Zhang J. Li Y. Li X. Ding X. Xiong Y. Yin, and Yang H.. 2018. The effects of dietary sulfur amino acids on growth performance, intestinal morphology, enzyme activity, and nutrient transporters in weaning piglets. J. Anim. Sci. 96:1130–1139. doi: 10.1093/jas/skx003 [DOI] [PMC free article] [PubMed] [Google Scholar]