Abstract
Context
A majority of women with polycystic ovary syndrome (PCOS) have metabolic abnormalities that result in an increased risk of developing type 2 diabetes and heart disease. Correlative studies have shown an association between changes in the gut microbiome and metabolic disorders. Two recent studies reported a decrease in α diversity of the gut microbiome in women with PCOS compared with healthy women.
Objective
We investigated whether changes in the gut microbiome correlated with specific clinical parameters in women with PCOS compared with healthy women. We also investigated whether there were changes in the gut microbiome in women with polycystic ovarian morphology (PCOM) who lacked the other diagnostic criteria of PCOS.
Participants
Subjects were recruited at the Poznan University of Medical Sciences. Fecal microbial diversity profiles of healthy women (n = 48), women with PCOM (n = 42), and women diagnosed with PCOS using the Rotterdam criteria (n = 73) were analyzed using 16S ribosomal RNA gene sequencing.
Results
Lower α diversity was observed in women with PCOS compared with healthy women. Women with PCOM had a change in α diversity that was intermediate between that of the other two groups. Regression analyses showed that hyperandrogenism, total testosterone, and hirsutism were negatively correlated with α diversity. Permutational multivariate analysis of variance in UniFrac distances showed that hyperandrogenism was also correlated with β diversity. A random forest identified bacteria that discriminated between healthy women and women with PCOS.
Conclusion
These results suggest that hyperandrogenism may play a critical role in altering the gut microbiome in women with PCOS.
A 16S rRNA gene sequencing analysis demonstrated that changes in the gut microbiome are highly correlated with hyperandrogenism in women with PCOS compared with healthy women.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age, with an estimated worldwide prevalence of 5% to 15% when the Rotterdam consensus criteria are used (1). Hyperandrogenism is a key feature of this disorder, and heritability studies indicate that there is a strong polygenic component (2). PCOS can result in profound, long-term health consequences (3). In addition to increased risks of infertility, miscarriage, and pregnancy complications, many women with PCOS have metabolic abnormalities that increase their risk of developing obesity, type 2 diabetes, and cardiovascular disease (4, 5).
A complex community of microorganisms that is important for human health resides within the large intestine (the gut microbiome) (6). Correlative studies demonstrated that the gut microbiome of individuals with metabolic disorders such as obesity and diabetes differs from that of healthy individuals (7–10). More recently, two studies reported differences in the gut microbiome of Caucasian or Han Chinese women with PCOS, including a decrease in the overall bacterial species richness (α diversity) of the gut microbial community and changes in several bacterial taxa, compared with that of healthy women (11, 12). Fecal microbiome transplantation from obese humans into germ-free mice also resulted in an obese phenotype, indicating a potential causative role of the gut microbiome in the development of metabolic disorders (13, 14).
Given that we previously observed changes in the gut microbiome in a hyperandrogenic mouse model of PCOS (15), we investigated whether changes in the gut microbiome in women with PCOS correlate with hyperandrogenism or other hallmarks of PCOS. We found that women with PCOS had a decrease in biodiversity in the gut microbiome and changes in specific bacterial taxa compared with healthy women. Women with polycystic ovarian morphology (PCOM) also had a change in gut microbial diversity that was intermediate between that of the other two groups. Furthermore, our analyses demonstrated that hyperandrogenism (total testosterone and hirsutism) was correlated with changes in the gut microbiome. A better understanding of the relationship between hyperandrogenism and the gut microbiome in women may lead to new therapeutic approaches for PCOS.
Subjects and Methods
Study cohort
A total of 163 premenopausal women were recruited at the Poznan University of Medical Sciences. Using the Rotterdam criteria, PCOS was diagnosed by the presence of at least two of the following conditions: clinical or biochemical hyperandrogenism (Ferriman-Gallwey score ≥8; testosterone level >0.5 ng/mL), oligomenorrhea or amenorrhea (<8 cycles/y), and polycystic ovaries. Congenital adrenal hyperplasia was excluded on the basis of a morning follicular phase 17-hydroxyprogesterone level <2 ng/mL. Diabetes mellitus was excluded on the basis of a fasting glucose level <100 mg/dL and a glucose tolerance test value <200 mg/dL at 30, 60, and 90 minutes and <140 mg/dL at 120 minutes. None of the subjects had elevated prolactin levels, thyroid disease, or Cushing disease. Study participants had no clinical signs or symptoms of any other endocrinopathy, a normal baseline renal function, and normal levels of bilirubin and aminotransferases. Exclusion criteria were the use of oral contraceptives, other steroid hormones, and metformin within the preceding 3 months. Subjects taking antibiotics, probiotics, or laxatives were excluded. All study participants were at least 18 years old and provided informed consent. The study was approved by the institutional review boards at the Poznan University of Medical Sciences and the University of California, San Diego.
Sampling and laboratory measurements
The study visits took place between 8:30 and 11 am. Clinical assessments included determination of body mass index (BMI) and hirsutism. Venous blood was collected after an overnight fast, and serum was stored at −80°C until the analyses were performed. A 2-hour oral glucose tolerance test was performed with determinations of glucose and insulin in the fasting state as well as after a 75-g glucose load at 30, 60, 90, and 120 minutes. Fecal samples were collected from the rectum using a cotton swab (CultureSwab 220135; Becton Dickinson). Samples were stored at −80°C within 20 minutes of collection. Transvaginal ultrasonographic evaluations were performed using the Aloka ProSound 7 (Aloka Co. Ltd.). The ovaries were measured in three perpendicular diameters. Ovarian volume was determined using the prolate ellipsoid formula. Glucose levels were determined using the enzymatic reference method with hexokinase. Serum testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), sex hormone-binding globulin, and insulin levels were determined using electrochemiluminescence assays (Roche Cobas 6000 System).
DNA isolation
Rectal swab samples were shipped on dry ice to the University of California, San Diego and stored at −80°C. Genomic DNA was extracted from samples in a class II biological safety cabinet using the PowerSoil DNA Isolation Kit (MoBio Laboratories, Inc.). Solution C1 (60 µL) was added to the bead beating tubes, and the cotton tips of the swabs were broken off directly into the tubes. Tubes were vortexed at maximum speed for 15 minutes using the PowerSoil Vortex Adaptor (MoBio Laboratories, Inc). The remaining steps were performed as directed by the manufacturer. Genomic DNA was quantified using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific), and the DNA was stored at −80°C.
16S ribosomal RNA amplicon sequencing
For each sample, the V4 hypervariable region of the 16S ribosomal RNA (rRNA) gene was polymerase chain reaction (PCR) amplified with primers 515F and 806R (16). The reverse primers contained unique 12-base-pair Golay barcodes that were incorporated into the PCR amplicons (17). PCR parameters were as follows: denaturing at 94°C for 3 minutes followed by amplification for 35 cycles at 94°C for 45 seconds, at 50°C for 60 seconds, at 72°C for 90 seconds, and with a final extension of 72°C for 10 minutes. Amplicon sequence libraries were prepared at The Scripps Research Institute Next Generation Sequencing Core Facility, where the libraries were sequenced on an Illumina MiSeq as previously described (15).
16S rRNA amplicon analysis
Raw sequences were imported into QIIME 2 (v.2017.6; https://docs.qiime2.org/2017.6/) (18) using the q2-tools-import script, and sequences were demultiplexed using the q2-demux emp-single script. This resulted in 5.6 million sequences with an average of 30,000 sequences per sample. The 16S rRNA sequences generated in this study were deposited into the European Nucleotide Archive (study accession no. PRJEB22972). DADA2 software was used to obtain a set of observed sequence variants (SVs) (19). DADA2 uses an Illumina sequence error correction algorithm to derive an abundance distribution of distinct SVs, which can differ by only a single nucleotide. On the basis of the quality scores, the forward reads were truncated at position 220 using the q2-dada2-denoise script. Taxonomy was assigned using a pretrained naive Bayes classifier [Greengenes 13_8 99% operational taxonomic units (OTUs)], and the q2-feature-classifier plug-in (20). Singletons and SVs present in <10% of samples were removed to minimize the effect of spurious, low abundance sequences using the q2-feature-table filter-features script. The resulting SVs were then aligned using MAFFT (21), and a phylogenetic tree was built using FastTree (22). Taxonomic distributions of the samples were calculated using the q2-taxa-barplot script. The rectal swabs contained bacteria representative of fecal samples, and there was no evidence of contamination with oral, skin, or vaginal bacteria.
α and β diversity metrics were computed using the q2-diversity core-metrics script at a rarefied sampling depth of 500. Rarefaction resulted in the removal of three samples (one control and two PCOS) that had <500 sequences per sample. Four α diversity metrics, observed SVs, Faith phylogenetic diversity (PD), Shannon, and Pielou were used to estimate fecal microbial community richness, PD, information content, and evenness, respectively (23–25). Two outliers (both PCOS) were identified in the measures of α diversity and removed from the analyses. UniFrac was used to compare the similarity (β diversity) among the microbial communities by calculating the shared PD between pairs of microbial communities (26). A cluster of outliers (four control and four PCOS) was identified in the principal coordinates analysis (PCoA) of unweighted UniFrac and removed from the analyses.
Statistical analysis
Statistical calculations were performed in the RStudio statistical package (version 0.99.893). Data were tested for normality via the Shapiro-Wilk test. Variables that were not normally distributed were transformed or ranked. Differences in the clinical characteristics of study participants and microbiome characteristics were analyzed using one-way or multifactor analysis of variance followed by a Tukey honest significant difference test for multiple post hoc comparisons. Multivariable linear regression models were generated by backward stepwise elimination implemented in R using the “step” library. Simple linear regression and Pearson rank correlation were also performed. PCoA and canonical correspondence analysis (CCA) plots were constructed using the phyloseq R package (V.1.19.1). PCoA plots were used to represent the similarity of fecal microbiome samples on the basis of multiple variables in the data set, whereas CCA was used to visualize the relationship of the fecal microbiome with specific clinical parameters. Permutational multivariate analysis of variance (PERMANOVA) used unweighted Unifrac distance measures to assess bacterial community compositional differences and relationships to patient clinical characteristics (999 permutations; “vegan” package). CCA combined with PERMANOVA was performed to single out significant variables driving microbiome composition and to orient the data for visualizing the differences among the factors of interest. A random forest classifier (27) was implemented in R using the “randomForest” library to identify α diversity factors and bacterial observed SVs that discriminate between healthy women and women with PCOS. Since random forest assumes that there are equal samples in each group, we sampled a random subset of the samples from the women with PCOS to compare with the control group.
Results
Clinical characteristics of study participants
Gut microbial diversity profiles were generated for a total of 163 women: 48 healthy controls, 42 with PCOM, and 73 diagnosed with PCOS. Of the 73 women with PCOS, 21 had all three criteria (hyperandrogenism, oligomenorrhea, and PCOM), 39 had hyperandrogenism and PCOM, two had hyperandrogenism and oligomenorrhea, and 11 had oligomenorrhea and PCOM. Table 1 summarizes the clinical characteristics of the study participants. Compared with healthy women in the control group and those with PCOM, the cohort of women with PCOS had higher levels of serum total testosterone and free testosterone as well as an increase in hirsutism and a decrease in the number of menses per year. Women with PCOS also had increased levels of serum LH, an increased ratio of LH/FSH, but no detectable change in serum FSH levels. In addition, although fasting glucose and insulin levels were not different, women with PCOS had higher homeostasis model assessment of insulin resistance (HOMA-IR) values. Although age and BMI did not differ between controls and women with PCOS, there was a small difference in BMI between women with PCOM and women with PCOS.
Table 1.
Clinical Characteristics of Study Participants
| Diagnosis |
ANOVA |
Tukey HSD (Adjusted
for Multiple Comparisons) |
|||||
|---|---|---|---|---|---|---|---|
| Control (n = 48) | PCOM Only (n = 42) | PCOS (n = 73) | P Value | Control vs PCOM | Control vs PCOS | PCOM vs PCOS | |
| Age, y | 29.4 ± 4.9 | 29.8 ± 5.3 | 27.4 ± 4.9 | 0.04 | 0.97 | 0.11 | 0.08 |
| BMI, kg/m2 | 23.7 ± 4.1 | 22.6 ± 4.2 | 25.6 ± 6.5 | 0.02 | 0.34 | 0.39 | 0.01 |
| Testosterone, ng/mL | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.56 ± 0.2 | <0.0001 | 0.98 | <0.0001 | <0.0001 |
| Free testosterone, V | 0.35 ± 0.2 | 0.3 ± 0.2 | 0.9 ± 0.5 | <0.0001 | 0.85 | <0.0001 | <0.0001 |
| Hirsutism (Ferriman-Gallwey) | 2.9 ± 1.3 | 3.5 ± 1.8 | 8.1 ± 4.3 | <0.0001 | 0.31 | <0.0001 | <0.0001 |
| Menses per y | 12.1 ± 0.5 | 10.9 ± 1.8 | 8.1 ± 3.4 | <0.0001 | 0.07 | <0.0001 | <0.0001 |
| LH, IU/L | 7.7 ± 5.8 | 10.8 ± 14.3 | 11.9 ± 8.4 | <0.0001 | 0.13 | <0.0001 | 0.02 |
| FSH, IU/L | 5.7 ± 1.9 | 6.4 ± 2.9 | 5.5 ± 1.9 | 0.29 | 0.52 | 0.91 | 0.25 |
| LH/FSH ratio | 1.4 ± 0.6 | 1.5 ± 0.8 | 2.3 ± 1.4 | <0.0001 | 0.59 | <0.0001 | 0.0006 |
| Fasting glucose, mmol/L | 4.86 ± 0.34 | 4.85 ± 0.4 | 5.14 ± 1.87 | 0.37 | 1 | 0.47 | 0.47 |
| Fasting insulin, pmol/L | 48.5 ± 18.5 | 46.6 ± 21.9 | 61.4 ± 38.2 | 0.05 | 0.83 | 0.2 | 0.06 |
| HOMA-IR | 1.75 ± 0.7 | 1.69 ± 0.84 | 2.27 ± 1.54 | 0.01 | 0.97 | 0.05 | 0.03 |
| Hyperandrogenism | 0 | 0 | 62 | <0.0001 | 1 | <0.0001 | <0.0001 |
| Oligomenorrhea | 0 | 0 | 34 | <0.0001 | 1 | <0.0001 | <0.0001 |
| PCOM | 0 | 42 | 71 | <0.0001 | <0.0001 | <0.0001 | 0.39 |
Data are presented as mean ± standard deviation. Nonnormal data were ranked and analyzed by one-way analysis of variance followed by the Tukey HSD test.
Abbreviations: HSD, honest significant difference; V, Vermeulen equation.
PCOS was associated with reduced biodiversity in the gut microbiome
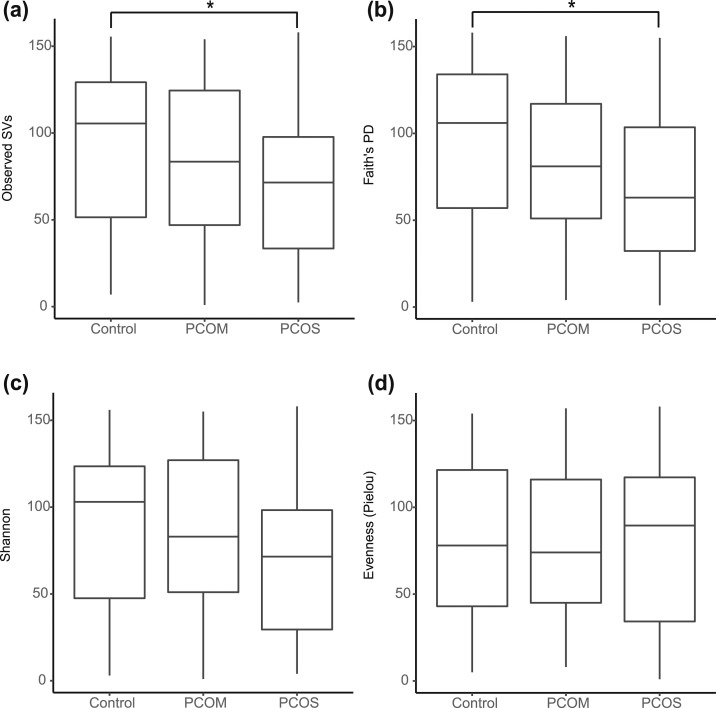
In total, 481 observed SVs (analogous to OTUs) were identified from the rectal swab samples. Women with PCOS had reduced gut microbiome α diversity compared with healthy women as measured by abundance (observed SVs; P = 0.04) and PD (Faith PD; P = 0.02) [Fig. 1(a) and 1(b)]. Women with PCOM displayed an intermediate phenotype in both observed SVs and Faith PD because the α diversity of their gut microbiome was not statistically different from that of healthy women or women with PCOS. Women with PCOS also tended to have lower Shannon diversity, which accounted for both abundance and evenness of SVs, than controls (P = 0.1) [Fig. 1(c)]. There was no difference in the evenness of the gut microbiome of women with PCOS compared with that of either controls or women with PCOM [Fig. 1(d)].
Figure 1.
Biodiversity of the gut microbiome was decreased in women with PCOS. Box plots of α diversity in fecal samples from healthy women (controls; n = 47), women with PCOM (n = 41), and women diagnosed with PCOS using the Rotterdam criteria (n = 70) are shown, with whiskers extending 1.5× past the interquartile range. (a‒d) α diversity was calculated using (a) the number of observed SVs as an estimate of species richness, (b) Faith PD as an estimate of species richness that takes phylogenetic relationships into account, (c) Shannon as an estimate of both species richness and evenness, and (d) Pielou as an estimate of the evenness of a community. One-way analysis of variance was performed on ranked data with the Tukey honest significant difference post hoc test to compare means among groups. *P < 0.05.
Higher total testosterone levels and hirsutism correlated with lower biodiversity in the gut microbiome
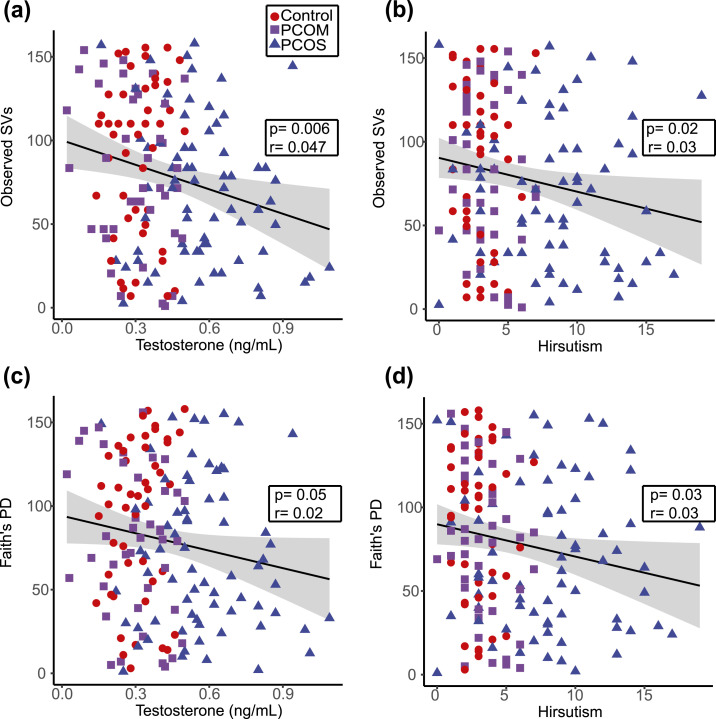
Backward stepwise regression was used to build multiple regression models for identification of clinical parameters that best predicted two measures of α diversity (i.e., observed SVs and Faith PD). Table 2 shows the factors that contributed to the multiple regression models. Total testosterone level, hyperandrogenism, and number of menses per year correlated with observed SVs in the multiple regression model, whereas total testosterone level, hirsutism, and hyperandrogenism correlated with Faith PD. The ratio of LH/FSH may also correlate with α diversity (observed SVs and Faith PD; P = 0.08). In contrast, age, BMI, and HOMA-IR did not correlate with α diversity and were not included in the models. In addition, simple linear regression was performed on clinical or biochemical hyperandrogenism to get a better understanding and visualization of the data. Both serum total testosterone level and hirsutism showed negative correlations with observed SVs (P = 0.006 and P = 0.02, respectively) and Faith PD (P = 0.05 and P = 0.03, respectively) (Fig. 2).
Table 2.
Summary of Multiple Regression Analysis Relating Patient Parameters to α Diversity
| Std. Error | t Value | P Value | |
|---|---|---|---|
| Observed SVs | |||
| Control vs PCOM | 9.61 | −1.49 | 0.13 |
| Control vs PCOS | 18.35 | −2.28 | 0.02a |
| Testosterone, ng/mL | 24.50 | −2.58 | 0.01a |
| Hirsutism | 1.25 | −1.70 | 0.09 |
| Hyperandrogenism | 18.67 | −2.28 | 0.02a |
| Menses per y | 2.27 | −2.05 | 0.04a |
| Oligomenorrhea | 16.67 | −1.51 | 0.13 |
| LH/FSH ratio | 3.20 | 1.75 | 0.08 |
| Faith PD | |||
| Control vs PCOM | 37.14 | −1.79 | 0.07 |
| Control vs PCOS | 48.13 | −2.55 | 0.01a |
| Testosterone, ng/mL | 24.38 | −2.00 | 0.04a |
| Hirsutism | 1.25 | −2.17 | 0.03a |
| Hyperandrogenism | 18.43 | −3.25 | 0.001b |
| Menses per y | 1.56 | −1.57 | 0.11 |
| PCOM | 43.77 | 1.42 | 0.16 |
| LH/FSH ratio | 3.17 | 1.76 | 0.08 |
Data were ranked and a backward stepwise regression was used to select the model that best predicted observed SVs (r = 0.14) and Faith PD (r = 0.16).
P < 0.05.
P < 0.01.
Figure 2.
A decrease in gut bacterial biodiversity correlated with an increase in testosterone level and hirsutism. (a‒d) Scatterplots and trend lines show the relationship between testosterone and (a) observed SVs or (c) Faith PD as well as the relationship between hirsutism and (b) observed SVs or (d) Faith PD. Results of a Pearson correlation (P value and correlation coefficient) are shown in the insets, with the gray shaded areas indicating the 95% confidence interval for the line of best fit. Healthy women (controls; n = 47), women with PCOM (n = 41), and women with PCOS (n = 70).
Hyperandrogenism was associated with changes in the gut microbiome
β diversity
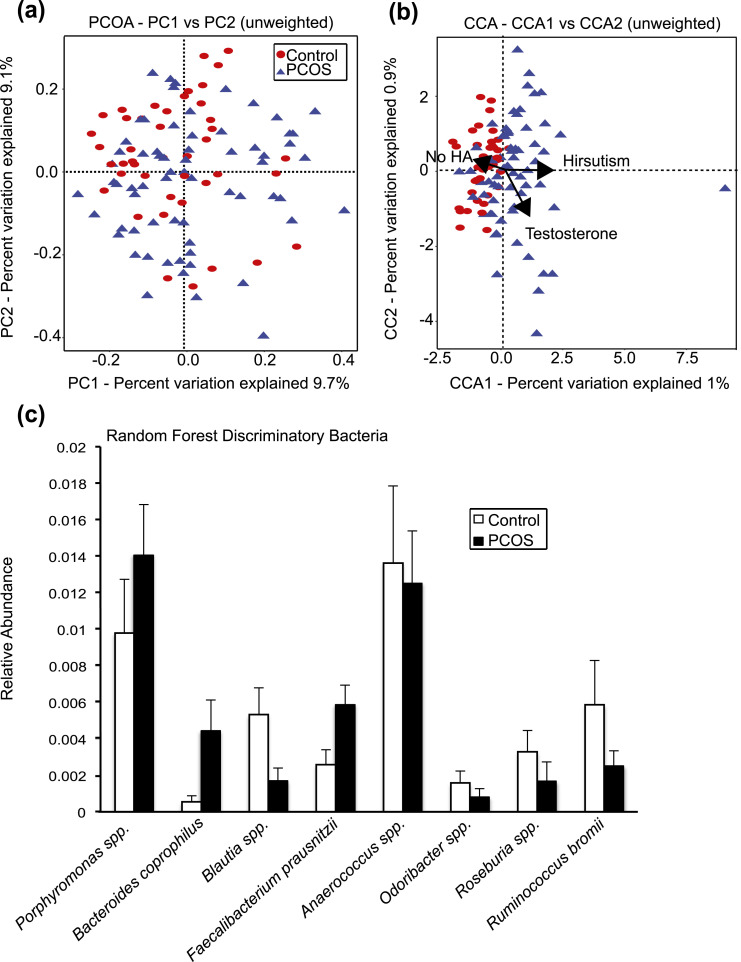
In addition to assessing α diversity, we used unweighted and weighted UniFrac analyses to compare the similarity of the gut microbial communities (β diversity) between healthy women and women with PCOS. α diversity estimates the within-sample biodiversity, whereas β diversity estimates the biodiversity between samples. PCoA and PERMANOVA were used to analyze the relationship between overall gut bacterial composition and clinical characteristics of the study participants. Although there was no distinct clustering between samples from controls and those from women with PCOS [Fig. 3(a)], PERMANOVA tests detected a highly significant effect of hyperandrogenism (P = 0.0009) and, to a lesser extent, diagnostic group (P = 0.08) on the microbial community composition (Table 3). When CCA was applied to visualize the relationship of the gut microbial community structure to clinical and biochemical hyperandrogenism, a separation between samples from controls and from women with PCOS that correlated with hirsutism was observed (P = 0.06) [Fig. 3(b)].
Figure 3.
β diversity of the gut bacterial community was influenced by hyperandrogenism (HA), and random forests identified bacterial taxa that distinguished between healthy women and women with PCOS. (a) PCoA of β diversity (unweighted UniFrac distances) of fecal samples from healthy women (controls; n = 43) and women with PCOS (n = 66). Proportion of variance explained by each principal coordinate (PC) axis is denoted on the corresponding axis. Permutation analysis of variance of the unweighted UniFrac distances indicated that hyperandrogenism had a strong influence on the gut microbial community (P = 0.0009). (b) CCA represents the relationship between β diversity and specific variables of interest, such as HA, testosterone, and hirsutism. CCA demonstrated that changes in the gut microbial communities between healthy women and women with PCOS correlated with hirsutism (permutation test; P = 0.06). Proportion of variance explained by each CCA axis is denoted on the corresponding axis. The arrows represent the direction and strength of the correlation between gut microbiome composition and specific variables of interest. (c) A random forest classifier was used to identify bacterial observed SVs that best distinguished between healthy women (controls) and women with PCOS. The relative mean abundances (mean ± standard error of the mean) of the top eight most discriminant observed SVs are identified to the genus and species level when possible.
Table 3.
Summary of Permutational Analysis of Variance Assessing the Effect of Patient Parameters on Unweighted UniFrac Distances (β Diversity)
| Mean Squares | Pseudo-F | P Value | |
|---|---|---|---|
| Control vs PCOS | 0.31 | 1.33 | 0.08 |
| Age, y | 0.22 | 1.01 | 0.42 |
| BMI, kg/m2 | 0.18 | 0.81 | 0.79 |
| Testosterone, ng/mL | 0.24 | 1.09 | 0.29 |
| Hirsutism | 0.18 | 0.81 | 0.77 |
| Menses per y | 0.24 | 1.08 | 0.31 |
| LH/FSH ratio | 0.19 | 0.85 | 0.71 |
| Fasting glucose, mmol/L | 0.24 | 1.1 | 0.37 |
| Fasting insulin, pmol/L | 0.21 | 0.97 | 0.51 |
| HOMA-IR | 0.19 | 0.89 | 0.67 |
| Hyperandrogenism | 0.47 | 2.11 | 0.0009a |
| Oligomenorrhea | 0.21 | 0.96 | 0.52 |
| PCOM | 0.20 | 0.95 | 0.52 |
P < 0.001.
Random forest identified bacterial taxa that distinguished between healthy women and women with PCOS
The random forest machine learning classifier was trained to determine how well healthy women and women with PCOS could be predicted on the basis of α diversity and bacteria represented by observed SVs. The random forest had the highest accuracy in distinguishing women with PCOS (65% accuracy) followed by controls (50% accuracy). The variable importance by mean decrease in accuracy was then calculated from the random forest model (Supplemental Fig. 1). The relative abundance of the eight bacterial genera whose removal caused the greatest decrease in model accuracy (i.e., the most important for classification) was graphed for healthy women and women with PCOS [Fig. 3(c)]. These bacteria included Porphyromonas spp., Bacteroides coprophilus, Blautia spp., Faecalibacterium prausnitzii, Anaerococcus spp., Odoribacter spp., Roseburia spp., and Ruminococcus bromii.
Discussion
This study demonstrated that Caucasian women diagnosed with PCOS using the Rotterdam criteria had a reduction in overall species richness (α diversity) of the gut microbiome compared with that of healthy women and changes in the composition of the microbial community (β diversity). Interestingly, our study found that the biodiversity of the microbiome strongly correlated with hyperandrogenism. More specifically, observed SVs and Faith PD were both negatively correlated with total testosterone level and hirsutism, whereas hyperandrogenism had a highly significant effect on the structure of the bacterial community as measured by unweighted UniFrac. We also observed an intermediate phenotype for women with PCOM regarding gut microbiome α diversity, suggesting that further studies are warranted to determine whether the gut microbiome of women with PCOM is significantly altered compared with that of healthy women.
α diversity metrics estimate the overall biodiversity of a community (i.e., the bacterial species in the gut microbiome). Compared with healthy controls, women with PCOS had a reduced overall number of bacterial species and lower PD (observed SVs and Faith PD), whereas there was no difference in community evenness (Pielou) [Fig. 1(a)‒1(d)]. This agrees with two previous studies that found a decrease in α diversity in women with PCOS compared with healthy women (11, 12). Reduced α diversity of the gut microbiome was also observed in humans with metabolic diseases compared with healthy individuals. Indeed, lower α diversity of the gut microbiome was consistently associated with human obesity according to several recent meta-analyses (28–30).
In the field of ecology, species richness has been proposed to correlate with the health of an ecosystem, as diverse communities may increase the stability and productivity of an ecosystem (31). In terms of the gut microbiome, it is possible that decreased bacterial diversity results in changes in gut function that can exacerbate diseases, including PCOS, though much work remains to be done to understand how changes in the gut microbiome influence host physiology.
Multiple and single linear regression analyses showed that the decrease in α diversity was associated with total testosterone level and hirsutism [Fig. 2(a)‒2(d); Table 2]. These results concur with the negative correlation we observed between α diversity and testosterone level in a hyperandrogenic, letrozole-induced PCOS mouse model (15). Interestingly, factors such as the number of menses per year and the LH/FSH ratio also contributed to the multiple regression models but did not have a significant association with α diversity in the single linear regression analysis. In contrast, free testosterone, LH, and FSH levels, as well as age, BMI, or HOMA-IR, did not contribute to the multiple regression models or correlate with α diversity.
In addition to α diversity, our study demonstrated a difference in the overall gut microbial composition (β diversity) between healthy women and women with PCOS. Our results agree with the two aforementioned studies that demonstrated changes in β diversity between healthy women and women with PCOS according to unweighted Unifrac and Bray-Curtis analyses (11, 12). In addition, using PERMANOVA, we demonstrated that hyperandrogenism was strongly correlated with changes in the gut microbiome (Table 3). CCA also identified a difference between the gut microbiome of women with PCOS and that of healthy women and showed that hirsutism was associated with the observed compositional differences (P = 0.06) [Fig. 3(b)]. Our results agree with the study by Liu et al. (12), who used SparCC to analyze the gut microbiome of Han Chinese women with PCOS and healthy women and found that a number of distinct bacterial OTUs correlated with both total testosterone level and hirsutism. Although one cannot infer causation from association studies, the accumulating data from studies of humans and rodent models suggest that androgen levels may have a significant effect on the composition of the gut microbiome in women with PCOS.
Kruskal-Wallis tests did not detect significant differences between the relative abundance of specific bacterial taxa in the gut microbiome of healthy women compared with women with PCOS after correction for multiple comparisons. However, a supervised learning approach using the random forest method identified several bacteria that distinguished the gut microbiome of healthy women from that of women with PCOS [Fig. 3(c)]. The relative abundance of Porphyromonas spp., B. coprophilus, Blautia spp., and F. prausnitzii was consistently higher in women with PCOS, whereas Anaerococcus spp., Odoribacter spp., Roseburia spp., and R. bromii were lower [Fig. 3(c)]. Porphyromonas has been reported to increase gut permeability and dysbiosis (32). The relative abundance of B. coprophilus was reported to be higher in obese individuals (33), whereas patients with type 2 diabetes and glucose intolerance had greater numbers of Blautia (34). Interestingly, F. prausnitzii is a commensal bacterium known to produce short-chain fatty acids (SCFAs), and in several reports, lower abundance of this bacterium was associated with obesity and Crohn disease (35), which is opposite to the pattern we observed in women with PCOS [Fig. 3(c)].
The four taxa identified by random forest that had lower abundance in women with PCOS [Fig. 3(c)] are all known to synthesize SCFAs. SCFAs are microbial metabolites that have distinct physiological effects on the host. Butyrate, in particular, is involved in a number of beneficial processes to the host, including downregulation of bacterial virulence; maintenance of colonic homeostasis, including acting as an energy source for intestinal epithelial cells; and anti-inflammatory effects (36). Decreased levels of certain strains of Odoribacter and Roseburia have been associated with Crohn disease and ulcerative colitis and were thought to increase the host’s inflammatory response via reduced SCFA production (37, 38). Specific strains of Anaerococcus are more abundant in obese individuals (33), whereas R. bromii was associated with a lower concentration of SCFAs and insulin sensitivity (39, 40).
Conclusion
In summary, our study demonstrated that hyperandrogenism was correlated with changes in the gut microbiome in women with PCOS. Our findings suggest that androgens may be an important factor in shaping the gut microbiome and that changes in the gut microbiome may influence the development and pathology of PCOS. If hyperandrogenism drives the microbial composition of the gut, it would be interesting to determine if treatment of PCOS with androgen antagonists or oral contraceptives results in recovery of the gut microbiome and improvement of the PCOS metabolic phenotype. Moreover, it would be informative to determine whether the gut microbiome of women diagnosed with PCOS using the criteria of oligomenorrhea and polycystic ovaries is distinct from that of women diagnosed with the other subtypes of PCOS that include hyperandrogenism.
Although many studies have reported that obesity was associated with changes in the gut microbiome, it is noteworthy that BMI or HOMA-IR did not correlate with changes in α or β diversity of the gut microbiome in our study. One possible explanation is that the average BMI of the women in this study was 24.32 ± 0.85 kg/m2. Further sampling of the gut microbiome of obese women with or without PCOS could address whether obesity and insulin resistance influence the gut microbiome in women with PCOS. However, given variations in the human gut microbiome, large clinical cohorts will likely be needed to address these questions. Future studies to determine whether specific gut bacterial species play a causative role in PCOS will also be important in determining whether probiotics are a treatment option for PCOS.
Supplementary Material
Acknowledgments
We thank Rob Edwards for the use of the Anthill computational cluster.
Financial Support: This work was funded by the National Institute of Child Health and Human Development through a cooperative agreement as part of the National Centers for Translational Research in Reproduction and Infertility (P50 HD012303; to V.G.T.).
Author Contributions: V.G.T., S.T.K., A.J.D., B.B., and L.P. conceived and designed the study. M.S., B.B., and L.P. supervised the enrollment of patients and the collection of clinical data and fecal samples. A.J.D. analyzed the clinical data. P.J.T. performed the DNA extractions and PCR amplifications. P.J.T., V.G.T., and S.T.K. analyzed the data. P.J.T. and V.G.T. wrote the manuscript. S.T.K., B.B., and A.J.D. provided comments, and P.J.T. and V.G.T. edited and revised the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- BMI
body mass index
- CCA
canonical correspondence analysis
- FSH
follicle-stimulating hormone
- HOMA-IR
homeostasis model assessment of insulin resistance
- LH
luteinizing hormone
- OTU
operational taxonomic unit
- PCoA
principal coordinates analysis
- PCOM
polycystic ovarian morphology
- PCOS
polycystic ovary syndrome
- PCR
polymerase chain reaction
- PD
phylogenetic diversity
- PERMANOVA
permutational multivariate analysis of variance
- rRNA
ribosomal RNA
- SCFA
short-chain fatty acid
- SV
sequence variant.
References
- 1. Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012;97(1):28–38.e25. [DOI] [PubMed] [Google Scholar]
- 2. Mykhalchenko K, Lizneva D, Trofimova T, Walker W, Suturina L, Diamond MP, Azziz R. Genetics of polycystic ovary syndrome. Expert Rev Mol Diagn. 2017;17(7):723–733. [DOI] [PubMed] [Google Scholar]
- 3. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 5. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E; American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE), Androgen Excess and PCOS Society . American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome - part 2. Endocr Pract. 2015;21(12):1415–1426. [DOI] [PubMed] [Google Scholar]
- 6. Walker AW, Lawley TD. Therapeutic modulation of intestinal dysbiosis. Pharmacol Res. 2013;69(1):75–86. [DOI] [PubMed] [Google Scholar]
- 7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. [DOI] [PubMed] [Google Scholar]
- 8. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. [DOI] [PubMed] [Google Scholar]
- 11. Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Leber B, Horvath A, Pieber TR, Gorkiewicz G, Stadlbauer V, Obermayer-Pietsch B. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLoS One. 2017;12(1):e0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, Ling Y, Fu H, Dong W, Shen J, Reeves A, Greenberg AS, Zhao L, Peng Y, Ding X. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. 2017;8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 14. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelley ST, Skarra DV, Rivera AJ, Thackray VG. The gut microbiome is altered in a letrozole-induced mouse model of polycystic ovary syndrome. PLoS One. 2016;11(1):e0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA. 2008;105(46):17994–17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6(3):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26(7):1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61(1):1–10. [Google Scholar]
- 24. Shannon CE. The mathematical theory of communication: 1963. MD Comput. 1997;14(4):306–317. [PubMed] [Google Scholar]
- 25. Pielou EC. Reviewed work:ecological diversity in theory and practice. Biometrics. 1980;36(4):742–743. [Google Scholar]
- 26. Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 28. Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588(22):4223–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finucane MM, Sharpton TJ, Laurent TJ, Pollard KS. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS One. 2014;9(1):e84689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome [published correction appears in MBio. 2017;8(6):6e01995-17] MBio. 2016;7(4):e01018-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C. Diversity and productivity in a long-term grassland experiment. Science. 2001;294(5543):843–845. [DOI] [PubMed] [Google Scholar]
- 32. Nakajima M, Arimatsu K, Kato T, Matsuda Y, Minagawa T, Takahashi N, Ohno H, Yamazaki K. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One. 2015;10(7):e0134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andoh A, Nishida A, Takahashi K, Inatomi O, Imaeda H, Bamba S, Kito K, Sugimoto M, Kobayashi T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr. 2016;59(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egshatyan L, Kashtanova D, Popenko A, Tkacheva O, Tyakht A, Alexeev D, Karamnova N, Kostryukova E, Babenko V, Vakhitova M, Boytsov S. Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect. 2016;5(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–119. [DOI] [PubMed] [Google Scholar]
- 37. Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. [DOI] [PubMed] [Google Scholar]
- 39. Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12(2):304–314. [DOI] [PubMed] [Google Scholar]
- 40. Yang X, Darko KO, Huang Y, He C, Yang H, He S, Li J, Li J, Hocher B, Yin Y. Resistant starch regulates gut microbiota: structure, biochemistry and cell signalling. Cell Physiol Biochem. 2017;42(1):306–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.