Abstract
Fermenting high fiber ingredients such as distillers dried grains with solubles (DDGS) with feed enzymes may improve their feeding value. We investigated the effects of fermenting corn DDGS with a blend of β-glucanase and xylanases (XB) on growth performance, gut parameters, and apparent total tract digestibility (ATTD) of nutrients and energy in growing pigs. Dietary treatments were: (1) corn soybean meal-based diet + 30% DDGS (control), (2) control + XB without fermentation (XBNS), and (3) control + DDGS fermented with XB (16% DM) for 3 to 10 d at 40 °C (XBS). Target activities in XB were 1,050 and 5,500 U/g of DDGS for XB, respectively. Diets contained phytase at 750 FTU/kg. Feed samples were taken during fermentation and at feeding for analysis of pH and organic acids. A total of 144 pigs (25 ± 1.0 kg BW) were assigned to pens (three barrows and three gilts) and allocated to the three diets in a two-phase feeding program (3 wk/phase). Diets were fed on ad libitum and were delivered by a computer-controlled liquid feeding system at a feed to water ratio of 1:4, four times per day. Pigs had free access to water. Fecal samples were taken in the final 3 d of phase 2 to determine ATTD using TiO2 marker method and one pig per pen was euthanized for gastrointestinal (GIT) measurements. The pH of diets at feeding time was lower (P < 0.01) for XBS (4.72) compared with control (5.45) and XBNS (5.45). Pigs fed XBNS had higher (P = 0.04) ADG than control in phase 1. In phase 2 and the overall (weeks 0 to 6), ADG and final BW were higher (P = 0.01) for XBNS than XBS but were not different (P > 0.05) from control. There were no diet effects (P > 0.05) on ADFI. Feed to gain (FCR) for XBNS (1.68) and XBS pigs (1.69) was better (P < 0.01) than for control pigs (1.78) in phase 1. There were no diet effects (P > 0.05) on FCR in phase 2 or in the overall. Pigs fed XBNS had lower (P < 0.01) ATTD of CP than control and XBS-fed pigs. Although not different (P > 0.05) from control, pigs fed XBNS had lower (P < 0.05) jejunal crypt depth and ATTD of gross energy than pigs fed XBS. In conclusion, treating corn DDGS with XB with or without liquid fermentation improved feed efficiency in phase 1, suggesting degradation of dietary fibrous components that may limit nutrient utilization in younger pigs. However, these benefits were not observed in phase 2.
Keywords: fiber degrading enzymes, growth performance and gut health, liquid fermentation, nutrients utilization, pigs
INTRODUCTION
Co-products from the ethanol industry such as corn distillers dried grains with solubles (DDGS) have been widely adopted by the swine industry (Stein and Shurson, 2009; Woyengo et al., 2014). However, utilization of DDGS is limited by high concentration of fiber that negatively influence feed intake, nutrient utilization, and health and metabolic processes (Jha and Leterme, 2012; Pedersen et al., 2014). Fiber-degrading enzymes have been shown to improve nutrient utilization and performance in pigs fed fibrous feedstuffs as they can hydrolyze fibrous components which can lead to increased nutrient release (Diebold et al., 2004; Nortey et al., 2008; Kiarie et al., 2016a). However, not all studies reported positive effects (e.g., Leek et al., 2007; Jacela et al., 2010). The reasons for inconsistent results are thought to be due to animal, diet, and bio-efficacy of enzymes (Slominski, 2000; Ndou et al., 2015; Kiarie et al., 2016b).
Liquid feeding systems allow feedstuffs to be soaked in water, which provides a favorable environment for endogenous enzymes present in feed ingredients and added exogenous enzymes to be activated for short or prolonged periods allowing enzymatic digestion prior to feeding. Improved nutrient digestibility and volatile fatty acid (VFA) production have been reported in pigs fed liquid fermented feed with supplemental enzymes (Jakobsen et al., 2015). Optimally fermented liquid feed can improve gastrointestinal health by reducing the number of pathogens as reduced pH from fermentation prevents growth of harmful microorganism such as Escherichia coli and Salmonella (Brooks et al., 2001; Canibe and Jensen, 2012), improving growth performance (Missotten et al., 2010; Wiseman et al., 2017). However, not all studies reported positive results when feeding fermented liquid feeds (Lawlor et al., 2002; Canibe and Jensen, 2003). The quality of fermented feed can be influenced by many factors such as temperature, dose and bio-efficacy of the enzyme, and duration of fermentation and substrate being fermented (Missotten et al., 2010; Wiseman, 2016).
The objective of this study was to evaluate nutritive value of corn DDGS treated with exogenous xylanase and β-glucanase (XB) in a fermented liquid feeding system on growth performance, gastrointestinal measurements, and apparent total tract digestibility (ATTD) of components in growing pigs. The dosage of the XB was based on preliminary fermentation evaluation using pH as a criterion. It was hypothesized that fermenting corn DDGS with fiber-degrading enzymes will enhance the nutritional value.
MATERIALS AND METHODS
Animal care and use protocols were approved by the University of Guelph Animal Care and Use Committee. Pigs were cared for in accordance with the Canadian Council on Animal Care guidelines (CCAC, 2009).
Animals and Housing
A total of 144 crossbred (Yorkshire*Landrace ♀ × Duroc ♂) pigs with an average initial BW of 25 ± 1.0 kg were used. Pigs (three barrows and three gilts) were randomly allocated to one of 24 pens in two identical temperature-controlled rooms (12 pens per room). Pens were then assigned to one of the three dietary treatments as described below, resulting in eight pen observations for each treatment. Feed was provided in stainless steel feed troughs that were connected to a liquid feeding system with transporter pipe (Squire, 2005) and each pen was equipped with a nipple drinker.
Fiber-Degrading Enzymes and Preparation of Fermented Corn DDGS
The enzyme product (XB) contained xylanase and β-glucanase (Axtra XB; Danisco Animal Nutrition-DuPont Industrial Biosciences, Marlborough, Wiltshire, UK). The inclusion level for XB was based on a preliminary in vitro study conducted in our lab (unpublished). Briefly, 50 g of corn DDGS was mixed with 250 mL of water with different ratios of XB, incubated, and pH measured after 48 h. Combination of XB in 1:1.01 ratio showed the lowest pH reduction after 48 h of incubation. Thus, in the current study, 296 g of xylanase and 300 g of β-glucanase were added to 1,000 kg of corn DDGS. Targeted activity levels for XB were 5,500 and 1,050 U/g, respectively.
The corn DDGS was fermented using a liquid feeding system (Big Dutchman HydroJet, Big Dutchman International, Vechta, Germany) by mixing a 1:4 ratio of corn DDGS to water with the enzymes to achieve approximately 16% DM (Wiseman, 2016). Once mixed in the fermentation tank, the mixture was left to ferment for 10 d at 40 °C. This temperature was selected based on previous optimization studies (Wiseman, 2016). A 15 min of agitation was programmed every hour during fermentation and immediately before feeding. Two fermentation tanks were used for the current study and were interchanged weekly. Between each batch of fermented corn DDGS, both base and acid rinses were performed sequentially with 90% l-lactic acid (pH of 1.2; Acros Organics, Geel, Belgium) and a 50% sodium hydroxide solution (pH 12.0; Fisher Scientific, Ottawa, ON, Canada), respectively, followed by a final rinse with water. Fermented corn DDGS were fed to pigs starting on day 3 of fermentation until the last day of fermentation (day 10). This was based on previous studies (Squire 2005; Wiseman, 2016) that established that after third day of fermentation a steady state (lactic and acetic acid concentration) was established up to 10 d. Immediately before feeding, fermented corn DDGS and basal diet were mixed with additional water to achieve 25% DM in the final liquid feed (Wiseman et al., 2017).
Diets and Feeding Schedules
All diets met or exceeded specifications for growing pigs (25 to 50 kg BW, NRC, 2012) and were prepared in two-phase feeding program (3 weeks per phase). Titanium dioxide was included at 0.3% in phase 2 diets as an indigestible marker (Table 1). All dietary treatments were based on a basal mix of corn and soybean meal along with supplemental vitamins, minerals, and synthetic lysine making up 70% of the diet to which 30% of unfermented or fermented corn DDGS was added to make the final diet (Table 1).
Table 1.
Composition of experimental diets, “as-fed basis”
| Item | Phase 1 | Phase 2 |
|---|---|---|
| Corn, yellow dent | 33.4 | 39.3 |
| Wheat, hard red winter | 10.0 | 10.0 |
| Poultry fat | 1.00 | 1.00 |
| Soybean meal, 47.5% CP | 22.6 | 16.7 |
| l-Lysine HCl | 0.29 | 0.31 |
| Salt | 0.50 | 0.50 |
| Limestone | 1.48 | 1.41 |
| Mono-calcium phosphate, 21% P | 0.12 | 0.00 |
| Vitamin and mineral premix1 | 0.50 | 0.50 |
| Corn DDGS2 | 30.0 | 30.0 |
| Phytase3 | 0.04 | 0.04 |
| Titanium dioxide | — | 0.30 |
| Calculated provisions | ||
| DM, % | 89.2 | 89.2 |
| Net energy, kcal/kg | 2,380 | 2,417 |
| CP, % | 23.4 | 21.0 |
| NDF, % | 15.7 | 15.8 |
| Crude fiber, % | 4.11 | 4.04 |
| Crude fat, % | 6.15 | 6.20 |
| SID Lys, % | 1.08 | 0.95 |
| SID Thr, % | 0.68 | 0.60 |
| SID Met, % | 0.35 | 0.32 |
| SID Met + Cys, % | 0.66 | 0.60 |
| SID Try, % | 0.20 | 0.17 |
| Ca, % | 0.70 | 0.63 |
| Total P, % | 0.52 | 0.47 |
| STTD P, % | 0.32 | 0.30 |
1Provided per kilogram of complete diet: vitamin A, 10,000 IU as retinyl acetate; vitamin D3, 1,000 IU as cholecalciferol; vitamin E, 40 IU as DL-α-tocopherol acetate; vitamin K, 2.5 mg as menadione; pantothenic acid, 15 mg; riboflavin, 5 mg; choline, 500 mg; folic acid, 2.0 mg; niacin, 25 mg; thiamine, 1.5 mg; pyridoxine, 1.5 mg; vitamin B12, 25 µg; biotin, 200 µg; Cu, 15 mg from CuSO4 × 5H2O; Fe, 100 mg from FeSO4; Mn, 20 mg from MnSO4; Zn, 105 mg from ZnO; Se, 0.30 mg from Na2SeO3; and I, 0.5 mg from KI (DSM Nutritional Products Canada Inc., ON, Canada).
2DDGS = distillers dried grains with solubles.
3 Buttiauxella spp. Phytase supplied 750 FTU/kg (Axtra PHY, Danisco UK Ltd, Marlborough, UK).
The three final liquid treatment diets were prepared as follows: (1) corn-soybean meal-based diet containing 30% corn DDGS (not steeped), prepared as a complete diet without XB and mixed with water immediately prior to feeding to achieve 25% DM (Control); (2) corn-soybean meal-based diet containing 30% corn DDGS (not steeped), prepared as a complete diet with XB (XBNS; similar activity as added in the fermentation tank) and was mixed with water to achieve 25% DM; and (3) corn-soybean meal-based basal diet mixed at the time of feeding with 30% corn DDGS that was separately fermented with XB (XBS; as described previously) and fed at 25% DM content.
Live Animal Measurements and Sample Collection
The experiment lasted for 6 weeks, and all three dietary treatments were mixed at feeding time and were delivered by a computer-controlled liquid feeding system as described by Squire (2005). Water was provided ad libitum throughout the trial. Pigs had ad libitum access to feed dispensed in four equal meals per day (0600, 1000, 1400, 1800; Squire, 2005; Wiseman, 2016). Weekly measurements included individual pig BW and feed intake on a pen basis. Feed intake was calculated based on the dispensed amount of feed recorded automatically by the liquid feeding computer system.
Fermented corn DDGS samples were taken on days 0, 3, 6, 7, and 10 of fermentation for pH and organic acid analyses. Briefly, samples were taken in a sterile container and pH was measured, after which they were transferred to a 15-mL centrifuge tube. Samples were centrifuged for 10 min at 30,000 g. The supernatant was transferred to a 2-mL micro centrifuge tube and was stored frozen at −20 °C until further analysis. Complete liquid feeds were sampled from the sample valve on the trough dispenser once every week for nutrient content and pH measurements. Complete liquid diets and fermented corn DDGS samples were kept frozen at −20 °C until analysis.
Fresh fecal grab samples were collected in each pen on a daily basis over the final 3 d of the experiment to determine apparent total tract digestibility (ATTD) of components. Fecal samples were pooled together by pen and stored at −20 °C until further analyses. At the end of the experiment, one pig per pen with a BW close to the average pen BW was selected and euthanized for gastrointestinal tract (GIT) weight, jejunum tissue for histomorphology, and ileal and cecal digesta for pH determination. Gastrointestinal tract weights were free of digesta. Jejunum tissues for histomorphology were collected and preserved according to the procedures of Carleton et al. (1980).
Laboratory Analyses
Supernatants of fermented corn DDGS were analyzed for organic acids (acetic acid and lactic acid) concentrations using HPLC (Agilent 1100 Series, Agilent Technologies, Santa Clara, CA) according to Wiseman et al. (2017) and pH was determined for fermented corn DDGS, complete feed, and digesta samples using Accumet 950 pH/ion meter (Fisher Scientific).
Diet and fecal materials were freeze dried and finely ground with a coffee grinder (KitchenAid, Benton Harbor, MI) for chemical analyses. Dry matter, CP, NDF, Ti, and crude fat were analyzed in diets, fecal, and digesta samples. Dry matter was determined according to standard procedures method 930.15 (AOAC, 2004). Crude protein was measured by analyzing N with the combustion method 968.06 (AOAC, 2004) using a CNS-2000 C, N, and S analyzer (Leco Corporation, St. Joseph, MI) and the obtained N value was multiplied by 6.25 to derive CP. The NDF contents were analyzed with an Ankom 200 Fiber Analyzer (Ankom Technology, Macedon, NY) using α-amylase and sodium sulfite according to ANKOM technology procedure 13 (Ankom Technology). Titanium content was measured with a UV spectrophotometer following the method of Myers et al. (2004). Crude fat content (without acid hydrolysis) was determined using an ANKOM XT 20 Extractor (Ankom Technology). Gross energy was determined using a bomb calorimeter (IKA Calorimeter System C 5000; IKA Works, Wilmington, NC).
Fixed jejunal tissues were embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin for morphological examinations. In each cross-sectioned tissue, at least five complete villous-crypt structures were examined under a Leica DMR microscope (Leica Microsystems, Wetzlay, Germany). Villous height and crypt depth were measured using a calibrated micrometer.
Calculations and Statistical Analyses
The ATTD of component was calculated according to Adeola (2001). Data were analyzed using the MIXED procedure of SAS 9.4 (SAS Institute Inc., Cary, NC). For animal data, pen was the experimental unit and treatment was the fixed effect. For pH and organic acids in samples collected during fermentation and at feeding, each sample was considered as experimental unit and sampling time was fixed effect. An α level of 0.05 was used to determine statistical significance among dietary treatment means, and means were separated using a Tukey’s test.
RESULTS AND DISCUSSION
In this study, a combination of XB was used because they target arabinoxylans, which are abundant in most of grains and co-products, followed by cellulose and β-glucans (De Vries et al., 2012; Jakobsen et al., 2015). Analyzed chemical composition of experimental diets is shown in Table 2. In phase 1, XBS diet showed lower CP than the other two diets and in phase 2, XBS diet showed slightly lower DM, GE, NDF, and CF than in the other two non-steeped treatments (Table 2). The data suggest that not much organic matter disappeared when corn DDGS was fermented.
Table 2.
Analyzed chemical composition of control, XBNS, and XBS diets, “air-dried basis”
| Item | Phase 1 | Phase 2 | ||||
|---|---|---|---|---|---|---|
| Control1 | XBNS2 | XBS3 | Control1 | XBNS2 | XBS3 | |
| DM, % | 86.9 | 87.5 | 86.5 | 87.2 | 87.8 | 86.3 |
| GE, kcal/kg | 4,026 | 4,124 | 4,183 | 4,279 | 4,288 | 4,173 |
| CP, % | 21.6 | 24.0 | 22.6 | 22.9 | 24.1 | 22.4 |
| NDF, % | — | — | — | 16.39 | 16.18 | 14.9 |
| Crude fat, % | — | — | — | 4.08 | 4.19 | 3.41 |
| Ca, % | 0.75 | 0.66 | 0.61 | 0.56 | 0.62 | 0.64 |
| TiO2 | — | — | — | 0.283 | 0.290 | 0.282 |
1Control, diet was mixed with water to 25% DM before feeding.
2XBNS, a complete diet containing DDGS and enzymes (xylanases and β-glucanase, XB) were mixed with water (25% DM).
3XBS, DDGS was steeped with enzymes XB (3 to 10 d at 40 °C), then it was mixed with the basal diet and more water (25% DM) immediately before feeding.
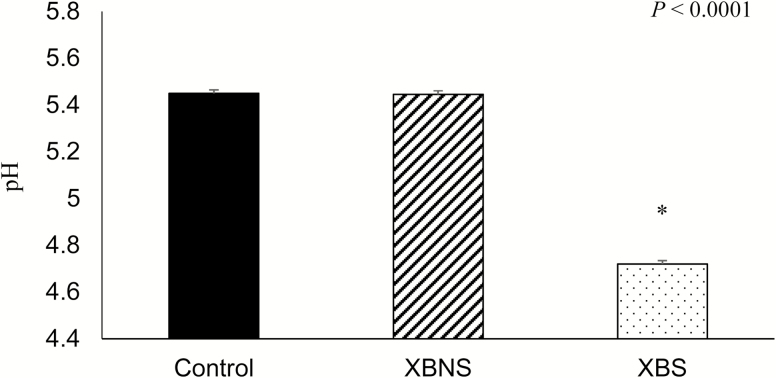
The corn DDGS fermentation at 40 °C was based on previous work conducted in our laboratory, which showed that this temperature had optimal fermentation in terms of lactic and acetic acid concentrations and pH (Wiseman, 2016). Moreover, previous fermentation studies that validated the liquid fermentation system used in the current study established that lactic acid and acetic acid concentrations remained steady and stable between days 3 and 10 of fermentation (Squire, 2005; Wiseman, 2016). Other studies have used different incubation temperature ranging between 20 and 30 °C (Brooks et al., 2001; Canibe and Jensen, 2003; Missotten et al., 2010). However, fermentation temperatures below 24 °C can result in the production of cold-shock proteins produced by Salmonella which allow bacteria to better survive various conditions (Beal et al., 2002). Also at lower temperatures, the prevalence of yeast development is greater, which contributes to poor palatability and low energy content of the feed (Brooks et al., 2001). Three parameters are considered to produce good quality, liquid feed: (1) pH below 4.5 to prevent pathogen growth (van Winsen et al., 2001) and to reduce incidence of diarrhea, (2) lactic acid concentrations above 100 mmol/L to prevent unwanted pathogens and reduce gastric pH (Brooks et al., 2003), and (3) acetic acid concentrations below 40 mmol/L because acetic acid has a distinct vinegar taste and smell at high concentrations, reducing the palatability of the feed (van Winsen et al., 2001; Beal et al., 2005). The pH of the three complete feeds (n = 6 samples for each diet) used in the present study were 5.45, 5.45, and 4.72 for control, XBNS, and XBS, respectively, with XBS pH being lower (P < 0.01) relative to the other dietary treatments (Figure 1). Changes in pH and organic acid concentrations in fermented corn DDGS over the 10 d of fermentation are illustrated in Figure 2. Corn DDGS fermented with enzymes at 40 °C had a decrease in pH from 4.42 on day 0 to 3.65 on day 3 (P < 0.05). The observed pH on day 0 was already below 4.5, suggesting that DDGS processing somewhat results in acidic final product (Wiseman, 2016). After day 3, pH slightly increased and remained stable with no detectable differences between days 3 and 10. Thus, the pH for the steeped corn DDGS decreased to an acceptable range from days 3 to 10 of fermentation. Canibe and Jensen (2003) reported that over-fermenting can result in a pH that may be too low (<4.2) which can reduce feed intake.
Figure 1.
pH of control2, XBNS3, XBS4 complete liquid feed prior to feeding1. 1Data are means of six batches of samples. 2Conrol, diet was mixed with water to 25% DM before feeding. 3XBNS, a complete diet containing DDGS and enzymes (XB) were mixed with water (25% DM) immediately before feeding. 4XBS, DDGS was steeped with enzymes (XB (3 to 10 d at 40 °C), then it was mixed with the basal diet and more water (25% DM) immediately before feeding. *Means are different at P < 0.05.
Figure 2.
Lactic acid and acetic acid concentrations and pH of DDGS treated with fiber degrading enzymes in a liquid fermentation tank at 40 °C for 10 d1. At day 0, DDGS and fiber degrading enzymes (XB) were mixed with water to achieve 16% DM and were held in fermentation tank for up to 10 d. Feeding steeped DDGS started on day 3. 1Data are means of six batches of samples. a,bMeans at time point with different superscripts are different at P < 0.05.
Lactic acid concentrations in fermented corn DDGS were 29 mM on day 0 and increased to 116 mM on day 3 (P < 0.05). After day 3, lactic acid concentration decreased (P < 0.05) to 53, 52, and 47 mM on days 6, 7, and 10, respectively, but these values were not different from day 0. Acetic acid concentrations were not different on day 0 vs. 3 but increased substantially (P < 0.05) from 6.9 and 14.2 mM on days 0 and 3, respectively, to 96.1, 117.5, and 115.5 mM on days 6, 7, and 10, respectively (Figure 2). Lactic acid and acetic acid concentrations were within the recommended ranges on day 3. However, fermentation of corn DDGS did not seem to reach stable conditions as indicated by the reduction of lactic acid concentrations and increase in acetic acid concentrations from days 3 to 10 of fermentation. The reason for the poor quality of fermented corn DDGS is unclear as acid and base cleaning of fermentation tanks was performed weekly to prevent contamination. However, these observations agreed with previous observations by Wiseman (2016) where lactic acid concentrations decreased below the optimum level and acetic acid increased above the acceptable level over time. Collectively, this suggested that the conditions in the tank changed after 3 d, indicating that 10 d of fermentation may have been too long of a period of fermentation. Demeckova et al. (2001) found that liquid feed treated with chlorine dioxide (ClO2) to eliminate all-natural microflora present in the feed was more palatable than a fermented diet. Treating fermented liquid feed with ClO2 has been shown to reduce the number of coliforms (Demeckova et al., 2001) and killed E. coli (Berg et al., 1986). Perhaps controlling fermentation by using acid or a lactic acid bacteria inoculant could be pivotal in sustaining acceptable levels of lactic acid concentration (Brooks et al., 2003; Niven et al., 2006).
In phase 1, pigs fed XBNS had greater (P < 0.05) ADG (0.959 kg/d) than pigs fed control diet (0.902 kg/d) with pigs fed XBS (0.918 kg/d) being intermediate and similar to the other treatments (Table 3). A better (P < 0.01) feed to gain ratio (FCR) was observed for XBNS and XBS fed pigs compared with the control in phase 1. Average BW at the end of phase 1 was greater (P < 0.05) for pigs fed XBNS than pigs fed control or XBS. In phase 2 and overall, pigs fed XBNS had higher (P < 0.05) ADG relative to control and XBS-fed pigs. Although ADFI was not influenced by treatments, lower ADG for XBS vs. XBNS may be related, in part, to reduce diet palatability associated with acetic acid concentration above 40 mmol/L in the fermented diet. Other studies have observed reduce ADFI related to acetic acid concentration >40 mmol/L in liquid diets (e.g., Beal et al., 2005). It may also be due to nutrient degradation as microorganisms such as E. coli can decarboxylate lysine to produce cadaverine during fermentation (Canibe and Jensen, 2012). Feed to gain ratio was not affected by treatments in phase 2 and for the overall study period (Table 3).
Table 3.
Growth performance of growing pigs fed corn-soybean meal-based diets in which corn DDGS were treated with fiber degrading enzymes with or without liquid fermentation
| Item | Control1 | XBNS2 | XBS3 | SEM | P-value |
|---|---|---|---|---|---|
| Phase 1, weeks 0 to 3 | |||||
| Initial BW, kg | 25.0 | 25.0 | 25.3 | 0.445 | 0.847 |
| ADG, kg/d | 0.902b | 0.959a | 0.918ab | 0.021 | 0.043 |
| ADFI, kg/d | 1.601 | 1.614 | 1.540 | 0.037 | 0.132 |
| Feed: gain, kg/kg | 1.779a | 1.680b | 1.681b | 0.026 | 0.001 |
| Final BW, kg | 44.0b | 45.3a | 44.4ab | 0.448 | 0.037 |
| Phase 2, weeks 3 to 6 | |||||
| ADG, kg/d | 1.146ab | 1.184a | 1.087b | 0.029 | 0.010 |
| ADFI, kg/d | 2.322 | 2.359 | 2.219 | 0.076 | 0.187 |
| Feed: gain, kg/kg | 2.031 | 1.997 | 2.042 | 0.049 | 0.634 |
| Final BW, kg | 68.1ab | 70.1a | 67.2b | 0.831 | 0.008 |
| Overall, weeks 0 to 6 | |||||
| ADG, kg/d | 1.024ab | 1.072a | 1.003b | 0.020 | 0.009 |
| ADFI, kg/d | 1.964 | 1.986 | 1.881 | 0.051 | 0.13 |
| Feed: gain, kg/kg | 1.918 | 1.857 | 1.875 | 0.033 | 0.185 |
Data are means of eight replicate pens of three barrows and three gilts.
a,bMeans within a row with similar superscripts are not different at P < 0.05.
1Conrol, diet was mixed with water to 25% DM before feeding.
2XBNS, a complete diet containing DDGS and enzymes (XB) were mixed with water (25% DM) immediately before feeding.
3XBS, DDGS was steeped with enzymes XB (3 to 10 d at 40 °C), then it was mixed with the basal diet and more water (25% DM) immediately before feeding.
At the end of the study, final BW of pigs fed XBNS were greater (P < 0.05) than pigs fed XBS, with the control being intermediate and not different. The improved ADG for pigs fed diets containing non-fermented corn DDGS with enzymes agrees with Canibe and Jensen (2003), who also observed higher ADG and ADFI in growing pigs when fed non-fermented diets with enzymes. Moran et al. (2016) found that feeding younger pigs a diet containing dry wheat middlings increased ADFI and ADG compared with feeding a diet containing 24 h steeped wheat middlings. In previous studies (Canibe and Jensen, 2003; Moran et al., 2016), pro-longed soaking or fermentation seemed to reduce feed intake, resulting in poorer ADG.
For intestinal measurements, there were no differences among dietary treatments for stomach, small intestinal, or combined cecum and colon weight (Table 4). Villus height and villus to crypt depth ratio were not different among treatments (Table 4). In contrast, pigs fed diets containing wheat middlings steeped for 24 h with xylanase improved villus height relative to addition of the same xylanase in dry feed containing wheat middlings (Moran et al., 2016). Pigs fed XBS had greater (P < 0.05) crypt depth (219 μm) relative to pigs fed XBNS (183 μm) but was not different from the control pigs. The production and shedding of enterocytes in crypts and villi has a direct influence on renewal of intestinal epithelium (Willing and Van Kessel, 2007). Therefore, to assess intestinal health and function, crypt depth is an effective measurement (Pluske et al., 1997). Increased crypt depth in pigs fed the XBS diet suggests that the pigs were experiencing greater intestinal cell renewal. However, as the difference between XBNS and XBS treatments was the fermentation process, the present study design did not allow discerning whether such effects were the result of the fermentation process per se or its interaction with XB. Interestingly and as shown below, ATTD of components was greater in XBS- vs. XBNS-fed pigs, suggesting that increased intestinal cell proliferation did not reach threshold of being detrimental to gut function (Pluske et al., 1997; Kiarie et al., 2013). Moreover, although not measured in the current study, the greater ATTD of components in XBS- vs. XBNS-fed pigs may have been due to the favorable impact on microbial activity (Kiarie et al., 2013).
Table 4.
Gastrointestinal tract weights, digesta pH and jejunum histomorphology for growing pigs when fed corn-soybean meal diets in which corn DDGS were treated with fiber degrading enzymes with or without liquid fermentation1
| Enzyme | |||||
|---|---|---|---|---|---|
| Item | Control2 | XBNS3 | XBS4 | SEM | P-value |
| Gastrointestinal weight5, g/kg | |||||
| Stomach, g/kg BW | 6.56 | 7.15 | 6.56 | 0.28 | 0.24 |
| Small intestine, g/kg BW | 31.0 | 31.7 | 31.7 | 1.25 | 0.89 |
| Large intestine, g/kg BW | 17.9 | 16.7 | 19.1 | 0.78 | 0.11 |
| Digesta pH | |||||
| Ileal | 6.83 | 6.70 | 6.96 | 0.13 | 0.54 |
| Caecal | 5.53 | 5.40 | 5.66 | 0.09 | 0.14 |
| Jejunum histomorphology, μm | |||||
| Villi height (VH), μm | 555 | 527.9 | 570.6 | 14.74 | 0.11 |
| Crypt depth (CD), μm | 210.5ab | 182.6b | 219.2a | 10.76 | 0.04 |
| VH:CD ratio | 3.768 | 3.817 | 3.491 | 0.258 | 0.62 |
abMeans within a row with similar superscripts are not different at P < 0.05.
1Data are means of eight replicate pens of three barrows and three gilts.
2Conrol, diet was mixed with water to 25% DM before feeding.
3XBNS, a complete diet containing DDGS and enzymes (XB) were mixed with water (25% DM) immediately before feeding.
4XBS, DDGS was steeped with enzymes XB (3 to 10 d at 40 °C), then it was mixed with the basal diet and more water (25% DM) immediately before feeding.
5Expressed as gram per kilogram live BW.
Apparent total tract digestibility of components was not affected by feeding XB-treated corn DDGS relative to pigs fed the control diet (Table 5). However, ATTD of DM (P = 0.05), OM (P = 0.02), CP (P < 0.01), and GE (P = 0.01) were greater for pigs fed XBS than pigs fed XBNS. Treatment differences were not (P > 0.05) observed for ATTD of NDF and crude fat. It is noteworthy that the ATTD of DM, OM, CP, and GE were (P ≤ 0.05) highest in pigs fed XBS compared to pigs fed the other two unfermented diets. Interestingly, digestibility of NDF was not improved in pigs fed XB-treated corn DDGS relative to the control diet. Similarly, previous studies reported no improvement in fiber utilization in pigs fed corn DDGS-based diets supplemented with fiber-degrading enzymes (Widyaratne et al., 2009; Asmus et al., 2012; Kerr and Shurson, 2013). Moreover, we recently reported that feeding growing pigs corn DDGS steeped with a combination of commercial fiber degrading enzymes for 24 h did not improve ileal or total digestibility of nutrients, fiber and energy relative to control corn DDGS steeped with no enzymes (Rho et al., 2018). The explanation for ineffectiveness of fiber-degrading enzymes is unclear; however, feed enzymes hydrolyze soluble fibers more readily than insoluble fiber (Urriola et al., 2010; Kiarie et al., 2016b). Because DDGS has greater concentrations of insoluble fiber which are highly resistant to degradation (Urriola et al., 2010; Moran et al., 2016), this may be the reason for the lack of an improvement in ATTD of NDF in the current study. Another possible explanation may be that the enzyme used in the current study was not effective for corn DDGS due to its complex fiber–starch–protein structure (Jha et al., 2015). This complex structure can limit the enzymes to access to hydrolyze fiber fractions (De Vries et al., 2012; Kiarie et al., 2016a, 2016b).
Table 5.
Apparent total tract digestibility of energy and nutrients in growing pigs fed corn-soybean meal with corn DDGS treated with fiber degrading enzymes with or without liquid fermentation1
| Item, % | Control2 | XBNS3 | XBS4 | SEM | P-value |
|---|---|---|---|---|---|
| Dry matter | 77.9ab | 76.5b | 78.7a | 0.58 | 0.05 |
| Organic matter | 79.5a | 77.8b | 80.2a | 0.55 | 0.02 |
| Crude protein | 79.3a | 75.5b | 78.7a | 0.58 | <0.001 |
| Crude fat | 64.1 | 66.2 | 67.9 | 3.46 | 0.73 |
| Neutral detergent fiber | 53.8 | 49.9 | 51.0 | 1.49 | 0.19 |
| Gross energy | 80.3a | 78.9b | 81.1a | 0.47 | 0.01 |
abMeans within a row with similar superscripts are not different at P < 0.05.
1Data are means of eight replicate pens of three barrows and three gilts.
2Conrol, diet was mixed with water to 25% DM before feeding.
3XBNS, a complete diet containing DDGS and enzymes (XB) were mixed with water (25% DM) immediately before feeding.
4XBS, DDGS was steeped with enzymes XB (3 to 10 d at 40 °C), then it was mixed with the basal diet and more water (25% DM) immediately before feeding.
In conclusion, treating corn DDGS with fiber-degrading enzyme without or with liquid fermentation improved feed efficiency in the first 3 wk of feeding, suggesting degradation of dietary fibrous components that may limit nutrient utilization in younger pigs. However, these benefits were not observed in phase 2, perhaps indicating that fiber was not limiting nutrient utilization potentially linked to microbial adaptation. Fermentation of feed ingredients has potential to improve the nutritive value of fibrous feedstuffs; however, the current study indicates no advantage compared to adding in dry feed. Therefore, the fermentation process needs to be carefully monitored and optimized as fermentation of feed ingredients is a complex process.
Footnotes
Presented in part at the 2017 ADSA–ASAS Midwest Meeting, Omaha, NE, March 13 to 17. Financial and technical support of Ontario Ministry of Agriculture, Food and Rural Affairs (Guelph, ON, Canada), DuPont Industrial Biosciences (Marlborough, Wiltshire, UK), and the National Pork Board (Des Moines, IA) is appreciated.
LITERATURE CITED
- Adeola O. 2001. Digestion and balance techniques in pigs. In: Lewis A. J. and L. L. Southern, editors, Swine nutrition. 2nd ed. CRC Press, Washington, DC: p. 903–916. [Google Scholar]
- AOAC 2004. Official methods of analysis AOAC offical methods.AOAC International, Gaithersburg, MD. [Google Scholar]
- Asmus M., DeRouchey J. M., Tokach M. D., Goodband R. D., Nelssen J. L., and Dritz S. S.. 2012. Effects of xylanase in high-co-product diets on nutrient digestibility in finishing pigs. Kansas Agricultural Experiment Station Research. Reports, Kansas State University, Manhattan. 335–342. doi: 10.4148/2378-5977.7089 [DOI] [Google Scholar]
- Beal J. D., Niven S. J., Brooks P. H., and Gill B. P.. 2005. Variation in short chain fatty acid and ethanol concentration resulting from the natural fermentation of wheat and barley for inclusion in liquid diets for pigs. J. Sci. Food Agric. 85:433–440. doi: 10.1002/jsfa.2013 [DOI] [Google Scholar]
- Beal J. D., Niven S. J., Campbell A., and Brooks P. H.. 2002. The effect of temperature on the growth and persistence of salmonella in fermented liquid pig feed. Int. J. Food Microbiol. 79:99–104. doi: 10.1016/S0168-1605(02)00183-6 [DOI] [PubMed] [Google Scholar]
- Berg J. D., Roberts P. V., and Matin A.. 1986. Effect of chlorine dioxide on selected membrane functions of Escherichia coli. J. Appl. Bacteriol. 60:213–220. [DOI] [PubMed] [Google Scholar]
- Brooks P., Beal J., and Niven S.. 2001. Liquid feeding of pigs: potential for reducing environmental impact and for improving productivity and food safety. Rec. Adv. Anim. Nutr. Australia 13:49–63. [Google Scholar]
- Brooks P., Beal J., Niven S., and Demeckova V.. 2003. Liquid feeding of pigs II. Potential for improving pig health and food safety. Anim. Sci. Pap. Rep. 21:23–39. doi: 10.1080/1828051X.2018.1438214 [DOI] [Google Scholar]
- Canibe N., and Jensen B. B.. 2003. Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. J. Anim. Sci. 81:2019–2031. doi: 10.2527/2003.8182019x [DOI] [PubMed] [Google Scholar]
- Canibe N., and Jensen B. B.. 2012. Fermented liquid feed-microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed Sci. Technol. 173:17–40. doi: 10.1016/j.anifeedsci.2011.12.021 [DOI] [Google Scholar]
- Carleton H. M., Drury R. A. B., and Wallington E. A.. 1980. Carleton’s histological technique. Oxford University Press, USA. [Google Scholar]
- CCAC, CCOAC.. 2009. The care and use of farm animals in research, teaching and testing. CCAC, Ottawa, ON: pp. 12–15. [Google Scholar]
- Demeckova V., Moran C., Cavenay C., Campbell A., Kuri V., and Brooks P.. 2001. The effect of fermentation and/or sanitization of liquid diets on the feeding preferences of newly weaned pigs. Digestive physiology of pigs. CABI Publishing, Wallingford, UK: pp. 291–293. [Google Scholar]
- De Vries S., Pustjens A., Schols H., Hendriks W., and Gerrits W.. 2012. Improving digestive utilization of fiber-rich feedstuffs in pigs and poultry by processing and enzyme technologies: a review. Anim. Feed Sci. Technol. 178:123–138. doi: 10.1016/j.anifeedsci.2012.10.004 [DOI] [Google Scholar]
- Diebold G., Mosenthin R., Piepho H. P., and Sauer W. C.. 2004. Effect of supplementation of xylanase and phospholipase to a wheat-based diet for weanling pigs on nutrient digestibility and concentrations of microbial metabolites in ileal digesta and feces. J. Anim. Sci. 82:2647–2656. doi: 10.2527/2004.8292647x [DOI] [PubMed] [Google Scholar]
- Jacela J., Dritz S., DeRouchey J., Tokach M., Goodband R., and Nelssen J.. 2010. Effects of supplemental enzymes in diets containing distillers dried grains with solubles on finishing pig growth performance1. Prof. Anim. Sci. 26:412–424. doi: 10.15232/S1080-7446(15)30623-9 [DOI] [Google Scholar]
- Jakobsen G. V., Jensen B. B., Knudsen K. B., and Canibe N.. 2015. Impact of fermentation and addition of non-starch polysaccharide-degrading enzymes on microbial population and on digestibility of dried distillers grains with solubles in pigs. Livest. Sci. 178:216–227. doi: 10.1016/j.livsci.2015.05.028 [DOI] [Google Scholar]
- Jha R., and Leterme P.. 2012. Feed ingredients differing in fermentable fibre and indigestible protein content affect fermentation metabolites and faecal nitrogen excretion in growing pigs. Animal 6:603–611. doi: 10.1017/S1751731111001844 [DOI] [PubMed] [Google Scholar]
- Jha R., Woyengo T. A., Li J., Bedford M. R., Vasanthan T., and Zijlstra R. T.. 2015. Enzymes enhance degradation of the fiber-starch-protein matrix of distillers dried grains with solubles as revealed by a porcine in vitro fermentation model and microscopy. J. Anim. Sci. 93:1039–1051. doi: 10.2527/jas.2014-7910 [DOI] [PubMed] [Google Scholar]
- Kerr B. J., and Shurson G. C.. 2013. Strategies to improve fiber utilization in swine. J. Anim. Sci. Biotechnol. 4:11. doi: 10.1186/2049-1891-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie E., Romero L. F., and Nyachoti C. M.. 2013. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 26:71–88. doi: 10.1017/S0954422413000048 [DOI] [PubMed] [Google Scholar]
- Kiarie E., Walsh M., and Nyachoti C.. 2016b. Performance, digestive function and mucosal responses to selected feed additives for pigs. J. Anim. Sci. 94 (Suppl. 3):169–180. doi: 10.2527/jas.2015-9835 [DOI] [Google Scholar]
- Kiarie E., Walsh M., Romero L., and Baidoo S.. 2016a. Digestibility responses of growing pigs fed corn plus corn distiller grains or wheat plus wheat coproduct-based diets without or with supplemental xylanase. J. Anim. Sci. 94 (Suppl. 3):211–214. doi: 10.2527/jas.2015-9736 [DOI] [Google Scholar]
- Lawlor P. G., Lynch P. B., Gardiner G. E., Caffrey P. J., and O’Doherty J. V.. 2002. Effect of liquid feeding weaned pigs on growth performance to harvest. J. Anim. Sci. 80:1725–1735. doi: 10.2527/2002.8071725x [DOI] [PubMed] [Google Scholar]
- Leek A. B. G., Callan J. J., Reilly P., Beattie V. E., and O’Doherty J. V.. 2007. Apparent component digestibility and manure ammonia emission in finishing pigs fed diets based on barley, maize or wheat prepared without or with exogenous non-starch polysaccharide enzymes. Anim. Feed Sci. Technol. 135:86–99. doi: 10.1016/j.anifeedsci.2006.03.024 [DOI] [Google Scholar]
- Missotten J. A., Michiels J., Ovyn A., De Smet S., and Dierick N. A.. 2010. Fermented liquid feed for pigs. Arch. Anim. Nutr. 64:437–466. doi: 10.1080/1745039X.2010.512725 [DOI] [PubMed] [Google Scholar]
- Moran K., de Lange C. F., Ferket P., Fellner V., Wilcock P., and van Heugten E.. 2016. Enzyme supplementation to improve the nutritional value of fibrous feed ingredients in swine diets fed in dry or liquid form. J. Anim. Sci. 94:1031–1040. doi: 10.2527/jas.2015-9855 [DOI] [PubMed] [Google Scholar]
- Myers W., Ludden P., Nayigihugu V., and Hess B.. 2004. A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. doi: 10.2527/2004.821179x [DOI] [PubMed] [Google Scholar]
- Ndou S. P., Kiarie E., Romero L. F., Arent S., Lorentsen R., and Nyachoti C. M.. 2015. Comparative efficacy of xylanases on growth performance and digestibility in growing pigs fed wheat and wheat bran- or corn and corn DDGS-based diets supplemented with phytase. Anim. Feed Sci. Technol. 209:230–239. doi: 10.1016/j.anifeedsci.2015.08.011 [DOI] [Google Scholar]
- Niven S. J., Beal J. D., and Brooks P. H.. 2006. The effect of controlled fermentation on the fate of synthetic lysine in liquid diets for pigs. Anim. Feed Sci. Technol. 129:304–315. doi: 10.1016/j.anifeedsci.2005.12.016 [DOI] [Google Scholar]
- Nortey T. N., Patience J. F., Sands J. S., Trottier N. L., and Zijlstra R. T.. 2008. Effects of xylanase supplementation on the apparent digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs. J. Anim. Sci. 86:3450–3464. doi: 10.2527/jas.2007-0472 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. National Academy of Sciences Press, Washington, DC. [Google Scholar]
- Pedersen M. B., Dalsgaard S., Knudsen K. E. B., Yu S., and Lærke H. N.. 2014. Compositional profile and variation of distillers dried grains with solubles from various origins with focus on non-starch polysaccharides. Anim. Feed Sci. Technol. 197(Suppl. C):130–141. doi: 10.1016/j.anifeedsci.2014.07.011 [DOI] [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Rho Y., Kiarie E., and de Lange C. K. F. M.. 2018. Nutritive value of corn distiller’s dried grains with solubles steeped without or with exogenous feed enzymes for 24 h and fed to growing pigs. J. Anim. Sci. 96:2352–2360. doi: 10.1093/jas/sky115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski B. 2000. A new generation of enzymes for animal feeds. In: Proc. 21st Western Nutrition Conference, Winnipeg, Manitoba, Canada. [Google Scholar]
- Squire J. M. 2005. Fermentation of an alternative feedstuff for use in swine liquid feeding. Master’s thesis, University of Guelph, Guelph, ON. [Google Scholar]
- Stein H., and Shurson G.. 2009. Board-invited review: the use and application of distillers dried grains with solubles in swine diets. J. Anim. Sci. 87:1292–1303. doi: 10.2527/jas.2008-1290 [DOI] [PubMed] [Google Scholar]
- Urriola P. E., Shurson G. C., and Stein H. H.. 2010. Digestibility of dietary fiber in distillers coproducts fed to growing pigs. J. Anim. Sci. 88:2373–2381. doi: 10.2527/jas.2009-2227 [DOI] [PubMed] [Google Scholar]
- Widyaratne G., Patience J., and Zijlstra R.. 2009. Effect of xylanase supplementation of diets containing wheat distiller’s dried grains with solubles on energy, amino acid and phosphorus digestibility and growth performance of grower-finisher pigs. Can. J. Anim. Sci. 89:91–95. doi: 10.4141/CJAS08103 [DOI] [Google Scholar]
- Willing B. P., and Van Kessel A. G.. 2007. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J. Anim. Sci. 85:3256–3266. doi: 10.2527/jas.2007-0320 [DOI] [PubMed] [Google Scholar]
- van Winsen R. L., Lipman L. J. A., Biesterveld S., Urlings B. A. P., Snijders J., and van Knapen F.. 2001. Mechanism of Salmonella reduction in fermented pig feed. J. Sci. Food Agric. 81:342–346. doi: 10.1002/1097-0010 [DOI] [Google Scholar]
- Wiseman M. 2016. Use of enzymes and inoculants to manipulate the feeding value of liquid fed DDGS for young pigs. Doctoral Dissertation, University of Guelph, Guelph, ON. [Google Scholar]
- Wiseman M., McBride B., Li J., Wey D., Zhu J., and de Lange C. F. M.. 2017. Effects of steeped or fermented distillers dried grains with solubles on growth performance in weanling pigs. J. Anim. Sci. 95:3563–3578. doi: 10.2527/jas.2017.1478 [DOI] [PubMed] [Google Scholar]
- Woyengo T., Beltranena E., and Zijlstra R.. 2014. Nonruminant nutrition symposium: Controlling feed cost by including alternative ingredients into pig diets: a review. J. Anim. Sci. 92:1293–1305. doi: 10.2527/jas.2013-7169 [DOI] [PubMed] [Google Scholar]