Abstract
Background
Breast cancer, a malignant tumor with its highest incidence in women, affects physical and mental health, and can even be life-threatening. In recent years, its incidence has continued to grow, accompanied by a trend of younger onset. XRCC1 is well known as a DNA-repair gene, and its abnormal expression is related to the occurrence of various malignant tumors.
Methods
In this study, we detected XRCC1 expression and investigated its association with the XRCC1 rs1799782 polymorphism. XRCC1 was overexpressed to investigate its effect on in breast cancer cells. CCK8 and clone formation efficiency assay were used to detect cell proliferation. Transwell assay was performed to confirm cell migration and invasion. Flow cytometry was used to detect cell apoptosis.
Results
In 118 breast cancer samples, CC genotype frequency was 49.15% (58 of 118), CT genotype frequency was 42.37% (50 of 118), and TT genotype frequency was 8.48% (ten of 118). Lymphatic metastasis was associated with a higher frequency of XRCC1 rs1799782 polymorphism (P<0.05), and breast cancer patients with positive PR, HER2, and negative ER had high XRCC1 rs1799782 frequency (P<0.05). Meanwhile, XRCC1 had low expression in breast cancer (74.6%, 88 of 118) and high expression in ER-negative, PR-negative, HER2-positive and Ki67-low-expression patients. XRCC1 rs1799782 may play an important role in the development and metastasis of breast cancer. These results differ from previous studies that did not suggest that rs1799782 is effective in breast cancer. We also investigated the role of XRCC1 in breast cancer progression.
Conclusion
We have proved that XRCC1 can inhibit proliferation and invasion and promote apoptosis of breast cancer cells. XRCC1 expression was regulated by the JNK pathway. We found that the JNK inhibitor SP600125 significantly inhibited the growth of breast cancer cells, and consider it a potential drug for breast cancer.
Keywords: MCF7, gene expression, proliferation, apoptosis, JNK pathway
Introduction
Tumor occurrence and development is a complex multistage process that is related to molecular-level variation in many genes. XRCC1 is well known as a DNA-repair gene, and its abnormal expression is related to the occurrence of various malignant tumors.1–3 Single-nucleotide polymorphisms (SNPs) of XRCC1 at multiple loci are closely related to tumor susceptibility and progression, including in lung cancer, breast cancer, bladder cancer, gastric cancer, skin cancer, colorectal cancer, prostate cancer, and head and neck cancer.4–7 However, due to the interference of genetic and individual factors, there is still wide divergence in the relationship between SNPs of the DNA-repair gene XRCC1 and tumor susceptibility. It is difficult to establish a clear correlation between XRCC1 SNPs and the susceptibility of a specific tumor on the basis of existing studies. In addition, related research on XRCC1 in tumors has been mostly focused on gene SNPs and tumor susceptibility, and specific roles and mechanisms in tumors have received less attention. Therefore, in this study, we not only explored SNPs and tumor susceptibility but also studied the specific function and mechanism of XRCC1 in breast cancer. Studies have shown that the XRCC1 rs1799782 (C194T) polymorphism may play an important role in the expression of XRCC1. Therefore, we focused on the relationship between this site and the pathological features of breast cancer in this study.
In our preceding studies, sequencing results of breast cancer samples showed a significant increase in the frequency of XRCC1 rs1799782 polymorphism in breast cancer samples, the role of which was controversial in breast cancer.8 In this study, we detected XRCC1 expression and investigated associations with the XRCC1 rs1799782 polymorphism to predict sensitivity to chemotherapy and prognosis, which has the potential to guide clinical diagnosis and treatment.
Methods
Agents and PCR primers
The genomic DNA-extraction and DNA-sequencing kits used in this research were purchased from Axygen (AP-96- BL-GDNA) and Beckman Coulter (Qiagen PyroMark PCR kit), respectively. SP600125 was obtained from the American MedChemExpress. PCR and sequencing primers were: XRCC1 sense, 5′-ATGCCGTCCCAGGTAAGC-3′; antisense, 5′-AGCCCCAAGACCCGGTCACT-3′.
Clinical specimens
A total of 118 breast cancer patients in Qianfoshan Hospital between March 2014 and December 2015 were selected. Patients were all female aged 31–88 years. The median age was 49 years. No patients received chemotherapy, endocrine therapy or radiotherapy before surgery, and all were confirmed to have aspecific invasive carcinoma. Peripheral blood (3–5 mL) was drawn from patients for genomic DNA extraction. All individuals supplied written informed consent to participate, and this was approved by the ethics committee of Shandong Provincial Qianfoshan Hospital.
Immunohistochemistry
Immunohistochemistry was performed to detect the XRCC1-expression levels in breast cancer. Patient specimens were fixed in 10% paraformaldehyde in PBS for 12 hours and then embedded in paraffin wax. Paraffin-block-embedded normal and tumor breast-tissue samples were cut into 4 µm sections. After dewaxing in xylene, sections were repaired in citrate buffer and blocked with 10% goat serum for 1 hour. XRCC1 (Abcam) antibody was added (1:100) to the sections, which were incubated in a humid chamber at room temperature for 1 hour. For the negative control, the XRCC1 antibody was replaced with PBS. Tissue in which ≤10% of cancer cells were positively stained was recorded as 0.1 point, with 10%–49% as 0.5 point and ≥50% as 1 point. Based on staining intensity, groups were divided into those with 0, 1, 2, and 3 points. XRCC1-expression levels of samples were then categorized as low (<1 point) and high (≥1 point). Results were analyzed independently by two pathologists.
Cell culture and transfection
The breast cancer cell lines MCF7 and MDA-MB231 were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 medium with 10% FBS, 0.1 mg/mL streptomycin, and 100 U/mL penicillin. Cells were seeded on six-well plates. XRCC1-OV transfection was applied using Lipofectamine 2000 following the manual. Cells were treated with the JNK-specific inhibitor SP600125 (MCE, 10 µM) for 8 hours for the next experiment.
Quantitative fluorescence PCR
Total RNA was isolated from MCF7 cells using an UltraPure RNA-extraction kit with reverse transcription and fluorescence quantitative PCR performed using a HiFiScript cDNA- synthesis kit and UltraSYBR mixture kit, respectively. Primers were: XRCC1 sense, 5′-CCCAGAAGGTGACAGTGACC-3′; antisense, 5′-AGACTGGAGAGGCTGAGGAG-3′; GAPDH sense, 5′-GATTTGGTCGTATTGGGCGC-3′; and antisense, 5′-TTCCCGTTCTCAGCCTTGAC-3′. mRNA-expression levels were calculated using the comparative CT method after normalization to GAPDH.
Western blot
After transfection, cells were lysed with RIPA buffer containing 1.0% NP-40, 150 Mm NaCl, 0.1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl pH 8.0, and protease inhibitors. Protein concentrations were detected using a BCA protein-assay kit. Protein samples were separated on 5%–10% SDS-PAGE gel. Membrane-contained target protein was incubated with primary antibodies at a dilution of 1:1,000. The primary antibodies XRCC1 (Abcam), Bcl2 (Proteintech), caspase 3 (Proteintech), Bax (Proteintech), tubulin (Proteintech), p70 (Proteintech), JNK (Proteintech), pJNK (Proteintech), STAT3 (Abcam), and pSTAT3 (Abcam) were used in this study. Assays were repeated three times. Relative protein-expression levels were calculated by determining a ratio between the amount of target protein and GAPDH.
Cell-proliferation assays
Cell vitality was detected every 24 hours after transfection by adding 10 µL cell counting kit-8 (CCK8) reagent. Cells were incubated at 37°C for 90 minutes and then OD values detected using a microplate reader at 450 nm.
Clone-formation-efficiency assays
Cells were collected and seeded in 10 cm plates at a density of 100 cells/mL medium. Cells were incubated at 37°C until formation of sufficiently large clones. After incubation for 7 days, cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The number of colonies was calculated and compared with experimental negative controls.
Cell-invasion and -migration assays
Transwell assays were performed to evaluate cell invasion and migration. Cells (104) were seeded in 24-well invasion chambers and inserts coated with Matrigel. Transwell assay for migration had no Matrigel coating. After incubation for 24 hours, noninvading cells were removed using a cotton swab. Chambers were washed with PBS twice. Cells were stained with 0.1% crystal violet, and total numbers of cells were averaged from triplicate determinations.
Cell-apoptosis analysis
Cells were incubated in serum-free medium for 24 hours and then resuspended in binding buffer (2.5 mM CaCl2, 140 mM NaCl, 10 mM HEPES/NaOH pH 7.4) at an intensity of 3−5×105 cells/mL. Annexin V–fluorescein isothiocyanate and propidium iodide were added to cell suspensions, incubated at room temperature in the dark, and analyzed with flow cytometry. Cell apoptosis was analyzed using BD FACSDiva software.
Statistical analysis
All data were analyzed using SPSS 18 statistical software (SPSS, Chicago, IL, USA). Pearson χ2 was performed to determine correlations between XRCC1 levels/mutation rate and clinical parameters. An ANOVA test was performed to analyze the changes between each group in cell line experiment. P<0.05 were considered significant.
Results
XRCC1 rs1799782 (C194T) polymorphism is correlated with molecular subtype and tumor metastasis in breast cancer
Sequencing of the 118 patient samples showed a higher frequency of XRCC1 rs1799782 polymorphism in breast cancer. XRCC1 (C194T) had three visible genotypes: CC, CT, and TT. In this study, CC frequency was 58.47% (69 of 118), CT 38.98% (46 of 118), and TT 2.54% (three of 118) in paracancerous tissues. However, in tumor tissue, CC frequency was 49.15% (58 of 118), CT 42.37% (50 of 118), and TT 8.48% (ten of 118). The sequencing results suggested a high frequency of XRCC1 rs1799782 polymorphism in breast cancer. Genotype distributions of XRCC1 rs1799782 were significantly different with molecular subtypes of breast cancer. XRCC1 rs1799782 showed high frequency in luminal A, HER2-positive, and triple-negative breast cancer (Table 1). As shown in Table 2, there was no significant difference for age, tumor size, pathological grade, or XRCC1 rs1799782 frequency (P>0.05). Lymphatic metastasis was associated with a higher frequency of XRCC1 rs1799782 polymorphism (P<0.05), and breast cancer patients with positive PR and HER2 and negative ER had high XRCC1 rs1799782 frequency (P<0.05).
Table 1.
XRCC1 rs1799782-polymorphism distribution and association of molecular subtypes of breast cancer
| Cases |
XRCC1 rs1799782 (n)
|
P-value | ||
|---|---|---|---|---|
| CC | CT/TT | |||
|
| ||||
| Luminal A | 21 | 9 | 12 | <0.05 |
| Luminal B | 56 | 37 | 19 | |
| HER2-positive | 32 | 6 | 16 | |
| Triple-negative | 19 | 6 | 13 | |
Table 2.
XRCC1 rs1799782-polymorphism distribution and association of clinicopathological features of breast cancer
| Cases |
XRCC1 rs1799782 (n)
|
P-value | ||
|---|---|---|---|---|
| CC | CT/TT | |||
|
| ||||
| Age, years | >0.05 | |||
| ≥49 | 71 | 35 | 36 | |
| <49 | 47 | 23 | 24 | |
| Tumor size | 2 | >0.05 | ||
| ≥2 cm | 73 | 40 | 33 | |
| <2 cm | 45 | 18 | 27 | |
| Pathological grade | >0.05 | |||
| I | 13 | 4 | 9 | |
| II–III | 105 | 54 | 51 | |
| Lymphatic metastasis | <0.05 | |||
| Positive | 47 | 34 | 13 | |
| Negative | 71 | 24 | 47 | |
| ER | <0.05 | |||
| Positive | 76 | 43 | 33 | |
| Negative | 42 | 15 | 27 | |
| PR | <0.05 | |||
| Positive | 68 | 28 | 40 | |
| Negative | 50 | 30 | 20 | |
| HER2 | <0.05 | |||
| Positive | 37 | 9 | 28 | |
| Negative | 81 | 49 | 32 | |
| Ki67 | <0.05 | |||
| High | 87 | 37 | 50 | |
| Low | 31 | 21 | 10 | |
XRCC1 expression is correlated with tumor metastasis and molecular subtypes in breast cancer
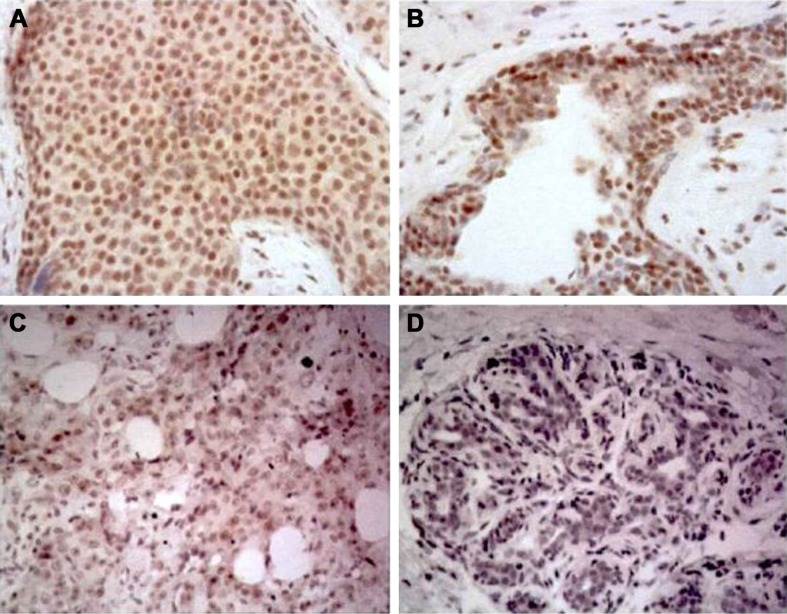
Immunohistochemistry was performed to investigate further the expression level of XRCC1 in breast cancer tissue. The results (Figure 1) showed that XRCC1 expression was low in breast cancer (74.6%, 88 of 118). We next evaluated correlations between XRCC1-expression and clinicopathological variables in breast cancer patients. XRCC1-expression levels in ALN+ were significantly higher than those in ALN− (P<0.05, Table 3). Meanwhile, XRCC1 showed high expression in ER−, PR−, HER2+ and low-Ki67-expression patients, but there was no significant correlation with age, tumor size, or pathological grade (Table 3). As shown in Tables 4 and 5, XRCC1 expression was not significantly correlated with XRCC1 rs1799782 polymorphism and molecular subtype of breast cancer.
Figure 1.
Immunohistochemistry staining showing XRCC1 expression in breast cancer tissue.
Notes: (A, B) High XRCC1 expression; (C, D) low XRCC1 expression. Magnification ×200.
Table 3.
XRCC1-expression association with clinicopathological features of breast cancer
| Cases |
XRCC1 expression (n)
|
P-value | ||
|---|---|---|---|---|
| Low | High | |||
|
| ||||
| Age, years | >0.05 | |||
| ≥49 | 71 | 58 | 13 | |
| <49 | 47 | 30 | 17 | |
| Tumor size | >0.05 | |||
| ≥2 cm | 73 | 50 | 23 | |
| <2 cm | 45 | 38 | 7 | |
| Pathological grade | >0.05 | |||
| I | 13 | 11 | 2 | |
| II | 72 | 47 | 25 | |
| III | 33 | 30 | 3 | |
| ALN | <0.05 | |||
| + | 50 | 24 | 26 | |
| − | 68 | 64 | 4 | |
| ER | <0.05 | |||
| Positive | 76 | 70 | 6 | |
| Negative | 42 | 18 | 24 | |
| PR | <0.05 | |||
| Positive | 68 | 65 | 3 | |
| Negative | 50 | 23 | 27 | |
| HER2 | <0.05 | |||
| Positive | 37 | 16 | 21 | |
| Negative | 81 | 72 | 9 | |
| Ki67 | <0.05 | |||
| High | 87 | 78 | 9 | |
| Low | 31 | 10 | 21 | |
Abbreviations: ALN, axillary lymph nodes; ER, estrogen receptor; PR, progesterone receptor.
Table 4.
XRCC1 rs1799782-polymorphism association of XRCC1 expression of breast cancer
| Cases |
XRCC1 rs1799782
|
P-value | ||
|---|---|---|---|---|
| CC | CT/TT | |||
|
| ||||
| High | 88 | 45 | 43 | >0.05 |
| Low | 30 | 13 | 17 | |
Table 5.
XRCC1-expression association with molecular subtypes of breast cancer
| Cases |
XRCC1 expression
|
P-value | ||
|---|---|---|---|---|
| Low | High | |||
|
| ||||
| Luminal A | 21 | 13 | 8 | >0.05 |
| Luminal B | 56 | 42 | 14 | |
| HER2-positive | 32 | 16 | 6 | |
| Triple-negative | 19 | 17 | 2 | |
XRCC1 inhibits proliferation and invasion and promotes apoptosis of breast cancer cells
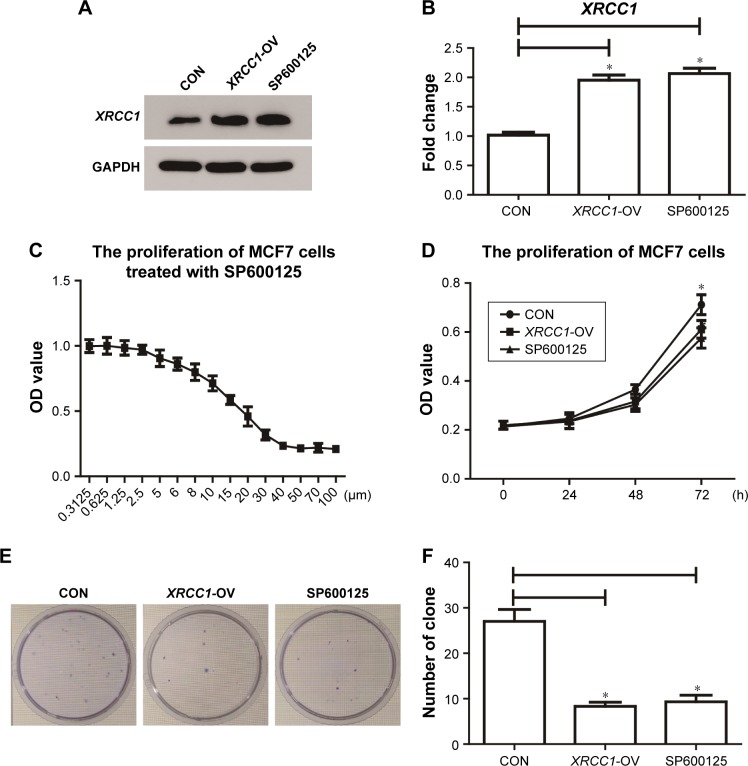
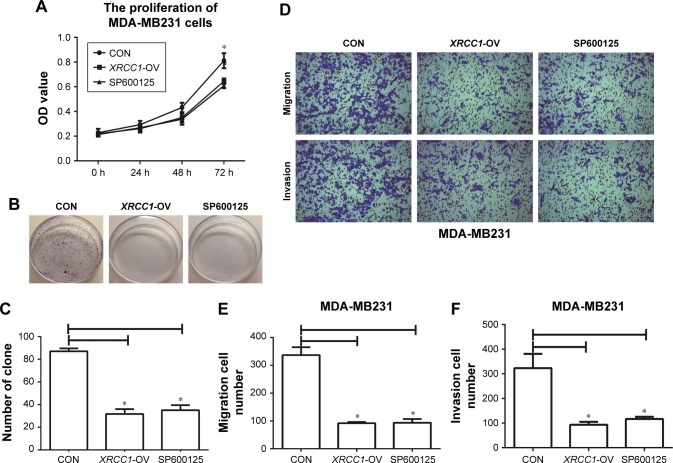
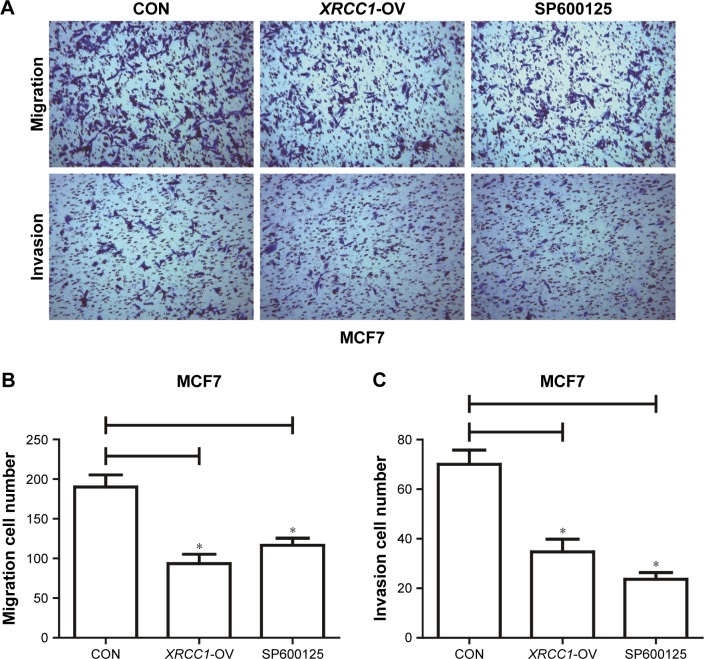
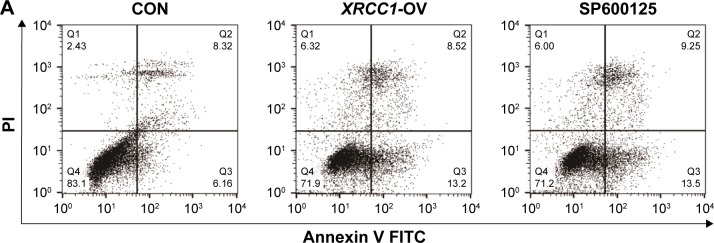
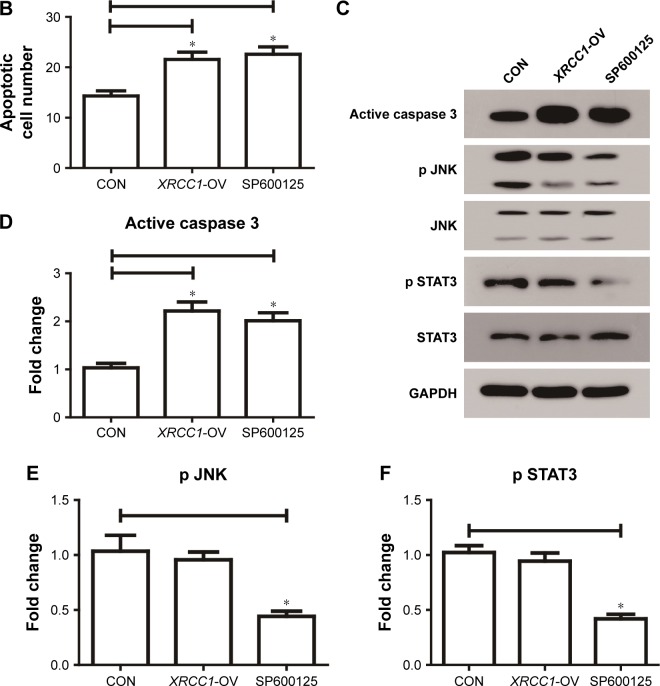
In addition to its DNA-repair function, XRCC1 has been less studied in oncology. In order to investigate whether XRCC1 has other effects on breast cancer progression, we constructed an overexpression plasmid of XRCC1 and overexpressed XRCC1 in breast cancer cell lines (Figure 2A and B). CCK8 assays showed that in both MCF7 (Figure 2D) and MDA-MB231 (Figure 3A) cell lines, OD values of XRCC1-OV cells had significantly decreased after 48 hours’ transfection, suggesting that cell proliferation ability decreased significantly. Clone-formation-efficiency assays were performed further to confirm the effect of XRCC1 in proliferation. From the results, the clone-formation efficiency of XRCC1-OV cells decreased significantly in MCF7 (Figure 2E and F) and MDA-MB231 (Figure 3B and C) cell lines. Transwell results showed that after transfection of the XRCC1-overexpression plasmid, the number of cells migrating to the lower chamber decreased significantly in MCF7 (Figure 4) and MDA-MB231 (Figure 3D–F) cells, suggesting XRCC1 inhibited the migration and invasion ability of breast cancer cells. Furthermore, we performed flow cytometry to estimate cell apoptosis. As shown in Figure 5A and B, cell-apoptosis in the XRCC1-OV group increased significantly, which proved that XRCC1 can promote apoptosis. All the results suggest that high XRCC1 expression can inhibit breast cancer progression.
Figure 2.
XRCC1 inhibited the proliferation of MCF7 cells.
Notes: (A, B) Effect of XRCC1-overexpression plasmid and SP600125. (C) Drug-sensitivity assay of SP600125 in MCF7 cells. (D) CCK8 assay to detect the proliferation of MCF7 cells. (E, F) Clone-formation-efficiency assay. *P<0.05.
Abbreviation: CON, negative control.
Figure 3.
XRCC1 inhibited the proliferation, migration, and invasion of MDA-MB231 cells.
Notes: (A) CCK8 assays were performed to detect the proliferation of MDA-MB231 cells. (B, C) Clone-formation-efficiency assay. (D) Representative images of transwell assays (×400). (E) Numbers of migration cells in CON (340±35), XRCC1-OV (95±5), and SP600125 (100±10) groups. (F) Numbers of invasion cells in CON (323±76), XRCC1-OV (95±5), and SP600125 (93±16) groups. *P<0.05.
Abbreviation: CON, negative control.
Figure 4.
XRCC1 inhibited migration and invasion of MCF7 cells.
Notes: Transwell assays were performed to estimate the cell migration and invasion. (A) Representative images of transwell assays. Magnification ×400. (B) Numbers of migration cells in CON (190±15), XRCC1-OV (90±10), and SP600125 (120±7) groups. (C) Numbers of invasion cells in CON (70±8), XRCC1-OV (35±5), and SP600125 (22±2) groups. *P<0.05.
Abbreviation: CON, negative control.
Figure 5.
XRCC1 promoted apoptosis of MCF7 cells.
Notes: (A) Apoptotic cell numbers of each group were examined by flow cytometry. (B) Number of apoptotic cells in CON, XRCC1-OV, and SP600125 group. (C–F) Western blot results of active caspase 3 and JNK pathway-related genes. *P<0.05.
Abbreviations: CON, negative control; FITC, fluorescein isothiocyanate; PI, propidium iodide.
XRCC1 expression increases during inhibition of JNK pathway
Studies have shown that JNK can regulate DNA repair, so we inhibited the JNK pathway in breast cancer and investigated its role in XRCC1. As shown in Figure 2A and B, cells treated with 10 µM SP600125 for 8 hours showed decreased XRCC1 levels and phosphorylation of JNK and STAT3 (Figure 5C–F). Meanwhile, XRCC1 overexpression had no effect on the JNK pathway (Figure 5C–F). Therefore, we hypothesized that XRCC1 expression is inhibited by the JNK pathway, which can affect tumor progression through XRCC1.
SP600125 inhibits growth of MCF7 cells
Corresponding to JNK-interference results, we found that the JNK inhibitor SP600125 was able to significantly inhibit the growth of breast cancer cells. The results showed that SP600125 inhibited the proliferation of MCF7 cells in a dose-dependent manner (Figure 2C). The results of CCK8 and clone-formation-efficiency assays revealed that proliferation of MCF7 (Figure 2D–F) and MDA-MB231 (Figure 3A–C) cells treated with SP600125 decreased significantly. Meanwhile, invasion and migration ability decreased significantly (Figures 3D–F and 4) and apoptosis was significantly induced in cells after treatment of SP600125 (Figure 5). These results suggested that SP600125 was a potential drug for breast cancer.
Discussion
Breast cancer, a malignant tumor with its highest incidence in women, affects physical and mental health, and can even be life-threatening. In recent years, its incidence has continued to grow, accompanied by a trend of younger onset.9 Polymorphism of metabolic enzyme genes can lead to differences in metabolic activity. This difference determines that individuals produce different biological effects on environmental chemical carcinogens and mutagens.
XRCC1 is an important component in base-excision and single-strand break repair, which are involved in the process of DNA repair of damage caused by cisplatin or carboplatin.10 The XRCC1 protein interacts with DNA ligase III to repair the broken DNA strand and with DNA polymerase P to conduct base-excision repair. Studies have demonstrated that the ability of DNA-repair genes (such as XRCC1) is correlated with the pathogenesis of malignant tumors and drug resistance.11 XRCC1 polymorphisms are associated with increased susceptibility of breast cancer, lung cancer, gastric cancer, and colorectal cancer.12–14 There have been three well-studied SNP loci on XRCC1: rs25487 (Arg399Gln), rs1799782 (Arg194Trp), and rs3810378 (Arg280His).15–17 rs1799782 can affect normal function and expression of the XRCC1 protein and reduce DNA-repair ability. Therefore, we focused on the relationship between this site and the pathological features of breast cancer in this study. In this study, there was no association between XRCC1 expression and rs1799782, but they were correlated with distinct molecular subtypes and tumor metastasis in breast cancer.
A case–control study of Egyptians demonstrated that XRCC1 Arg399Gln and Arg194Trp genotypes were associated with an increased risk of breast cancer.18 Two significant SNP loci – XRCC1 Arg399Gln and Arg194Trp – have been found in XRCC1. Changes of amino acid in these two sites were able ultimately to affect XRCC1 protein function and sensitivity of the individual to chemotherapeutic drugs. Wang et al found that patients with Arg194Trp were more sensitive to platinum-based chemotherapy than patients with wild-type alleles in non-small-cell lung cancer.19 In this study, XRCC1 CT and TT genotypes were in 42.37% and 8.48%, respectively, in 118 patients with breast cancer.
In the present study, we investigated the role of XRCC1 in breast cancer progression. We have proved that XRCC1 can inhibit proliferation and invasion and promote apopto-sis of breast cancer cells. XRCC1 expression was regulated by the JNK pathway. Furthermore, we found that the JNK inhibitor SP600125 significantly inhibited the growth of breast cancer cells, marking it as a potential drug for breast cancer. Abnormal activation of JNK-signaling pathway was proved to promote the development of various tumors. Studies have shown that the JNK pathway is overactivated in triple-negative human breast cancer, and this activation can promote tumor growth and metastasis. As such, research on JNK-targeted therapy is one of the foci in clinical transformation medicine for breast cancer.20 Human breast cancer is the result of a combination of environmental and genetic factors. With the continuous development in sequencing technology, it is possible to detect the genetic background of patients. Therefore, the results of XRCC1-polymorphism research have great application potential in clinical treatment. According to our results, the XRCC1 polymorphism can be used as a marker for differentiating the susceptibility of different types of breast cancer, and its effect on breast cancer can be inhibited by the JNK-signaling pathway.
Conclusion
Polymorphisms of susceptibility genes in breast cancer have positive clinical implications for our understanding of gene mutations in regional breast cancer patients.21,22 We expect case–control studies with large samples, high quality, good uniformity, and multicenter collaboration to evaluate the relationship between these polymorphisms and susceptibility to breast cancer, and to provide more reliable evidence and basic research for the prevention and treatment of breast cancer.
Obviously, the failure of chemotherapy is not caused by a single factor. It is a complex network and a combination of multiple cellular pathways and molecular mechanisms. Therefore, there is still much for us to explore.
Acknowledgments
This research was supported by the Natural Science Foundation of Shandong Province (ZR2012HL04) and the Shandong Provincial Science and Technology Development Plan (2012YD18062).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Seibold P, Behrens S, Schmezer P, et al. XRCC1 Polymorphism Associated With Late Toxicity After Radiation Therapy in Breast Cancer Patients. Int J Radiat Oncol Biol Phys. 2015;92(5):1084–1092. doi: 10.1016/j.ijrobp.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Zeng X. Association between XRCC1 polymorphisms and the risk of cervical cancer: a meta-analysis based on 4895 subjects. Oncotarget. 2017;8(2):2249. doi: 10.18632/oncotarget.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou BH, Jian ZX, Cui P, Li SJ, Tian RQ, Ou JR. Association and Intragenic Single-Nucleotide Polymorphism Interactions of the XRCC1 Polymorphisms for Pancreatic Cancer Susceptibility. Pancreas. 2016;45(4):546–551. doi: 10.1097/MPA.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 4.Takeshita H, Fujihara J, Yasuda T, Kimura-Kataoka K. Worldwide Distribution of Four SNPs in X-Ray and Repair and Cross-Complementing Group 1 (XRCC1) Clin Transl Sci. 2015;8(4):347–350. doi: 10.1111/cts.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu AQ, Wang WM, Chen WY, et al. Int J Clin Exp Med. 2015;8(1):145p–154p. XRCC1 genetic polymorphisms and sensitivity to platinum-based drugs in non-small cell lung cancer: an update meta-analysis based on 4708 subjects. [PMC free article] [PubMed] [Google Scholar]
- 6.Seibold P, Behrens S, Schmezer P, et al. XRCC1 Polymorphism Associated With Late Toxicity After Radiation Therapy in Breast Cancer Patients. Int J Radiat Oncol Biol Phys. 2015;92(5):1084–1092. doi: 10.1016/j.ijrobp.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Forat-Yazdi M, Gholi-Nataj M, Neamatzadeh H, Nourbakhsh P, Shaker-Ardakani H. Association of XRCC1 Arg399Gln Polymorphism with Colorectal Cancer Risk: A HuGE Meta Analysis of 35 Studies. Asian Pac J Cancer Prev. 2015;16(8):3285–3291. doi: 10.7314/apjcp.2015.16.8.3285. [DOI] [PubMed] [Google Scholar]
- 8.Patel AV, Calle EE, Pavluck AL, Feigelson HS, Thun MJ, Rodriguez C. A prospective study of XRCC1 (X-ray cross-complementing group 1) polymorphisms and breast cancer risk. Breast Cancer Res. 2005;7(6):R1168. doi: 10.1186/bcr1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 10.Caldecott KW, Aoufouchi S, Johnson P, Shall S. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res. 1996;24(22):4387–4394. doi: 10.1093/nar/24.22.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirschner K, Melton DW. Multiple roles of the ERCC1-XPF endonuclease in DNA repair and resistance to anticancer drugs. Anticancer Res. 2010;30(9):3223–3232. [PubMed] [Google Scholar]
- 12.Xue H, Ni P, Lin B, Xu H, Huang G. X-ray repair cross-complementing group 1 (XRCC1) genetic polymorphisms and gastric cancer risk: A HuGE review and meta-analysis. Am J Epidemiol. 2011;173(4):363–375. doi: 10.1093/aje/kwq378. [DOI] [PubMed] [Google Scholar]
- 13.Ricci S, Antonuzzo A, Galli L, et al. Multicentric randomized phase II study with gemcitabine (GEM) plus cisplatin (CDDP) in patients with stage IIIB-IV NSCLC. Lung Cancer. 1998;21(98):S48. [Google Scholar]
- 14.Nissar S, Lone TA, Banday MZ, et al. Arg399Gln polymorphism of XRCC1 gene and risk of colorectal cancer in Kashmir: a case control study. Oncol Lett. 2013;5(3):959–963. doi: 10.3892/ol.2013.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tengström M, Mannermaa A, Kosma VM, Hirvonen A, Kataja V. XRCC1 rs25487 polymorphism predicts the survival of patients after postoperative radiotherapy and adjuvant chemotherapy for breast cancer. Anticancer Res. 2014;34(6):3031–3037. [PubMed] [Google Scholar]
- 16.Huang Y, Li L, Yu L. XRCC1 Arg399Gln, Arg194Trp and Arg280His polymorphisms in breast cancer risk: a meta-analysis. Mutagenesis. 2009;24(4):331–339. doi: 10.1093/mutage/gep013. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Ghosh S, Bankura B, et al. Association of DNA repair and xenobiotic pathway gene polymorphisms with genetic susceptibility to gastric cancer patients in West Bengal, India. Tumour Biol. 2016;37(7):9139–9149. doi: 10.1007/s13277-015-4780-5. [DOI] [PubMed] [Google Scholar]
- 18.Ramadan RA, Desouky LM, Elnaggar MA, Moaaz M, Elsherif AM. Association of DNA repair genes XRCC1 (Arg399Gln), (Arg194Trp) and XRCC3 (Thr241Met) polymorphisms with the risk of breast cancer: a case-control study in Egypt. Genet Test Mol Biomarkers. 2014;18(11):754–760. doi: 10.1089/gtmb.2014.0191. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZH, Miao XP, Tan W, Zhang XR, Xu BH, Lin DX. Single nucleotide polymorphisms in XRCC1 and clinical response to platin-based chemotherapy in advanced non-small cell lung cancer. Ai Zheng. 2004;23(8):865–868. [PubMed] [Google Scholar]
- 20.Xie X, Kaoud TS, Edupuganti R, et al. Abstract 3241: Preclinical development of JNK-targeted therapy for triple-negative breast cancer. Cancer Res. 2013;73(8 Supplement):3241–3241. [Google Scholar]
- 21.Romanowicz H, Pyziak Ł, Jabłoński F, et al. Analysis of DNA Repair Genes Polymorphisms in Breast Cancer. Pathology & Oncology Research. 2016:1–7. doi: 10.1007/s12253-016-0110-5. [DOI] [PubMed] [Google Scholar]
- 22.Lin W, Lin HD, Guo XY, et al. Allelic expression imbalance polymorphisms in susceptibility chromosome regions and the risk and survival of breast cancer. Mol Carcinog. 2017;56(1):300–311. doi: 10.1002/mc.22493. [DOI] [PubMed] [Google Scholar]