Abstract
STUDY QUESTIONS
We aim to produce, disseminate and implement a core outcome set for future infertility research.
WHAT IS KNOWN ALREADY
Randomized controlled trials (RCTs) evaluating infertility treatments have reported many different outcomes, which are often defined and measured in different ways. Such variation contributes to an inability to compare, contrast and combine results of individual RCTs. The development of a core outcome set will ensure outcomes important to key stakeholders are consistently collected and reported across future infertility research.
STUDY DESIGN, SIZE, DURATION
This is a consensus study using the modified Delphi method. All stakeholders, including healthcare professionals, allied healthcare professionals, researchers and people with lived experience of infertility will be invited to participate.
PARTICIPANTS/MATERIALS, SETTING, METHODS
An international steering group, including people with lived experience of infertility, healthcare professionals, allied healthcare professionals and researchers, has been formed to guide the development of this core outcome set. Potential core outcomes have been identified through a comprehensive literature review of RCTs evaluating treatments for infertility and will be entered into a modified Delphi method. Participants will be asked to score potential core outcomes on a nine-point Likert scale anchored between one (not important) and nine (critical). Repeated reflection and rescoring should promote convergence towards consensus ‘core’ outcomes. We will establish standardized definitions and recommend high-quality measurement instruments for individual core outcomes.
STUDY FUNDING/COMPETING INTEREST(S)
This project is funded by the Royal Society of New Zealand Catalyst Fund (3712235). BWM reports consultancy fees from Guerbet, Merck, and ObsEva. R.S.L. reports consultancy fees from Abbvie, Bayer, Fractyl and Ogeda and research sponsorship from Ferring. S.B. is the Editor-in-Chief of Human Reproduction Open. The remaining authors declare no competing interests.
Keywords: infertility, consensus study, modified Delphi method, core outcome set, randomized controlled trials, systematic review, clinical practice guidelines
Introduction
Infertility is a disease of the reproductive system defined by the failure to achieve a clinical pregnancy following 12 months or more of regular unprotected sexual intercourse (Zegers-Hochschild et al., 2009). It is estimated that infertility affects one in six couples (Thoma et al., 2013). The main categories of infertility include ovulatory disorders, tubal damage, uterine or peritoneal disorders, reduced semen quantity and quality, and unexplained infertility (Collins and van Steirteghem, 2004). Treatment falls into two main categories: interventions to restore fertility when a clear cause is established, such as ovulation induction, and assisted reproductive technology (ART). Potential infertility treatments require careful evaluation.
What does this mean for patients?
Research studies testing new treatments for infertility often measure different outcomes.
Some use pregnancy as their mark of success, while others count live births, and they may, or may not, collect information about risks or side effects. When complete, the results from different research studies cannot be easily compared or combined, to see which treatments work best. This is a barrier to improving the care people with infertility receive.
The article explains that an international group of healthcare professionals, researchers and fertility patients has been set up to overcome this barrier by developing a core set of outcomes that would be common to all future infertility research. They started by collecting all the different outcomes currently reported by infertility research and will go on to ask healthcare professionals, allied healthcare professionals, researchers and fertility patients what outcomes they think are important in a three-round questionnaire. Anyone, anywhere can participate. The results will be used to select the most meaningful outcomes which will consistently be collected and reported in future infertility research.
Randomized controlled trials (RCTs), where the inclusion criteria are broad, and the outcomes are patient-centred, are the best way of establishing the comparative efficacy and safety of treatments. While individual RCTs are useful, pooling results from all available RCTs is likely to provide the best evidence to inform clinical practice (Duffy et al., 2017a). Considerable attention has been paid to standardizing the methods of conducting RCTs and systematic reviews. However, the selection, collection and reporting of outcomes has been largely overlooked and there is currently limited consensus regarding the outcomes and outcome measures infertility research should collect and report (Duffy et al., 2017c).
In the absence of a standardized approach, researchers have made arbitrary decisions, and this has resulted in many different outcomes and outcome measures collected and reported in infertility RCTs (Dapuzzo et al., 2011; Braakhekke et al., 2014; Wilkinson et al., 2016). A recent systematic review of 910 RCTs evaluating fertility treatments identified that only a fifth of included trials reported live birth (182/910; 20%). Singleton live birth was the primary outcome in 68 trials (7.4%). Only a minority of included trials reported maternal outcomes (52/910; 5.7%) and neonatal outcomes (44/910; 4.8%) (Braakhekke et al., 2014). Adverse outcomes, including ovarian hyperstimulation syndrome, congenital anomalies and long-term health risks, were infrequently reported (Braakhekke et al., 2015). The complexity of the situation is further increased because individual numerators can be associated with numerous different denominators: for example, a recent review of infertility RCTs identified 15 different denominators for clinical pregnancy (Wilkinson et al., 2017).
The lack of consensus regarding the collection and reporting of outcomes limits the comparison and pooling of individual trial data and directly impacts the usefulness of research to inform patients and professionals in clinical practice. Such diversity of outcome collection and reporting has been demonstrated in many different reproductive conditions, including endometriosis, pre-eclampsia and preterm birth (Hirsch et al., 2016; Duffy et al., 2017b, 2017c, 2017d; van’t Hooft et al., 2016).
With so many different outcomes and outcome measures in common use, there is considerable scope for selective emphasis and reporting of results. Outcome reporting bias is defined as the selection for publication of a subset of the original recorded outcome variables on the basis of the results (Kirkham et al., 2010). Several systematic reviews evaluating interventions for infertility have reported the possibility of outcome reporting bias (Duffy et al., 2009, 2010, 2014). Several studies have confirmed outcome reporting bias and quantified its impact when pooling data from individual RCTs in a meta-analysis (Chan et al., 2004; Chan and Altman, 2005; Kirkham et al., 2010; Smyth et al., 2011; Hart et al., 2012; Page et al., 2014; Saini et al., 2014). A systematic review of 157 Cochrane systematic reviews, published in 2007, revealed that over a third had at least one randomized trial at high risk of outcome reporting bias (Kirkham et al., 2010). A sensitivity analysis, excluding those with reporting bias, demonstrated a relative reduction of over 20% in the treatment effect of the primary outcome (Clark et al., 1998).
The research community has engaged with the process of standardizing the reporting of RCTs of infertility treatments. An international working group established a consensus process to modify the Consolidated Standards of Reporting Trials (CONSORT) statement. The improving the reporting of RCTs of infertility treatments (IMPRINT) statement made several recommendations including a preferred primary outcome for all infertility RCTs of live birth, defined as delivery of a live infant >20 weeks gestation, or cumulative live birth, defined as where more than one cycle occurs or where frozen embryos are transferred (Legro et al., 2014).
The next challenge is to address the unwarranted variation in outcome collection and reporting (Dapuzzo et al., 2011; Braakhekke et al., 2014; Wilkinson et al., 2016). The development and implementation of a minimum data set, termed a core outcome set, would help to address these issues. Core outcome sets are minimum collections of outcomes with standardized measurement and reporting (Williamson et al., 2012). They are identified using consensus science methods, thereby enabling key stakeholders, including healthcare professionals, researchers and patients, to suggest and prioritize outcomes (Duffy et al., 2017e).
We aim to produce, disseminate and implement a core outcome set for infertility RCTs, systematic reviews and clinical practice guidelines.
Materials and Methods
Prospective registration
This study has been prospectively registered with the Core Outcome Measures in Effectiveness Trials (COMET) initiative; the registration number is 1023 and is available online (www.comet-initiative.org/studies/details/1023).
Steering group
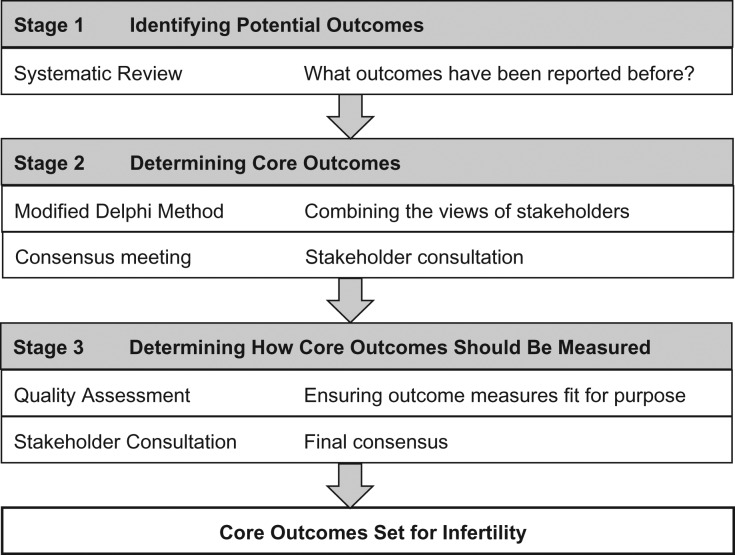
An international steering group, including people with lived experience of infertility, healthcare professionals and researchers, has been formed to guide the development of this core outcome set (Fig. 1). Members of the steering group represent various disciplines, geographical areas and expertise.
Figure 1.
Developing a core outcome set for infertility trials.
Core outcome set scope
The steering group recommend the core outcome set should apply to RCTs, systematic reviews and clinical practice guidelines evaluating interventions to restore or circumvent infertility.
The study’s methods have been informed by reviewing previous core outcome sets in women’s and newborn health (Duffy et al., 2016a; Hirsch et al., 2016b; Khalil et al., 2017; Webbe et al., 2017; Whitehouse et al., 2017).
Stage 1: Identifying potential core outcomes
We have performed a systematic review of infertility RCTs and extracted the reported outcomes and outcome measures (Wilkinson et al., 2016). A comprehensive inventory of outcomes will be developed in consultation with key stakeholders including healthcare professionals, researchers and people with lived experience of infertility (Duffy and McManus, 2016b). During the iterative development of the inventory of outcomes, consideration will be given to efficacy and safety outcomes, the aim of the intervention, for example, interventions aiming to circumvent infertility, and the timing of the intervention, for example, preconception interventions. Lay definitions will be developed for individual outcomes. The outcome inventory and lay definitions will be entered into a modified Delphi method.
Stage 2: Identifying core outcomes
The modified Delphi method assesses the extent of agreement and then resolves disagreement (Sinha et al., 2011). The survey will be piloted to ensure the ease of completion. Stakeholders including healthcare professionals, researchers and people with lived experience of infertility, will be invited to participate. There is no robust method for calculating the required sample size. We aim to recruit a minimum of 16 participants for each stakeholder group.
During round one, participants will provide their demographic details and will be allocated a unique identifier, which facilitates future anonymity. Potential core outcomes will be presented within each domain and participants will be asked to score individual outcomes using a nine-point scale from one (not important for decision making) to nine (critical for decision making) (Guyatt et al., 2011). Participants will be invited to suggest additional outcomes before completing the first-round survey.
All outcomes will be carried forward from round one into round two. For each outcome, the percentage of participants scoring individual outcomes during round one at each possible response from one to nine will be calculated and tabulated for their own stakeholder group. Additional outcomes will be considered by the steering group, prompting the reformulation of round one outcomes and the inclusion of additional outcomes in round two.
During the round two survey, participants will receive their own scores and stakeholder group feedback for each round one outcome. Participants will be invited to reflect upon summarized stakeholder group feedback and their own score before rescoring round one outcomes as well as scoring additional outcomes suggested by participants in round one.
All outcomes will be carried forward from round two into round three. For each outcome, the percentage of participants scoring individual outcomes during round two at each possible response from one to nine will be calculated and tabulated for each individual stakeholder group. During the round three survey, participants will receive their own scores and stakeholder group feedback for each round two outcome. Participants will be invited to reflect upon summarized stakeholder group feedback and their own score before rescoring round two outcomes.
Following round three, a standardized definition will be applied to the results to identify prioritized outcomes, defined by a median score of eight in each stakeholder group. A consensus development workshop will review the round three results. The objective of the consensus workshop will be to develop a final core outcome set for infertility.
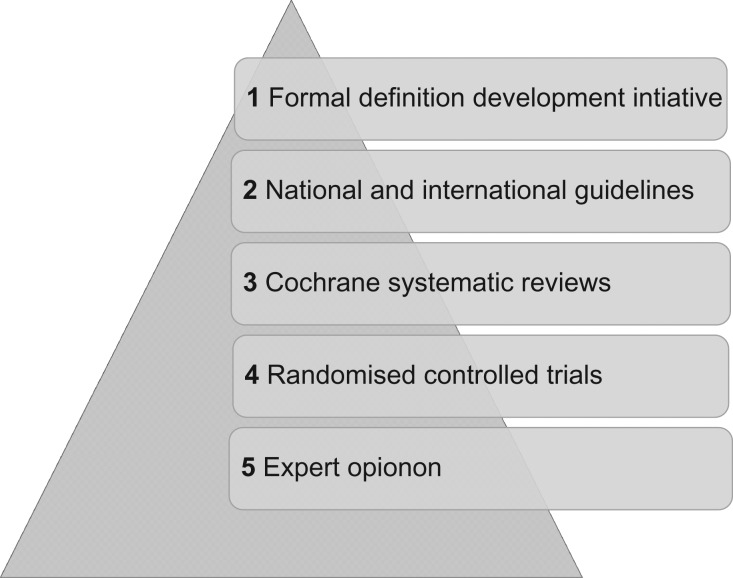
Stage 3: Identifying and standardizing core outcome measures
Once core outcomes are agreed upon, we will determine how the outcomes should be defined and measured. Potential definitions will be inventoried across formal definition development initiatives, national and international guidelines, Cochrane systematic reviews and RCTs (Fig. 2). Potential definitions will be entered into a consensus development workshop including healthcare professionals, researchers and people with lived experience of infertility. The objective of the consensus workshop will be to identify definitions for individual core outcomes. Careful attention will be paid to the appropriate selection of both numerators and denominators. Potential measurement instruments will be inventoried across national and international guidelines, Cochrane systematic reviews and RCTs. Potential measurement instruments will be quality assessed using the COMET initiative and the Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) initiative quality assessment framework (Prinsen et al., 2016). High-quality measurement instruments will be associated with core outcomes.
Figure 2.
Definition hierarchy: a framework for evaluating potential definitions for individual core outcomes.
Ethical review
We asked the advice of the National Research Ethics Service about whether this study required ethical review by an NHS Research Ethics Committee, and they advised that this study should be considered as service evaluation and development, and therefore not require ethical review.
Discussion
Implementing core outcome sets in future RCTs, systematic reviews and clinical guidelines could reduce research waste and advance the relevance of research to inform clinical practice.
Improving outcome selection
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement, implemented by funders of health research including the National Institutes of Health, European Commission, and the National Institute of Health Research, recommend the use of core outcome sets where they exist. A core outcome set ensures that outcomes relevant to all stakeholders, including healthcare professionals, researchers and patients, are collected and reported (Duffy et al., 2017e).
Improving outcome reporting
The Core Outcomes in Women’s and Newborn Health (CROWN) initiative, a consortium of 84 speciality journals, including the Cochrane Gynaecology and Fertility Group, Human Reproduction and Human Reproduction Update has been established to support the development, dissemination, and implementation of core outcome sets (Khan, 2014; Duffy et al., 2017e). Participating journals will require researchers to report core outcomes and outcome measures (Khan, 2014).
Infrastructure to support future research
Developing a core outcome set will establish an international network of organizations and stakeholders with experience of contributing to a collaborative consensus study. This infrastructure could be leveraged in other settings, for example, prioritizing research uncertainties.
Conclusion
Rigorous implementation of a core outcome set should ensure that outcomes important to all stakeholders, including healthcare professionals, researchers and people with infertility, will be collected and reported in a standardized fashion, advancing the usefulness of research to inform clinical practice.
Acknowledgements
We would like to thank colleagues at the Cochrane Gynaecology and Fertility Group, University of Auckland, New Zealand and Mr David J. Mills for administrative and material support.
Authors’ roles
Study concept and design: J.M.D. and C.M.F. Acquisition of data: J.M.D., S.B., C.C., J.L.E., R.G.F., S.X.F., Y.K., R.E.S.L., S.L., B.W.M., C.N., N.H.Y., S.R., A.S., T.H.L., A.V., M.v.W., N.L.V., A.Y.W., R.W., M.A.Y. and C.M.F. Analysis and interpretation of data: J.M.D., S.B., C.C., J.L.E., R.G.F., S.X.F., R.E.S.L., S.L., B.W.M., C.N., N.H.Y., S.R., A.S., T.H.L., A.V., M.v.W., N.L.V., A.Y.W., R.W., M.A.Y. and C.M.F. Drafting of the article: J.M.D. and C.M.F. Critical revision of the article for important intellectual content: S.B., C.C., J.L.E., R.G.F., S.X.F., Y.K., R.E.S.L., S.L., B.W.M., C.N., N.H.Y., S.R., A.S., T.H.L., A.V., M.v.W., N.L.V., A.Y.W., R.W. and M.A.Y. Statistical analysis: A.V., R.W. and J.W. Study supervision: C.M.F.
Funding
This project is partly funded by the Royal Society of New Zealand Catalyst Fund (3712235). The funder has no role in the design and conduct of the study, the collection, management, analysis, or interpretation of data, or article preparation. B.W.M. is supported by a National Health and Medical Research Council (Australia) Practitioner Fellowship (GNT1082548)
Conflict of interest
B.W.M. reports consultancy fees from Guerbet, Merck and ObsEva. R.S.L. reports consultancy fees from Abbvie, Bayer, Fractyl and Ogeda and research sponsorship from Ferring. S.B. is Editor-in-Chief of Human Reproduction Open The remaining authors declare no competing interests.
References
- Braakhekke M, Kamphuis EI, Mol R, Norman RJ, Bhattachcharya S, van der Veen F, Mol BW. Effectiveness and safety as outcome measures in reproductive medicine. Hum Reprod 2015;30:2249–2251. [DOI] [PubMed] [Google Scholar]
- Braakhekke M, Kamphuis EI, van Rumste MM, Mol F, van der Veen F, Mol BW. How are neonatal and maternal outcomes reported in randomised controlled trials (RCTs) in reproductive medicine? Hum Reprod 2014;29:1211–1217. [DOI] [PubMed] [Google Scholar]
- Chan AW, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. Br Med J 2005;330:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Krleža-Jerić K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ 2004;171:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 1998;59:1540–1548. [DOI] [PubMed] [Google Scholar]
- Collins JA, Van Steirteghem A. Overall prognosis with current treatment of infertility. Hum Reprod Update 2004;10:309–316. [DOI] [PubMed] [Google Scholar]
- Dapuzzo L, Seitz FE, Dodson WC, Stetter C, Kunselman AR, Legro RS. Incomplete and inconsistent reporting of maternal and fetal outcomes in infertility treatment trials. Fertil Steril 2011;95:2527–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilisation. Cochrane Database Syst Rev 2010;1:CD000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, Arambage K, Correa FJS, Olive D, Farqhuar C, Garry R, Barlow DH, Jacobson TZ. Laparoscopic surgery for endometrisosis. Cochrane Database Syst Rev 2014;4:CD011031. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Bhattacharya S, Herman M, Mol B, Vail A, Wilkinson J, Farquhar C. Reducing research waste in benign gynaecology and fertility research. BJOG 2017. a;124:366–369. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Hirsch M, Gale C, Pealing L, Kawsar A, Showell M, Williamson PR, Khan KS, Ziebland S, McManus RJ. A systematic review of primary outcomes and outcome measure reporting in randomized trials evaluating treatments for pre-eclampsia. Int J Gynaecol Obstet 2017. c;139:262–267. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Hirsch M, Kawsar A, Gale C, Pealing L, Plana MN, Showell M, Williamson PR, Khan KS, Ziebland S et al. Outcome reporting across randomised controlled trials evaluating therapeutic interventions for pre-eclampsia. BJOG 2017. b;124:1829–1839. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Hirsch M, Pealing L, Showell M, Khan KS, Ziebland S, McManus RJ. Inadequate safety reporting in pre-eclampsia trials: a systematic evaluation. BJOG 2017. d. 10.1111/1471-0528.14969; in press. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Johnson N, Ahmad G, Watson A. Postoperative procedures for improving fertility following pelvic reproductive surgery. Cochrane Database Syst Rev 2009;1:CD001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, McManus RJ. Influence of methodology upon the identification of potential core outcomes: recommendations for core outcome set developers are needed. BJOG 2016. b;123:1599. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Rolph R, Gale C, Hirsch M, Khan KS, Ziebland S, McManus RJ. Core outcome sets in women’s and newborn health: a systematic review. BJOG 2017. e;124:1481–1489. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, van ‘t Hooft J, Gale C, Brown M, Brogman W, Fitzpatrick R, Karumanchi SA, Lucas N, Magee L, Mol B et al. A protocol for developing, disseminating, and implementing a core outcome set for pre-eclampsia. Preg Hyper 2016. a;6:274–278. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- Hart B, Lundh A, Bero L. Effect of reporting bias on meta-analyses of drug trials: reanalysis of meta-analyses. Br Med J 2012;344:d7202. [DOI] [PubMed] [Google Scholar]
- Hirsch M, Duffy JMN, Kusznir JO, Davis CJ, Plana MN, Khan KS, International Collaboration to Harmonize Outcomes and Measures for Endometriosis . Variation in outcome reporting in endometriosis trials: a systematic review. Am J Obstet Gynecol 2016;214:452–464. [DOI] [PubMed] [Google Scholar]
- Khalil A, Perry H, Duffy JMN, Reed K, Baschat A, Deprest J, Hecher K, Lewi L, Lopriore E, Oepkes D. Twin-Twin Transfusion Syndrome: a study protocol for developing, disseminating, and implementing a core outcome set. Trials 2017;18:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KS. The CROWN Initiative: journal editors invite researchers to develop core outcomes in women’s health. BJOG 2014;121:1181–1182. [DOI] [PubMed] [Google Scholar]
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, Williamson PR. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. Br Med J 2010;340:c365. [DOI] [PubMed] [Google Scholar]
- Legro RS, Wu X, Barnhart KT, Farquhar C, Fauser BCJM, Mol B, Legro RS, Wu X, Barnhart K, Niederberger C et al. Improving the Reporting of Clinical Trials of Infertility Treatments (IMPRINT): modifying the CONSORT statement. Hum Reprod 2014;29:2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Kirkham J, Dwan K, Kramer S, Green S, Forbes A. Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions. Cochrane Database Syst Rev 2014;1:MR000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, Williamson PR, Terwee CB. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials 2016;17:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini P, Loke YK, Gamble C, Altman DG, Williamson PR, Kirkham JJ. Selective reporting bias of harm outcomes within studies: findings from a cohort of systematic reviews. BMJ 2014;349:g6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med 2011;8:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth RMD, Kirkham JJ, Jacoby A, Altman DG, Gamble C, Williamson PR. Frequency and reasons for outcome reporting bias in clinical trials: interviews with trialists. Br Med J 2011;342:c7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ‘t Hooft J, Duffy JMN, Daly M, Williamson PR, Meher S, Thom E, Saade GR, Alfirevic Z, Mol BW, Khan KS. A core outcome set for evaluation of interventions to prevent preterm birth. Obstet Gynecol 2016;127:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse KC, Kim CR, Ganatra B, Duffy JMN, Blum J, Brahmi D, Creinin MD, PePineres T, Gemzell-Danielsson K, Grossman D et al. Standarizing abortion research outcomes (STAR): a protocol for developing, disseminating and implementing a core outcome set for medical and surgical abortion. Contraception 2017;95:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J, Roberts SA, Showell M, Brison DR, Vail A. No common denominator: a review of outcome measures in IVF RCTs. Hum Reprod 2016;31:2714–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J, Vail A, Roberts SA. Direct to consumer advertising of success rates for medically assisted reproduction: a review of national clinic websites. BMJ Open 2017;7:e012218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, Tugwell P. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webbe J, Brunton G, Ali S, Duffy JMN, Modi N, Gale C. Developing, implementing and disseminating a core outcome set for neonatal medicine. BMJ Peads Open 2017;1:e00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S, for the International Committee for Monitoring Assisted Reproductive and World Health Organiztion . International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009;92:1520–1524. [DOI] [PubMed] [Google Scholar]