Abstract
Context
Hypothalamic kisspeptin signaling plays a critical role in the initiation and maintenance of reproductive function. Biallelic mutations in the coding sequence of KISS1R (GPR54) have been identified in patients with idiopathic hypogonadotropic hypogonadism, but it is unknown whether biallelic variants can also be associated with related reproductive disorders.
Case Description
A missense homozygous variant (c.890G>T p.R297L) in KISS1R was identified in a child who presented with microphallus and bilateral cryptorchidism. This variant has been reported to reduce, but not abolish, postreceptor signaling in vitro. Biochemical evaluation during the neonatal period revealed low testosterone levels. By 11 years and 8 months, the boy began demonstrating increases in testicular volume. By 17 years and 3 months, his testicular volume was 20 mL; his penile length was 7.3 cm; and he had adult levels of circulating gonadotropins and testosterone.
Conclusion
This case report associates biallelic loss-of-function mutations in KISS1R with normal timing of adolescent puberty. Because these coding sequence variants occurred in a patient with microphallus and cryptorchidism, they demonstrate different levels of dependence of the hypothalamic-pituitary-gonadal cascade on kisspeptin signaling at distinct times in the reproductive life span. The suppression of the hypothalamic-pituitary-gonadal cascade during early life but not adolescence suggests that the mini puberty of infancy depends more on kisspeptin-induced, gonadotropin-releasing hormone–induced luteinizing hormone secretion than does adolescent puberty.
A patient with biallelic KISS1R mutations had an abnormal mini puberty and a normal adolescent puberty.
Loss-of-function mutations in KISS1R (also known as GPR54) have been described in idiopathic hypogonadotropic hypogonadism (IHH) (1, 2), a disease characterized by deficient release of gonadotropin-releasing hormone (GnRH). Despite the importance of kisspeptin signaling in modulating GnRH secretion, coding sequence mutations in KISS1R are relatively rare causes of IHH compared with mutations in other genes associated with the disease (3, 4). To determine the breadth of reproductive phenotypes associated with KISS1R variants, we examined the coding sequence of KISS1R in >1600 patients with a spectrum of reproductive phenotypes.
Subjects and Methods
All activities were approved by the Partners Human Research Committee. All subjects provided written informed consent.
Subjects (N = 1337 probands) with IHH were referred to the Reproductive Endocrine Unit at Massachusetts General Hospital for participation in genetic studies. Laboratory studies, olfactory testing (40-item University of Pennsylvania Smell Identification Test) (5), and brain imaging (when available) were reviewed to ensure proper medical diagnosis. A questionnaire was administered to all subjects to assess family history and phenotype. Clinical assays were used to measure gonadotropin levels. One radioimmunoassay (in-house tritium assay with World Health Organization external quality assessment; sensitivity: 0.01 ng/mL; intra-assay coefficient of variation, 7.9; interassay coefficient of variation, 10.5) was used from 0 to 9 years; then a second radioimmunoassay (Diagnostic Products Corp., Los Angeles, CA) was used from 10 years on.
Genomic DNA was extracted from peripheral blood leukocytes or cultured lymphoblastoid cells (6). All exons of KISS1R (NM_032551) and 50 bp of intronic DNA flanking each exon were either sequenced by Sanger sequencing or whole-exome sequencing performed using the Broad Institute’s Illumina Capture platform (Cambridge, MA). Rare sequence variants were identified on the basis of a minor allele frequency <1%, as determined by the Exome Aggregation Consortium (7) database with special consideration for the ethnicity of the patient. All sequence variants were confirmed in a separate polymerase chain reaction assay by direct sequencing.
Of the 1337 individuals, nine probands (eight male, one female) were identified with biallelic rare variants in the coding sequence of KISS1R (eight homozygous, one compound heterozygous). Eight of the nine probands had IHH; however, one proband, the subject of this case report, did not have IHH and is described in Results.
Results
Subject 233 is of Chilean descent, which is consistent with his ethnicity as determined by principal component analysis. He is the only child of nonconsanguineous parents and was born at term. He had normal body weight (3.02 kg) at birth, microphallus (<1 cm) with a normally placed urethral meatus, and bilateral cryptorchidism (inguinal left testis and no palpable mass in the right scrotum or inguinal canal) with a hypoplastic scrotum.
He also had somatic abnormalities, including a high-arched palate, clinodactyly, an atrioseptal defect (i.e., ostium secundum with operative closing using a patch at 10 years), and flat feet. The origin of these somatic abnormalities is unknown. However, the boy was noted to have a normal karyotype, no rare sequence variants in KAL, normal olfaction (University of Pennsylvania Smell Identification Test score: 35 of 40), and normal olfactory bulbs by brain imaging.
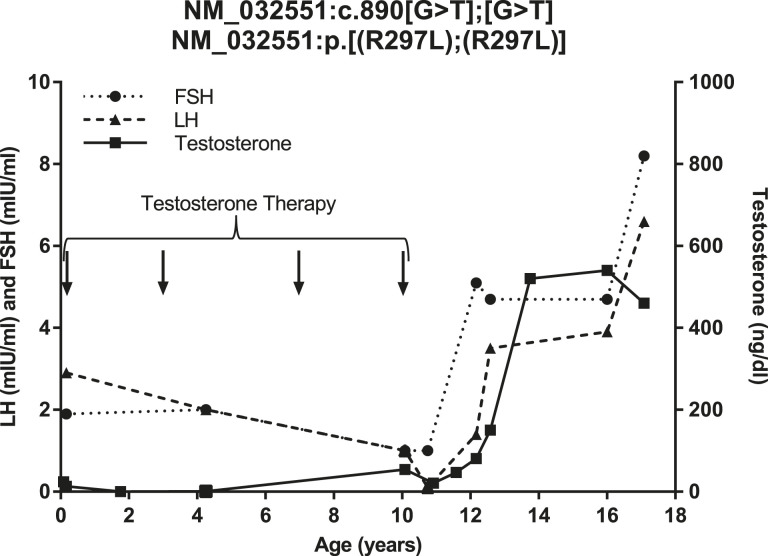
At 2 months, the boy had low testosterone (13 ng/dL), with a luteinizing hormone (LH) level of 2.9 mIU/mL and follicle-stimulating hormone (FSH) level of 1.9 mIU/mL (Fig. 1). [For comparison, the normal range of testosterone levels in Chilean infant males has been reported to be 60 to 230 ng/dL (8).] The subject underwent a human chorionic gonadotropin (hCG) stimulation test with a normal testosterone response (pretest level, 13 ng/dL; posttest level, 240 ng/dL). The subject was subsequently treated with testosterone enanthenate (75 mg/mo for 3 months); his penile length increased to 1.7 cm.
Figure 1.
LH, FSH, and testosterone levels of subject 233. Arrows indicate age at exogenous testosterone administration. Values below the assay limit of detection were plotted at the assay limit of detection.
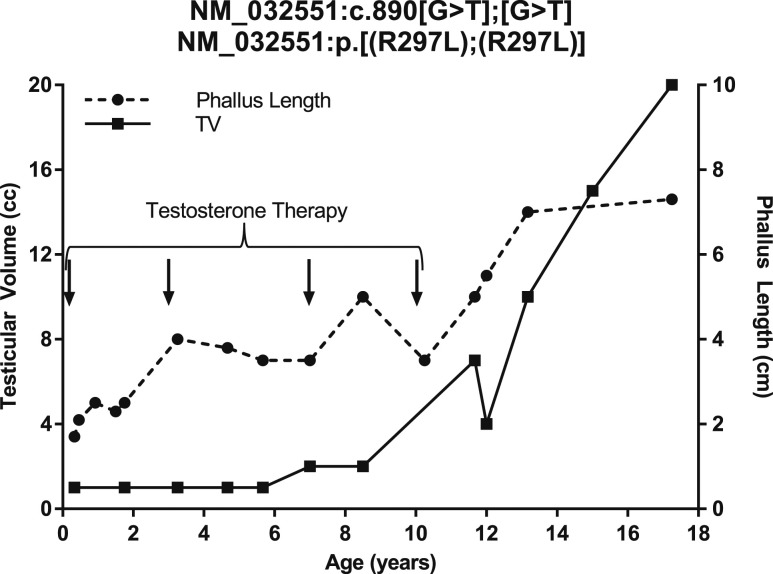
Subject 233 underwent further treatment throughout childhood. At 1 year and 6 months, he received hCG (1000 IU twice weekly for 3 weeks), which caused partial descent of the left testis into the scrotum. He underwent right orchidopexy at 2 years and 5 months and left orchidopexy at age 4 years. He received additional testosterone treatment for microphallus at 3 years (50 mg/mo for 3 months), 7 years (75 mg/mo for 3 months), and 10 years (100 mg/mo for 3 months) (Figs. 1 and 2).
Figure 2.
Testicular volume (TV) and phallus length of subject 233. Arrows indicate age at exogenous testosterone administration.
Beginning at 10 years, laboratory studies demonstrated the initiation of reproductive hormone secretion (Fig. 1). At 10 years and 9 months, a GnRH stimulation test showed FSH predominance suggestive of early puberty (LH level pretest, <1.0 mIU/mL; at 30 minutes, 2.0 mIU/mL; and at 60 minutes, 2.0 mIU/mL; FSH level pretest, 1.0 mIU/mL; at 30 minutes, 8.0 mIU/mL; at 60 minutes, 12.0 mIU/mL). At 10 years and 11 months, results of an hCG stimulation test showed a marked increase in testosterone levels (pretest, 21 ng/dL; posttest, 700 ng/dL), demonstrating mature hormonal response at the level of the Leydig cell.
The patient began experiencing erections and ejaculations at 13 years and 14 years, respectively. By age 13 years and 2 months, he reached a testicular volume of 10 mL and continued to grow such that by 17 years and 3 months, his testes were 20 mL and his phallus length was 7.3 cm (Fig. 2). He had a semen analysis at age 19 years with a volume of 1.3 mL and 5.8 million sperm/mL. Subject 233 fell short of his midparental target height; his height plateaued at age 14 years.
Subject 233 later underwent screening of 14 genes associated with IHH, which revealed a rare homozygous missense variant (NM_032551:c.890[G>T];[G>T]; NM_032551:p.[(R297L);(R297L)]; rs144670595) in KISS1R. This variant has a frequency of 0.02315% (i.e., one in 4320) and is not present in the homozygous form in the Exome Aggregation Consortium database (7). A R297L mutant construct was shown to lead to a modest but significant reduction in kisspeptin-dependent Ca++ mobilization in vitro (9). R297L had an increased half-minimal effective concentration (7 nM) compared with wild type (3.2 nM), with a maximal activity of 85% of the wild type. Both unaffected parents carried the same mutation in a heterozygous form.
Discussion
Despite increasing understanding of adolescent puberty, the mini puberty of infancy remains poorly understood. Mini puberty in male infants is characterized by robust secretion of gonadotropins and testosterone between 1 and 3 months of age (10). Although a fetus’ hypothalamic-pituitary-gonadal cascade is thought to be active during the second half of gestation (11), the mini puberty has been suggested to be critical for further genital growth and neurobehavioral development (10, 12).
Loss-of-function variants in KISS1R were initially identified in patients with hypogonadotropic hypogonadism (1, 2). Two case reports have also described KISS1R variants with an abnormal mini puberty (9, 13). Taken together, the literature appears to support a role for kisspeptin as a powerful stimulus of GnRH-induced LH secretion across reproductive life.
The current index subject challenges the notion that mini puberty and adolescent puberty are equally dependent on kisspeptin signaling. Subject 233 had an abnormal mini puberty, as evidenced by low testosterone levels; however, he underwent normal timing of adolescent puberty. The discordance between the mini puberty and adolescent puberty demonstrates that the functioning of GnRH neurons in mini puberty may be more sensitive to the integrity of kisspeptin signaling compared with the functioning of GnRH neurons during adolescent puberty.
Previous reports have documented discordance between the mini puberty and adolescent puberty outside the setting of KISS1R mutations. The gene NROB1 encodes an orphan nuclear hormone receptor superfamily and, when mutated, can cause adrenal hypoplasia congenita, a disease associated with IHH. However, newborn males with adrenal hypoplasia congenita have a normal mini puberty, as evidenced by normal serum gonadotropin and testosterone levels. Although NROB1 is biologically distinct from KISS1R, both genes point to the disparate mechanisms involved in mini puberty and adolescent puberty (14).
It is noteworthy that the subject case in this report displayed clinical features including clinodactyly, flat feet, and a high-arched palate. Many such syndromic features have been associated with Kallman syndrome and have not been observed in patients with homozygous loss-of-function mutations in KISS1R. The etiology of the syndromic features observed in subject 233 is not clear and a separate underlying genetic defect cannot be excluded.
In summary, this report demonstrates diversity in the phenotypic expression of biallelic KISS1R mutations and demonstrates that such mutations can be associated with absent mini puberty but with normal onset of adolescent puberty. Furthermore, this report suggests that although pubertal activation of the hypothalamic-pituitary-gonadal cascade requires kisspeptin signaling, even more robust kisspeptin drive is necessary for activation of the cascade in infancy.
Acknowledgments
We thank Michael H. Guo for his role in the principal component analysis.
Financial Support: This work was supported by the Eunice K. Shriver National Institute for Child Health and Human Development Grants R01 HD043341 and P50 HD-28138 (to S.S.). S.S. is a Robert and Laura Reynolds Research Scholar. Y.-M.C. was supported by a Doris Duke Clinical Scientist Development Award (Grant 2013110). M.L. was supported by a Catalyst Medical Research Investigator Training Award from Harvard Catalyst. M.S. was supported by the Fulbright Visiting Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Current Affiliation: M. Shahab’s current affiliation is the Laboratory of Reproductive Neuroendocrinology, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, 45320, Pakistan.
Disclosure Summary: Y.-M.C. was on a scientific advisory board for Abbvie and gave a presentation at an educational symposium sponsored by Endo Pharmaceuticals. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- hCG
human chorionic gonadotropin
- IHH
idiopathic hypogonadotropic hypogonadism
- LH
luteinizing hormone
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 3. Silveira LG, Latronico AC, Seminara SB. Kisspeptin and clinical disorders. Adv Exp Med Biol. 2013;784:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breuer O, Abdulhadi-Atwan M, Zeligson S, Fridman H, Renbaum P, Levy-Lahad E, Zangen DH. A novel severe N-terminal splice site KISS1R gene mutation causes hypogonadotropic hypogonadism but enables a normal development of neonatal external genitalia. Eur J Endocrinol. 2012;167(2):209–216. [DOI] [PubMed] [Google Scholar]
- 5. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2 Pt 1):176–178. [DOI] [PubMed] [Google Scholar]
- 6. Anderson MA, Gusella JF. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984;20(11):856–858. [DOI] [PubMed] [Google Scholar]
- 7. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Recabarren SE, Sir-Petermann T, Rios R, Maliqueo M, Echiburú B, Smith R, Rojas-García P, Recabarren M, Rey RA. Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J Clin Endocrinol Metab. 2008;93(9):3318–3324. [DOI] [PubMed] [Google Scholar]
- 9. Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O’rahilly S, Aparicio SA. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90(3):1849–1855. [DOI] [PubMed] [Google Scholar]
- 10. Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82(2):73–80. [DOI] [PubMed] [Google Scholar]
- 11. Kaplan SL, Grumbach MM. The ontogenesis of human foetal hormones. II. Luteinizing hormone (LH) and follicle stimulating hormone (FSH). Acta Endocrinol (Copenh). 1976;81(4):808–829. [DOI] [PubMed] [Google Scholar]
- 12. Lamminmäki A, Hines M, Kuiri-Hänninen T, Kilpeläinen L, Dunkel L, Sankilampi U. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm Behav. 2012;61(4):611–616. [DOI] [PubMed] [Google Scholar]
- 13. Teles MG, Trarbach EB, Noel SD, Guerra-Junior G, Jorge A, Beneduzzi D, Bianco SD, Mukherjee A, Baptista MT, Costa EM, De Castro M, Mendonça BB, Kaiser UB, Latronico AC. A novel homozygous splice acceptor site mutation of KISS1R in two siblings with normosmic isolated hypogonadotropic hypogonadism. Eur J Endocrinol. 2010;163(1):29–34. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi T, Shoji Y, Shoji Y, Haraguchi N, Takahashi I, Takada G. Active hypothalamic-pituitary-gonadal axis in an infant with X-linked adrenal hypoplasia congenita. J Pediatr. 1997;130(3):485–488. [DOI] [PubMed] [Google Scholar]