Abstract
Context
Little is known about reproductive function in girls with youth-onset type 2 diabetes.
Objectives
To characterize girls with irregular menses and effects of glycemic treatments on menses and sex steroids in the Treatment Options for Type 2 Diabetes in Youth (TODAY) study.
Design
Differences in demographic, metabolic, and hormonal characteristics between regular- vs irregular-menses groups were tested; treatment group (metformin with or without rosiglitazone, metformin plus lifestyle) effect on menses and sex steroids over time in the study was assessed. This is a secondary analysis of TODAY data.
Setting
Multicenter study in an academic setting.
Patients
TODAY girls not receiving hormonal contraception and those at least 1-year postmenarche were included. Irregular menses was defined as three or fewer periods in the prior 6 months.
Results
Of eligible participants with serum measurement of sex steroids (n = 190; mean age, 14 years), 21% had irregular menses. Those with irregular vs regular menses had higher body mass index (BMI) (P = 0.001), aspartate aminotransferase (AST) (P = 0.001), free androgen index (P = 0.0003), and total testosterone (P = 0.01) and lower sex hormone–binding globulin (SHBG) (P = 0.004) and estradiol (P = 0.01). Differences remained after adjustment for BMI. There was no treatment group effect on menses or sex steroids at 12 or 24 months, and no association of sex steroids was seen with measures of insulin sensitivity or secretion.
Conclusions
Menstrual dysfunction is common in girls with recently diagnosed type 2 diabetes and associated with alterations in sex steroids, SHBG, and AST but not with alteration in insulin sensitivity or β-cell function and did not improve with 2 years of antihyperglycemic treatment.
This study of reproductive function in girls with youth-onset type 2 diabetes suggests that menstrual dysfunction is common in such girls and is associated with sex steroids, SHBG, and AST.
Obesity is associated with irregular menses and decreased fertility (1), partly because of its co-occurrence with polycystic ovarian syndrome (PCOS) (2). In adolescents, PCOS is characterized by clinical and/or biochemical evidence of elevated testosterone and oligomenorrhea (2, 3). PCOS is also associated with insulin resistance, particularly in women who are obese, and increased risk for type 2 diabetes in adults (4, 5). Systemic insulin resistance is presumed to result in elevated testosterone through preserved ovarian responsiveness to compensatory hyperinsulinemia, which excessively stimulates ovarian androgen production (6) and suppresses hepatic sex hormone–binding globulin (SHBG) production by the liver (7). A similar pathophysiological schema has been proposed for adolescents with PCOS who have decreased insulin sensitivity and compensatory hyperinsulinemia compared with obese controls (8). Obesity is also thought to be associated with functional hypogonadotropic hypogonadism in some women (9), but this disorder has not been well characterized. The frequency of menstrual dysfunction among adolescent girls with type 2 diabetes has not been reported. Furthermore, although metformin treatment has been demonstrated to improve glucose tolerance in adolescent girls with PCOS and impaired glucose tolerance (10), little is known about treatment effects in girls with type 2 diabetes and PCOS or other menstrual disorders.
The Treatment Options for Type 2 Diabetes in Youth (TODAY) study was designed to assess the addition of lifestyle improvement or rosiglitazone to standard metformin therapy on durability of glycemic control. This secondary analysis takes advantage of the largest reported adolescent type 2 diabetes cohort in the world to (1) assess the frequency of self-reported menstrual dysfunction in girls with recently diagnosed type 2 diabetes, (2) compare metabolic and hormonal characteristics in TODAY girls with and without menstrual dysfunction, (3) assess the effect of treatment on menstrual function, and (4) evaluate associations of sex steroids with estimates of insulin sensitivity, insulin secretion, and oral disposition index (oDI, β-cell function in relation to insulin sensitivity).
Methods
Study cohort
The TODAY study was designed to evaluate the effects of three treatment arms (metformin alone, metformin plus rosiglitazone, and metformin plus lifestyle) on time to failure to maintain glycemic control [hemoglobin A1c (HbA1c) ≥8% for 6 months or inability to wean from temporary insulin started for acute metabolic decompensation]. Detailed methods are published elsewhere (11, 12).
Briefly, TODAY study participants were identified from 15 participating diabetes centers. Eligibility criteria included negativity for diabetes autoantibodies (glutamic acid decarboxylase-65 and tyrosine phosphatase), measurable C-peptide, body mass index (BMI) in or above the 85th percentile, age at entry 10 to 17 years, and <2 years’ duration of type 2 diabetes. After an initial screening visit, those who met eligibility criteria participated in a 2- to 6-month run-in period, which consisted of mastery of standardized diabetes education, titration of metformin to a maximum dose of 1000 mg twice daily (minimum of 500 mg twice daily), discontinuation of all other oral diabetes medications, and discontinuation of insulin. To successfully complete the run-in period, participants were required to maintain an HbA1c of 8% or less. Participants were followed for an average of 3.86 years. For the following analyses, we present data from baseline (randomization) and months 12 and 24. Measures performed at the baseline and through follow-up included anthropometrics (height, weight, and waist circumference), blood pressure, fasting laboratory studies (collected before 10 am after a 10- to 14-hour fast), and a 2-hour oral glucose tolerance test (OGTT). All participants provided both informed parental consent and minor-child assent.
Assessment of menstrual dysfunction
Menstrual history was assessed retrospectively by self-report. At baseline (after run-in, and just before randomization), the following questions were asked: (1) “Have you had your first period? (2) If yes, how old were you when your periods began? and (3) How many periods have you had in the past 6 months?” Regular periods were defined as a response ofyes" to the question about ever having a period at baseline, having had five periods or more in the past 6 months, and having had the first period at least 1 year before baseline. The timeframe of 1 year was chosen on the basis of evidence that most girls achieve a regular menstrual cycle within 1 year after menarche (13). Irregular menses was defined as a response ofyes" to the question about having had a period at least 1 year before baseline and having had three periods or fewer in the past 6 months.
At every follow-up visit, the following questions were asked: (1) “Have you had a period since the last visit?” and (2) “If yes, how many have you had?” This information was collected every 2 months during the first year of TODAY and every 3 months thereafter. Therefore, to determine the total number of periods in the 6-month period before the year 1 and year 2 visits, we combined information from visits at months 8, 10, and 12 for year 1 and from visits at months 21 and 24 for year 2.
Assays and calculations
Measurements of lipids (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides), alanine aminotransferase, aspartate aminotransferase (AST), glucose, insulin, C-peptide, HbA1c, and sex steroids/SHBG were performed at a centralized laboratory (Northwest Lipid Research Laboratory, University of Washington, Seattle, WA) in stored morning serum samples using chemiluminescent immunoassays. The analytical range for testosterone is 10 to 1600 ng/dL, with intra-assay and interassay coefficients of variation (CVs) of 4.4% to 9.4% and 3.2% to 5.3%, respectively. The analytical range for SHBG is 1 to 200 nmol/L, with intra-assay and interassay CVs of 3.1% to 4.9% and 6.6% to 7.5%, respectively. The analytical range for estradiol is 20 to 4800 pg/mL, with intra-assay and interassay CVs of 7.9% to 19.9% and 4.7% to 8.9%, respectively.
Free androgen index was used as an estimate of free testosterone and was calculated as follows: 100 × [total testosterone (nmol/L)/SHBG (nmol/L)]. Free testosterone was also calculated, assuming normal albumin, using the Vermeulen equation (14). Insulin sensitivity was estimated by using 1/fasting insulin (1/IF) (15). Insulin secretion was estimated from 30-minute glucose (G30), insulin (I30), and C-peptide (C30) samples collected during the OGTT by using the insulinogenic index (ΔI30/ΔG30) and C-peptide index (ΔC30/ΔG30) (16). The oDI was used as an estimate of β-cell function in relation to insulin sensitivity from 30-minute samples obtained during an OGTT. Two methods were used to calculate oDI: 1/IF × ΔI30/ΔG30 and 1/IF ×ΔC30/ΔG30 (17).
Statistical analyses
Baseline demographic and metabolic characteristics were compared between girls with regular and irregular menses by using the Student t test or Wilcoxon rank-sum test for quantitative variables and the χ2 test for categorical variables. Additional adjustments were made for waist circumference as a surrogate for adiposity. Separate generalized linear mixed models were used to evaluate the effects of original treatment group assignment on the mean of each sex steroid over repeated time points. The models assumed an unstructured covariance structure. The TODAY study visits (baseline and months 12 and 24) were included as a class effect and the interaction between treatment group and visit was evaluated. Separate linear regression models were used to evaluate the association between each of the sex steroid measurements at baseline with markers of insulin resistance and β-cell function. Of note, those who were started on hormonal contraception (n = 26 between baseline and month 12 and n = 28 between months 12 and 24) during the study were removed from analysis at subsequent visits. This is a secondary exploratory analysis; thus, adjustment for multiple comparisons was not made. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
Frequency of menstrual dysfunction
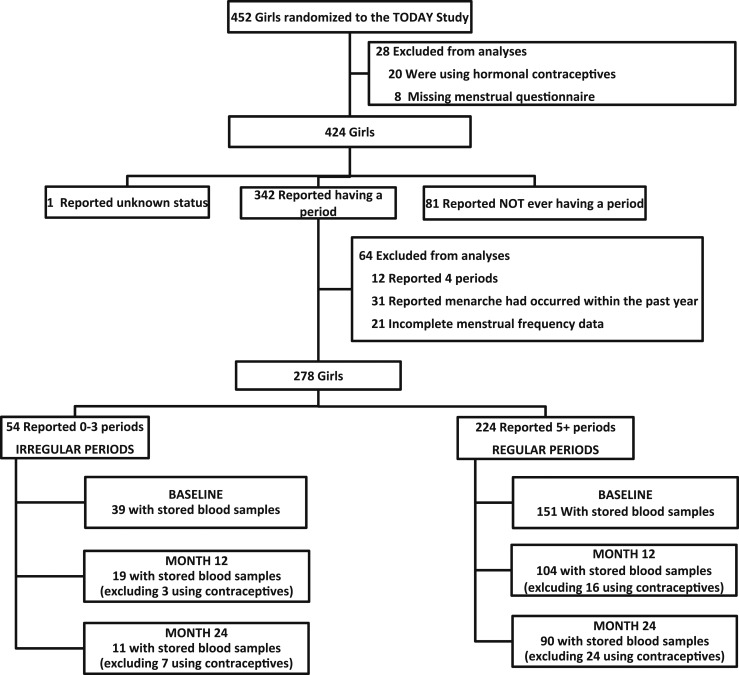
A total of 432 girls from the TODAY study were not receiving hormonal contraception in the 6 months before randomization; of those, 278 met the remaining inclusion criteria and had adequate menstrual data (Fig. 1), and 190 of those girls with adequate stored serum for measurement of sex steroids and SHBG were included for the remainder of the analyses. At months 12 and 24, a total of 123 and 101 girls, respectively, had adequate stored samples and were not receiving hormonal contraception. In the final cohort of 190 participants, 151 (79.5%) reported having had at least five periods in the previous 6 months (regular menses) and 39 (20.5%) had three or fewer periods in the previous 6 months (irregular menses).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram for inclusion in analysis.
Compared with the girls (n = 262) who were not included in the analysis, the final cohort was older [14.2 insulin; (mean) ± 1.7 insulin; (standard deviation) vs 13.4 ± 2.2 years; P < 0.0001] and had lower insulin secretion, as measured by insulinogenic index and C-peptide index, but similar disposition indexes and slightly lower AST levels (21.3 ± 9.6 vs 24.0 ± 11.0 U/L; P = 0002). They also had higher total and free testosterone and estradiol (data not shown). Compared with those included in the study, those who were excluded because of hormonal contraceptive use were, on average, older (baseline: 14.2 ± 1.7 vs 15.2 ± 1.6 years, P = 0.0308; month 12: 15.0 ± 1.6 vs 16.2 ± 1.2 years, P = 0.0033; month 24: 16.1 ± 1.6 vs 16.9 ± 1.5 years, P = 0.0138). Those who were receiving hormonal contraceptives did not differ from the included cohort in terms of BMI, HbA1c, or treatment group at any time point.
Clinical, metabolic, and hormonal characteristics associated with menstrual dysfunction
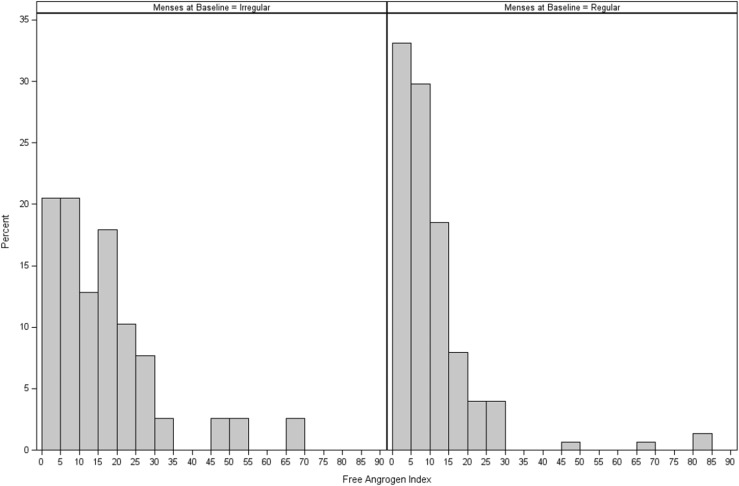
Demographic and metabolic characteristics at baseline are presented in Table 1. TODAY girls with menstrual dysfunction were similar to those with regular menses in terms of age, race/ethnicity, diabetes duration, and diabetes control. However, those with irregular menses showed greater clinical evidence of metabolic dysfunction, as indicated by higher BMI, waist circumference, and liver aminotransferase values. The difference in mean AST remained significant after adjustment for BMI. Mean insulin sensitivity (1/IF) and oDI did not significantly differ between the two groups. The mean insulinogenic index was higher in those with irregular menses, but this difference was not statistically significant after adjustment for BMI. Mean total testosterone and free androgen index were higher and mean SHBG and estradiol lower in girls with menstrual dysfunction; differences remained significant after adjustment for BMI. Most girls with irregular menses (79%) and a substantial proportion of those with regular menses (58%) had a free androgen index in a range (cut point > 6), consistent with previous reports of girls with clinical evidence of hyperandrogenism and women with PCOS (18, 19). The distribution of free androgen index in those with and without irregular menses is shown in Fig. 2. Of note, the frequency of irregular menses at baseline did not differ between girls who did not reach primary outcome by the end of TODAY (20% irregular menses) and those who had failure of glycemic control on oral therapy (21% irregular menses) (P = 0.75).
Table 1.
Baseline Characteristics of Female TODAY Study Participants (n = 190) by Menstrual Status
| Characteristics | Regular Periodsa (n = 151) | Irregular Periodsb (n = 39) | P Value | Adjusted P Value |
|---|---|---|---|---|
| Age, y | 14.1 ± 1.7 | 14.3 ± 1.7 | 0.7150 | — |
| Gynecological age, y | 2 (1, 4) | 2 (1, 3) | 0.3704 | |
| Diabetes duration, mo | 6 (4, 11) | 5 (4, 9) | 0.3155 | — |
| Race/ethnicity, % | ||||
| Black non-Hispanic | 39.1 | 25.6 | 0.1793 | — |
| Hispanic | 42.4 | 41.0 | ||
| White non-Hispanic | 15.2 | 25.6 | ||
| Other | 3.3 | 7.7 | ||
| Ever smoked, % | 13.9 | 12.8 | 0.8603 | — |
| Treatment group, % | ||||
| Metformin only | 36.4 | 38.5 | 0.9375 | — |
| Metformin + rosiglitazone | 29.8 | 30.8 | ||
| Metformin + lifestyle | 33.8 | 30.8 | ||
| Metformin use at screening | 80.1 | 79.5 | 0.9284 | — |
| Physical examination | ||||
| BMI, kg/m2 | 34.2 ± 6.8 | 37.6 ± 7.9 | 0.0097 | — |
| Waist circumference, cm | 106.6 ± 14.6 | 112.4 ± 15.7 | 0.0374 | — |
| Systolic BP, mm Hg | 111.4 ± 9.9 | 112.9 ± 9.5 | 0.4063 | — |
| Diastolic BP, mm Hg | 67.2 ± 8.3 | 67.1 ± 6.5 | 0.9773 | — |
| Lipid/liver | ||||
| Total cholesterol, mg/dL | 146.2 ± 29.9 | 144.6 ± 27.2 | 0.7742 | — |
| LDL cholesterol, mg/dL | 83.3 ± 26.3 | 82.9 ± 23.2 | 0.9219 | — |
| HDL cholesterol, mg/dL | 40.7 ± 9.7 | 37.9 ± 8.6 | 0.1064 | — |
| Triglycerides, mg/dL | 113.2 ± 81.6 | 118.9 ± 55.5 | 0.1757 | — |
| ALT, U/L | 25.5 ± 18.1 | 33.1 ± 21.7 | 0.0066 | 0.0695 |
| AST, U/L | 20.3 ± 8.8 | 25.1 ± 11.6 | 0.0012 | 0.0034 |
| Metabolic | ||||
| HbA1c, % | 6.1 ± 0.7 | 6.1 ± 0.7 | 0.9132 | 0.8941 |
| Fasting glucose, mg/dL | 113.2 ± 23.2 | 109.8 ± 21.1 | 0.4078 | 0.4085 |
| IF, µU/mLc | 28.7 ± 18.9 | 36.7 ± 28.2 | 0.1474 | 0.1870 |
| Fasting C-peptide, ng/mLc | 3.6 ± 1.4 | 4.1 ± 1.5 | 0.0415 | 0.3372 |
| Insulin sensitivity [1/IF], mL/µUc | 0.052 ± 0.049 | 0.043 ± 0.028 | 0.1263 | 0.4593 |
| Insulinogenic index [ΔI30/ΔG30], µU/mL per mg/dLc | 1.140 ± 1.018 | 1.507 ± 1.289 | 0.0226 | 0.0777 |
| C-peptide index [ΔC30/ΔG30], ng/mL per mg/dLc | 0.061 ± 0.052 | 0.077 ± 0.067 | 0.0977 | 0.1871 |
| oDI [1/IF × ΔI30/ΔG30]c | 0.049 ± 0.055 | 0.061 ± 0.073 | 0.2336 | 0.2579 |
| oDI [1/IF × ΔC30/ΔG30]c | 0.003 ± 0.003 | 0.003 ± 0.004 | 0.7976 | 0.5934 |
| Sex steroids | ||||
| Free androgen indexc | 10.6 ± 12.1 | 16.9 ± 14.5 | 0.0003 | 0.0019 |
| Free testosterone, nmol/Lc | 0.035 ± 0.038 | 0.045 ± 0.023 | 0.0011 | 0.0062 |
| Testosterone, ng/mLc | 0.39 ± 0.38 | 0.44 ± 0.20 | 0.0129 | 0.0422 |
| SHBG, nmol/Lc | 20.1 ± 23.1 | 13.6 ± 10.0 | 0.0042 | 0.0124 |
| Estradiol, pg/mLc | 70.5 ± 56.4 | 50.5 ± 34.8 | 0.0114 | 0.0021 |
Gynecological age represents years since menarche. Data are mean ± standard deviation, median (interquartile range), or percentage. The P values are calculated from the Student t test or Wilcoxon rank-sum test for continuous variables and from the χ2 test for categorical variables. P values were also adjusted for waist circumference.
Abbreviations: ALT, alanine aminotransferase; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Defined as ayes" response to the question at baseline about ever having a period and, if yes, having had five periods or more in the past 6 months.
Defined as ayes" response to the question at baseline about ever having a period and, if yes, having three or fewer periods in the past 6 months.
Variables were log-transformed before testing.
Figure 2.
Free androgen index at randomization by menstrual status in female youth with type 2 diabetes in the TODAY study: 39 girls with irregular menses and 151 girls with regular menses at randomization. There was a high frequency of elevated free androgen index (>6) in both groups, despite treatment with metformin for at least 2 months during the run-in period.
There were no significant associations between sex steroids or SHBG and insulin sensitivity, insulin secretion, or oDI estimates at baseline (data not shown).
Effect of treatment group on menstrual irregularity and sex steroids/SHBG
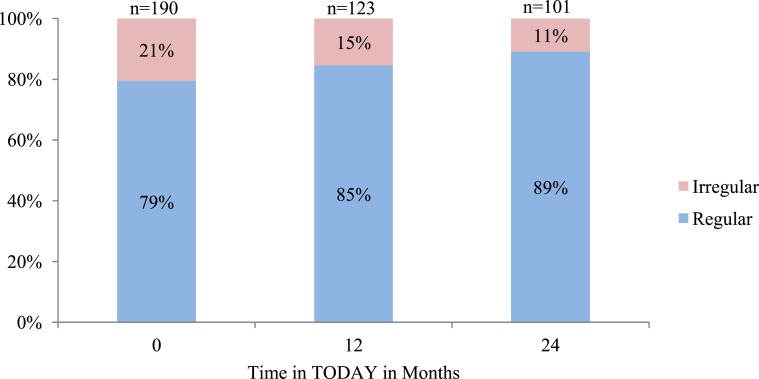
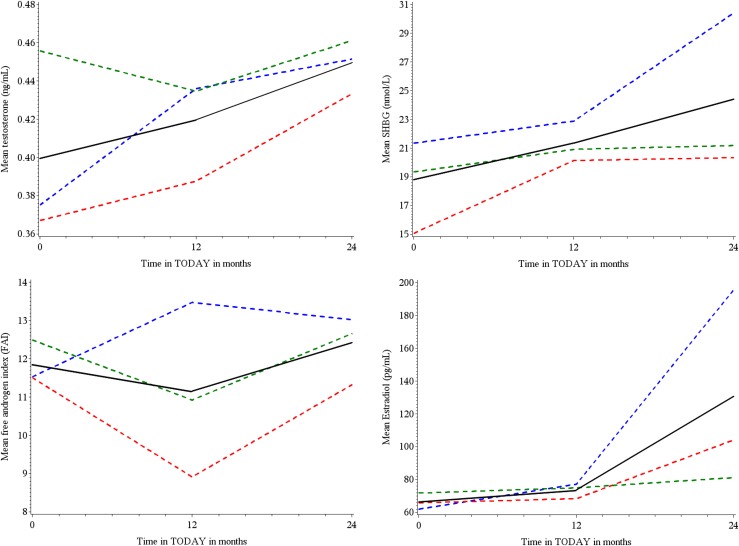
Treatment group had no significant effect on menstrual irregularity between baseline and at 12 and 24 months (data not shown). The frequency of menstrual irregularity did not significantly improve over time (Fig. 3). Menstrual frequency was also assessed at 12 and 24 months, including the girls who did not have adequate stored samples, and results were similar (data not shown). In the overall cohort, there was a significant increase in testosterone (P = 0.0095), SHBG (P = 0.0017), and estradiol (P = 0.001) across treatment visits, but there were no effects of treatment group on sex steroids or SHBG over time (Fig. 4).
Figure 3.
Menstrual irregularity at annual visits. Proportion of TODAY girls not receiving hormonal contraception with irregular menses at baseline, 12 months, and 24 months. Prevalence of irregular menses over time did not significantly differ (P = 0.10). There was also no significant treatment effect on menstrual irregularity (data not shown; P = 0.60)
Figure 4.
Mean sex steroid levels at annual visits overall and by treatment group. In the overall cohort, there was a significant increase in testosterone (P = 0.0095), SHBG (P = 0.0017), and estradiol (P = 0.001) across treatment visits, but there were no effects of treatment group on sex steroids or SHBG over time. Blue lines, metformin only; red lines, metformin plus rosiglitazone; green lines, metformin plus lifestyle; black lines, overall.
Discussion
This study reports on the frequency of menstrual dysfunction and associated metabolic defects in adolescent girls with type 2 diabetes. The 20.5% frequency of irregular menses in the TODAY girls who are >1 year postmenarche appears to be higher than in the general adolescent population, although reported prevalence of irregular menses in normal adolescents varies widely. One study suggests that 75% of healthy girls achieve a normal adolescent cycle length (21 to 45 days) within 1 year of menarche and that the frequency increases to at least 80% in the second postmenarchal year (20); however, the proportion of these girls with more strictly defined oligomenorrhea is unknown. Data from two large studies that include a total of 3358 girls and 307,592 menstrual cycles suggest that <5% of girls have an average cycle length of ≥3 months in the first year after menarche (20, 21). The prevalence rate of menstrual dysfunction in obese nondiabetic girls is not well studied, although a study of 835 Serbian girls suggests that BMI is higher in those with irregular menses (22). In one smaller study of girls randomly selected from an adolescent medicine clinic, the frequency of irregular periods, defined as fewer than nine menses per year, in obese girls was 46% (19); another very small study of severely obese girls undergoing bariatric surgery reported a 32% frequency of oligomenorrhea (23). Although the frequency of menstrual dysfunction in the TODAY study is much lower than in these other obese populations, substantial differences in the definitions of irregular menses in these studies and in the populations themselves make exact comparisons with TODAY difficult. Moreover, 80% of the TODAY girls were already receiving metformin at the time of screening (Table 1), and all were receiving a maximized metformin dose at baseline, so that the girls with PCOS would be considered treated at that time. This may have masked some of the menstrual dysfunction that would have been present if the girls had been untreated.
In the TODAY cohort, girls with irregular menses showed some differences in concentrations of circulating sex steroids compared with those with regular menses. Notably, they had significantly higher total testosterone (despite significantly lower SHBG) and free androgen index and lower estradiol. These differences persisted after adjustment for BMI. Elevated testosterone is a hallmark of the diagnosis of PCOS, a condition that affects 10% to 20% of the normal adult female population and is commonly associated with obesity and increased risk for type 2 diabetes. However, PCOS can be diagnosed only 2 years after menarche, and youth-onset type 2 diabetes is often apparent before a diagnosis of PCOS can be made. As pointed out above, because the standard first-line treatment of type 2 diabetes is also a treatment of PCOS, underlying PCOS may be masked in these youth. These factors make the relationship between PCOS and type 2 diabetes in adolescents particularly challenging to elucidate. Characterization of PCOS in the TODAY cohort is further complicated by the fact that clinical hyperandrogenism was not assessed. However, studies in adult (18) and adolescent (19) women suggest that indices of free testosterone, including free androgen index, are excellent markers of clinical hyperandrogenism and irregular menses. Receiver-operating characteristic analysis in a study of 120 lean and obese adolescent girls was used to define a free androgen index cut-point of 6.0 to indicate high risk for clinical hyperandrogenism. The proportion of TODAY girls with free androgen index >6.0 is high both in the regular menses group (58%) and in those with menstrual dysfunction (79%), indicating that many girls with treated diabetes still show biochemical and, therefore, likely clinical, hyperandrogenism. Thus, although PCOS cannot be clearly defined in the TODAY sample, key features of PCOS are common. Of note, a subset of TODAY girls with menstrual dysfunction did not have biochemical hyperandrogenism (20.5%), although this could potentially reflect previous treatment with metformin. Obesity itself is associated with menstrual disruption through suppression of gonadotropins (24, 25), which may explain some of menstrual dysfunction seen in TODAY (although gonadotropins were not measured).
The differences in sex steroid levels between those who were included in the analysis and those who were excluded probably reflect the relative reproductive immaturity of those excluded because a substantial proportion of them were premenarchal or had only recently experienced menarche (n = 112), and they were significantly younger than those included. Earlier pubertal status could also explain the higher insulin secretion in the excluded cohort, as insulin secretion is known to increase significantly during puberty in lean and obese youth without diabetes (26, 27).
On the basis of known effects of obesity on reproductive factors in adults and animal evidence regarding the role of sex steroids in metabolism, we hypothesized that there may be some interaction between sex steroids during adolescence and metabolic function in obese girls with diabetes. However, we did not find any interactions among sex steroids, insulin sensitivity, and β-cell function in our cohort. Evidence from ovariectomized rats (28, 29) and postmenopausal women (30–35) suggests that estrogen may improve insulin sensitivity. However, when estrogen concentrations are physiologically elevated, as in the midluteal phase of the menstrual cycle (36), insulin sensitivity worsens. Even less is known about estrogen effects on β-cell function; however, animal evidence suggests that estrogen promotes β-cell survival, insulin synthesis, and insulin secretion (37). Elevated androgens as seen in girls with PCOS may also attenuate the favorable effects of estradiol in obese females during puberty. This body of evidence suggests a complex role for sex hormones in the regulation of insulin action and secretion that needs further study. However, our study is limited by the fact that it does not include measurements before puberty, that all girls were receiving metformin at the time of sex steroid measurement, and that sex steroids were not measured by the most sensitive method (tandem mass spectrometry/mass spectroscopy). Further longitudinal studies are needed to better characterize the effect of obesity on reproductive hormones and β-cell function during the pubertal transition period and, thus, to better understand potential sex differences in the pathophysiology of youth-onset type 2 diabetes.
Although the TODAY participants with irregular menses were similar to those with regular menses in terms of insulin sensitivity and most metabolic markers, they did have a higher BMI and liver aminotransferase levels. Even after adjustment for BMI, AST remained significantly higher in the TODAY girls with menstrual dysfunction. This may specifically represent a link between PCOS and fatty liver disease. One recent study of nondiabetic obese adolescent girls with and without PCOS demonstrated a high frequency of hepatic steatosis, as measured by magnetic resonance imaging, in girls with PCOS (49%) compared with those without (14%) (38). As new treatments for nonalcoholic fatty liver disease emerge, developing better ways to clinically identify patients at the greatest risk will be important, with menstrual dysfunction being one such marker that can be used in combination with others, such as liver aminotransferases.
We did not find any added treatment effects of lifestyle or rosiglitazone on the biochemical hyperandrogenism in TODAY participants, who were previously treated with metformin. Studies of women with PCOS treated with metformin or thiazolidinediones demonstrate improvements in insulin sensitivity and reductions in total or free testosterone, and improvement in fertility (39–41). Fewer studies have compared the addition of thiazolidinediones to metformin treatment in women with PCOS. One study that compared metformin and rosiglitazone monotherapy reported improvement in androgens, insulin sensitivity, and menstruation in both groups; however, improvements in menstruation were greater in the rosiglitazone group (42). A Chinese study showed a better therapeutic effect of metformin plus rosiglitazone compared with metformin alone on menstrual function/fertility and also demonstrated a reduction in triglycerides, testosterone, and insulin resistance in the metformin plus rosiglitazone group (40). In the TODAY cohort, menstrual regularity and free androgen index did not significantly improve over time. Furthermore, although metformin plus rosiglitazone showed beneficial effects compared with metformin monotherapy in terms of glycemic control, the combination did not have added benefit for menstrual regularity or serum testosterone concentrations. Of note, the TODAY participants had already been treated with metformin for a minimum of 2 months when the baseline sex steroids and SHBG were measured, which may have somewhat masked the true treatment effects on these measures.
In summary, in girls with recent-onset type 2 diabetes, we found a higher frequency of irregular menses than in previous reports of healthy adolescents; however, it is difficult to quantify how much of this menstrual irregularity is related to obesity alone because of the lack of an obese nondiabetic comparison group. Irregular menses were associated with higher testosterone and AST and lower estradiol concentrations. This study examined sex steroids and menstrual health in a large cohort of girls with obesity and recent youth-onset type 2 diabetes treated with metformin with or without rosiglitazone. Further studies are needed to better understand the role of reproductive hormones on sex differences in pathophysiology of type 2 diabetes in youth.
Supplementary Material
Acknowledgments
Additional information about the TODAY study is available in the Supplemental Data.
Financial Support: This work was completed with funding from the National Institute of Diabetes and Digestive and Kidney Disease/National Institutes of Health grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254; from the National Center for Research Resources General Clinical Research Centers Program grants M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Children’s Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the National Center for Research Resources Clinical and Translational Science Awards grants UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver).
Clinical Trial Information: ClinicalTrials.gov no. NCT00081328 (registered 12 April 2004).
Disclosure Summary: M.M.K. is a site principal investigator for Merck and Daiichi-Sankyo. M.E.G. is a clinical trial steering committee member for Daiichi-Sankyo. L.L.L. is a consultant for Eli Lilly. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AST
aspartate aminotransferase
- BMI
body mass index
- C30
30-minute C-peptide
- CV
coefficient of variation
- G30
30-minute glucose
- HbA1c
hemoglobin A1c
- I30
30-minute insulin
- IF
fasting insulin
- oDI
oral disposition index
- OGTT
oral glucose tolerance test
- PCOS
polycystic ovarian syndrome
- SHBG
sex hormone–binding globulin
References
- 1. Hartz AJ, Barboriak PN, Wong A, Katayama KP, Rimm AA. The association of obesity with infertility and related menstural abnormalities in women. Int J Obes. 1979;3(1):57–73. [PubMed] [Google Scholar]
- 2. Harwood K, Vuguin P, DiMartino-Nardi J. Current approaches to the diagnosis and treatment of polycystic ovarian syndrome in youth. Horm Res. 2007;68(5):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blank SK, Helm KD, McCartney CR, Marshall JC. Polycystic ovary syndrome in adolescence. Ann N Y Acad Sci. 2008;1135(1):76–84. [DOI] [PubMed] [Google Scholar]
- 4. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774–800. [DOI] [PubMed] [Google Scholar]
- 5. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. [DOI] [PubMed] [Google Scholar]
- 6. Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62(5):904–910. [DOI] [PubMed] [Google Scholar]
- 7. Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67(3):460–464. [DOI] [PubMed] [Google Scholar]
- 8. Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138(1):38–44. [DOI] [PubMed] [Google Scholar]
- 9. Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B, Santoro N. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468–2473. [DOI] [PubMed] [Google Scholar]
- 10. Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87(4):1555–1559. [DOI] [PubMed] [Google Scholar]
- 11. Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, Kaufman F; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, Wilfley D; TODAY Study Group . Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8(2):74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab. 2000;85(3):1021–1025. [DOI] [PubMed] [Google Scholar]
- 14. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 15. George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab. 2011;96(7):2136–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25(11):2081–2087. [DOI] [PubMed] [Google Scholar]
- 17. Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr. 2012;161(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bui HN, Sluss PM, Hayes FJ, Blincko S, Knol DL, Blankenstein MA, Heijboer AC: Testosterone, free testosterone, and free androgen index in women: Reference intervals, biological variation, and diagnostic value in polycystic ovary syndrome. Clin Chim Acta. 2015;450:227–232. [DOI] [PubMed] [Google Scholar]
- 19. Rieder J, Santoro N, Cohen HW, Marantz P, Coupey SM.. Body shape and size and insulin resistance as early clinical predictors of hyperandrogenic anovulation in ethnic minority adolescent girls. J Adolesc Health. 2008;43(2):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Treloar AE, Behn BG, Cowan DW. Analysis of gestational interval. Am J Obstet Gynecol. 1967;99(1):34–45. [DOI] [PubMed] [Google Scholar]
- 21. Vollman RF. The menstrual cycle. Major Probl Obstet Gynecol. 1977;7:1–193. [PubMed] [Google Scholar]
- 22. Radivojević T, Anselmi J, Scalas E. Ergodic transition in a simple model of the continuous double auction. PLoS One. 2014;9(2):e88095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hillman JB, Miller RJ, Inge TH. Menstrual concerns and intrauterine contraception among adolescent bariatric surgery patients. J Womens Health (Larchmt). 2011;20(4):533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenfield RL, Bordini B. Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity. Brain Res. 2010;1364:186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod. 2004;19(2):383–392. [DOI] [PubMed] [Google Scholar]
- 26. Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. [DOI] [PubMed] [Google Scholar]
- 27. Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and β-cell function in overweight Latino youth. J Pediatr. 2011;158(3):442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2002;282(5):E1139–E1146. [DOI] [PubMed] [Google Scholar]
- 29. Kumagai S, Holmäng A, Björntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand. 1993;149(1):91–97. [DOI] [PubMed] [Google Scholar]
- 30. Cucinelli F, Paparella P, Soranna L, Barini A, Cinque B, Mancuso S, Lanzone A. Differential effect of transdermal estrogen plus progestagen replacement therapy on insulin metabolism in postmenopausal women: relation to their insulinemic secretion. Eur J Endocrinol. 1999;140(3):215–223. [DOI] [PubMed] [Google Scholar]
- 31. Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA, Bush TL. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Diabetes Care. 1998;21(10):1589–1595. [DOI] [PubMed] [Google Scholar]
- 32. Evans EM, Van Pelt RE, Binder EF, Williams DB, Ehsani AA, Kohrt WM. Effects of HRT and exercise training on insulin action, glucose tolerance, and body composition in older women. J Appl Physiol (1985). 2001;90(6):2033–2040. [DOI] [PubMed] [Google Scholar]
- 33. Jensen MD, Martin ML, Cryer PE, Roust LR. Effects of estrogen on free fatty acid metabolism in humans. Am J Physiol. 1994;266(6 Pt 1):E914–E920. [DOI] [PubMed] [Google Scholar]
- 34. O’Sullivan AJ, Ho KKY. A comparison of the effects of oral and transdermal estrogen replacement on insulin sensitivity in postmenopausal women. J Clin Endocrinol Metab. 1995;80(6):1783–1788. [DOI] [PubMed] [Google Scholar]
- 35. Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003;285(2):E311–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Escalante Pulido JM, Alpizar Salazar M. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30(1):19–22. [DOI] [PubMed] [Google Scholar]
- 37. Mauvais-Jarvis F. Role of sex steroids in β cell function, growth, and survival. Trends Endocrinol Metab. 2016;27(12):844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohman-Hanson RA, Cree-Green M, Kelsey MM, Bessesen DH, Sharp TA, Pyle L, Pereira RI, Nadeau KJ. Ethnic and sex differences in adiponectin: from childhood to adulthood. J Clin Endocrinol Metab. 2016;101(12):4808–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baillargeon JP, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil Steril. 2004;82(4):893–902. [DOI] [PubMed] [Google Scholar]
- 40. Liao L, Tian YJ, Zhao JJ, Xin Y, Xing HY, Dong JJ. Metformin versus metformin plus rosiglitazone in women with polycystic ovary syndrome. Chin Med J (Engl). 2011;124(5):714–718. [PubMed] [Google Scholar]
- 41. Steiner CA, Janez A, Jensterle M, Reisinger K, Forst T, Pfützner A. Impact of treatment with rosiglitazone or metformin on biomarkers for insulin resistance and metabolic syndrome in patients with polycystic ovary syndrome. J Diabetes Sci Technol. 2007;1(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yilmaz M, Biri A, Karakoç A, Törüner F, Bingöl B, Cakir N, Tiras B, Ayvaz G, Arslan M. The effects of rosiglitazone and metformin on insulin resistance and serum androgen levels in obese and lean patients with polycystic ovary syndrome. J Endocrinol Invest. 2005;28(11):1003–1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.