Abstract
STUDY QUESTION
Have seasonal variations in births by factors related to maternal education, age, parity and re-partnering changed over a 72-year period?
SUMMARY ANSWER
Seasonal variation in births has been reduced overall but also changed its pattern over the last seven decades.
WHAT IS KNOWN ALREADY
The number of births varies markedly by season, but the causes of this variation are not fully understood. Seasonality of births is, in some populations, strongly influenced by sociodemographic factors.
STUDY DESIGN SIZE, DURATION
A longitudinal study design was used by analysing the seasonal variation in live births between 1940 and 2012, and relating it to mothers’ sociodemographic characteristics at the time of childbirth (maternal education, age, parity and re-partnering).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Register data on 6 768 810 live births in Sweden between 1940 and 2012 were used. Information on biological parents are available for more than 95% of all births. Multinomial logistic regressions were used to calculate predicted probabilities of giving birth for each calendar month.
MAIN RESULTS AND THE ROLE OF CHANCE
Between 1940 and 1999, Swedish birth rates showed the typical seasonal variation with high numbers of births during the spring, and low numbers of births during the last quarter of the year. However, during the 21st century, the seasonal variation in fertility declined so that only minor variation in birth rates between February and September now remains. Still, the pattern of low birth rates at the end of the year remains and has even become more pronounced from the 1980s onwards. The characteristic ‘Christmas effect’ that used to be visible in September has vanished over the last 30 years. The roles in seasonal variation of maternal education, the mother’s age, parity and instances where the mother has re-partnered between subsequent births changed during the second half of the 20th century. From 1980s onwards, the decline in birth rates during the last quarter of the year became particularly pronounced among highly educated mothers. Over the 72 years studied, the seasonal variation among first-time mothers declined steadily and has almost disappeared at the end of the study period. Using data that cover ~180 000 births in each month, all meaningful results are statistically significant.
LIMITATIONS REASONS FOR CAUTION
The study uses data from one Nordic country only, making it difficult to draw conclusions that may hold for other countries.
WIDER IMPLICATIONS OF THE FINDINGS
The typical seasonal variation reported for Sweden between 1940 and 1999, with high numbers of births during the spring and low numbers of births during the last quarter of the year, is in line with results from most other European countries during the same time period. However, the significant decline in seasonal variation in the early 21st century is a novel development. The study underlines that in a society with low fertility and efficient birth control, active choices and behaviours associated with an individual’s sociodemographic characteristics tend to matter more for the seasonal timing of childbearing than environmental factors related to the physiological ability to reproduce and cultural–behavioural factors related to the frequency of intercourse.
STUDY FUNDING/COMPETING INTEREST(S)
The study was funded by the Swedish Research Council (Vetenskapsrådet) via the Swedish Initiative for Research on Microdata in the Social and Medical Sciences (SIMSAM): Stockholm University SIMSAM Node for Demographic Research (grant registration number 340-2013-5164). The authors declare no conflicts of interest.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: birth seasonality, fertility, socioeconomic factors, register data, family planning
Introduction
Almost all human populations exhibit seasonal variation in births. Most European countries show seasonal variation that usually peak in the spring and are the lowest during the last quarter of the year. In contrast, most US states show patterns with high numbers of births during the summer and autumn, and low numbers of births during spring. Some countries, such as Israel, Australia, New Zealand and South Africa, show almost no seasonal variation in birth rates (Lam and Miron, 1994; Bronson, 1995). Research has repeatedly found that a child’s month of birth is associated with later outcomes in life, such as those related to health (Reffelmann et al., 2011) and mortality (Ueda et al., 2013).
WHAT DOES THIS MEAN FOR PATIENTS?
This paper looks at the way the number of births differs during each season and explores whether this is changing. There are seasonal patterns for birth across the world, and in much of Europe there have traditionally tended to be more births in spring and fewer towards the end of the year. It is thought that these variations may be partly to do with seasonal changes which may affect sperm quality, ovulation and coital frequency but may also be related to social and cultural factors.
For this study, data were collected from the register of births in Sweden from 1940 to 2012 and matched with information about each mother’s education, her age at childbirth, how many children she had and if the mother had a new partner.
The researchers found that the seasonal variation in birth rates has declined in recent years as some of the factors which made a difference in the past have altered. When considering more detailed data about the mothers, they concluded that the roles these factors play in birth rates have also changed. The researchers say their results reflect the fact that in societies with efficient birth control and low overall fertility rates, individual social and demographic factors are now more important in the timing of birth.
Although frequently studied, the causes of the seasonal variation are not fully understood. Explanations for human birth seasonality can be grouped into three categories: seasonality due to climatological factors, energetic factors and social and cultural factors (Ellison et al., 2005). Changes in temperature and light have been suggested to affect the ability to conceive via hormonal changes linked to the quality of semen, the frequency of ovulation or length of the menstrual cycle (Lam and Miron, 1996). The energetic factors comprise variation in female nutritional status that may also be linked to ovulation rates and length of the menstrual cycle (Ellison and Ellison, 2009). Another set of factors that have been shown to affect humans’ ability to reproduce are pesticides (Rupa et al., 1991) and air pollution (Shah et al., 2011), factors that in some regions vary by season and thus could contribute to seasonal variation in human births. Among the social and cultural factors that may influence the seasonal variation in human birth rates, those associated with the probability of intercourse have received the most attention (Udry and Morris, 1967). In many countries, the highest birth rates can be observed around 9 months after major secular and religious holidays (Lam and Miron, 1994). Most European countries that celebrate Christmas exhibit a minor peak of births in September (James, 1990). Also, seasonality in marriage has been shown to explain some of the seasonal variation in births (Stolwijk et al., 1996). However, it is likely that the effect of social patterning in the frequency of conception is relatively low in contemporary society due to effective methods of contraception and more deliberate decisions about family planning (Bobak and Gjonca, 2001).
Another set of factors that has been studied in relation to seasonal variations in births is that which relates to different sociodemographic characteristics. Several studies have examined the impact of social class on the seasonal variation in births (Pasamanick et al., 1960; Zelnik, 1969; Erhardt et al., 1971; James, 1971; Chaudhury, 1972; Warren and Tyler, 1979; Bobak and Gjonca, 2001; Buckles and Hungerman, 2013; Azcorra et al., 2017). Most of these studies show that the seasonal variation in childbearing differs by the sociodemographic characteristics of the mother. However, most studies rely on aggregated and sometimes quite outdated sources of data. All previous studies used bivariate analyses and thus did not control for the potential role of any confounding factors. Additionally, hardly any previous study reports on changes in the seasonal variation in births over time, the main exceptions being a univariate study on Sweden by Cassel (2002) and a parity-specific study on the Netherlands by Haandrikman and van Wissen (2008). The present study contributes to the previous literature by reporting on clear changes over time regarding the impact of four sociodemographic factors in the seasonal variation in childbearing, i.e. those of maternal education, age, parity and re-partnering.
Materials and Methods
Data were retrieved from the Swedish multigenerational register, with information on all Swedes born from 1932 onwards, who have been registered as residents in Sweden at any time since 1961. The data contain high-accuracy information on vital events such as childbirths. Using the unique personal registration number assigned to all Swedish residents, the data on births could be linked to those of other administrative registers, such as the Educational and Total Population Registers. The information used in the present study includes all Swedish births of Nordic-born mothers that occurred between 1940 and 2012, for which both the biological mother and father were known. Mothers with adopted children were excluded from the study. Biological parents were identified for 90% of children born in the 1940 cohort, 95% in the 1945 cohort and more than 99% for children born in 1950 onwards. In total, 6 768 810 live births were included in the analyses. The main independent variables were the mother’s education, her age at childbirth, parity and if the mother had re-partnered between previous births. Three categories of educational attainment were used: compulsory schooling, upper secondary education and post-secondary education. Parity was categorized into three groups of birth orders: first birth, second birth and third or higher order births. Four categories of mother’s age at childbirth were used: younger than 25, 25–29, 30–34, and 35 years or older. The re-partnering variable measured whether the mother had re-partnered between consecutive births. All four sociodemographic factors were measured at the time of childbirth. To analyse the changes by sociodemographic factors over time, the calendar years that were studied were divided into four groups: 1940–1959 (N = 1 909 300), 1960–1979 (N = 2 009 475), 1980–1999 (N = 1 802 088) and 2000–2012 (N = 1 047 947). When analysing the role of re-partnering, only women with at least one prior delivery were included: 1940–1959 (N = 1 072 983), 1960–1979 (N = 1 132 224), 1980–1999 (N = 1 051 421) and 2000–2012 (N = 569 358).
Multinomial logistic regressions were used to calculate predicted probabilities of giving birth in each calendar month of a year. The results are reported as the observed vs expected number of childbirths for each month. Each month’s number of days was taken into consideration (February is assumed to have 28.25 days on average). When analysing each variable, all other variables were also included in a set of multinomial logistic regressions. Using multinomial logistic regression rather than simply comparing expected and observed numbers of births allows for multivariate analyses with the controls for potentially confounding factors. Two-tailed P-values <0.05 were considered significant. All statistical analyses were performed utilizing the statistical software package STATA 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX, USA: StataCorp LLC).
Results
The aggregate differences between the expected and observed probability of childbearing for each month during the four calendar periods are shown in Fig. 1. The differences for women of different sociodemographic characteristics are shown in Figs 2–5. For most calendar periods and all sociodemographic categories, we find some seasonal variation in childbearing. However, the seasonal variation is most pronounced for women with certain specific characteristics, and has, in most cases, changed its pattern over time.
Figure 1.
Seasonal variation in births in Sweden by year. –––– 1940–1959, ······ 1960–1979, - - - - 1980–1999,  2000–2012 (error bars are 95% CI). Total number of births between 1940 and 2012, N = 6 768 810.
2000–2012 (error bars are 95% CI). Total number of births between 1940 and 2012, N = 6 768 810.
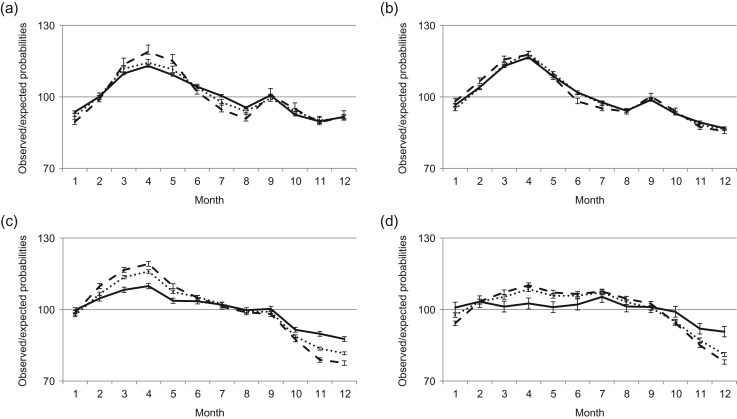
Figure 2.
Seasonal variation in births by mothers’ education. Data are for period (a) 1940–1959 (total number of births (N = 1 909 300), (b) 1960–1979 (N = 2 009 475), (c) 1980–1999 (N = 1 802 088) and (d) 2000–2012 (N = 1 047 947). –––– Compulsory, ······ upper secondary, - - - - post-secondary (error bars are 95% CI).
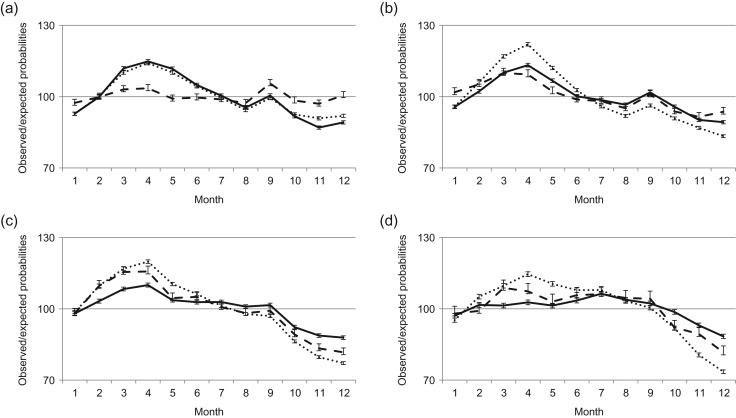
Figure 5.
Seasonal variation in births by instance of re-partnering between subsequent births. Data are for period (a) 1940–1959 (total number of births (N = 1 072 983), (b) 1960–1979 (N = 1 132 224), (c) 1980–1999 (N = 1 051 421) and (d) 2000–2012 (N = 569 358). –––– Same partner and ······ new partner (error bars are 95% CI).
Between 1940 and 1999, most births took place during the spring and the least between October and December. However, during the most recent period of the 21st century, the seasonal variation was visibly reduced, with relatively small variation in outcomes over the months between February and September. Still, the pattern of low birth rates during the end of the year remained and even become more pronounced from the 1980s onwards. During the first four decades under study, a clear increase in the number of births occurred in September. However, this Christmas effect vanished in the 1980s (Fig. 1).
Between 1940 and 1979, there were only minor differences in the seasonal variation in births between mothers with different education attainment. During 1940s and 1950s, the pattern of higher birth rates in the spring and fewer during the second half of the year was somewhat more pronounced among mothers with post-secondary education (Fig. 2a and b). During 1980–1999, mothers with upper secondary education or higher education had a more pronounced seasonal variation than mothers with low education level. During the 2000s, the seasonal variation declined for mothers of all educational levels, although the decline in birth rates during the last quarter of the year became even more pronounced among mothers with upper secondary or higher education (Fig. 2c and d).
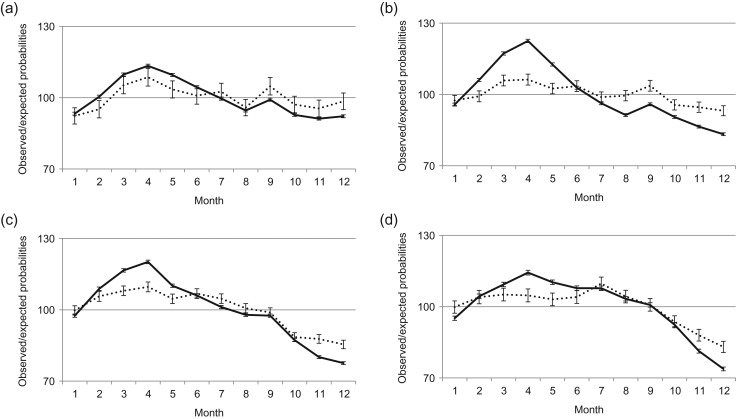
During the first four decades we study, differences in seasonal variation between mothers at different ages were relatively small, and mothers of all ages showed high birth rates in the spring and lower rates during the second half of the year (Fig. 3a and b). However, the characteristic Christmas peaks were substantially clearer among younger mothers (<25 years) in the 1940s and 1950s. From the 1980s onwards, mothers aged 35 years and above show only minor seasonal variation in childbirths. In 2000s, the seasonal variation for this age group of mothers continued to decline (Fig. 3c and d).
Figure 3.
Seasonal variation in births by mothers’ age. Data are for period (a) 1940–1959 (total number of births N = 1 909 300), (b) 1960–1979 (N = 2 009 475), (c) 1980–1999 (N = 1 802 088) and (d) 2000–2012 (N = 1 047 947). –––– <25, ······ 25–29, - - - - 30–34,  35+ (error bars are 95% CI).
35+ (error bars are 95% CI).
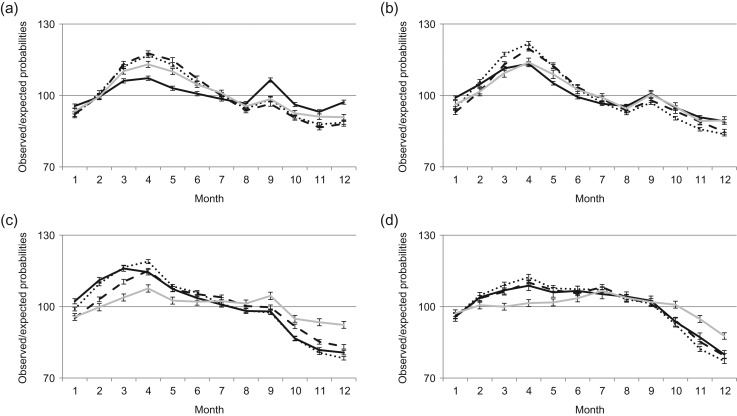
During 1940–1979, mothers of third and higher order births showed the least seasonal variation, while mothers of first- and second-order births showed more pronounced patterns of seasonality. From the 1960s onwards, mothers with second births had the most pronounced seasonal variation in childbearing. From the 1940s and throughout the study period, the seasonal variation among first-time mothers declined steadily, and from the 2000s the seasonal variation for this group of mothers was quite minor (Fig. 4a, b, c and d).
Figure 4.
Seasonal variation in births by parity. Data are for period (a) 1940–1959 (total number of births N = 1 909 300), (b) 1960–1979 (N = 2 009 475), (c) 1980–1999 (N = 1 802 088) and (d) 2000–2012 (N = 1 047 947). –––– First, ······ second, - - - - third or higher order birth (error bars are 95% CI).
During the first two decades under study, there were no meaningful differences in seasonal variation between mothers with the same or a new co-parent for subsequent births (Fig. 5a). However, during 1960s and 1970s, mothers who had not re-partnered between subsequent births exhibited much clearer seasonal variation than mothers who had re-partnered between previous births. In 1980s and 1990s, the difference between re-partnered and other mothers remained intact, although at a somewhat reduced level. Also, in the 21st century, the differences between the seasonal variation for mothers who had re-partnered and those who had not done so were smaller than those seen in the 1960s– 1990s. We note that the reduction in the differences between re-partnered and other mothers is primarily driven by the weakened seasonal variability of mothers not re-partnering. The seasonal variation among re-partnered mothers was relatively small during the entire study period.
All reported differences are statistically significant. Dividing calendar years into groups other than those presented here does not affect the main results. When single decades are used instead of 20-year birth cohorts, some patterns are amplified, but overall, the results remain the same.
Discussion
Our study showed that the seasonal variation in childbearing in Sweden has declined during the first decade of the 21st century, and also that it has changed in its sociodemographic structure during the course of the 20th century. During the six decades of the 20th century that we study, Swedish birth rates showed the typical seasonal variation with high numbers of births during the spring and low numbers of births during the last quarter of the year. However, during the new century, the seasonal variation clearly declined to a situation where there was only minor variation in birth rates between February and September. The pattern of low birth rates at the end of the year still remained and even gained in magnitude. Additionally, the previously characteristic Christmas peak in September has vanished over the last three decades.
The role of mother’s educational attainment began to matter during the 1980s, when the seasonal variation among mothers with only compulsory education started to decrease drastically. In the 21st century, the effect of the mother’s education on the birth month primarily manifests itself in a reduced birth rate during the last quarter of the year among mothers with high- or medium-level education. From the 1960s onwards, second-time mothers exhibit the most pronounced seasonal variation. In the 21st century, second-order births spike in spring and decline sharply at the end of the year. The seasonal variation for first-time mothers has become less pronounced over time. Throughout the study period, mothers who re-partnered between subsequent births displayed less seasonal variation than mothers who resumed childbearing with the same partner. The differences between re-partnered and other mothers were strongest during 1960s–1970s.
Much previous research on the causes of human birth seasonality has focused on the physical environment, such as temperatures and photoperiods (Jongbloet, 1983; Kallan and Udry, 1989; Roenneberg and Aschoff, 1990a; 1990b; Lam and Miron, 1991, 1996; Smits et al., 1998). It has been argued that both social and biological factors account for seasonal variation in births, but that the latter matter the most (Roenneberg and Aschoff, 1990a). These factors may matter more in contexts located at extreme latitudes, such as the Nordic countries, with their much larger seasonal variation in temperatures and daylight exposure. However, in keeping with the findings of the present study, it is likely that the effect of biological factors has become weaker in contemporary advanced societies where childbearing is much more strongly determined by factors related to conscious planning and individual choice (Goldin and Katz, 2000).
Childbearing decisions today take place in a very different context than those of the agricultural and industrial societies of the past, where factors related to harvests and vacation times (James, 1971; Basso et al., 1995) had huge bearing. During the last half a century, the labour market in developed societies underwent two fundamental changes; women’s increased activity (Hoem, 2000; Stanfors and Goldscheider, 2017) and the transition from an industry-dominated to a service-dominated occupational structure (Esping-Andersen, 1993). At the same time, family patterns in the Nordic countries have changed so that men have become increasingly engaged in issues related to childrearing (Goldscheider et al., 2015). Not only has women’s participation in the labour force increased (in Sweden; from 50% in 1960 to 80% in 1990), but their occupational aspirations have increased as well. Women have come to dominate many fields of higher education and the gender wage gap has decreased. In dual-earner dual-carer couples where both partners’ career developments might be affected by childbearing decisions it may be less optimal to follow traditional seasonality patterns and more rational instead to plan childbearing so that it suits both partners’ labour market careers. Another factor to consider is that Swedish fathers’ uptake of parental leave has increased steadily over time so that they now use about one-quarter of all parental leave days (Swedish Social Insurance Agency, 2017). Couples that share their parental leave more equally may have incentives to plan their childbearing so that it suits both parents’ occupational trajectories.
Evidently, the transition to a services-dominated society has created a much more diverse labour force in terms of work schedules, working hours and employment types (Kalleberg, 2000). In a society with less standardized employment structures, behavioural factors related to the frequency of intercourse and standardized vacation schedules may become less important for seasonal variations in childbearing. These changes have occurred in tandem with the observed decline in elevated birth rates during the spring season.
Still, some of the late 20th century patterns in seasonality remain intact. These are also the ones that appear most related to the conscious planning of the timing of births. Our main finding is that of distinct differences between the number of births that happen at the end of a calendar year and those that take place in the first few months of a year. The increasingly strong pattern of depressed birth rates in November and December is likely explained by the December–January cut-off threshold for Swedish pupils’ school entry and their parents increasing awareness of the negative effects on school outcomes for children who are juniors in the school entry cohort they belong to (Bedard and Dhuey, 2006). In other countries, other cut-off rules may produce other patterns of seasonality. Well-educated parents tend to be more likely to invest in their children’s education (Useem, 1992) and thus be more concerned over matters of this nature. The results show that mothers with higher education are significantly more likely to avoid births late in the year.
Previous research has shown that well-educated Japanese parents make an effort to have their children born on the right side of the cut-off date for school entry in order to give their children an advantage within the education system. Based on the data on 50 million births during 1974–2010, Shigeoka (2015) shows that more than 1800 births per year are delayed by about 1 week in order to occur after the cut-off date, by means of postponed Caesarean sections, mostly by highly educated mothers.
Our study shows that patterns of seasonality vary by parents’ sociodemographic characteristics in ways that are related to their incentives for, and possibilities to plan for, the timing of their births. For example, women with previous births are equipped with a better understanding of their fecundity and therefore better able to plan their next birth so that it does not occur at the end of a year. Also mothers with no re-partnering interruptions in their childbearing career should be better able to plan the timing of any next birth. However, the seasonal variation in higher order births may also relate to relationship quality, as frequencies of sex (Call et al., 1995; Liu, 2003) decline with relationship duration, net of age. Consequently, higher order births may more often be conceived during times when parents spend more time together—summer vacation and holidays—and thereby maintain the more traditional seasonal pattern in those births.
Factors such as ART may also have a marginal role to play, with about 2.5% of all births during the first decade of the 21st century stemming from IVF (Källén et al., 2010). Women who had IVF are generally older and with a higher educated attainment than other mothers and often of first parity (Källén et al., 2005). IVF may marginally contribute to alter the seasonal variation in births among these groups.
Further, high maternal age and different risk behaviours, such as high alcohol consumption, unemployment or economic stress, may be correlated with the risk of spontaneous abortions (Abel, 1997; de La Rochebrochard and Thonneau, 2002; Catalano et al., 2005a; 2005b; Bruckner et al., 2016). However, the evidence of any seasonal variation in miscarriages is inconclusive (Warren et al., 1980). Any seasonal variation in spontaneous and planned abortions is also related to and distorted by the gestational age at which they occur.
Our study supports the notion that cultural norms regarding the timing of childbearing decisions may be relatively weak in contemporary societies, and that increased individual autonomy and self-realization have contributed to more diversity in childbearing behaviour (Surkyn and Lesthaeghe, 2004; Billari et al., 2011). Sweden was among the first countries to enter what is often termed the ‘second demographic transition’, with declining marriage rates, increased childbearing outside marriage, increases in divorce and cohabitation, as well as a value shift towards more individualistic and expressive values (van de Kaa, 2002; Sobotka and Toulemon, 2008; Lesthaeghe, 2010). In a society with below-replacement and highly controlled fertility due to efficient contraception, active choices and behaviours associated with individual sociodemographic characteristics may matter more than the physiological ability to reproduce (Bobak and Gjonca, 2001). To a large extent, increased individual autonomy and self-realization might have overridden the role of factors that influence the physiological ability to reproduce and cultural behaviours that affect the likelihood of sexual intercourse.
Considering the consequences of birth month for individual health and socioeconomic success these findings have clear implications for parents and their children. Also, if (dis)advantageous family demographic behaviours are shaped by the family of origin, they can affect the intergenerational reproduction of (dis)advantage (Dahlberg, 2015). Highly educated mothers show an increasingly strong pattern of depressed birth rates in November and December. This could potentially help their children to perform even better in school and thereby continue reproducing their high socioeconomic status across generations.
Authors’ roles
J.D. conceived the original idea, conducted and interpreted the statistical analysis and authored the manuscript. G.A. contributed to the interpretation of statistical analysis and co-authored the manuscript.
Funding
The Swedish Research Council (Vetenskapsrådet) via the Swedish Initiative for Research on Microdata in the Social and Medical Sciences, grant registration number 340-2013-5164.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abel EL. Maternal alcohol consumption and spontaneous abortion. Alcohol Alcohol 1997;32:211–219. [DOI] [PubMed] [Google Scholar]
- Azcorra H, Vázquez-Vázquez A, Méndez N, Salazar-Rendón JC, Munguía-Rosas MA, Banik SD. Birth Seasonality in Yucatan, Mexico. Hum Ecol 2017;45:409–415. [Google Scholar]
- Basso O, Olsen J, Bisanti L, Juul S, Boldsen J. The European study group on infertility and subfecundity. Are seasonal preferences in pregnancy planning a source of bias in studies of seasonal variation in reproductive outcomes? Epidemiology 1995;6:520–524. [DOI] [PubMed] [Google Scholar]
- Bedard K, Dhuey E. The persistence of early childhood maturity: international evidence of long-run age effects. Q J Econ 2006;121:1437–1472. [Google Scholar]
- Billari FC, Goisis A, Liefbroer AC, Settersten RA, Aassve A, Hagestad G, Spéder Z. Social age deadlines for the childbearing of women and men. Hum Reprod 2011;26:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak M, Gjonca A. The seasonality of live birth is strongly influenced by socio-demographic factors. Hum Reprod 2001;16:1512–1517. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Seasonal variation in human reproduction: environmental factors. Q Rev Biol 1995;70:141–164. [DOI] [PubMed] [Google Scholar]
- Bruckner TA, Mortensen LH, Catalano RA. Spontaneous pregnancy loss in Denmark following economic downturns. Am J Epidemiol 2016;183:701–708. [DOI] [PubMed] [Google Scholar]
- Buckles KS, Hungerman DM. Season of birth and later outcomes: old questions, new answers. Rev Econ Stat 2013;95:711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call V, Sprecher S, Schwartz P. The incidence and frequency of marital sex in a national sample. J Marriage Fam 1995;57:639–652. [Google Scholar]
- Cassel PG. Changing seasonality of births in Sweden 1900–1999. Nordic Demography: Trends and Differentials. Scandinavian Population Studies2002;13:97–110.
- Catalano R, Bruckner T, Anderson E, Gould JB. Fetal death sex ratios: a test of the economic stress hypothesis. Int J Epidemiol 2005. b;34:944–948. [DOI] [PubMed] [Google Scholar]
- Catalano R, Bruckner T, Hartig T, Ong M. Population stress and the Swedish sex ratio. Paediatr Perinat Epidemiol 2005. a;19:413–420. [DOI] [PubMed] [Google Scholar]
- Chaudhury RH. Socioeconomic and seasonal variations in births: a replication. Soc Biol 1972;19:65–68. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. Social background and becoming a parent in Sweden: a register-based study of the effect of social background on childbearing in Sweden. Eur J Popul 2015;31:417–444. [Google Scholar]
- de La Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod 2002;17:1649–1656. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Ellison PT. On Fertile Ground: A Natural History of Human Reproduction. Cambridge, MA: Harvard University Press, 2009. [Google Scholar]
- Ellison PT, Valeggia C, Sherry D. Human birth seasonality In: Brockman D, Schaik C (eds). Seasonality in Primates: Studies of Living and Extinct Human and Non-Human Primates. Cambridge, UK: Cambridge University Press, 2005, 379–400. [Google Scholar]
- Erhardt CL, Nelson FG, Pakter J. Seasonal patterns of conception in New York City. Am J Public Health 1971;61:2246–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esping-Andersen G. Post industrial class structures: an analytical framework In: Esping-Andersen G (ed). Changing Classes: Stratification and Mobility in Post Industrial Societies. London, UK: Sage, 1993, 7–31. [Google Scholar]
- Goldin C, Katz LF. Career and marriage in the age of the pill. Am Econ Rev 2000;90:461–465. [DOI] [PubMed] [Google Scholar]
- Goldscheider F, Bernhardt E, Lappegård T. The gender revolution: a framework for understanding changing family and demographic behavior. Popul Dev Rev 2015;41:207–239. [Google Scholar]
- Haandrikman K, van Wissen L. Effects of the fertility transition on birth seasonality in the Netherlands. J Biosoc Sci 2008;40:655–672. [DOI] [PubMed] [Google Scholar]
- Hoem B. Entry into motherhood in Sweden: the influence of economic factors on the rise and fall in fertility, 1986–1997. Demogr Res 2000;2:1–28. [Google Scholar]
- James WH. Social class and season of birth. J Biosoc Sci 1971;3:309–320. [DOI] [PubMed] [Google Scholar]
- James WH. Seasonal variation in human births. J Biosoc Sci 1990;22:113–119. [DOI] [PubMed] [Google Scholar]
- Jongbloet PH. Menses and moon phases, ovulation and seasons, vitality and month of birth. Dev Med Child Neurol 1983;25:527–531. [DOI] [PubMed] [Google Scholar]
- Kallan JE, Udry JR. Demographic components of seasonality of pregnancy. J Biosoc Sci 1989;21:101–108. [DOI] [PubMed] [Google Scholar]
- Kalleberg AL. Nonstandard employment relations: part-time, temporary and contract work. Annu Rev Sociol 2000;26:341–365. [Google Scholar]
- Källén B, Finnström O, Lindam A, Nilsson E, Nygren KG, Otterblad Olausson P. Trends in delivery and neonatal outcome after in vitro fertilization in Sweden: data for 25 years. Hum Reprod 2010;25:1026–1034. [DOI] [PubMed] [Google Scholar]
- Källén B, Finnström O, Nygren KG, Otterblad Olausson P. In vitro fertilization in Sweden: maternal characteristics. Acta Obstet Gynecol Scand 2005;84:1185–1191. [DOI] [PubMed] [Google Scholar]
- Lam DA, Miron JA. Temperature and the seasonality of births In: Zorgniotti AW (ed). Temperature and Environmental Effects on the Testis. New York, NY: Plenum Press, 1991, 73–88. [DOI] [PubMed] [Google Scholar]
- Lam DA, Miron JA. Global patterns of seasonal variation in human fertility. Ann N Y Acad Sci 1994;709:9–28. [DOI] [PubMed] [Google Scholar]
- Lam DA, Miron JA. The effects of temperature on human fertility. Demography 1996;33:291–305. [PubMed] [Google Scholar]
- Lesthaeghe R. The unfolding story of the second demographic transition. Popul Dev Rev 2010;36:211–251. [DOI] [PubMed] [Google Scholar]
- Liu C. Does quality of marital sex decline with duration? Arch Sex Behav 2003;32:55–60. [DOI] [PubMed] [Google Scholar]
- Pasamanick B, Dinitz S, Knobloch H. Socio-economic and seasonal variations in birth rates. Milbank Mem Fund Q 1960;38:248–254. [PubMed] [Google Scholar]
- Reffelmann T, Ittermann T, Empen K, Dörr M, Felix SB. Is cardiovascular mortality related to the season of birth? J Am Coll Cardiol 2011;57:887–888. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Aschoff J. Annual rhythm of human reproduction: I. Biology, sociology, or both? J Biol Rhythms 1990. a;5:195–216. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Aschoff J. Annual rhythm of human reproduction: II. Environmental correlations. J Biol Rhythms 1990. b;5:217–239. [DOI] [PubMed] [Google Scholar]
- Rupa DS, Reddy PP, Reddi OS. Reproductive performance in population exposed to pesticides in cotton fields in India. Environ Res 1991;55:123–128. [DOI] [PubMed] [Google Scholar]
- Shah PS, Balkhair T, Knowledge Synthesis Group on Determinants of Preterm/LBW births . Air pollution and birth outcomes: a systematic review. Environ Int 2011;37:498–516. [DOI] [PubMed] [Google Scholar]
- Shigeoka H. School Entry Cutoff Date and the Timing of Births (No. w21402). Cambridge, MA: National Bureau of Economic Research, 2015. [Google Scholar]
- Smits LJ, Zielhuis GA, Jongbloet PH, Straatman H. Seasonal variation in human fecundability. Hum Reprod 1998;13:3520–3524. [DOI] [PubMed] [Google Scholar]
- Sobotka T, Toulemon L. Overview chapter 4: changing family and partnership behaviour: common trends and persistent diversity across Europe. Demogr Res 2008;19:85–138. [Google Scholar]
- Stanfors M, Goldscheider F. The forest and the trees: industrialization, demographic change, and the ongoing gender revolution in Sweden and the United States, 1870–2010. Demogr Res 2017;36:173–226. [Google Scholar]
- Stolwijk AM, Straatman H, Zielhuis GA, Jongbloet PH. Seasonal variation in the time to pregnancy: avoiding bias by using the date of onset. Epidemiology 1996;7:156–160. [DOI] [PubMed] [Google Scholar]
- Surkyn J, Lesthaeghe R. Value orientations and the second demographic transition (SDT) in Northern, Western and Southern Europe: an update. Demogr Res 2004;3:45–86. [Google Scholar]
- Swedish Social Insurance Agency Social Insurance in Figures 2017. 2017. https://www.forsakringskassan.se/wps/wcm/connect/6fa0e434-a212-4e6b-8c8d-5d7a498a253d/socialforsakringen-i-siffror-2017-engelsk.pdf?MOD=AJPERES&CVID=
- Udry JR, Morris NM. Seasonality of coitus and seasonality of birth. Demography 1967;4:673–679. [DOI] [PubMed] [Google Scholar]
- Ueda P, Bonamy AKE, Granath F, Cnattingius S. Month of birth and mortality in Sweden: a nation-wide population-based cohort study. PLoS One 2013;8:e56425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Useem EL. Middle schools and math groups: parents’ involvement in children’s placement. Sociol Educ 1992;65:263–279. [Google Scholar]
- van de Kaa DJ. The idea of a second demographic transition in industrialized countries. Birth 2002;35:45. [Google Scholar]
- Warren CW, Gold J, Tyler CW Jr, Smith JC, Paris AL. Seasonal variation in spontaneous abortions. Am J Public Health 1980;70:1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CW, Tyler CW. Social status and season of birth: a study of a metropolitan area in the southeastern United States. Soc Biol 1979;26:275–288. [DOI] [PubMed] [Google Scholar]
- Zelnik M. Socioeconomic and seasonal variations in births: a replication. Milbank Mem Fund Q 1969;47:159–165. [PubMed] [Google Scholar]