Abstract
Study question
What is the effect of endometriosis compared to unexplained subfertility on live birth rate in women undergoing IVF and embryo transfer (ET)?
Summary answer
Endometriosis decreases live birth rate in women undergoing IVF-ET treatment, particularly with increasing severity of the disease.
What is known already
Endometriosis affects up to 50% of women seeking fertility treatment and is known to reduce fecundity. There remains a debate as to effects of endometriosis on the outcomes of IVF treatment, with live birth being a secondary outcome or not reported in most studies.
Study design, size, duration
A retrospective cohort study analyzing data of IVF treatment cycles from January 2000 to December 2014 was carried out.
Participants/materials, setting, methods
Women with endometriosis (n = 531) and women with unexplained subfertility (n = 737) undergoing a first cycle of IVF-ET in a tertiary fertility treatment center were included in the study. The primary outcome was live birth. Other outcome measures were response to ovarian stimulation, embryo development and implantation rate. Bivariate and multivariate logistic regression analysis was performed and differences compared using Chi squared test of Student’s t-test as appropriate.
Main results and the role of chance
Women with endometriosis had 24% less likelihood of a live birth when compared to those with unexplained subfertility [odds ratio (OR) 0.76 (95% CI, 0.59–0.98) P = 0.035]. This effect became more apparent with increasing severity of endometriosis. Using multivariable logistic regression analysis, the trend for lower live birth rate remained but did not reach statistical significance [adjusted OR 0.76 (95% CI 0.56–1.03), P = 0.078]. Women with endometriosis were as likely as those with unexplained subfertility to have a singleton live birth when two embryos were transferred as opposed to a single ET [OR 1.38 (95% CI 0.73–2.62), P = 0.32 and OR 3.22 (95% CI 1.7–6.05), P = 0.0003, respectively]. Compared to women with unexplained subfertility, those with endometriosis had fewer oocytes retrieved [(10.54 (95% CI 10.13–0.95) and 9.15 (95% CI 8.69–9.6), respectively], lower blastocyst transfer [OR 0.24 (95% CI 0.12–0.5), P = 0.0001] and a significantly reduced implantation rate [OR 0.73 (0.58–0.92), P = 0.007].
Limitations reasons for caution
The study is limited by a retrospective design. By limiting the study to a single ET cycle, it was not possible to assess the cumulative outcome including use of all frozen embryos.
Wider implications of the findings
Endometriosis has similar phenotypes among women in different populations and would be expected to have a similar effect on fertility. These results are therefore generalizable to other populations of women.
Study funding/competing interest(s)
None.
Trial registration number
Not applicable.
Keywords: endometriosis, ART, live birth, ovarian response, embryo development, implantation, IVF
Introduction
Endometriosis is a chronic inflammatory condition characterized by presence of ectopic endometrial glands and stroma that respond to ovarian steroid hormone action (de Ziegler et al., 2010). Commonly, symptoms present during the reproductive years, being most widespread at 25–35 years of age, and with an estimated prevalence of ~5–10% women of reproductive age (Macer and Taylor, 2012).
WHAT DOES THIS MEAN FOR PATIENTS?
This study looks at the impact endometriosis has on live birth rates after IVF and compares the chances of success of women who have endometriosis with those who have unexplained infertility. The researchers looked back over the data from treatment cycles of women having their first IVF cycle at one clinic over a 14-year period.
Endometriosis affects up to half of women having fertility treatment. It is a condition where tissue similar to the womb lining is found elsewhere in the pelvic area. Endometriosis is known to reduce fertility, but there has been some debate as to whether it affects the chances of getting pregnant with treatment. Some of the studies which have investigated this were carried out some time ago and the techniques used in IVF have changed.
The researchers found that endometriosis does have an impact on the chances of successful fertility treatment, and that the more severe the endometriosis is, the more it reduces the likelihood of a successful outcome. The study suggests that more research is needed to find techniques to help overcome this.
The association between endometriosis and infertility is well recognized; ~25–50% of infertile women are diagnosed with endometriosis and ~30–50% of endometriosis patients are thought to suffer from infertility (Missmer et al., 2004). Yet, the possible mechanisms of endometriosis-associated infertility remain only poorly understood.
ART using IVF/ET is a well-established and effective technique in the management of infertility for a variety of different causes (Van Voorhis, 2007). However at present, it is unclear whether endometriosis negatively impacts on the outcomes of IVF and this issue remains highly debated (Barnhart et al., 2002; Harb et al., 2013).
Most studies looking at this matter were conducted more than a decade ago, since when the techniques for IVF have evolved and changed, with improvements made to clinical protocols and laboratory techniques. Consequently, the findings of previous studies looking at IVF and endometriosis may not represent the current state of practice. Additionally, while live birth rate is considered the most important ART outcome, this has not been the primary outcome measure in previous studies. When assessed, live birth rate had tended to be a secondary outcome, and studies lacked sufficient statistical power to evaluate the association (Barnhart et al., 2002;Harb et al., 2013). As a result, it remains unclear whether women with endometriosis have compromised live birth rate following IVF treatment.
This study aimed to assess the impact of endometriosis on live birth rate in women undergoing their first IVF cycle compared to those with unexplained subfertility. Secondary outcome measures included response to controlled ovarian stimulation (COS) as assessed by total dose of gonadotrophins, duration of stimulation, number of mature oocytes retrieved, fertilization rate, blastocyst development, ongoing pregnancy, miscarriage and ectopic pregnancy rates.
Materials and Methods
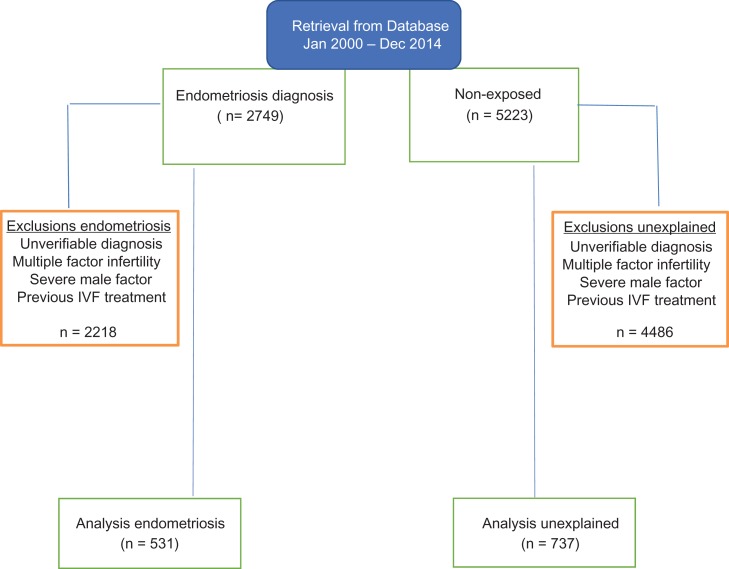
We undertook a comprehensive cohort study by analyzing the data of patients who completed fertility treatment. These were data routinely collected during the course of a patient’s clinical care that had been electronically stored since 1987 in a database (Paradox®, Borland, Scott Valley, CA, USA). To avoid heterogeneity, we only included data from participants who had undergone their first cycle of ART at one site, Oxford Fertility, between January 2000 and December 2014 (Fig. 1). We compared the treatment data for two cohort groups, which are women with endometriosis and those with unexplained subfertility. All women analyzed had a diagnostic laparoscopy performed during work-up investigations for subfertility in a UK National Health Service secondary care hospital prior to referral for IVF/ICSI treatment. In the endometriosis group, the distribution between different disease stages (defined according to the revised American Society of Reproductive Medicine (r-ASRM) classification (ASRM, 1997) was as follows; minimal (26.5%), mild (21.5%), moderate (24.9%) and severe (27.1%) disease. Appropriate approval was obtained from the University of Oxford, Central University Research Ethics Committee (MSD 892) to conduct the study.
Figure 1.
Flow diagram showing data retrieval, verification and selection of the cases (endometriosis) and controls (unexplained).
Definition and selection of exposed and non-exposed participants
Patients in the exposed group were defined as women who had endometriosis confirmed and staged at laparoscopy prior to starting IVF treatment (ASRM, 1997). Women with an ultrasound diagnosis of ovarian endometrioma would also have had laparoscopy and endometriosis staging. Ultrasound features of ovarian endometrioma included unilocular or multi-locular cyst with ground-glass appearance and no vascular papillary projections. We did not collect information on the interval between the diagnosis of endometriosis and start of IVF treatment. Those in the non-exposed group were defined as women with confirmed ovulatory cycles with mid-luteal progesterone of at least 16 pmol/l, patent Fallopian tubes demonstrable with dye test at laparoscopy and no evidence of endometriosis on laparoscopy, confirming a diagnosis of unexplained subfertility. Those with multiple diagnoses, severe male factor subfertility, undergoing donor ART treatment, choosing to freeze embryos or returning for a subsequent cycle were excluded. We also excluded cases of treatment protocols other than the long GnRH agonist.
Sample size calculation
It was estimated that the live birth rate in the unexplained subfertility group would be 30% (Harb et al., 2013). To detect 20%, relative risk difference between the two groups, we determined that a sample size of 472 participants would have 90% power with a significance threshold of 5% (http://www.openepi.com/SampleSize/SSCohort.htm). We included all the 531 records of confirmed endometriosis and 737 records for the unexplained subfertility in the study period.
Treatment protocol
The standard IVF treatment for the study was the long GnRH agonist protocol, as described previously, using either Buserelin; Suprefact®; Aventis Pharma, Kent, UK; or Nafarelin; Synarel®; Pharmacia, Milton Keynes, UK (Swanton et al., 2010). Gonadotrophin stimulation with Gonal-F®; Serono Pharmaceuticals Ltd., Feltham, UK, Puregon® Organon Laboratories Ltd., Hoddesdon, UK, or Menopur® Ferring Pharmaceuticals Ltd., West Drayton, UK was started at a daily dose ranging from 150 to 375 IU depending on patient characteristics including age, early follicular phase FSH, antral follicle count and BMI. When at least three leading follicles measuring ≥18 mm were seen on ultrasound scan the trigger injection of hCG 6500 IU (Ovitrelle®; Serono Pharmaceuticals Ltd., Feltham, UK) was administered, followed 37 h later by oocyte retrieval. Depending on sperm parameters following preparation on the day of oocyte retrieval, fertilization was achieved either by conventional IVF or ICSI (Jones et al., 2012). ET was performed between Days 2 and 5 of development depending on morphological assessment and the woman’s age (Cutting et al., 2008). Criteria for blastocyst culture were the presence of two top quality embryos on Day 3 in women younger than 38 years and three top quality embryos for women aged 38 years and older. Top quality embryos were defined as having between 6 and 10 blastomeres of regular size and <20% fragmentation. All embryos reaching blastocyst stage were considered for transfer regardless of grade. Standard practice was the transfer of up to two embryos.
Luteal support was initiated 1 day after oocyte retrieval with 800 mg micronized progesterone (Cyclogest®; Shire Pharmaceuticals Ltd., Basingstoke, UK) administered vaginally in two divided doses daily and continued for 14 days, when a urine pregnancy test was taken. If pregnant, luteal support continued until 8 weeks of pregnancy when a transvaginal ultrasound scan was arranged to confirm a clinical pregnancy, detected by the presence of fetal heart activity. Women were then discharged from the care of the IVF unit back to their general practitioner and midwife for follow-up in accordance with national guidelines (National Collaborating Centre for Women’s and Children’s, 2008). Requirements of the Human Fertilisation and Embryology Authority ensured recording of comprehensive pregnancy outcome and birth data.
Outcome measures
The primary outcome measure was live birth rate per transfer, defined as live birth at ≥24 weeks of gestation. We also analyzed the cumulative dose of gonadotrophin used for COS, number of days of stimulation, number of mature metaphase II oocytes (MII), fertilization rate (defined as number of normally fertilized oocytes divided by total number of oocytes retrieved), blastocyst transfer rate (number of cycles reaching blastocyst transfer), clinical pregnancy rate (number of patients with confirmed pregnancy on ultrasound scan divided by patients with positive pregnancy test) and miscarriage rate (defined as pregnancy loss prior to viability scan and including those confirmed on ultrasound scan up to ≤23+6 weeks of gestation) and number of ectopic pregnancies.
Statistical analysis
Bivariate associations for categorical variables between the outcomes and identified risk factors were evaluated using a Chi-square or Fisher’s exact test as appropriate. The Students t-test was used for continuous variables to compare mean differences between two groups in the outcomes. Following univariate analysis, independent variables were incorporated into a multivariable logistic regression model using backward and stepwise procedure. The variables included age, baseline FSH, endometriosis stage, number of mature oocytes and development stage at ET. All statistical analysis was performed in statistical software STATA® version 12 (College Station, TX, USA). A P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline characteristics are shown in Table I. Women with endometriosis (exposed group) were younger compared to those with unexplained subfertility with a median age 35 years (range 23–44) and 36 years (range 19–44), respectively, P < 0.0001. Compared with the unexplained subfertility group, women with endometriosis had a median FSH 6.7 IU/ml (2.2–17.6) and 6.3 IU/ml (1.5–15.4), P = 0.53, respectively. Within the grades of endometriosis, the proportion of women with FSH >10 IU/ml was equally distributed, however this was higher than in women with unexplained subfertility, P = 0.03. There was no statistically significant difference in the duration of subfertility between the two groups as well as the history of a previous birth. Male partners of women unexplained subfertility had a higher sperm concentration 74.8 million/ml (95% CI, 70–79.7) compared to those with endometriosis 66.6 million/ml (95% CI, 62.5–70.8), however this was not clinically significant.
Table I.
Baseline characteristics of women with endometriosis and unexplained subfertility
| Characteristics | Endometriosis (n = 531) | Unexplained (n = 737) | P-value | |||
|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | |||||
| Median Age (years, range) | 35 (23–44) | 36 (19–44) | <0.0001 | |||
| BMI (Kg/m2) | 23.4 (23.1–23.7) | 23.9 (23.6–24.2) | 0.04 | |||
| median FSH (range) IU/ml | 6.3 (1.5–15.4) | 6.7 (2.2–17.6) | 0.53 | |||
| Baseline FSH (%) | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Non-exposed | 0.03 |
| <10 IU/ml | 102 (19.2) | 71 (13.4) | 94 (17.7) | 84 (15.8) | 458 (62.7) | |
| ≥10 IU/ml | 39 (7.3) | 43 (8.1) | 38 (7.2) | 60 (11.3) | 273 (37.3) | |
| Duration of Infertility (Years) | 3.9 (3.5–4.3) | 3.6 (3.3–3.9) | 0.08 | |||
| Previous Live Birth (%) | 56/531 (10.7) | 99/737 (13.5) | 0.2 | |||
| Partner’s Sperm Count (×106 per ml) | 66.6 (62.5–70.8) | 74.8 (70–79.7) | 0.01 | |||
Outcome parameters
Table II shows comparisons of the treatment cycle characteristics between women in the endometriosis and unexplained subfertility groups. Overall, women with endometriosis required a higher total dose of gonadotrophin for ovarian stimulation for a comparable duration compared to those with unexplained subfertility. Conversely, compared with the unexplained subfertility group, women with endometriosis had one less mature oocyte and two less total oocytes collected at oocyte retrieval. There was, however, no statistically significant difference in the percentage fertilization rate between the two groups. In terms of embryo development, due to variable embryo development and grading we analyzed the treatment cycles according to whether they reached blastocyst transfer rather than the proportion of embryos reaching blastocyst development. There were significantly fewer cycles reaching the blastocyst transfer in women with endometriosis compared to those with unexplained subfertility.
Table II.
Response to controlled ovarian stimulation, fertilization and embryo development for cases with endometriosis and unexplained subfertility
| Characteristics | Endometriosis (n = 531) | Unexplained (n = 737) | P-value |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | ||
| Duration of Stimulation (Days) | 11 (10.8–11.2) | 11.2 (11–11.4) | 0.92 |
| Total Gonadotrophin Dose (Units) | 2504.18 (2400.21–2608.15) | 2214.37 (2142.08–2286.65) | <0.0001 |
| Total Oocytes Retrieved | 9.15 (8.69–9.6) | 10.54 (10.13-0.95) | <0.0001 |
| Mature Oocytes (MII) | 7.56 (7.16–7.96) | 8.84 (8.48–9.2) | <0.0001 |
| Number Fertilized (2PN) | 5.96 (5.6–6.3) | 6.6 (6.3–6.9) | 0.07 |
| Percentage fertilization | 78.8 (77.6–80.1) | 74.7 (73.6–75.7) | <0.0001 |
| Development at Embryo Transfer (%) | |||
| Cleavage Stage | 462 (86.96) | 460 (62.43) | <0.001 |
| Blastocyst Stage | 69 (13.04) | 277 (37.57) |
N/S = not significant, MII: metaphase II, PN: pronucleii. Chi-square test for categorical and Student’s t-test for continuous data.
Table III shows the bivariate analysis of treatment outcomes between women with endometriosis and those with unexplained subfertility. Women with endometriosis were 24% less likely to have a live birth compared to those without endometriosis [OR 0.76 (95% CI 0.59–0.98), P = 0.035]. The odds of a live birth in women with endometriosis decreased with endometriosis disease severity compared to those with unexplained subfertility (Score test for trend of odds P = 0.008) (Table IV). However, using a stepwise multivariable logistic regression analysis and correcting for age, baseline FSH, endometriosis stage, maturity of retrieved oocytes and ET day, this difference was no longer maintained, [adjusted OR 0.76 (95% CI 0.56–1.03), P = 0.08] (data in the explanatory text of Table III). Women with endometriosis were less likely to achieve a positive pregnancy test when compared to those with unexplained subfertility [OR 0.65 (0.53–0.8), P < 0.0001] (Table III). There was, however, no significant difference in the clinical pregnancy rate between the two groups.
Table III.
Bivariate analysis comparing IVF treatment outcomes between women with endometriosis and the non-exposed group
| Outcome | Endometriosis (%) | Unexplained (%) | Crude OR (95% CI) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Live birth rate | 128 (24.1) | 217 (29.4) | 0.76 (0.59–0.98)a | 0.035 | |||||||
| Gestation at birth | |||||||||||
| ≥37 Weeks | 109 (85.2) | 187 (86.2) | 0.09 (0.58–2.02) | 0.46 | |||||||
| <37 Weeks | 19 (14.8) | 30 (13.8) | |||||||||
| Clinical pregnancy rate | 142 (26.7) | 220 (29.9) | 0.86 (0.67–1.1) | 0.23 | |||||||
| Miscarriage rateb | 50/183 (27.3) | 173/392 (44.1) | 0.62 (0.43–0.89) | 0.009 | |||||||
| Positive pregnancy test | 183/531 (34.5) | 392/737 (53.2) | 0.65 (0.53–0.8) | <0.0001 | |||||||
| Implantation Rate Per Embryo Transferred (%) | 37.6 (33.5–41.8) | 45.3 (41.7–48.9) | 0.73 (0.58–0.92) | 0.007 | |||||||
| Ectopic pregnancy | 5 | 2 | 5.36 (1.03–27.86) | 0.046 | |||||||
| Birth per embryos transferred | Embryo(s) transferred | No birth | Single | Twin | Triplet | No birth | Single | Twin | Triplet | ||
| 1 | 51 (78.5) | 13 (20) | 1 (1.5) | 0 | 76 (54.3) | 61 (43.6) | 3 (2.1) | 0 | 0.31 (0.16–0.58) | 0.0002 | |
| 2 | 264 (70.8) | 88 (23.6) | 21 (5.6) | 0 | 252 (62.2) | 118 (29.1) | 32 (7.9) | 3 (0.7) | 0.72 (0.38–1.36) | 0.32 | |
| 3 | 25 (83.3) | 4 (13.3) | 0 | 1 (3.3) | 12 (100) | 0 | 0 | 0 | n/a | ||
aMultivariable stepwise logistic regression analysis (adjusted for age, ovarian reserve (FSH), endometriosis stage, number of mature oocytes and embryo developmental stage) adjusted OR 0.76 (95% CI 0.56–1.03), P = 0.08.
bIncludes both biochemical and clinical miscarriage.
n/a = not applicable.
Table IV.
Odds ratio for live birth with endometriosis disease severity
| Endometriosis status | OR | 95% CI | P-value |
|---|---|---|---|
| aUnexplained | 1 | ||
| Minimal endometriosis | 0.89 | 0.6–1.33 | 0.58 |
| Mild endometriosis | 0.65 | 0.4–1.04 | 0.07 |
| Moderate endometriosis | 0.87 | 0.57–1.31 | 0.5 |
| Severe endometriosis | 0.56 | 0.35–0.87 | 0.009 |
aWomen with unexplained infertility are the reference group.
Score test for trend of odds P = 0.008.
We sought to explore the chance of achieving a live birth against the number of embryos transferred by comparing single versus double ET (Table III). Using descriptive statistics, there was no statistically significant difference in the chance of achieving a singleton live birth with double ET in either the endometriosis or unexplained subfertility groups [OR 0.72 (0.38–1.36), P = 0.32, however, with single ET the chance of achieving a singleton live birth was significantly lower in the endometriosis group [OR 0.31 (0.16–0.58)), P = 0.0002]. This was unsurprising as this group was more likely to achieve blastocyst transfer.
Discussion
The association between endometriosis and infertility has been known for many years, even though the mechanisms are only poorly understood and remain largely speculative. However, the effect of endometriosis on outcomes of infertility treatment using ART continues to be a subject of debate and interest (Dunselman et al., 2013). Our study demonstrates that women with endometriosis undergoing IVF treatment have a lower live birth rate when compared to those with unexplained subfertility, this effect being greater with increasing disease severity. Similarly, other outcome parameters were also compromised in women with endometriosis when compared to women with unexplained subfertility. Despite requiring a comparable duration of time for COS, women with endometriosis compared with those with unexplained subfertility were given a higher total dose of gonadotrophin yet still produced fewer total and mature oocytes. Despite having similar oocyte fertilization rates, women with endometriosis had fewer blastocyst transfer cycles compared to women with unexplained subfertility. This observation was surprising as women with endometriosis were younger than those with unexplained subfertility with both groups having a comparable ovarian reserve.
These observations confirm in a large cohort using modern IVF methods the earlier trends noted from smaller studies as well as national databases (Barnhart et al., 2002; Harb et al., 2013; Senapati et al., 2016). We believe this is the first study to look at live birth as the primary outcome measure using a large sample size with appropriate power calculation. Live birth rate is the ideal outcome variable for ART (Maheshwari et al., 2016). Previous authors have reported live birth as a secondary outcome measure in smaller and more heterogeneous studies (Harb et al., 2013). Since we only included women who had laparoscopy to exclude endometriosis in the unexplained subfertility group, our study overcomes the diagnostic limitation of other trials, which included heterogeneous comparator groups. Our study reported on treatment outcomes only for the first cycle of IVF treatment to limit repeat observations that would introduce bias. We acknowledge that it would have been ideal to assess the cumulative live birth per cycle started since this would be more clinically relevant to current practice as cryopreservation protocols have significantly improved over the years. However, due to Health Authority funding policy variations in the UK, this was not possible to ascertain in this study because not all patients were in a position to utilize cryopreservation of embryos.
The study demonstrates that endometriosis impacts negatively on the chance of a live birth in a ‘dose-response relationship’ as the effect is more pronounced with increasing severity of disease based on the r-ASRM staging system (ASRM, 1997). It appears that endometriosis affects all aspects of IVF outcomes including folliculogenesis, embryo development and implantation. It is postulated that impaired folliculogenesis results from an abnormal intra-ovarian cytokine milieu that may also cause perturbations in endometrial decidualisation (Garrido et al., 2000; Sallam et al., 2006). In this study, we demonstrate the association between endometriosis and reduced follicular recruitment, decreased likelihood of blastocyst ET and impaired implantation. These effects of endometriosis are therefore not only restricted to ovarian function but include endometrial function. The distribution of various disease stages of endometriosis was well balanced in the cohort, negating any bias for selecting cases with more severe disease and skewing the observations. However, it appears the impact of endometriosis becomes more apparent with increasing disease severity. The exact mechanism behind this effect is unknown, however, it has been observed that donor oocytes from women with endometriosis have a lower developmental potential and achieve lower pregnancy rates when given to women without endometriosis, an effect which cannot be explained entirely by a difference in ovarian reserve (Garrido et al., 2002). Such oocytes have also been reported to have abnormal cytoskeletal and molecular characteristics (Da Broi et al., 2014). It has been shown both in human and in animal models that oocytes exposed to endometriosis had reduced potential to form blastocyst embryos and demonstrated a higher rate of blastomere apoptosis and developmental arrest (Da Broi et al., 2014; Xu et al., 2015). Several mechanisms are thought to underlie these observations including abnormal nitric oxide activation, formation of reactive oxygen species and lipid peroxidation, and an abnormal cytokine milieu (Goud et al., 2014; Dong et al., 2013;Stilley et al., 2012). It is also possible that the presence of endometriosis may have epigenetic effects on the oocyte resulting in alterations in the expression of genes responsible for aromatase synthesis in cumulus cells, leading to reduced intrafollicular oestradiol production (Baumann et al., 2015; Hosseini et al., 2016). This cumulus–oocyte cross talk is essential for cytoplasmic and nuclear maturation of the oocyte to render it competent.
Reduced implantation in women with endometriosis compared to those with unexplained subfertility points towards altered endometrial function. This observations were also made by previous authors and seem to be more pronounced in women conceiving by ART (Omland et al., 2005). Basic science studies demonstrate abnormal inflammation-mediated oestradiol production and progesterone resistance, rendering the endometrium less receptive in endometriosis (Bulun et al., 2010). Whereas other studies demonstrated increased risk of miscarriage in women with endometriosis, we were unable to show this effect (Santulli et al., 2016). Rather surprisingly, women with endometriosis had reduced odds of miscarriage compared to those with unexplained subfertility [OR 0.62 (95% CI, 0.43–0.89), P = 0.009]. It may well be that women with unexplained subfertility were older than those with endometriosis, this being a known risk factor for miscarriage. This finding should be interpreted with caution as the study was not powered for this outcome.
The global effects of endometriosis at both ovarian and endometrial levels have been shown to be mitigated by long-term suppression of pituitary function with GnRH analogs before IVF treatment to improve outcomes (Sallam et al., 2006). Whereas this is a promising development, more focussed research is urgently needed, particularly to elucidate the mechanisms underlying endometrial dysfunction in women with endometriosis. Implantation failure does not appear to be overcome by double ET and certainly the risk of multiple births cannot be overlooked. Other ways of improving embryo selection, such as use of morphokinetic parameters by time-lapse technology, need to be urgently evaluated in similar studies in the future. (Bhide et al., 2017).
Conclusion
We have demonstrated that endometriosis reduces the chance of a live birth in women undergoing ART, an effect that is more pronounced with increasing disease severity. The effects of endometriosis are associated with both reduced oocyte quality and embryo developmental potential, as well as reduced implantation. Whereas ART has proved effective in overcoming subfertility in many couples, research is urgently needed in techniques that could further improve treatment outcomes in women with endometriosis.
Acknowledgements
The authors wish to acknowledge Ginny Mounce, PhD for kindly proof-reading the manuscript and offering valuable critique.
Authors’ roles
C.M., E.O., T.C. and C.B. participated in study design, execution, analysis, manuscript drafting and critical discussion.
Funding
None.
Conflict of interest
The authors have no conflict of interest to declare.
References
- ASRM Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril 2002;77:1148–1155. [DOI] [PubMed] [Google Scholar]
- Baumann C, Olson M, Wang K, Fazleabas A, De La Fuente R. Arginine methyltransferases mediate an epigenetic ovarian response to endometriosis. Reproduction 2015;150:297–310. [DOI] [PubMed] [Google Scholar]
- Bhide P, Maheshwari A, Cutting R, Seenan S, Patel A, Khan K, Homburg R. Time lapse imaging: is it time to incorporate this technology into routine clinical practice? Hum Fertil 2017;20:74–79. [DOI] [PubMed] [Google Scholar]
- Bulun S, Cheng Y, Pavone M, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med 2010;28:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A. Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb) 2008;11:131–146. [DOI] [PubMed] [Google Scholar]
- Da Broi MG, Malvezzi H, Paz CCP, Ferriani RA, Navarro P. Follicular fluid from infertile women with mild endometriosis may compromise the meiotic spindles of bovine metaphase II oocytes. Hum Reprod 2014;29:315–323. [DOI] [PubMed] [Google Scholar]
- de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet 2010;376:730–738. [DOI] [PubMed] [Google Scholar]
- Dong XY, Liao XH, Wang R, Zhang HW. The impact of endometriosis on IVF/ICSI outcomes. Int J Clin Exp Pathol 2013;6:1911–1918. [PMC free article] [PubMed] [Google Scholar]
- Dunselman G, Vermeulen N, Nelen W. The 2013 ESHRE guideline on the management of women with endometriosis. Hum Reprod 2013;28:86. [DOI] [PubMed] [Google Scholar]
- Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, Simon C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update 2002;8:95–103. [DOI] [PubMed] [Google Scholar]
- Garrido N, Navarro J, Remohi J, Simon C, Pellicer A. Follicular hormonal environment and embryo quality in women with endometriosis. Hum Reprod Update 2000;6:67–74. [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Joshi N, Puscheck E, Diamond MP, Abu-Soud HM. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil Steril 2014;102:151–159.e5. [DOI] [PubMed] [Google Scholar]
- Harb HM, Gallos ID, Chu J, Harb M, Coomarasamy A. The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta-analysis. BJOG 2013;120:1308–1320. [DOI] [PubMed] [Google Scholar]
- Hosseini E, Mehraein F, Shahhoseini M, Karimian L, Nikmard F, Ashrafi M, Afsharian P, Aflatoonian R. Epigenetic alterations of CYP19A1 gene in Cumulus cells and its relevance to infertility in endometriosis. J Assist Reprod Genet 2016;33:1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J, Horne G, Fitzgerald C. Who needs ICSI? A nationwide UK survey on ICSI use. Hum Fertil 2012;15:144–149. [DOI] [PubMed] [Google Scholar]
- Macer M, Taylor H. Endometriosis and infertility a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am 2012;39:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Hamilton M, Bhattacharya S. Should we be promoting embryo transfer at blastocyst stage? Reprod Biomed Online 2016;32:142–146. [DOI] [PubMed] [Google Scholar]
- Missmer S, Hankinson S, Spiegelman D, Barbieri R, Marshall L, Hunter D. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 2004;160:784–796. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Women’s and Children’s H. National Institute for Health and Clinical Excellence: Guidance Antenatal Care: Routine Care for the Healthy Pregnant Woman. London: RCOG Press, 2008. [PubMed] [Google Scholar]
- Omland A, Abyholm T, Fedorcsak P, Ertzeid G, Oldereid N, Bjercke S, Tanbo T. Pregnancy outcome after IVF and ICSI in unexplained, endometriosis-associated and tubal factor infertility. Hum Reprod 2005;20:722–727. [DOI] [PubMed] [Google Scholar]
- Sallam H, Garcia-Velasco J, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev 2006:CD004635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli P, Marcellin L, Menard S, Thubert T, Khoshnood B, Gayet V, Goffinet F, Ancel P, Chapron C. Increased rate of spontaneous miscarriages in endometriosis-affected women. Hum Reprod 2016;31:1014–1023. [DOI] [PubMed] [Google Scholar]
- Senapati S, Sammel M, Morse C, Barnhart K. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil Steril 2016;106:164–171.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilley JAW, Birt JA, Sharpe-Timms KL. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res 2012;349:849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton A, Storey L, McVeigh E, Child T. IVF outcome in women with PCOS, PCO and normal ovarian morphology. Eur J Obstet Gynecol Reprod Biol 2010;149:68–71. [DOI] [PubMed] [Google Scholar]
- Van Voorhis B. In vitro fertilization. N Engl J Med 2007;356:379–386. [DOI] [PubMed] [Google Scholar]
- Xu B, Guo N, Zhang XM, Shi W, Tong XH, Iqbal F, Liu YS. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci Rep 2015;5:10779. [DOI] [PMC free article] [PubMed] [Google Scholar]