Abstract
STUDY QUESTION
What is the evidence pertaining to availability, effectiveness and safety of ART in sub-Saharan Africa?
SUMMARY ANSWER
According to overall limited and heterogeneous evidence, availability and utilization of ART are very low, clinical pregnancy rates largely compare to other regions but are accompanied by high multiple pregnancy rates, and in the near absence of data on deliveries and live births the true degree of effectiveness and safety remains to be established.
WHAT IS KNOWN ALREADY
In most world regions, availability, utilization and outcomes of ART are monitored and reported by national and regional ART registries. In sub-Saharan Africa there is only one national and no regional registry to date, raising the question what other evidence exists documenting the status of ART in this region.
STUDY DESIGN, SIZE, DURATION
A systematic review was conducted searching Pubmed, Scopus, Africawide, Web Of Science and CINAHL databases from January 2000 to June 2017. A total of 29 studies were included in the review. The extracted data were not suitable for meta-analysis.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The review was conducted according to Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines. All peer-reviewed manuscripts irrespective of language or study design that presented original data pertaining to availability, effectiveness and safety of ART in sub-Saharan Africa were eligible for inclusion. Selection criteria were specified prior to the search. Two authors independently reviewed studies for possible inclusion and critically appraised selected manuscripts. Data were analysed descriptively, being unsuitable for statistical analysis.
MAIN RESULTS AND THE ROLE OF CHANCE
The search yielded 810 references of which 29 were included based on the predefined selection and eligibility criteria. Extracted data came from 23 single centre observational studies, two global ART reports, two reviews, one national data registry and one community-based study. ART services were available in 10 countries and delivered by 80 centres in six of these. Data pertaining to number of procedures existed from three countries totalling 4619 fresh non-donor aspirations in 2010. The most prominent barrier to access was cost. Clinical pregnancy rates ranged between 21.2% and 43.9% per embryo transfer but information on deliveries and live births were lacking, seriously limiting evaluation of ART effectiveness. When documented, the rate of multiple pregnancy was high with information on outcomes similarly lacking.
LIMITATIONS, REASONS FOR CAUTION
The findings in this review are based on limited data from a limited number of countries, and are derived from heterogeneous studies, both in terms of study design and quality, many of which include small sample sizes. Although representing best available evidence, this requires careful interpretation regarding the degree of representativeness of the current status of ART in sub-Saharan Africa.
WIDER IMPLICATIONS OF THE FINDINGS
The true extent and outcome of ART in sub-Saharan Africa could not be reliably documented as the relevant information was not available. Current efforts are underway to establish a regional ART data registry in order to report and monitor availability, effectiveness and safety of ART thus contributing to evidence-based practice and possible development strategies.
STUDY FUNDING/COMPETING INTERESTS
No funding was received for this study. The authors had no competing interests.
TRIAL REGISTRATION NUMBER
PROSPERO CRD42016032336
Keywords: ART, availability, IVF/ICSI outcome, infertility, sub-Saharan Africa, registry
Introduction
Infertility is a disease that affects the reproductive health of many millions of women and men worldwide and erodes their human right to have a family. A review of current regulatory arrangements for ART in European countries stated that to combat this pandemic required both prevention and ART (Gianaroli et al., 2016). The importance of prevention is arguably nowhere greater than in sub-Saharan Africa where preventable causes of infertility are common and resources for interventions are limited. Despite limited resources, the need for ART is equally undisputable. Firstly, ART is one of the most effective infertility interventions and the only one that can overcome severe tubal and male factor infertility. In addition, access to and availability of ART has been shown to reduce the social stigma of infertility which is highly prevalent in Africa (Inhorn and Patrizio, 2015).
ART export to Africa started in the early 1980s and continues to expand both within and between countries. Although the extent of this expansion is poorly documented, it seems to fall well short of the need for ART according to a recent review of global infertility and reproductive technologies (Inhorn and Patrizio, 2015). ART export to Africa has also not been accompanied by the development of data registries, which monitor and report utilization and outcomes in most world regions. In the absence of systematically collected data on availability, effectiveness and safety it is in turn difficult to ascertain the scope, practice and success of ART in sub-Saharan Africa.
To narrow this information gap, or alternatively to document its size, we conducted a systematic review on ART in sub-Saharan Africa. We searched for peer-reviewed papers that satisfied the following PICOS requirements: women and/or infertile women/couples accessing gynaecological or specialized infertility services, or health care providers who practiced or referred patients for ART (Participants); ART (Intervention); no or any comparison with a reference or control group (Comparisons); any information on availability, effectiveness and safety (Outcomes); and any qualitative or quantitative study design that presented or included original data (Study design). With the exception of ART, these criteria were purposefully broad in order to maximize data capture.
Materials and Methods
Following protocol registration with PROSPERO (Registration number CRD42016032336), a systematic review was conducted according to Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines. No approval from the Institutional Research Ethics Committee was sought as the study did not involve contact with patients or their records.
Selection criteria
Selection criteria were established prior to the search. Articles that satisfied the above PICOS criteria were included using the following indicators for outcomes:
- Availability: expressed quantitatively as number of ART centres or cycles in a country or region; or qualitatively describing the presence of ART services, settings (public, academic or private), their accessibility, referral pathways and funding.
- Effectiveness: expressed quantitatively as singleton live birth delivery rates or pregnancy, delivery or birth rates per cycle initiated, aspiration, embryo transfer or woman; or qualitatively describing ART success.
- Safety: expressed quantitatively as rates of multiple pregnancies, deliveries or births; stimulation and procedure-related complications, and maternal/neonatal complications; or qualitatively describing safety, risks and risk management of ART.
Where applicable, the definition of outcome measures was based on the International Glossary of Infertility and Fertility Care (Zegers-Hochschild et al., 2017).
Search strategy
We searched Pubmed, Scopus, AfricaWide, Web of Science and CINAHL databases with the assistance of a trained medical librarian. The preselected MeSH and keywords words were: sub-Saharan Africa, including MeSH terms for specific countries and regions; outcome plus keywords for clinical efficacy, treatment efficacy, availability and available, access, accessibility, treatment outcomes; as well as Reproductive Techniques, Assisted, Fertilization in Vitro, Assisted Reproductive Technique, assisted reproductive techniques, assisted reproductive technology, embryo transfer, single embryo transfer, intracytoplasmic sperm injections, in vitro oocyte maturation techniques, assisted reproductive technologies, fertilization in vitro, gamete intrafallopian transfer. All titles and abstracts identified by the search and published between 2000 and June 2017 were screened and relevant papers retrieved in full text. These were scrutinized for additional keywords and index terms which were added to the search strategy. The reference list of all included papers was evaluated for further publications. Lastly, articles that cited manuscripts selected for the review were screened for possible inclusion.
Study eligibility
All peer reviewed papers regardless of language and study design that provided original data on the predefined selection criteria as well as systematic reviews that included such data were eligible for inclusion. Publications prior to 2000 were excluded in order to capture relatively recent information. We also excluded laboratory-based studies that provided intermediary measures of effectiveness such as fertilization rates without stating pregnancy rates; non-systematic reviews because of the risk of selection bias after searching their references for eligible studies; and opinion-based papers, reports without original data, theses, and other ‘grey literature’. Study selection was conducted independently by two reviewers (BB and SD) with disagreement resolved by consensus. No attempt was made to contact authors for additional information.
Data extraction
A standardized critical appraisal and data extraction tool was generated for each of the different study designs (observational studies, systematic reviews, RCTs) using criteria from CASP (Critical Appraisal Skills Programme) and statements from CONSORT (Consolidated Standards of Reporting Trials), PRISMA, STARD (Standards for Reporting Diagnostic accuracy studies), MOOSE (Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies) and STROBE (Strengthening The Reporting of Observational Studies in Epidemiology) as appropriate. Two reviewers independently appraised the articles and extracted data (BB and SD; as well as BB and DS for papers authored by SD). Results were compared and differences resolved by consensus.
Risk of bias
To reduce selection bias, the abstracts and full text papers were evaluated by masking the authors as far as possible and by basing decisions regarding relevance and eligibility on the independent appraisal by two reviewers. Bias in studies included was assessed independently by the two reviewers through use of the predesigned critical appraisal tool.
Data analysis
The included articles contained highly diverse settings and findings which did not allow for combination or comparison by statistical means and were amenable to descriptive analysis only. Studies were grouped by outcome measures differentiating between quantitative and qualitative information. Some studies provided quantitative results related to effectiveness and safety of ART that were generated according to different study criteria to the ones set out in this review while at the same time providing sufficient information to re-calculate outcomes in keeping with those applied here. These results are marked with a footnote in the relevant tables.
Results
Study selection
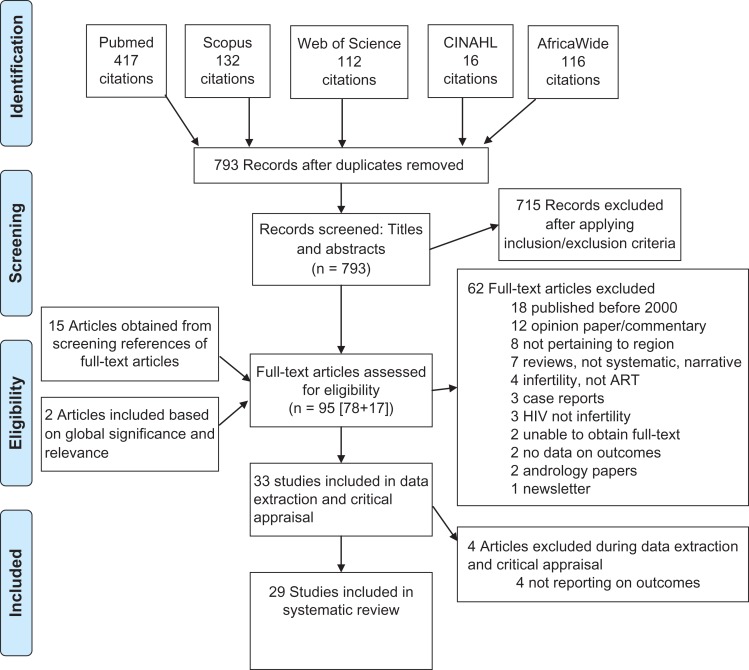
The initial search revealed 793 non-duplicate citations. All abstracts were screened and 715 were excluded. Seventeen additional papers were included: 15 from reference search and two manuscripts not meeting the search criteria but based on their global significance and relevance to the review. The citation search yielded no additional entries. Ninety-five full text manuscripts were reviewed of which 62 were excluded with reason (Fig. 1). Four papers were subsequently withdrawn from the analysis since upon close scrutiny no relevant information could be extracted. This left 29 manuscripts for final inclusion. The initial search yielded publications in English and French only, and all included manuscripts were in English.
Figure 1.
Flow chart of the systematic review of ART in sub-Saharan Africa.
Characteristics of included studies
Tables I and II provide an overview of the charactersitics of included studies. Seventeen studies originated from Nigeria, eight from South Africa, two from Kenya, and one each from Rwanda and the Netherlands (review). The two global surveys had no country of origin. There were 21 cohort studies of which four were propsective, seven retrospective, eight cross sectional, and two case–control. The remainder study designs included RCT (n = 1), systematic review (n = 1), registry data (n = 2), survey (n = 2), case report (n = 1), and letter to the editor (n = 1). In 24 manuscripts, ART was the primary focus. The remaining five papers addressed infertility more generally with ART included (Adeyemi et al., 2009; Gerrits and Shaw, 2010; Dhont et al., 2011; Adegbola and Akindele, 2013; Menuba et al., 2014). Information on the specified outcomes of availability, effectiveness and safety was provided by 21, 15 and 20 manuscripts, respectively (Table I).
Table I.
Summary of peer-reveiwed papers published from January 2000 to June 2017 included in the review of ART in sub-Saharan Africa: study design, setting, size and outcomes reported.
| Region/country | References | Study design and quality | Setting | Study population/Sample size | Outcomes reported | ||
|---|---|---|---|---|---|---|---|
| Availability | Effectiveness | Safety | |||||
| Global | Dyer et al. (2016) | Registry report (H) | NA | Data from Cameroon, Mali, South Africa | x | x | x |
| Ory et al. (2016) | Survey (H) | Na | Data from Cameroon, Kenya, Mali, Nigeria, Senegal, South Africa | x | x | ||
| Sub-Saharan Africa | Gerrits and Shaw (2010) | Systematic reviewa (L) | NA | 68 publications | x | x | x |
| Giwa-Osagie (2002) | Survey (L) | NA | ART practitioners from 10 countries. | x | x | x | |
| Kenya | Murage et al. (2011) | Cross-sectional (M) | NA | 188 Obstetricians and Gynaecologists | x | x | |
| Noreh et al. (2009) | Retrospective Cohort (L) | Private ART centre | 362 initiated fresh IVF cycles | x | x | x | |
| 430 fresh and frozen ET | |||||||
| Nigeria | Adegbola and Akindele (2013) | Retrospective Cohort (L) | University hospital | 2724 women attending GOC including 730 infertile couples | x | ||
| Adenike et al. (2014) | Cross-sectional (H) | University hospitals (public) | 250 women attending GOC | x | |||
| Adesiyun (2011) | Letter to Editor (L) | University hospital | 23 couples in 8 years | x | x | x | |
| Adeyemi et al. (2009) | Retrospective Cohort (L) | University hospital | 208 women attending GOC | x | |||
| Ajayi and Dibosa-Osadolor (2013) | Cross-sectional (M) | Conference | 102 obstetricians and gynaecologists attending academic meeting | x | x | ||
| Ezechi et al. (2008) | Case–control (H) | Private hospital | 52 ART pregnancies in 48 women | x | x | x | |
| 2160 pregnancies in non-infertile women | |||||||
| Jegede and Fayemiwo (2010) | Cross-sectional (M) | Community setting | 23 informants from diverse background incl two health care professionals | x | x | ||
| Melie (2003) | Cross-sectional (M) | Private ART centre | 452 aspirations with ≥ one oocyte | x | x | ||
| 421 fresh non-donor ET | |||||||
| Menuba et al. (2014) | Prospective Cohort (M) | University hospital | 1983 women attending GOC including 218 infertile couples | x | |||
| Okohue et al. (2009) | Prospective Cohort (M) | Private ART centres (n = 2) | 298 women < 36 y | x | x | x | |
| 276 fresh non-donor ET | |||||||
| Okohue (2010a) | Case report (M) | Private ART centre | 5 women with ectopic pregnancies after ART | x | |||
| Okohue (2010b) | Cross-sectional (M) | Private ART centre | 54 women undergoing ART | x | x | ||
| Okohue et al. (2013) | Retrospective Cohort (M) | Private ART centre | 72 women < 36 y | x | x | ||
| 76 embryo transfers | |||||||
| Okwelogu et al. (2012) | Cross-sectional (M) | Public hospitals | 500 women attending infertility clinics | x | x | ||
| Oloyede et al. (2012) | Retrospective cohort (H) | Private ART centre | 55 intrauterine ART pregnancies | x | x | ||
| Orhue et al. (2012) | Retrospective Cohort (M) | University hospital | 600 fresh non-donor ART cycles | x | x | x | |
| 504 fresh embryo transfers | |||||||
| Rwanda | Dhont et al. (2011) | Prospective Cohort (H) | University hospital (public) | 312 Infertile women and partners attending infertility clinicb | x | ||
| South Africa | Abels (2007) | Randomized controlled trial (M) | University hospital (public) | 85 women | x | x | |
| 45 GIFT cycles with ≥ three oocytes, 44 IUI cycles | |||||||
| Bosman et al. (2015) | Case-control (L) | Private ART centre | 82 couples with female partners < 38 y and ≥5 oocytes/aspiration | x | x | ||
| 82 ETs after IVF | |||||||
| Dyer and Kruger (2012) | Registry report (M) | Private and public-academic ART Centres | 12 ART centres | x | x | x | |
| 4512 aspirations | |||||||
| 3872 embryo transfers | |||||||
| Dyer et al. (2013) | Cross-sectional (H) | University hospital (public) | 135 couples | x | |||
| 135 initiated cycles | |||||||
| Raja and Franken (2006) | Prospective Cohort (H) | Private ART centre | 39 couples requiring ICSI. No. cycles not specified. | x | |||
| Windt et al. (2002) | Retrospective Cohort (M) | Not specified | 142 aspiration cycles with ≥ one oocytes; fertilisation by ICSI with testicular extracted sperm | x | |||
ET, embryo transfer; GIFT, gamete intrafallopian transfer; GOC, gynaecology outpatient clinic; H, high quality; low quality; M, medium quality; No., number.
aNot adherent to PRISMA guidelines.
bTemporary infertility clinic in a research setting.
Table II.
Summary of peer-reveiwed papers published January 2000 to June 2017 included in the review: study characteristics and outcomes.
| Region/ country | References | Characteristics | Outcomes |
|---|---|---|---|
| Global | Dyer et al. (2016) | Retrospective summary data of ART utilization, effectiveness and safety stratefied by countries and world regions. | In 2010, mean ART utilization in sub-Saharan Africa was 87 cycles/million population/annum; mean fresh non-donor CPR/asp was 31.3%; near absence of data pertaining to deliveries, births and multiple pregnancies. |
| Ory et al. (2016) | Global data on ART availability, regulation and practice stratefied by countries. | Limited number of ART centres; ART largely unregulated; near absence of ART reimbursement policies/state funding. | |
| Sub-Saharan Africa | Gerrits and Shaw (2010) | Systematic reviewa evaluating infertility services with focus on counselling, male involvement and ART availability/acceptability. | Weak infertility services in public sector. ART acceptable but mostly located in private sector and unavailable/unaffordable for most people. Limited male partner involvement. Overall lack of data acknowledged. |
| Giwa-Osagie (2002) | Survey complemented by published scientific data and information from site visits and media. | ART services in six countries, largely unregulated, sustainable only in the private sector. Collaborations with non-African centres. Fresh non-donor PR/ET 21%, MPR 14.3–36% (data from three centres). | |
| Kenya | Murage et al. (2011) | Online survey to determine frequency of consultations for infertility and access to ART. | Response rate 25.0% (n = 47). Infertility accounted for 1:4 of gynaecological consultations. 50.3% due to tubal factor, 14.8% due to male factor. Three ART centres. Access severely limited due to cost and treatment capacity. |
| Noreh et al. (2009) | Case note review to determine outcomes of ART. | Cumulative CPR/initiated cycle 27.3%b,a. MPR 23.2%b. Mean of three embryos transferred. | |
| Nigeria | Adegbola and Akindele (2013) | Retrospective evaluation of 264 couples followed up for 24–48/12, of which 90 were fully investigated, including characteristics, diagnosis and occurrence of pregnancy. | Infertility prevalence 26.8%. Female factor 37.8%, male factor 11.1%, combined factor 40.0%, unexplained 11.1%. Two couples referred for ART with outcome not specified. Non-ART treatment not further specified. PR/couple 4.9%, spontaneous versus treatment-related pregnancies not specified. |
| Adenike et al. (2014) | Administered questionnaires to determine infertility prevalence, and knowledge and acceptability of ART. | 40.8% of women presented with infertility, 46.0% aware of ART of whom 73.5% would accept ART if required. Higher educational status, being married, and longer duration of infertility predictors of ART awareness and acceptability. | |
| Adesiyun (2011) | Description of patient characteristics and treatment outcome in couples. | Indication for ART female (n = 7), male (n = 5) and combined factor (n = 11). Nine couples underwent ART with nine pregnancies in seven couples resulting in 13 live births. | |
| Adeyemi et al. (2009) | Case note review to determine pattern of gynaecological consultations, investigations and treatment. | 48.1% of women presented with infertility; 60.1% did not receive definite treatment. Infertility associated with higher odds for no treatment versus other conditions (OR 6.0; 2.99–12.05). ART only available in private sector at high cost. | |
| Ajayi and Dibosa-Osadolor (2013) | Questionnaire survey to determine opinions re ethical issues in ART. | Majority of respondents considered ART services necessary (99%), supported egg and sperm donation (84.3% and 80.4%) as well as surrogacy (82.4%), favoured transfer of > 3 embryos (78.4%) and opposed SET (71.6%) and ART in same sex couples (87.3%) and single women (53.9%). | |
| Ezechi et al. (2008) | Case note review of pregnancy and neonatal outcome in ART and non-ART pregnancies. | More adverse outcomes in ART pregnancies vs controls (30.8% vs 12.6%) including MPRs (17.3% vs 4.3%), preterm delivery (23.1% vs 4.8%) and low birthweight (13.5% vs 2.5%). | |
| Jegede and Fayemiwo (2010) | In-depth interviews to elicit cultural and ethical challenges of ART. | Main barriers to ART lack of female decision-making power, perceived risk (foetal abnormality, lack of social acceptance), religious reasons and cost of treatment. | |
| Melie (2003) | Retrospective review of ART outcome stratified by number of oocytes retrieved. | Four groups by number of oocytes after conventional ovarian stimulation (1–5; 6–10; 11–15; >15). Significantly lower PRs with <6 oocytes, no difference between other groups. CPR/asp 19.7%b,c and CPR/ET 21.1%b,c. | |
| Menuba et al. (2014) | Prospective evaluation of infertile couples including characteristics, investigations, recommended treatment, and outcomes following non-ART interventions. | Infertility prevalence 12.1%. Female factor 32.1%, male factor 26.1%, combined factor 29.4%, unexplained 12.4%. CPR/couple after non-ART treatment 12.8%b. 47 couples (21.6%) required ART but unable to access. | |
| Okohue et al. (2009) | Prospective evaluation of ART outcome stratified by endometrial thickness. | Significantly higher CPR in women with endometrial thickness 7–14 mm compared to <7 mm or >14 mm. Centres conducted ±75 cycles/month. | |
| Okohue (2010a) | Case studies. | 64 clinical pregnancies after ART over 3 yrs. Of these, presentation and outcome of five ectopic pregnancies reported. | |
| Okohue (2010b) | Questionnaire-based interviews to determine preference for number of embryos transferred. | After patient education on risk of multiple pregnancies, the number of women choosing transfer of 1, 2 and 3 embryos was 3(5.6%), 36 (66.6%) and 15 (27.8%). 31 women desired twins mostly due to ART cost. | |
| Okohue et al. (2013) | Case note review to determine outcome of fresh IVF in women with PCOS and tubal factor infertility. | Lower fertilization rates, higher risk of OHSS, but comparable CPR and miscarriage rates in women with PCOS (n = 30) vs tubal infertility (n = 42). | |
| Okwelogu et al. (2012) | Questionnaire-based interviews to determine awareness and perceptions of ART. | 40.4% of participants had tertiary and 52.8% secondary education. 312 women (62.4%) had not heard of ART. Of the other 188 women, 118 (62.8%) would reject ART because babies perceived to be abnormal (n = 94) or treatment cost (n = 15). Of those in favour of ART (n = 70), 64 women desired multiple pregnancies. | |
| Oloyede et al. (2012) | Case note review to determine frequency of spontaneous foetal reduction in multiple pregnancies. | MPR 34.5% (19/55) with 11 twin, six triplet and two quadruplet pregnancies. Spontaneous reduction in 47.7% of multiples. Average of three embryos transferred. | |
| Orhue et al. (2012) | Case note review to determine outcomes of ART. | CPR 30.0%, MPR 20.0%b. Up to three embryos/transfer. Public ART feasible with WHO grant. Cost for good responders 3000USD/cycle (60–75% of cost in private). | |
| Rwanda | Dhont et al. (2011) | Prospective evaluation of 244 fully investigated couples including characteristics, diagnosis, recommended treatment, and occurrence of pregnancy (spontaneous and treatment related). | Infertility due to combined factors 50%, female factor 31%, male factor 16%, unexplained 3%. After 12–18 months, 40 pregnancies including 17 after hysterosalpingography and 9 after non-ART treatment (CPR/couple 16.4%) and 1 after ART (crossborder care; CPR/couple 0.4%). |
| South Africa | Abels (2007) | RCT comparing PRs after GIFT vs IUI with ovarian stimulation in couples with unexplained infertility. | Higher ongoing PR after GIFT. Three oocytes per GIFT. Mean number of cycles per pregnancy 7.3 (IUI) vs 2.0 (GIFT). |
| Bosman et al. (2015) | Propsective observational study comparing ART outcome in couples with hyperinsulinaemic and normoinsulinaemic men (all normozoospermic). | Higher CPR/ET in normoinsulinaemic versus hyperinsulinaemic group (57.9% vs 31.8%). | |
| Dyer and Kruger (2012) | Registry data. | 4512 aspirations for IVF,ICSI and OD. CPR/asp 28.9%; 12.8% SETsb and 54.5% DETsb. No data on FET, MPR, deliveries or births. | |
| Dyer et al. (2013) | Questionnaire-based interviews to determine extent and impact of out-of-pocket payment for 1 ART cycle in a government institution. | Out-of-pockey payment caused catastrophic expenditure for 22% of all households and 51% of the poorest. Couples activated multiple financial coping strategies. Mean annual CPR/ET ± 31%. | |
| Raja and Franken (2006) | Prospective evaluation of sperm morphometrics and paternal sex chromosomes on ICSI outcome. | Sperm selection using morphometrics associated with good fertilsation and PRs. Of 16 randomly selected embryos with no development 68.7% were XY. | |
| Windt et al. (2002) | Retrospective evaulation of use of fresh, cryopreserved and/or pre-incubated sperm on fertilisation and PRs after ICSI/TESE. | Similar fertilisation and PRs in couples with obstructive vs non-obstructive azoospermia. Preincubated sperm for associated with similar ongoing PRs vs non-preincubation. |
Asp, aspiration; CRP, clinical pregnancy rate; DET, double embryo transfer; FET, frozen embryo transfer; MPR, multiple pregnancy rate; No., number; OD, oocyte donation; OHSS, ovarian hyperstimualtion syndrome; PCOS, polycystic ovary syndrome; PR, pregnancy rate; SET, single embryo transfer; TESE, testicular sperm extraction; USD, United States Dollar; WHO, world health organization.
aNot adherent to PRISMA guidelines.
bNot presented as part of original study results but calculated from original study data for purpose of this review.
cClinical pregnancy defined as intrauterine pregnancy with foetal heart.
dTemporary infertility clinic in a research setting.
The World Report of the International Committee Monitoring ART (ICMART) presented global registry data pertaining to ART utilization and outcomes in 2008, 2009 and 2010 (Dyer et al., 2016). Only data from the most recent year (2010) were included in this review. The paper presenting registry data from South Africa reported summary data on a few outcome indicators for 2009 which were submitted voluntarily by 12 of 18 ART centres (Dyer and Kruger, 2012). The Surveillance of the International Federation of Fertility Societies (IFFS) provided global survey data on ART availability and safety as well as extensive information on ART practices (Ory et al., 2016). The survey by Giwa-Osagie (2002) comprised original information through personal enquiry together with scientific studies and grey literature on ART in sub-Saharan Africa but excluding South Africa. The systematic review by Gerrits and Shaw (2010) comprised peer-reviewed English language publications idenitifed via Pubmed, included years not being stipulated, on psycho-social or cultural information pertaining to infertility or infertility care in sub-Saharan Africa.
Risk of bias and study quality
All included manuscripts were evaluated vis-à-vis the criteria specified in the critical appraisal tool. Specifically, a percentage score of number of criteria satisfied in the data extraction tool was generated. Manuscripts were rated as medium quality if the score was 50–65% (n = 14) or as high (>65%; n = 7) and low quality (<50%; n = 8) as agreed by the reviewers.
Availability
Number of countries, centres and cycles
Quantitative information pertaining to ART availability and utilization (e.g. annual number of procedures) is summarized in Table III. The number of ART cycles included in the studies, where applicable, is captured in Table I. According to qualitative data, the number of private ART clinics was slowly increasing in some countries, but these facilities were largely unavailable to rural communities (Giwa-Osagie, 2002; Gerrits and Shaw, 2010; Okwelogu et al., 2012).
Table III.
Summary of ART availability and utilization in sub-Saharan Africa.
| Year of referencea | References | No. countries | No. centres | Annual no. aspirations (fresh IVF and ICSI) |
|---|---|---|---|---|
| 2016 | Ory et al. (2016) | 6 | Cameroon 2, Kenya 5, Mali 1, Nigeria 50, Senegal 2, South Africa 20 | NA |
| 2011 | Murage et al. (2011) | NA | Kenya 3 | NA |
| 2010 | Dyer et al. (2016) | 3 | Cameroon 2, Mali 1, South Africa 20 | Cameroon 96; Mali 171; South Africa 4352 |
| 2009 | Dyer and Kruger (2012) | NA | South Africa 18 | 4512 incl OD |
| 2008 | Ezechi et al. (2008) | NA | Nigeria 9 | NA |
| 2002 | Giwa-Osagie (2002) | 7 | Cameroon (NA), Ghana (NA), Nigeria (NA), Senegal (NA), South Africa (NA), Togo (NA), Zimbabwe (NA) | NA |
NA, not assessed; OD, oocyte donation.
aThe year to which reported availability pertains.
Public-sector facilities
Availability of ART in public sector facilities was scant. In the six countries that participated in the IFFS Surveillance, only seven of 80 ART facilities were outside the private health sector: six in university hospitals (public or private) and one in a public hospital (Ory et al., 2016). A review on biomedical infertility care in sub-Saharan Africa concluded that whilst some sort of infertility care was offered in public health care systems, it was often unco-ordinated and rarely included ART (Gerrits and Shaw, 2010). Similarly, three papers described that public-sector care in Nigeria and Kenya had some capacity for infertility investigations but no or few options for treatment (Giwa-Osagie, 2002; Murage et al., 2011; Menuba et al., 2014). Discrepant access to treatment was documented in a retrospective review conducted at a gynaecological outpatient clinic in Nigeria (Adeyemi et al., 2009). Although infertility was the commonest diagnosis (48.1%), it was significantly less likely to result in treatment compared to other gynaecological conditions.
Barriers
Cost was the most frequently cited barrier (Gerrits and Shaw, 2010; Jegede and Fayemiwo, 2010; Okohue et al., 2010b; Adesiyun, 2011; Murage et al., 2011; Dyer and Kruger, 2012; Orhue et al., 2012; Adegbola and Akindele, 2013; Dyer et al., 2013; Adenike et al., 2014; Menuba et al., 2014). Only Kenya reported the presence of regulations for ART reimbursement in the IFFS surveillance (Ory et al., 2016). Describing a grant-based introduction of ART in a public-academic hospital in Nigeria, Orhue et al. (2012) documented a cost of 3000USD for good responders, which prevented many couples from undergoing ART or undergoing more than 1 cycle. Higher costs for one private ART cycle were described by the authors: 4000–5000USD in Nigeria and Mali, and 3500USD in Uganda. The cost of one cycle in Ghana was reported as equivalent to a nurse’s salary over 18 months (Gerrits and Shaw, 2010). The large discrepancy between ART cost and average household income was also discussed qualitatively (Giwa-Osagie, 2002; Gerrits and Shaw, 2010; Okohue et al., 2010b). The concern was expressed that even a dramatic cost reduction would still keep ART out of reach for most people, especially in countries where more than 70% of the population lived below the poverty line (Giwa-Osagie, 2002).
One study from South Africa explored out-of-pocket co-payment among 135 couples undergoing one ART cycle with conventional ovarian stimulation at a public-academic institution (Dyer et al., 2013). All couples used multiple financial coping strategies to offset the impact, which created catastrophic expenditure, defined as out-of-pocket payment exceeding 40% of the annual non-food household expenditure, for 22% of all households and for 51% among the poorest third.
Several papers referred to geographical barriers affecting people in countries with no or limited ART services as well as people in rural communities (Giwa-Osagie, 2002; Gerrits and Shaw, 2010; Dhont et al., 2011; Murage et al., 2011; Okwelogu et al., 2012).
According to the IFFS surveillance, in Cameroon, Mali and Senegal ART was only accessible to heterosexual, married couples. Nigeria also offered ART to single women but not others in non-traditional relationships, while South Africa had no access restrictions based on relationship structures (Ory et al., 2016). In contrast, two papers from Nigeria reported that the majority of surveyed infertility specialists (n = 102) opposed the use of ART in single women and that such practice contravened societal norms (Adesiyun, 2011; Ajayi and Dibosa-Osadolor, 2013).
Two surveys explored ART knowledge and acceptability among 250 women attending general gynaecological clinics and 500 women attending infertility clinics in Nigeria (Okwelogu et al., 2012; Adenike et al., 2014). Knowledge of ART existence was 37.2% and 46% with an acceptability among those aware of ART of 37.6% and 73.5%, respectively. Cost and religious reasons were the most prominent barriers, followed by the belief that ART babies were socially unacceptable or ‘abnormal’. These results are aligned with qualitative data from Nigeria which highlighted that, according to a variety of informants, ART was against religion and interfered with concepts of paternity, hence offspring would be discriminated against by both kin and community (Jegede and Fayemiwo, 2010). Gerrits and Shaw (2010) concluded that little was known about consumer ART acceptability in sub-Saharan Africa.
Lastly, the role of the male partner in facilitating or preventing access was identified. According to qualitative data from Nigeria, women had no financial or decision-making autonomy regarding use of ART (Jegede and Fayemiwo, 2010). The importance but lack of male involvement was also reported by Gerrits and Shaw (2010). Reasons were diverse and included conceptualizing infertility as a female condition, polygamy, cultural barriers to semen analysis, fear of diagnosis of male factor infertility, and health system failure to include men.
Effectiveness
Fourteen studies provided data pertaining to the effectiveness of ART (Table IV). With one exception (Abels et al., 2007), ovarian stimulation was conventional or not stipulated (n = 4). Different indicators were used, the most frequent being clinical pregnancy rate (CPR) per embryo transfer (ET) which ranged from 19.3% to 43.9%. The ICMART World Report documented a mean CPR per aspiration for fresh IVF and ICSI in sub-Saharan Africa of 31.3% in 2010 (Dyer et al., 2016). The concomitant delivery rate (DR) was 20.8% in Cameroon and not assessed in Mali and South Africa. Together, these three countries reported 1445 pregnancies with pregnancy outcome data for only 39 of these (Dyer et al., 2016). Only two other studies provided delivery data, documenting a DR per fresh non-donor ET of 16.2% (Orhue et al., 2012) and a cumulative live birth DR of 14.4% per initiated cycle with fresh and frozen ETs (Noreh et al., 2009).
Table IV.
Summary of clinical and multiple pregnancy rates.
| Region/ Country | References | Study population/Sample size | Ovarian stimulation | Clinical PR | Multiple PRa |
|---|---|---|---|---|---|
| Sub-Saharan Africa | Dyer et al. (2016) | 4619 fresh non-donor asp (Cameroon 96; Mali 171; South Africa 4352) | Not specified | CPR/asp 31.3% | MBR 20% Cameroon, NA in South Africa and Mali |
| Kenya | Noreh et al. (2009) | 362 initiated fresh ART cycles | Conventional | Cumulative CPR 27.3%/cycle(99/362)b | 27.3% (27/99)b |
| 430 fresh and frozen ETs | CPR 23.0%/fresh & frozen ET (99/430)b | ||||
| Nigeria | Adesiyun (2011) | 9 couples treated with ART. Number of cycles, asp or ET not specified | Not specified | 77.8%/pt (7/9) | 44.4% (4/9) |
| Ezechi et al. (2008) | 52 ART pregnancies | Not specified | NA | 17.3% (9/52) vs 4.3% (93/2160) (P < 0.05) | |
| 2160 non-ART pregnancies | |||||
| Melie (2003) | 452 asp with ≥1 oocyte | Conventional | 19.7%/aspb (89/452) | NA | |
| 421 fresh non-donor ETs | 21.1%/ETb (89/421) | ||||
| Okohue et al. (2009) | 298 women <36 y | Conventional | 39.0%/aspb (110/282) | NA | |
| 276 fresh non-donor ETs | 39.9%/ETb (110/276) | ||||
| Okohue et al. (2013) | 72 women <36 y | Conventional | 43.4%/ETb (33/76) | NA | |
| 76 ETs | |||||
| Oloyede et al. (2012) | 55 ART multiple pregnancies | Not specified | NA | 34.5% (19/55)c | |
| Orhue et al. (2012) | 600 fresh non-donor ART cycles | Conventional | 30%/cycle (180/600) | 20.0% (36/180) | |
| 35.7%/ET (180/504) | |||||
| 504 fresh ETs | |||||
| South Africa | Abels (2007) | 85 women, age 22–40 y | Clomiphene Citrate plus uHMG | Ongoing PR 35.6%/GIFT cycle (16/45) | 12.5% (3/16) |
| 45 GIFT cycles | |||||
| Bosman et al. (2015) | 82 asp in women < 38 with ≥5 oocytes; 82 ETs | Conventional | 43.9%/ETb (36/82) | 38.9% (14/36) | |
| Dyer and Kruger (2012) | 4512 aspd | Not specified | 28.9%/asp | NA | |
| 3872 fresh ETsd | 33.6%/ET | ||||
| Raja and Franken (2006) | 39 couples requiring ICSI; no. cycles not specified | Not specified | PR 51.3%e/pt | NA | |
| Windt et al. (2002) | 142 ICSI asp with ≥1 oocyte; 135 ETs | Conventional | 18.3%/asp (26/142)b,f; 19.3%/ET (26/135)b,f | NA |
PT, patient; uHMG, urinary HMG.
aDenominator: all clinical pregnancies.
bNot original study result but calculated from original data.
cSix triplets and two quadruplets.
dIncludes donor and non-donor cycles.
eNot clarified whether PR or CPR.
fDefined as intrauterine pregnancy with foetal heart.
Qualitative review data referred to ART pregnancy rates around 21% per ET with a ‘take home baby rate’ of 15% (Giwa-Osagie, 2002; Gerrits and Shaw, 2010).
Safety
Twelve manuscripts provided information on the number of embryos transferred. According to the ICMART World Report, the mean number of embryos transferred in sub-Saharan Africa in fresh non-donor IVF and ICSI cycles was 2.31 in 2010. This number was, however, derived from only two countries (Cameroon and South Africa) and fewer than 4000 ETs (Dyer et al., 2016). Data from the South African registry, when calculated for the purpose of this review, yielded the same number in 2009 (Dyer and Kruger, 2012). In three studies from Nigeria and Kenya, the mean number of embryos transferred ranged between 2.9 and 3.2 with no upper limit specified (Noreh et al., 2009; Okohue et al., 2009, 2013). Patient and doctor preference for the transfer of multiple embryos was reported by three studies (Okohue et al., 2010b; Okwelogu et al., 2012).
Information on the rate of multiple pregnancies was provided in seven papers (Table IV). The majority documented a rate ≥20% although the range was wide (12.5–44.4%). The rate of triplet pregnancies ranged from 4.0% to 14.5% (Noreh et al., 2009; Adesiyun, 2011; Oloyede et al., 2012; Orhue et al., 2012; Bosman et al., 2015); that of miscarriages from 11.8% to 22.2% per clinical pregnancy (Abels et al., 2007; Ezechi et al., 2008; Noreh et al., 2009; Okohue et al., 2013; Orhue et al., 2012); and that of ectopic pregnancies from 2.2% to 4.2% per clinical pregnancy (Abels et al., 2007; Noreh et al., 2009; Okohue et al., 2009; Orhue et al., 2012).The frequency of ovarian hyperstimulation syndrome ranged widely, from 0.3% per initiated cycle to 18.2% per woman with polycystic ovary syndrome, without severity being specified (Noreh et al., 2009; Orhue et al., 2012; Okohue et al., 2013). No information on aspiration related complications was identified.
Information on maternal and neonatal outcomes was similarly scarce. Only one study provided reasonably comprehensive documentation but based on a small sample size of 52 ART pregnancies (Ezechi et al., 2008). Compared to >2000 spontaneous pregnancies, the authors found statistically significant increases in early pregnancy bleeding, placenta praevia, Caesarean sections, as well as rates of multiple pregnancy and preterm delivery. Differences remained significant after controlling for confounding variables. Low preterm DRs were reported in two other small samples but rising to 22.5% in a subgroup of multiple pregnancies with a concomitant high rate of Caesarean deliveries (Noreh et al., 2009; Orhue et al., 2012). Lastly, five papers made reference to ART regulations. Ory et al. (2016) documented absence of any form of government regulations in all the African countries participating in the IFFS Surveillance except South Africa. In the absence of these, ART practice was frequently based on voluntary adherence to international guidelines (Giwa-Osagie, 2002; Gerrits and Shaw, 2010; Ory et al., 2016). Qualitative data captured the concern that the global recognition for the need of regulation had not included sub-Saharan Africa where lack of regulations hampered ART practice and potentially exposed patients to substandard and costly care (Murage et al., 2011; Ajayi and Dibosa-Osadolor, 2013).
Discussion
To our knowledge, this is the first review to systematically identify, analyse and present published, peer-reviewed evidence on the availability, effectiveness and safety of ART in sub-Saharan Africa. Although information on ART in Africa is captured at a global level by both the IFFS Surveillance and ICMART World Report, our review focuses on Africa while contributing additional information. It highlights what we know – and all we do not.
There are 48 states in sub-Saharan Africa (https://data.worldbank.org). Our review documented availability of ART in 10 of these of which four provided papers included here. Services were delivered by a total of 80 centres but this number missed data from Ghana, Sierra Leone, Togo and Zimbabwe. Only six countries (Cameroon, Kenya, Mali, Nigeria, Senegal and South Africa) participated in the IFFS Surveillance and only three (Cameroon, Mali and South Africa) submitted data to the latest ICMART world report (Dyer et al., 2016; Ory et al., 2016). Collectively this implies that around 80% of African states do not offer ART or do not reveal related information in the scientific literature; and that the remaining countries publish little of their own data and have low participation in global ART monitoring.
Our review highlights that the need for ART far exceeds what is available and accessible. One thousand five hundred cycles per million population per year are required to meet the need for ART, but in 2010 the regional mean for sub-Saharan Africa – albeit based on only three countries – was 87 cycles/million (Collins, 2002; Dyer et al., 2016). This was the lowest of all regions and less than one-fifth of global utilization, which was reported as 474 cycles/million population. In comparison, the mean utilization in Latin America was 152 (second lowest), while Europe reported 932 cycles/million.
Unsurprisingly, this review identified cost as the single biggest barrier. Underlying reasons are many and include the high prevalence of poverty and low income in sub-Saharan African countries, the lack of government or insurance scheme funding, the lack of resource allocation to capacity building (causing high expenditures for those acquiring skills and infrastructure which need to be recuperated once ART is being offered), and the lack of public sector infertility and ART services. Other barriers included lack of services overall, outside large urban settings, and for people not in heterosexual relationships; and lack of patient education, public awareness, and acceptability of ART. Our findings are in keeping with reports on access to health care, which identify availability, affordability and acceptability as the key determinants of access (Thiede and McIntyre, 2008; McIntyre et al., 2009). These authors emphasize that affordability has traditionally dominated the concept of access to care, while acceptability of service provision – for both users and providers – has received relatively little attention. Moreover, even in Europe, where ART usage is high compared to sub-Saharan Africa, cultural norms and values have been shown to powerfully influence access to ART over and above country wealth (Präg and Mills, 2017). Improving ART access thus does not only require generating fiscal space for third party funding to improve affordability – the difficulty of which is acknowledged and beyond the scope of this discussion – but also must, for example, address reproductive health illiteracy, societal attitudes and improving ART availability through training and infrastructure development.
Half of the selected manuscripts provided information on ART effectiveness. The predominant indicator was CPR per ET following fresh IVF and ICSI, which ranged from 15% to 57%, with an average of 31.3% according to the ICMART World Report in 2010. Internationally, live birth – and preferably a single live birth at term – is considered the most important indicator of ART success, although increasingly the need to report cumulative live birth rates is recognized in view of the rising contribution from frozen ETs (Maheshwari et al., 2015). Our review found almost no data on live births with even the ART registry in South Africa failing to provide this information. Information on frozen ETs and oocyte donation was similarly lacking. Collectively this implies that the true success of ART is currently not documented.
Multiple pregnancy is recognized as the leading risk of ART. Our findings indicate a preference of transferring multiple embryos with resultant multiple pregnancy rates of ±20–30%. Given the information deficit on live births as well as obstetrical complications, this approach to ‘optimizing’ ART success is unchallenged by any local evidence pertaining to possible detrimental outcomes. The inverse relationship between lack of third party funding and single ET is recognized and not unique to sub-Saharan Africa (Chambers et al., 2014). Our review also highlights the information deficit on ART-related maternal and neonatal complications, be it singleton or multiple pregnancies. How the recognized complications of prematurity, low birthweight and operative deliveries impact on mothers and their offspring, how effectively they are managed, and who carries the burden of their costs is therefore also unknown.
Strengths and limitations
The strength of this review lies in the pertinence and novelty of the research question in sub-Saharan Africa, and in the rigorous process of finding an answer. In addition, the authors had expertise in research methodology and infertility in sub-Saharan Africa. The main limitation was the broad definition of our outcome indicators. This resulted in data that were both highly heterogeneous and that frequently contributed mere basics to the research question; for example, documenting ART availability in terms of existing facilities rather than national, total number of cycles. More stringent criteria might have improved data quality but would have lessened the amount of information, which was already scant. Data heterogeneity also prevented the combination or comparison of data through statistical analysis let alone conduct a meta-analysis. Manuscripts also lacked consistency in the definition of clinical terms. The glossary for ART, recently revised and expanded, provides international consensus definitions as an important prerequisite for data collection, analysis and comparison (Zegers-Hochschild et al., 2009, 2017): it was referenced by two manuscripts in this review. Additional limitations include restricting the review to peer-reviewed manuscripts with original data, and not attempting to contact authors for primary data or further information. The decision to exclude grey-literature, non-peer-reviewed manuscripts and opinion-based papers attempted to generate a minimum of scientific homogeneity in otherwise diverse data.
Conclusion
While this systematic review provides best evidence on availability and outcomes of ART in sub-Saharan Africa, it failed to adequately answer the research question. This was due to the overall lack of data that are generally accepted as being key components of evidence-based practice and development strategies. Evidently, such data need to be derived from and be applicable to local settings. They cannot be extrapolated from studies conducted in other parts of the world.
Fortunately, the need to close the ART information gap in sub-Saharan Africa is increasingly recognized. Both regional and national fertility organizations are calling for greater research output. Moreover, the African Network and Registry for ART (ANARA; www.anara-africa.com) has evolved over the last 3 years receiving support and recognition from many stakeholders. Its mission is to collect, analyse and report ART data at regional level while simultaneously providing participating centres with confidential reports of their own data and countries with their national data. ANARA was initiated under the auspices of ICMART and received developmental support from the Latin American Registry including donation of its data collection software. It is anticipated that ANARA will build capacity in data collection, thereby addressing the main limitation highlighted in this review. The ANARA-ICMART initiative has already resulted in increasing participation from African countries in the ICMART World Reports, with two countries having submitted 2009 data and seven countries 2012 data (Dyer et al., 2016; ICMART, unpublished data). The collected data, which are growing in width and depth, will strengthen our regional understanding of the role of scientific data. It will then be the role of clinicians, scientists, fertility organizations, governments and other stakeholders to use the data for sound decision and policy-making.
Acknowledgements
The authors wish to thank Ms Dilshaad Brey for her expert assistance in the literature search.
Authors’ role
All authors conceptualized the study and designed the protocol. B.B. undertook the literature search. B.B. and S.D. identified and all authors critically appraised selected papers. All authors analysed and interpreted the data. B.B. drafted the manuscript which was revised and approved in its final version by all authors.
Funding
None.
Conflict of interest
None of the authors declared a conflict of interest.
References
- Abels PR, Kruger TF, Merwe JPvd, Norsaka S, Kitilla T, Lombaard CJ. Gamete intrafallopian transfer versus super-ovulation with intrauterine insemination for the treatment of infertility: a prospective randomised study on pregnancy outcome. S Afr J Obstet Gynaecol 2007;13:104–109. [Google Scholar]
- Adegbola O, Akindele MO. The pattern and challenges of infertility management in Lagos, Nigeria. Afr Health Sci 2013;13:1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adenike OB, Adebimpe Wasiu O, Olarewaju Sunday O, Olaniyan B. Prevalence of infertility and acceptability of assisted reproductive technology among women attending gynecology clinics in tertiary institutions in southwestern Nigeria. Gynecol Obstet 2014;4:1–7. [Google Scholar]
- Adesiyun AG. Referral for assisted reproductive technology: indications and treatment outcome. Ann Afr Med 2011;10:316–318. [DOI] [PubMed] [Google Scholar]
- Adeyemi AS, Adekanle DA, Afolabi AF. Pattern of gynaecological consultations at Ladoke Akintola University of Technology Teaching Hospital. Niger J Clin Pract 2009;12:47–50. [PubMed] [Google Scholar]
- Ajayi RA, Dibosa-Osadolor OJ. Opinion of obstetricians and gynaecologists on ethical issues in the practice of in-vitro fertilisation and embryo transfer in Nigeria. Afr J Reprod Health 2013;17:130–136. [PubMed] [Google Scholar]
- Bosman E, Esterhuizen AD, Rodrigues FA, Becker P, Hoffmann WA. Influence of male hyperinsulinaemia on IVF outcome. Andrologia 2015;47:91–96. [DOI] [PubMed] [Google Scholar]
- Chambers GM, Lee E, Hansen M, Sullivan EA, Bower C, Chapman M. Hospital costs of multiple-birth and singleton-birth children during the first 5 years of life and the role of assisted reproductive technology. JAMA Pediatr 2014;168:1045–1053. [DOI] [PubMed] [Google Scholar]
- Collins JA. An international survey of the health economics of IVF and ICSI. Hum Reprod Update 2002;8:265–277. [DOI] [PubMed] [Google Scholar]
- Dhont N, van de Wijgert J, Vyankandondera J, Busasa R, Gasarabwe A, Temmerman M. Results of infertility investigations and follow-up among 312 infertile women and their partners in Kigali, Rwanda. Trop Doct 2011;41:96–101. [DOI] [PubMed] [Google Scholar]
- Dyer S, Chambers G, De Mouzon J, Nygren K, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson G. International committee for monitoring assisted reproductive technologies world report: assisted reproductive technology 2008, 2009 and 2010. Hum Reprod 2016;31:1588–1609. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Kruger TF. Assisted reproductive technology in South Africa: first results generated from the South African Register of Assisted Reproductive Techniques. S Afr Med J 2012;102:167–170. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Sherwood K, McIntyre D, Ataguba JE. Catastrophic payment for assisted reproduction techniques with conventional ovarian stimulation in the public health sector of South Africa: frequency and coping strategies. Hum Reprod 2013;28:2755–2764. [DOI] [PubMed] [Google Scholar]
- Ezechi OC, Ndububa VI, Loto OM, Ezeobi PM, Kalu BK, Njokanma OF, Nwokoro CA. Pregnancy, obstetric and neonatal outcome after assisted reproduction in Nigerians. J Matern Fetal Neonatal Med 2008;21:261–266. [DOI] [PubMed] [Google Scholar]
- Gerrits T, Shaw M. Biomedical infertility care in sub-Saharan Africa: a social science – review of current practices, experiences and view points. Facts Views Vis Obgyn 2010;2:194–207. [PMC free article] [PubMed] [Google Scholar]
- Gianaroli L, Ferraretti AP, Magli MC, Sgargi S. Current regulatory arrangements for assisted conception treatment in European countries. Eur J Obstet Gynecol Reprod Biol 2016;207:211–213. [DOI] [PubMed] [Google Scholar]
- Giwa-Osagie OF. ART in developing countries with particular reference to sub-Saharan Africa In: Vayena E, Rowe PJ, Griffin D (eds). Current Practices and Controversies in Assisted Reproduction: Report of a WHO Meeting. Geneva, Switzerland: WHO Library, 2002, 22–27. [Google Scholar]
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–426. [DOI] [PubMed] [Google Scholar]
- Jegede AS, Fayemiwo AS. Cultural and ethical challenges of assisted reproductive technologies in the management of infertility among the Yoruba of southwestern Nigeria. Afr J Reprod Health 2010;14:115–127. [PubMed] [Google Scholar]
- Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod 2015;30:2703–2707. [DOI] [PubMed] [Google Scholar]
- McIntyre D, Thiede M, Birch S. Access as a policy-relevant concept in low-and middle-income countries. Health Econ Policy Law 2009;4:79–193. [DOI] [PubMed] [Google Scholar]
- Melie NA, Adeniyi OA, Igbineweka OM, Ajayi RA. Predictive value of the number of oocytes retrieved at ultrasound-directed follicular aspiration with regard to fertilization rates and pregnancy outcome in intracytoplasmic sperm injection treatment cycles. Fertil Steril 2003;80:1376–1379. [DOI] [PubMed] [Google Scholar]
- Menuba IE, Ugwu EO, Obi SN, Lawani LO, Onwuka CI. Clinical management and therapeutic outcome of infertile couples in southeast Nigeria. Ther Clin Risk Manag 2014;10:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murage A, Muteshi MC, Githae F. Assisted reproduction services provision in a developing country: time to act? Fertil Steril 2011;96:966–968. [DOI] [PubMed] [Google Scholar]
- Noreh LJ, Tucs O, Sekadde-Kigondu CB, Noreh JA. Outcomes of assisted reproductive technologies at the Nairobi In Vitro Fertilisation Centre. East Afr Med J 2009;86:156–161. [DOI] [PubMed] [Google Scholar]
- Okohue JE, Ikimalo JI, Omoregie OB. Ectopic pregnancy following in vitro fertilisation and embryo transfer. West Afr J Med 2010. a;29:349–351. [PubMed] [Google Scholar]
- Okohue JE, Onuh SO, Ebeigbe P, Shaibu I, Wada I, Ikimalo JI, Okpere EE. The effect of endometrial thickness on in vitro fertilization (IVF)-embryo transfer/intracytoplasmic sperm injection (ICSI) outcome. Afr J Reprod Health 2009;13:113–121. [PubMed] [Google Scholar]
- Okohue JE, Onuh SO, Ikimalo JI. Comparison of IVF/ICSI outcome in patients with polycystic ovarian syndrome or tubal factor infertility. Niger J Clin Pract 2013;16:207–210. [DOI] [PubMed] [Google Scholar]
- Okohue JE, Onuh SO, Ikimalo JI, Wada I. Patients’ preference for number of embryos transferred during IVF/ICSI: a Nigerian experience. Niger J Clin Pract 2010. b;13:294–297. [PubMed] [Google Scholar]
- Okwelogu I, Azuike E, Ikechebelu J, Nnebue C. In-vitro fertilization practice: awareness and perceptions among women attending fertility clinics in Okija, Anambra State, Nigeria. Afrimedic J 2012;3:5–10. [Google Scholar]
- Oloyede OA, Iketubosin F, Bamgbopa K. Spontaneous fetal reduction and early pregnancy complications in multiple pregnancies following in vitro fertilization. Int J Gynaecol Obstet 2012;119:57–60. [DOI] [PubMed] [Google Scholar]
- Orhue AA, Aziken ME, Osemwenkha AP, Ibadin KO, Odoma G. In vitro fertilization at a public hospital in Nigeria. Int J Gynaecol Obstet 2012;118:56–60. [DOI] [PubMed] [Google Scholar]
- Ory SJ, Miller K, Horton M, Allan S, Balabam B, Banker M, Brinsden P, Buster J, Mocanu E, Pai H et al. . IFFS Surveillance 2016. Glob Reprod Health 2016;1:1–143. [Google Scholar]
- Präg P, Mills MC. Cultural determinants influence assisted reproduction usage in Europe more than economic and demographic factors. Hum Reprod 2017;32:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja K, Franken DR. The role of paternal chromosomes and sperm morphology on the outcome of intracytoplasmic sperm injection. Andrologia 2006;38:179–185. [DOI] [PubMed] [Google Scholar]
- Thiede M, McIntyre D. Information, communication and equitable access to health care: a conceptual note. Cad Saude Publica 2008;24:1168–1173. [DOI] [PubMed] [Google Scholar]
- Windt ML, Coetzee K, Kruger TF, Menkveld R, van der Merwe JP. Intracytoplasmic sperm injection with testicular spermatozoa in men with azoospermia. J Assist Reprod Genet 2002;19:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Hum Reprod 2009;24:2683–2687. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod 2017;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]