Abstract
STUDY QUESTION
Are there any differences in live birth rates (LBR) following fresh blastocyst transfer in natural or clomiphene-stimulated cycles, or after elective blastocyst freezing in clomiphene-stimulated cycles followed by thawing and transfer at different time-points?
SUMMARY ANSWER
Clomiphene citrate (CC) administration adversely affected the LBR after single fresh blastocyst transfer (SBT) in CC cycles compared with that in natural cycles, while this adverse effect of CC is not present when a single vitrified-warmed blastocyst transfer (SVBT) is performed in subsequent natural ovulatory cycles, regardless of the duration between CC administration and the day of SVBT.
WHAT IS KNOWN ALREADY
CC affects uterine receptivity associated with a thinning of the uterine endometrium through an antioestrogenic effect. However, the duration that this adverse effect of CC on uterine endometrium persists after initial use is still unknown.
STUDY DESIGN, SIZE, DURATION
A retrospective cohort study of 157 natural cycle IVFs followed by SBT and 1496 minimal ovarian stimulation with CC IVF cycles followed by SBT (n = 24) or SVBT (n = 1472) from January 2010 to December 2014 was conducted. SVBT cycles were classified into two groups according to the period between the last day of CC administration and the day of SVBT (A: ≤60 d and B: ≥61 d). All groups were then compared based on pregnancy outcomes (natural-SBT group: n = 157, CC-SBT group: n = 24, SVBT-A: n = 1143, SVBT-B: n = 329).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women were aged 30–39 years at oocyte retrieval. In SVBT cycles, blastocysts were vitrified and warmed using a Cryotop safety kit. SVBT was performed in subsequent natural ovulatory cycles. The main outcomes were LBR and neonatal outcome, and both were compared among the groups.
MAIN RESULTS AND THE ROLE OF CHANCE
The LBR in the CC-SBT group (29.2%, 7/24) was significantly lower compared with the natural-SBT (56.1%, 88/157) (P = 0.01) and SVBT-A (50.0%, 572/1143) (P = 0.04), but not SVBT-B (47.4%, 156/329), groups. Furthermore, multivariate logistic regression analysis revealed that the LBR was comparable among the natural-SBT and SVBT groups, but was significantly lower in the CC-SBT group (adjusted odds ratio: 0.324, 95% CI: 0.119–0.800, P = 0.01). No significant differences among all groups were observed for gestational age (P = 0.19), birthweight (P = 0.41) and incidence of malformation (P = 0.53).
LIMITATIONS, REASONS FOR CAUTION
In this study we analysed a biased sample, based on clinical judgement regarding endometrial thickness, and the study was limited by its retrospective nature. The low statistical power caused by the group size disparity was also a limitation, especially in the CC-SBT group. Although the outcome showing inferiority of CC-SBT compared to natural-SBT is consistent with general findings in the literature, further large-scale clinical studies, ideally RCTs, are necessary to validate our results and clarify the prolonged effect of CC in SVBT cycles on pregnancy and neonatal outcomes.
WIDER IMPLICATIONS OF THE FINDINGS
Our observation suggests that CC administered in minimal ovarian stimulation cycles affects adversely the pregnancy outcomes when SBT is performed. Therefore, for a CC-based minimal stimulation IVF cycle, we suggest that frozen embryo transfer should be performed in a subsequent natural ovulatory cycle to avoid the possibility of implantation failure associated with CC administration.
STUDY FUNDING/COMPETING INTERESTS
The authors have no conflicts of interest to declare. No external funding was either sought or obtained.
Keywords: clomiphene citrate, endometrium, live birth, single blastocyst transfer, uterine receptivity, vitrification
Introduction
Clomiphene citrate (CC) is often administered in minimal ovarian stimulation IVF treatment (Kousta et al., 1997; Bodri et al., 2014; Song et al., 2016; Zhang et al., 2016). CC binds hypothalamic oestrogen receptors and induces GnRH secretion by altering the negative feedback effect of oestrogen on the hypothalamus. Clinically, in minimal ovarian stimulation IVF, CC is administered alone or in combination with a GnRH antagonist or FSH and HMG. Recent studies have shown that minimal ovarian stimulation with CC yielded comparable embryonic outcomes, such as fertilization and development, and pregnancy outcomes to conventional IVF (Pilehvari et al., 2016), reduced the rate of multiple pregnancy, and decreased gonadotrophin use (Zhang et al., 2016). On the other hand, it has been demonstrated that endometrial thickness is significantly reduced in cycles with CC administration compared to that in non-CC cycles (Randall and Templeton, 1991; Dehbashi et al., 2003; Zollner et al., 2003; Bromer et al., 2009). Endometrial cell growth is essential for uterine receptivity, and endometrial thickness correlates with embryo implantation and subsequent pregnancy (Friedler et al., 1996; Dey et al., 2004; Cha et al., 2012; Mahajan and Sharma, 2016). Therefore, we hypothesized that cryopreservation of all embryos and performance of a single embryo transfer (ET) in a subsequent cycle is preferable to avoid the adverse effects of CC administration.
WHAT DOES THIS MEAN FOR PATIENTS?
This study looks at pregnancy outcomes after fresh and frozen blastocyst transfer in both natural cycle IVF and in cycles using clomiphene citrate to stimulate the ovaries.
Clomiphene citrate is sometimes used to mildly stimulate the ovaries in IVF cycles, but it is known that it is associated with a thinning of the womb lining. The researchers wanted to find out whether using frozen rather than fresh blastocysts would avoid this drawback of clomiphene citrate.
This study looked back at the outcomes of cycles carried out at a clinic in Japan over a 5-year period. They found that clomiphene citrate was linked to lower pregnancy rates in fresh cycles, but this impact was not seen in the frozen cycles, which would suggest that it is better to freeze embryos if clomiphene is used for stimulation. Not many clinics freeze blastocysts after a cycle using clomiphene citrate and looking back over existing data is the limitation of this study. The researchers have advised that further research is needed to confirm their findings.
Uncertainty remains regarding the duration of the effect of CC on uterine receptivity in the endometrium. Nakagawa et al. (2015) previously demonstrated the long-term adverse effects of minimal ovarian stimulation with CC on pregnancy outcomes after either single or multiple ETs in a hormone replacement cycle; however, FSH and HCG were also used for ovarian stimulation, which may have affected their results. Moreover, their study included single and multiple ETs; thus, an evaluation of the actual effect of CC administration on uterine receptivity is challenging. Therefore, debate remains regarding whether treatment with CC alone affects pregnancy outcomes after ET in subsequent cycles. In the present study, we aimed to examine pregnancy and neonatal outcomes in patients treated with CC alone during oocyte retrieval cycles, followed by a single fresh blastocyst transfer (SBT) during a spontaneous natural cycle. Patients were stratified by the duration between the last day of CC administration and the day of single vitrified-warmed blastocyst transfer (SVBT).
Materials and Methods
Ethical approval
The present study was approved by the institutional review board at the Kato Ladies Clinic (approval number: 13-24, 16-30). Written informed consent was obtained from all patients who were undergoing IVF treatment at the centre after they were informed that de-identified data could be used for a retrospective analysis.
Study patients
In total, 1653 treatment cycles from 1653 women who underwent IVF treatment and ET between January 2010 and December 2014 at the Kato Ladies Clinic were retrospectively reviewed. ET protocols included the following: oocyte retrieval during a natural cycle, followed by SBT (natural-SBT group); oocyte retrieval during a CC-administered cycle, followed by SBT (CC-SBT group) or SVBT (SVBT group). The present study only included patients aged 30–39 years at oocyte retrieval. All patients used their own oocytes during the treatment. Patients who did not obtain a blastocyst or did not receive ET were excluded. Cycles that were cancelled before ET because of endometrial thickness below the cut-off value (<6 mm) were excluded. Patients who previously underwent ET treatment were excluded. In addition, patients who underwent PGD and women with hypothalamus–pituitary gland-related amenorrhoea were excluded.
Natural cycle and minimal ovarian stimulation cycle IVF
In our clinic, both natural cycle IVF and the CC-based minimal stimulation cycle IVF are applicable for patients who have normal pituitary gland function and menstrual cycles. The ovarian stimulation method is usually determined through consultation with patients; their preferences are often taken into account.
The detailed protocol for minimal stimulation with CC alone, which is the main IVF treatment strategy at our clinic, has been previously reported (Kato et al., 2012, 2014; Kawachiya et al., 2012; Fukuda et al., 2016). Briefly, CC (50–100 mg/d; Fuji Pharma Co., Ltd, Saitama, Japan) was orally administered with an extended regimen, from Day 3 of the retrieval cycle to the day before the induction of final oocyte maturation. Monitoring, which involved an ultrasound scan and hormonal profiles, was usually initiated on Day 8, and was continuously performed every other day until the ovulation-triggering day. Ovulation triggering was performed using a nasal spray containing the GnRH agonist buserelin (Suprecur; Mochida Pharmaceutical Co., Ltd, Tokyo, Japan or Buserecur; Fuji Pharma Co., Ltd, Saitama, Japan).
In the natural cycle IVF protocol, the only pharmaceutical intervention involved the induction of final oocyte maturation with a GnRH agonist. Monitoring consisted of an ultrasound scan and hormonal profile, which was usually initiated on the morning of Day 10 and/or 12, according to the length of the patient’s cycle. When the leading follicle reached 18 mm diameter, with a concomitant oestradiol level of ≥250 pg/mL, oocyte retrieval was scheduled.
Oocyte retrieval was usually performed 30–36 h after triggering, and was performed using a 21 g needle (Kitazato Corporation, Shizuoka, Japan). Anaesthesia and follicular flushing were not utilized during oocyte retrieval. The absence of follicles on ultrasound before initiating oocyte retrieval was considered as premature ovulation.
Conventional insemination, intracytoplasmic sperm injection and embryo culture
Conventional insemination or ICSI was performed ~3 and 5 h after oocyte retrieval, respectively (Kato et al., 2014). A P1 medium supplemented with 10% serum substitute supplement (Irvine Scientific, Santa Ana, CA, USA) was used as a fertilization medium. Fertilization assessment was performed 16–20 h after insemination or ICSI (Day 1). Normally fertilized zygotes with two pronuclei were individually cultured in 20 μL of Quinn Advantage Protein Plus cleavage medium (Origio Aktieselskab, Måløv, Denmark) on Days 1–3. Subsequently, the embryos were transferred to a Quinn Advantage Protein Plus blastocyst medium (Origio Aktieselskab, Måløv, Denmark) for Days 4–6. All embryos were cultured at 37°C (gas phase: 5% O2, 5% CO2 and 90% N2), with 100% humidity in a water jacket or non-humidified incubators (Astec Co. Ltd, Fukuoka, Japan). Blastocysts were evaluated using a blastocyst grading system based on the woman’s age at oocyte retrieval and embryo developmental speed, in accordance with our previous study (Kato et al., 2014).
Cryopreservation
During the study period SVBTs were exclusively performed. The embryos were cultured to the blastocyst stage and vitrified for subsequent use in ET cycles. The embryo vitrification was performed using Cryotop® (Kitazato Corporation, Shizuoka, Japan), as described previously (Kato et al., 2014, Mori et al., 2015).
Embryo transfer
SBTs were performed on Day 5 after oocyte retrieval and SVBTs were performed on Day 5 after confirmation of ovulation in an ovulation-induced natural cycle (Kato et al., 2012, 2014; Fukuda et al., 2016). SBTs were performed if the embryo meets the following criteria: the embryo developed to the expanded blastocyst stage by 110 h post-insemination; the embryo had a good morphology (Gardner’s criteria: >4AA) (Gardner and Schoolcraft, 1999). If the embryo did not meet these criteria, the fresh embryo transfer was cancelled and the embryo was vitrified and used for SVBT in subsequent cycles. In SVBT cycles, the day of SVBT was not strictly specified; thus, patients returned for SVBT according to their own circumstances (between 29 and 180 days after the last day of CC administration). If the endometrial thickness was <6 mm, ET was cancelled. Dydrogesterone (30 mg/d) was routinely administered orally during the early luteal phase after the blastocyst transfer procedures. Additionally, in cases with insufficient luteal function, progesterone was administered intramuscularly (125 mg/d) or intravaginally (100 mg/d) until the ninth week of pregnancy.
Statistical analyses
Patients who underwent SVBT were further classified into two groups according to the duration between the last day of CC administration and the SVBT day (Group A: ≤60 d, Group B: ≥61 d).
All analyses were performed using JMP software (SAS, Cary, NC, USA). Statistical analyses were conducted in two steps. The first step comprised a descriptive analysis of patient characteristics and pregnancy and neonatal outcomes. Age at oocyte retrieval, age at ET, BMI, infertility cause, endometrial thickness, clinical pregnancy rate based on observation of a gestational sac, ongoing pregnancy rate based on observation of foetal heart beat, live birth rate (LBR), miscarriage rate, gestation age, sex ratio, and the infant’s height, weight, and malformation rate were evaluated for each group. Nominal variables were analysed using an ANOVA and Tukey’s test, Kruskal–Wallis test, Cochran–Armitage test for trends, or chi-squared test, as appropriate.
The second step of the analysis assessed the prolonged effect of CC administration on the LBR after ET using a logistic regression model adjusting for confounders. A test for trends was performed in the univariate logistic analysis. Adjusted odds ratios (OR) are reported with 95% CI for each group, stratified by the duration between the last day of CC administration and the day of SVBT, using the natural-SBT group as a reference. A receiver operating characteristic (ROC) curve analysis was also performed to evaluate the association of LBR with the duration between the last CC administration and day of SVBT. A P-value <0.05 was considered statistically significant.
Results
Inclusion criteria and embryo developmental outcomes
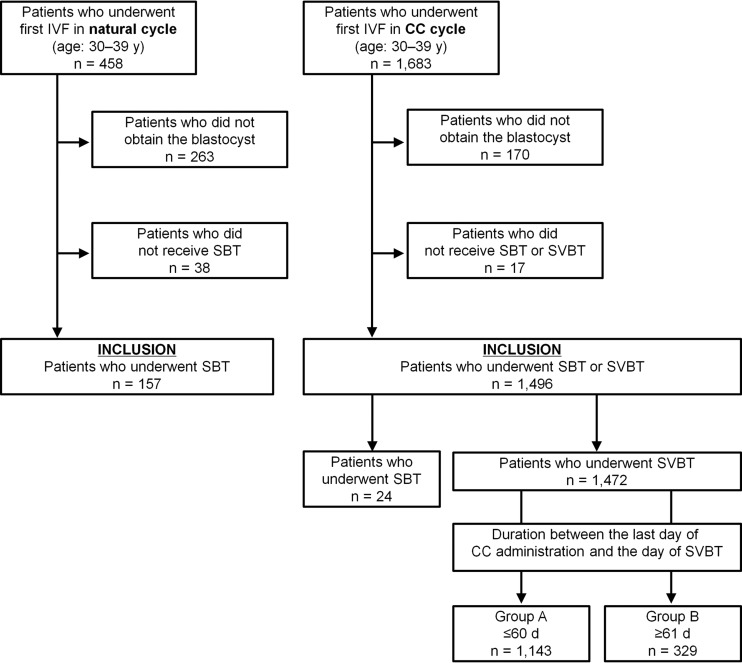
The patient selection flowchart is shown in Fig. 1. Among 458 patients who underwent the first oocyte retrieval in a natural cycle, 157 patients met the inclusion criteria. Among 1683 patients who underwent the first oocyte retrieval in a CC cycle, 1496 patients met the inclusion criteria. The outcomes of oocyte retrieval and IVF are shown in Supplementary Table SI. The serum oestradiol level at the time of trigger and number of retrieved oocytes were significantly greater in the CC cycle than in the natural cycle. The distribution of insemination method was significantly different between natural cycle and CC cycle. The rates of fertilization and blastocyst formation in the natural (81.0 and 52.2%, respectively) and CC cycles (80.2 and 53.3%, respectively) were comparable (Supplementary Table SI). The survival rate after blastocyst warming was 99.8% (1496/1499).
Figure 1.
Patient selection flowchart, including inclusion and exclusion criteria, in a comparison of pregnancy outcomes after fresh and electively frozen single blastocyst transfer in natural cycle and clomiphene-stimulated IVF cycles. CC, clomiphene citrate; SBT, single fresh blastocyst transfer; SVBT, single vitrified-warmed blastocyst transfer.
Baseline patient characteristics
Demographic characteristics of the women are shown in Table I. During the study period, 1653 patients were scheduled for 1653 ET cycles (natural-SBT group: n = 157, CC-SBT group: n = 24, SVBT-A group: n = 1143, SVBT-B group: n = 329). The baseline patient characteristics were compared among groups. The patients’ age at both oocyte retrieval and embryo transfer was significantly lower in the CC-SBT group compared with the natural-SBT group (A: P < 0.01, B: P < 0.01), whereas the ages were comparable between the CC-SBT and SVBT groups. There was no significant difference in the patient age between the natural-SBT and SVBT groups. BMI was comparable among groups. The main causes of infertility were identified as other factors, followed by tubal factor, combined factors, male factor, endometriosis and unexplained infertility. There were significant differences in the proportion of the infertility cause, male factor (P < 0.01) and unexplained (P < 0.01), among the groups (Table I).
Table I.
Demographic characteristics of study patients in a comparison of pregnancy outcomes following fresh and electively frozen single blastocyst transfer in natural cycle and clomiphene-stimulated IVF cycles.
| Total | Natural cycle SBT | CC cycle | |||
|---|---|---|---|---|---|
| SBT | SVBT | ||||
| A | B | ||||
| No. of patients | 1653 | 157 | 24 | 1143 | 329 |
| No. of cycles | 1653 | 157 | 24 | 1143 | 329 |
| Patient age at oocyte retrieval (y) | 35.4 ± 0.1 | 35.9 ± 0.2a | 34.1 ± 0.5b | 35.3 ± 0.1b,c | 35.4 ± 0.1a,c |
| Patient age at embryo transfer (y) | 35.4 ± 0.1 | 35.9 ± 0.2a | 34.1 ± 0.5b | 35.3 ± 0.9b,c | 35.5 ± 0.1a,c |
| BMI (kg/m2) | 20.4 ± 0.1 | 20.3 ± 0.2 | 20.6 ± 0.5 | 20.4 ± 0.1 | 20.6 ± 0.1 |
| Cause of infertility | |||||
| Tubal factor | 506 (30.6) | 43 (27.4) | 11 (45.8) | 339 (29.7) | 113 (34.4) |
| Endometriosis | 37 (2.2) | 3 (1.9) | 1 (4.2) | 23 (2.0) | 10 (3.0) |
| Male factor | 72 (4.4) | 14 (8.9)a | 1 (4.2)a,b | 42 (3.7)b | 15 (4.6)a,b |
| Other | 24 (1.5) | 3 (1.9) | 0 (0) | 15 (1.3) | 6 (1.8) |
| Combined | 80 (4.8) | 8 (5.1) | 4 (16.7) | 49 (4.3) | 19 (5.8) |
| Unexplained | 934 (56.5) | 86 (54.8)a,b | 7 (29.2)c | 675 (59.1)a | 166 (50.5)b |
CC, clomiphene citrate; SBT, single fresh blastocyst transfer; SVBT, single vitrified-warmed blastocyst transfer; No., number. Data are presented as n, n (%), or mean ± SEM. Data with distinct superscripts (a, b, c) are significantly different from each other within each row (P < 0.05). Age and BMI were normally distributed, and were analysed using one-way ANOVA, with significance determined using Tukey’s test for post-hoc analysis. Cochran–Armitage test for trends and chi-squared tests were used to evaluate group differences for infertility cause.
Pregnancy outcomes after single blastocyst transfer
A total of 1653 SBTs or SVBTs were performed. The grades of transferred blastocysts are shown in Table II. The number of grade A blastocysts was notably higher in the natural-SBT and CC-SBT groups compared to that in the other groups (P < 0.01). Although endometrial thickness in the natural-SBT and CC-SBT groups was higher than that in the SVBT groups, the range of endometrial thickness was not different among the groups. The clinical pregnancy and ongoing pregnancy rates were significantly higher in the natural-SBT group compared with the CC-SBT and SVBT groups. The LBR in the CC-SBT group was notably lower than those in the natural-SBT (P = 0.01) and SVBT-A groups (P = 0.04), whereas the rate in the CC-SBT group was comparable with the SVBT-B group (P = 0.09).
Table II.
Pregnancy outcomes after single fresh or vitrified-warmed blastocyst transfer in natural cycle and CC cycles.
| Total | Natural cycle SBT | CC cycle | |||
|---|---|---|---|---|---|
| SBT | SVBT | ||||
| A | B | ||||
| No. of total embryos transferred | 1653 | 157 | 24 | 1143 | 329 |
| Average no. of embryos transferred per patient | 1 | 1 | 1 | 1 | 1 |
| Blastocyst grade | |||||
| A | 1040 (62.9) | 157 (100)a | 24 (100)a | 667 (58.4)b | 192 (58.4)b |
| B | 186 (11.3) | 0 (0)a | 0 (0)a,b | 154 (13.5)b | 32 (9.7)b |
| C | 347 (21.0) | 0 (0)a | 0 (0)a | 272 (23.8)b | 75 (22.8)b |
| D | 80 (4.8) | 0 (0)a | 0 (0)a,b | 50 (4.4)b | 30 (9.1)b |
| Endometrial thickness (mm) (range, median) | 9.2 ± 0.0 (6–20, 9) | 9.6 ± 0.2a (6–20, 9) | 10.3 ± 0.4a (7–16, 9) | 9.2 ± 0.1b (6–20, 9) | 9.1 ± 0.1b (6–16, 8) |
| Clinical pregnancy | 996 (60.3) | 109 (69.4)a | 11 (45.8)b | 692 (60.5)b | 184 (55.9)b |
| Ongoing pregnancy | 920 (55.7) | 102 (65.0)a | 10 (41.7)b | 639 (55.9)b | 169 (51.4)b |
| Live birth | 823 (49.8) | 88 (56.1)a | 7 (29.2)b | 572 (50.0)a | 156 (47.4)a,b |
| Miscarriage | 173 (17.4) | 21 (19.3) | 4 (33.3) | 120 (17.3) | 28 (15.2) |
Data are presented as n, n (%), or mean±SEM. Data with distinct superscripts (a, b) are significantly different from each other within each row (P < 0.05). Cochran–Armitage test for trends and chi-squared tests were used to evaluate group differences for blastocyst grade, clinical pregnancy, ongoing pregnancy, live birth and miscarriage. Endometrial thickness was analysed using Kruskal–Wallis test.
The univariate logistic analysis revealed a significant association between the LBR and age at oocyte retrieval, age at ET, the proportion of infertility cause, and the grade of the transferred blastocysts (Grades A, B, C and D) (Table III). To prevent adjustment reduplication in the multivariate logistic regression analysis, only blastocyst grade and infertility cause were used as confounders, since our blastocyst grading system was categorized using embryo development speed and the woman’s age at oocyte retrieval. The analysis revealed that the adjusted OR for the CC-SBT group was significantly lower than that for the natural-SBT group (reference group, P = 0.01). However, the ratios for both SVBT subgroups were comparable with that for the natural-SBT group (Table IV). Furthermore, the logistic regression and ROC analysis demonstrated that the LBR after SVBT was not associated with the duration between the last day of CC administration and the day of SVBT (OR: 1.000, 95% CI: 0.997–1.001, P = 0.96; AUC: 0.60, 95% CI: 0.57–0.63).
Table III.
Univariate logistic analysis for live birth rate after single blastocyst transfer.
| OR | 95% CI | P-value | AUC | |
|---|---|---|---|---|
| Patient age at oocyte retrieval (y) | 0.897 | 0.862–0.934 | <0.001 | 0.577 |
| Patient age at embryo transfer (y) | 0.900 | 0.865–0.936 | <0.001 | 0.576 |
| BMI (kg/m2) | 0.982 | 0.944–1.021 | 0.365 | 0.515 |
| Cause of infertility | 0.516 | |||
| Tubal factor | – | – | – | |
| Endometriosis | 0.940 | 0.479–1.839 | 0.856 | |
| Male factor | 1.049 | 0.639–1.723 | 0.850 | |
| Other | 0.408 | 0.156–0.964 | 0.041 | |
| Combined | 0.733 | 0.453–1.177 | 0.200 | |
| Unexplained | 1.018 | 0.820–1.264 | 0.872 | |
| Blastocyst grade | 0.587 | |||
| A | – | – | – | |
| B | 0.906 | 0.663–1.240 | 0.537 | |
| C | 0.448 | 0.348–0.575 | <0.001 | |
| D | 0.265 | 0.154–0.439 | <0.001 | |
| Endometrial thickness (mm) | 0.989 | 0.941–1.039 | 0.662 | 0.505 |
OR, odds ratio. OR and AUC were obtained through univariate logistic regression analysis and receiver operating characteristic curve analysis for live birth rate after single blastocyst transfer.
Table IV.
Adjusted OR for live birth rate after single fresh or vitrified-warmed blastocyst transfer in natural cycle and CC cycles.
| Adjusted OR | 95% CI | P-value | AUC | |
|---|---|---|---|---|
| Natural cycle | 0.607 | |||
| SBT (Reference) | – | – | – | |
| CC cycle | ||||
| SBT | 0.324 | 0.119–0.800 | 0.014 | |
| SVBT | ||||
| A | 1.016 | 0.715–1.439 | 0.929 | |
| B | 0.960 | 0.645–1.427 | 0.843 |
Adjusted OR was obtained through multivariate logistic regression analysis for live birth rate after single blastocyst transfer. Blastocyst grade and infertility cause were used as confounders.
Neonatal outcomes after single blastocyst transfer
Gestational age was similar among groups (Table V). Additionally, the sex ratio was comparable across groups. Furthermore, there were no group differences in the height, birthweight or the incidence of malformation.
Table V.
Neonatal outcomes after single fresh or vitrified-warmed blastocyst transfer in natural cycle and CC cycles.
| Total | Natural cycle SBT | CC cycle | |||
|---|---|---|---|---|---|
| SBT | SVBT | ||||
| A | B | ||||
| No. of patients delivered | 823 | 88 | 7 | 572 | 156 |
| No. of babies delivered | 832 | 91 | 7 | 575 | 159 |
| Gestational age (days) | 273.8 ± 0.5 | 273.8 ± 1.5 | 265.6 ± 5.4 | 274.3 ± 0.6 | 272.3 ± 1.1 |
| Sex | |||||
| Male | 445 (53.5) | 45 (49.5) | 4 (57.1) | 314 (54.6) | 82 (51.6) |
| Female | 387 (46.5) | 46 (50.5) | 3 (42.9) | 261 (45.4) | 77 (48.4) |
| Height (cm) | 48.4 ± 0.2 | 47.4 ± 0.7 | 47.8 ± 2.4 | 48.6 ± 0.3 | 48.1 ± 0.5 |
| Weight (g) | 3035 ± 17 | 3004 ± 52 | 2810 ± 187 | 3050 ± 21 | 3005 ± 40 |
| Malformation (%) | 27 (3.2) | 4 (4.4) | 0 (0) | 16 (2.8) | 7 (4.4) |
Data are presented as n, n (%), or mean ± SEM. Gestational age, height and weight were normally distributed, and were analysed using one-way ANOVA, with significance determined using Tukey’s test for post-hoc analysis. Chi-squared test was used to analyse the group difference for sex ratio. Cochran–Armitage test for trends was used to evaluate group differences for infertility cause.
Discussion
The present study evaluated whether CC administration for follicular development adversely affected LBR after SBT, compared with that in patients without CC administration. In addition, the prolonged effect of CC administration was determined by comparing the LBR after SBT and SVBT at different time points of replacement. Our results showed that an adversely prolonged effect of CC administration was not observed after SVBT in subsequent cycles, regardless of the timing of replacement. The results also revealed that there was no correlation between neonatal outcomes and CC administration.
CC belongs to a class of selective oestrogen receptor modulators that bind to oestrogen receptors (Ernst et al., 1976; Goldstein et al., 2000). Although CC is a safe and effective oral agent, it has been known to have antioestrogenic side effects, such as endometrial thinning (Eden et al., 1989; Gonen and Casper, 1990; Nakamura et al., 1997). Reduced endometrial thickness is caused by CC-induced inhibition of epithelial cell proliferation and oestrogen response element transactivation in the endometrium (Amita et al., 2010). Furthermore, ovarian stimulation using CC adversely affects uterine receptivity by reducing the endometrial expression of Homeobox gene 10, integrin αvβ3 and leukaemia inhibitory factor during the implantation period (Bao et al., 2009; Chen et al., 2016). A recent systematic review (Gadalla et al., 2018) reported the adverse effects of CC administration on the endometrium, which resulted in lower pregnancy and LBR. In the present study, multivariate logistic regression analysis revealed that SBT in the CC cycle led to a significant decrease in LBR, which supported the results in the Gadall et al. (2018) systematic review.
In contrast, no adverse effect of CC administration on pregnancy outcomes was observed after SVBT. A previous study reported that a cleaved ET, which included single and double embryo transfer, within 90 days after the last CC treatment resulted in a lower pregnancy rate compared with that for transfers at more than 90 days after CC treatment (Nakagawa et al., 2015). However, in their study, FSH and HCG were also used for ovarian stimulation. Ovarian stimulation using exogenous gonadotrophins impairs blastocyst implantation and decidualization by altering steroid signalling, and the adverse effect of exogenous gonadotrophins remains in consecutive cycles (Bourgain and Devroey, 2003; Horcajadas et al., 2007; Ezoe et al., 2014; Fukuda et al., 2016). Therefore, the observed lower pregnancy rate could be related to the administration of exogenous gonadotrophins, rather than CC administration. In addition, the present results indicate that neonatal outcomes were not adversely affected by CC administration, which is consistent with the results of a previous study (Correy et al., 1982).
Concern exists regarding zuclomiphene accumulation after CC-based ovarian stimulation. CC is a mixture of two diastereoisomers: enclomiphene (62%) and zuclomiphene (38%) (Ernst et al., 1976). The trans-isomer, enclomiphene, is the more potent isomer, and is the element primarily responsible for the ovulation-inducing actions of CC (Van Campenhout et al., 1973; Clark and Markaverich, 1981). Enclomiphene levels rapidly rise after administration and fall to undetectable concentrations soon thereafter (half-life: 10.5 h) (Mikkelson et al., 1986). The cis-isomer, zuclomiphene, is eliminated far more slowly. Levels of the less-active isomer remain detectable in circulation for more than 30 days after administration, and may accumulate over consecutive cycles of treatment; however, there is no evidence suggesting an important clinical consequence (Mikkelson et al., 1986; Young et al., 1999). In the present study, no adverse effect due to CC administration was observed in the SVBT cycle. Therefore, pregnancy and neonatal outcomes do not appear to be affected by zuclomiphene accumulation in the consecutive cycles.
The strength of the present study was that it dealt with a topic that has a relatively rare cohort in current IVF practice. Very few clinics use a clomiphene stimulation protocol along with elective freezing; therefore, the findings would provide beneficial knowledge to improve clinical outcomes in CC-based minimal stimulation IVF cycles. However, there were also several limitations. In the present study, the outcomes of patients who underwent a single blastocyst transfer were exclusively evaluated; thus, we analysed the cohort that was very biased towards good prognosis, based on clinical judgement regarding endometrial thickness. Another limitation concerns the study’s retrospective nature. The low statistical power caused by the differences in sample size was also a limitation, especially in the CC-SBT group. Although the outcome showing inferiority of CC-SBT compared to natural-SBT is consistent with the general findings reported in the previous literature (Gadalla et al., 2018), further large-scale clinical studies are necessary to validate our results. Furthermore, characteristics of patients and grade of transferred embryos were not uniform in the present study because of our clinical decisions. Therefore, further RCTs are required to confirm the lack of a prolonged effect of CC on pregnancy and neonatal outcomes in SVBT.
In conclusion, CC administration in minimal ovarian stimulation IVF cycles affected pregnancy outcomes when fresh blastocyst transfer was performed. This adverse effect of CC did not occur with SVBT in subsequent natural ovulatory cycles, regardless of the period between the administration of CC and the day of SVBT. Therefore, when a CC-based minimal stimulation cycle IVF is applied, we suggest that frozen embryo transfer should be performed in a subsequent natural ovulatory cycle, instead of a fresh embryo transfer, to avoid the possibility of implantation failure associated with CC administration.
Supplementary Material
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Authors’ roles
K.K., K.E. and A.Y. designed the study; K.E., T.K. and S.U. analysed the data; K.K., K.E. and A.Y. wrote the article; and J.F., H.F. and T.K. revised the article.
Funding
Funding was neither sought nor obtained for this study.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Amita M, Takahashi T, Tsutsumi S, Ohta T, Takata K, Henmi N, Hara S, Igarashi H, Takahashi K, Kurachi H. Molecular mechanism of the inhibition of estradiol-induced endometrial epithelial cell proliferation by clomiphene citrate. Endocrinology 2010;151:394–405. [DOI] [PubMed] [Google Scholar]
- Bao SH, Sheng SL, Peng YF, Lin QD. Effects of letrozole and clomiphene citrate on the expression of HOXA10 and integrin alpha v beta 3 in uterine epithelium of rats. Fertil Steril 2009;91:244–248. [DOI] [PubMed] [Google Scholar]
- Bodri D, Kawachiya S, De Brucker M, Tournaye H, Kondo M, Kato R, Matsumoto T. Cumulative success rates following mild IVF in unselected infertile patients: a 3-year, single-centre cohort study. Reprod Biomed Online 2014;28:572–581. [DOI] [PubMed] [Google Scholar]
- Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update 2003;9:515–522. [DOI] [PubMed] [Google Scholar]
- Bromer JG, Aldad TS, Taylor HS. Defining the proliferative phase endometrial defect. Fertil Steril 2009;91:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012;18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Yan Q, Liu K, Zhou X, Xian Y, Liang D, Zhao X, Guo X, Quan S. Endometrial receptivity markers in mice stimulated with raloxifene versus clomiphene citrate and natural cycles. Reprod Sci 2016;23:748–755. [DOI] [PubMed] [Google Scholar]
- Clark JH, Markaverich BM. The agonistic-antagonistic properties of clomiphene: a review. Pharmacol Ther 1981;15:467–519. [DOI] [PubMed] [Google Scholar]
- Correy JF, Marsden DE, Schokman FC. The outcome of pregnancy resulting from clomiphene-induced ovulation. Aust NZ J Obstet Gynaecol 1982;22:18–21. [DOI] [PubMed] [Google Scholar]
- Dehbashi S, Parsanezhad ME, Alborzi S, Zarei A. Effect of clomiphene citrate on endometrium thickness and echogenic patterns. Int J Gynaecol Obstet 2003;80:49–53. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev 2004;25:341–373. [DOI] [PubMed] [Google Scholar]
- Eden JA, Place J, Carter GD, Jones J, Alaghband-Zadeh J, Pawson ME. The effect of clomiphene citrate on follicular phase increase in endometrial thickness and uterine volume. Obstet Gynecol 1989;73:187–190. [PubMed] [Google Scholar]
- Ernst S, Hite G, Cantrell JS, Richardson A Jr, Benson HD. Stereochemistry of geometric isomers of clomiphene: a correction of the literature and a reexamination of structure-activity relationships. J Pharm Sci 1976;65:148–150. [DOI] [PubMed] [Google Scholar]
- Ezoe K, Daikoku T, Yabuuchi A, Murata N, Kawano H, Abe T, Okuno T, Kobayashi T, Kato K. Ovarian stimulation using human chorionic gonadotrophin impairs blastocyst implantation and decidualization by altering ovarian hormone levels and downstream signaling in mice. Mol Hum Reprod 2014;20:1101–1116. [DOI] [PubMed] [Google Scholar]
- Friedler S, Schenker JG, Herman A, Lewin A. The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: a critical review. Hum Reprod Update 1996;2:323–335. [DOI] [PubMed] [Google Scholar]
- Fukuda J, Abe T, Okuno T, Kobayashi T, Kato K. Administering human chorionic gonadotropin injections for triggering follicle maturation could impact fertility during the subsequent menstrual cycle. Int J Gynaecol Obstet 2016;132:309–313. [DOI] [PubMed] [Google Scholar]
- Gadalla MA, Huang S, Wang R, Norman RJ, Abdullah SA, El Saman AM, Ismail AM, van Wely M, Mol BWJ. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:64–76. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol 1999;11:307–311. [DOI] [PubMed] [Google Scholar]
- Goldstein SR, Siddhanti S, Ciaccia AV, Plouffe L Jr. A pharmacological review of selective oestrogen receptor modulators. Hum Reprod Update 2000;6:212–224. [DOI] [PubMed] [Google Scholar]
- Gonen Y, Casper RF. Sonographic determination of a possible adverse effect of clomiphene citrate on endometrial growth. Hum Reprod 1990;5:670–674. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Pellicer A, Simon C. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum Reprod Update 2007;13:77–86. [DOI] [PubMed] [Google Scholar]
- Kato K, Takehara Y, Segawa T, Kawachiya S, Okuno T, Kobayashi T, Bodri D, Kato O. Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-centre, Japanese cohort. Reprod Biol Endocrinol 2012;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Ueno S, Yabuuchi A, Uchiyama K, Okuno T, Kobayashi T, Segawa T, Teramoto S. Women’s age and embryo developmental speed accurately predict clinical pregnancy after single vitrified-warmed blastocyst transfer. Reprod Biomed Online 2014;29:411–416. [DOI] [PubMed] [Google Scholar]
- Kawachiya S, Matsumoto T, Bodri D, Kato K, Takehara Y, Kato O. Short-term, low-dose, non-steroidal anti-inflammatory drug application diminishes premature ovulation in natural-cycle IVF. Reprod Biomed Online 2012;24:308–313. [DOI] [PubMed] [Google Scholar]
- Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update 1997;3:359–365. [DOI] [PubMed] [Google Scholar]
- Mahajan N, Sharma S. The endometrium in assisted reproductive technology: how thin is thin? J Hum Reprod Sci 2016;9:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelson TJ, Kroboth PD, Cameron WJ, Dittert LW, Chungi V, Manberg PJ. Single-dose pharmacokinetics of clomiphene citrate in normal volunteers. Fertil Steril 1986;46:392–396. [DOI] [PubMed] [Google Scholar]
- Mori C, Yabuuchi A, Ezoe K, Murata N, Takayama Y, Okimura T, Uchiyama K, Takakura K, Abe H, Wada K et al. . Hydroxypropyl cellulose as an option for supplementation of cryoprotectant solutions for embryo vitrification in human assisted reproductive technologies. Reprod Biomed Online 2015;30:613–621. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Kaneyama M, Nishi Y, Sugiyama R, Motoyama H, Sugiyama R. Clomiphene citrate affects the receptivity of the uterine endometrium. Reprod Med Biol 2015;14:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Ono M, Yoshida Y, Sugino N, Ueda K, Kato H. Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium. Fertil Steril 1997;67:256–260. [DOI] [PubMed] [Google Scholar]
- Pilehvari S, ShahrokhTehraninejad E, Hosseinrashidi B, Keikhah F, Haghollahi F, Aziminekoo E. Comparison pregnancy outcomes between minimal stimulation protocol and conventional GnRH antagonist protocols in poor ovarian responders. J Fam Reprod Health 2016;10:35–42. [PMC free article] [PubMed] [Google Scholar]
- Randall JM, Templeton A. Cervical mucus score and in vitro sperm mucus interaction in spontaneous and clomiphene citrate cycles. Fertil Steril 1991;56:465–468. [DOI] [PubMed] [Google Scholar]
- Song D, Shi Y, Zhong Y, Meng Q, Hou S, Li H. Efficiency of mild ovarian stimulation with clomiphene on poor ovarian responders during IVF/ICSI procedures: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 2016;204:36–43. [DOI] [PubMed] [Google Scholar]
- Van Campenhout J, Borreman E, Wyman H, Antaki A. Induction of ovulation with cisclomiphene. Am J Obstet Gynecol 1973;115:321–327. [DOI] [PubMed] [Google Scholar]
- Young SL, Opsahl MS, Fritz MA. Serum concentrations of enclomiphene and zuclomiphene across consecutive cycles of clomiphene citrate therapy in anovulatory infertile women. Fertil Steril 1999;71:639–644. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Merhi Z, Yang M, Bodri D, Chavez-Badiola A, Repping S, van Wely M. Minimal stimulation IVF vs conventional IVF: a randomized controlled trial. Am J Obstet Gynecol 2016;214:96 e91–96 e98. [DOI] [PubMed] [Google Scholar]
- Zollner U, Zollner KP, Blissing S, Pohls U, Steck T, Dietl J, Muller T. Impact of three-dimensionally measured endometrial volume on the pregnancy rate after intrauterine insemination. Zentralbl Gynakol 2003;125:136–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.