Abstract
STUDY QUESTION
Do the uterine leiomyoma driver events – mediator complex subunit 12 (MED12) mutations, high mobility group AT-hook (HMGA2) overexpression, and fumarate hydratase (FH) inactivation – also contribute to the development of uterine adenomyomas?
SUMMARY ANSWER
MED12 mutations and FH deficiency occur in a subset of uterine adenomyomas, but at lower frequencies than in leiomyomas.
WHAT IS KNOWN ALREADY
Uterine adenomyomas are benign tumours with clinical features very similar to uterine leiomyomas. Mutations affecting MED12, HMGA2 and FH account for up to 80–90% of leiomyomas, but their contribution to adenomyomas is not known.
STUDY DESIGN, SIZE, DURATION
Formalin-fixed paraffin-embedded adenomyoma samples from 21 patients operated on during 2012–2014 were collected at the pathology department’s archives and analysed for uterine leiomyoma driver events.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Adenomyoma diagnoses were verified by a specialized pathologist and representative areas were marked on haematoxylin-eosin slides. DNA was extracted from the tissue samples and sequenced to detect mutations in MED12. Expression levels of HMGA2 and 2SC, a robust indirect method to detect FH inactivation, were analysed by immunohistochemistry (IHC). The coding region of FH was sequenced in one adenomyoma sample showing strong 2SC staining as well as in the same patient’s normal tissue sample. All patients’ medical histories were collected and reviewed.
MAIN RESULTS AND THE ROLE OF CHANCE
MED12 mutation c.131G > A, p.G44D, the most common mutation in uterine leiomyomas, was identified in two samples (2/21; 9.5%). One adenomyoma displayed strong 2SC positivity and subsequent sequencing revealed a frameshift FH mutation c.911delC, p.P304fs in the tumour. The mutation was also present in the patient’s normal tissue sample, indicating that she has a hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome. HMGA2 protein expression was normal in all adenomyomas.
LIMITATIONS, REASONS FOR CAUTION
Restricted sample size limits the determination of exact mutation frequencies of the studied aberrations in adenomyomas.
WIDER IMPLICATIONS OF THE FINDINGS
Uterine leiomyoma driver mutations do contribute to the development of some adenomyomas. We also report an adenomyoma in the context of hereditary HLRCC syndrome. Despite clinical similarities, the pathogenic mechanisms of adenomyomas and leiomyomas are likely different. Large-scale genomic analyses are warranted to elucidate the complete molecular background of adenomyomas.
STUDY FUNDING/COMPETING INTERESTS
This study was supported by The Academy of Finland, the Sigrid Jusélius Foundation, and the Cancer Society of Finland. The authors declare no conflict of interest.
Keywords: uterine adenomyoma, uterine leiomyoma, MED12 mutations, mediator complex subunit 12, high mobility group AT-hook, HMGA2, fumarate hydratase, FH, hereditary leiomyomatosis and renal cell cancer, HLRCC, immunohistochemistry
Introduction
Uterine adenomyosis is characterized by the presence of ectopic endometrial glands in myometrial stroma. It is surrounded by smooth muscle cell hyperplasia and often produces a diffusely enlarged uterus (Benagiano and Brosens, 2006). It is a common condition with the mean prevalence of ~30% at hysterectomy. Adenomyosis can be separated into two categories: diffuse and focal. In the more common diffuse adenomyosis, ectopic endometrial glands are spread diffusely throughout the myometrium, whereas in the focal form there is one large or several small foci of adenomyosis in the myometrium. Foci of adenomyosis within a tumour like mass of smooth muscle are called adenomyomas. Adenomyomas are not usually sharply demarcated from the myometrium, but the border of adenomyoma merges with the myometrium. Adenomyomas can also exhibit a polypoid growth pattern (Taran et al., 2013).
WHAT DOES THIS MEAN FOR PATIENTS?
Adenomyomas are benign tumour-like masses of the uterus. They may cause symptoms like heavy menstrual bleeding, painful periods, and infertility, and surgical treatment may be needed. Adenomyomas resemble uterine leiomyomas, another benign uterine tumour, and sometimes it is difficult for a gynecologist to make a correct diagnosis.
To get a better understanding of why and how adenomyomas develop, we have studied DNA changes in them. Tissue samples from 21 patients were analysed.
This study shows that DNA changes typical for uterine leiomyomas can also be found in a subset of adenomyomas. They explain, however, only a minority of tumours and additional studies are needed to clarify the alterations leading to adenomyoma formation. Understanding the molecular mechanisms underlying tumour development paves the way for better treatment options.
Adenomyosis can be a challenging entity for a clinician, and traditionally the diagnosis is confirmed only after hysterectomy. Common symptoms of adenomyosis and adenomyomas include menorrhagia, chronic pelvic pain, dysmenorrhoea and infertility (Taran et al., 2013; Vercellini et al., 2014). These symptoms are typical also in other gynaecological conditions, such as leiomyomas and endometriosis. Moreover, adenomyosis has been reported to co-exist frequently (15–57% of patients) with uterine leiomyomas (Taran et al., 2013), which are common benign smooth muscle tumours of the uterus. Differentiating adenomyomas from leiomyomas and other uterine conditions can be challenging. Typical features in transvaginal ultrasound include diffuse thickening of myometrium, poorly defined myometrial heterogeneity, and cystic or glandular structures inside the myometrium (Shwayder and Sakhel, 2014). MRI can be used to further improve the diagnostics.
In recent years, extensive studies have brought new key insight into the genetics and pathophysiology of uterine leiomyomas (Stewart, 2015). Uterine leiomyomas are clonal tumours with their growth driven by mutations. The most frequent driver alterations are specific mutations in mediator complex subunit 12 (MED12) and chromosomal rearrangements leading to high mobility group AT-hook (HMGA2) overexpression (Mehine et al., 2014). The third major driver in uterine leiomyomas is biallelic inactivation of fumarate hydratase (FH) encoding an enzyme fumarase of the tricarboxylic acid cycle, typically in patients with hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome (Tomlinson et al., 2002). These three driver mutations, which underlie 80–90% of all uterine leiomyomas, are mutually exclusive (Kämpjärvi et al., 2016; Mäkinen et al., 2017) and tumours with different driver alterations present distinct gene expression profiles (Mehine et al., 2016).
The molecular background of adenomyomas is not well known and most studies looking into their genetic and molecular features have focused on the rare subtype of atypical polypoid adenomyoma (APA) (Takahashi et al., 2014; Nemejcova et al., 2015; Yuan et al., 2017). Here, we have analysed the contribution of uterine leiomyoma driver events—MED12 mutations, HMGA2 overexpression, and FH inactivation—in 21 adenomyoma samples from 21 patients. Identification of the key driver mutations in uterine adenomyomas would significantly contribute to the understanding of their tumorigenesis mechanisms and biological properties. This may, in the future, help the clinical decision making and the development of novel treatment options.
Materials and Methods
Patient samples
The study was approved by the appropriate ethics review board of Hospital District of Helsinki and Uusimaa, Finland (88/13/03/03/2015) and carried out in accordance with the Declaration of Helsinki. All experiments were performed in accordance with relevant guidelines and regulations. The sample series comprises 21 adenomyomas diagnosed at 2012–2014 at the Department of Pathology, Helsinki University Hospital, Finland. The samples were identified from the pathology report database, and archival formalin-fixed paraffin-embedded (FFPE) adenomyoma samples with sufficient amount of representative adenomyoma tissue were collected from the pathology department’s archives. The adenomyoma diagnosis was confirmed by a pathologist (AP) through inspection of haematoxylin-eosin stained histological tissue sections (Fig. 1). Leiomyoma and normal endometrium samples were collected from one patient (patient 1) whose adenomyoma indicated FH deficiency. All samples were collected according to Finnish laws and regulations, with authorization from National Supervisory Authority for Welfare and Health (Valvira; 8522/06.01.03.01/2015).
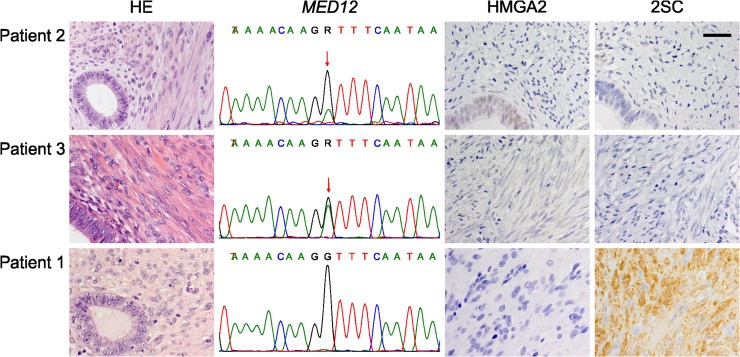
Figure 1.
Analysis of leiomyoma driver mutations reveals MED12 mutations and FH inactivation in adenomyomas. Two adenomyomas (Patients 2 and 3) display mediator complex subunit 12 (MED12) mutations (c.131 G > A, p.G44D) and one (Patient 1) shows 2SC positivity indicating fumarate hydratase (FH) inactivation. HE: haematoxylin-eosin staining, HMGA2: high mobility group AT-hook, 2SC: a robust indirect method to detect FH inactivation. Image magnification 40×, scale bar 50 μm.
Collection of clinical data
Medical records concerning the adenomyoma operation were collected and reviewed for all the patients (Table I). The youngest patient was 34 years old and the oldest 84 years. Endometriosis was diagnosed in five of the 21 patients. Majority of the patients (15/21) were premenopausal at the time of diagnosis. Hormonal treatment had been administered to six patients. Most patients (11/21) had multiple pregnancies, but there were more women that only had one delivery (7/21) than multiparous women (6/21). The most common preoperative symptom that had led to a gynaecologic exam was pelvic pain (10/21), followed by menorrhagia (7/21). Nearly all (18/21) patients had a hysterectomy; three patients who were under 41 years old had undergone myomectomy preserving the uterus and thus fertility. The weight of the removed uterus varied from 25 g to 602 g. The majority of uteri (14/21) contained only one adenomyoma, the diameter of which varied from 1.2 cm to 15 cm. Most of the uteri (11/21) simultaneously had at least one leiomyoma. For four of the patients the postoperative diagnosis was malignant; these patients were all over 65 years old.
Table I.
Clinical characteristics of adenomyoma patients.
| Patient ID | Age (years) | Age at menopause | BMI (kg/m2) | Hormonal treatment | Smoking | Main symptom | Number of adenomyomas | Adenomyoma diameter (cm) | Diffuse adenomyosis | Number of leiomyomas | Uterine weight (g) | Pregnancies (Deliveries) | Endometriosis diagnosed | Cancer diagnosis at operation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | pre | 23 | No | No | Menorrhagia | 1 | 3.5 | No | 1 | na | 1 (0) | No | No |
| 2 | 66 | 53 | 32 | No | No | Postmenopausal bleeding, abdominal resistence | 2 | na | No | Multiple | 490 | na | No | OEC |
| 3 | 34 | pre | 26 | No | na | Pelvic paina | 1 | 5.5 | No | 0 | na | 1 (1) | Yes | No |
| 4 | 40 | pre | na | No | na | Menorrhagia, pelvic pain | Multiple | na | Yes | Multiple | 602 | 2 (1) | No | No |
| 5 | 84 | 50 | 28 | No | No | Otherb | 1 | 1.5 | No | 0 | 52 | 5 (3) | No | No |
| 6 | 53 | pre | 25 | LNG-IUD | No | Menorrhagia, pelvic pain | 1 | 8 | Yes | 0 | 486 | 5 (1) | No | No |
| 7 | 49 | pre | 19 | LNG-IUD | na | Dysmenorrhoea, metrorragia, pelvic pain | Multiple | na | No | Multiple | 331 | 2 (0) | Yes | No |
| 8 | 43 | pre | 30 | COC | Yes | Pelvic pain | 1 | 3.5 | No | 0 | 146 | 0 | Yes | No |
| 9 | 67 | 48 | 40 | No | No | Postmenopausal bleeding | 2 | 4.5; 2 | No | 0 | 123 | 3 (1) | Yes | EEC |
| 10 | 53 | pre | na | HRT | na | Menorrhagia | Multiple | na | Yes | 0 | 188 | 2 (0) | No | No |
| 11 | 71 | 53 | 24 | No | No | Other | 1 | 1.2 | Yes | Multiple | 25 | 0 | No | FTC |
| 12 | 60 | 53 | 31 | No | No | Other | 1 | 1.2 | Yes | 0 | 95 | 4 (1) | No | No |
| 13 | 47 | pre | 30 | No | na | Dysmenorrhoea, pelvic pain | Multiple | na | No | Multiple | 140 | 1 (1) | Yes | No |
| 14 | 46 | pre | na | LNG-IUD | na | Pelvic pain | 1 | 1.2 | Yes | 1 | 114 | 4 (3) | No | No |
| 15 | 50 | pre | na | No | na | Menorrhagia, dysmenorrhoea | 1 | na | Yes | Multiple | 195 | 1 (1) | No | No |
| 16 | 51 | pre | 21 | No | No | Dysmenorrhoea, pelvic pain, urinary frequence | 1 | 15 | No | 0 | 476 | 4 (2) | No | No |
| 17 | 46 | pre | 26 | No | No | Dysmenorrhoea, pelvic pain | 1 | 4 | Yes | 0 | 116 | 3 (2) | No | No |
| 18 | 40 | pre | 18 | No | No | Pelvic pain, menorrhagia | 1 | 8 | No | 0 | na | 0 | No | No |
| 19 | 66 | 60 | 30 | No | na | Postmenopausal bleeding | 1 | na | Yes | Multiple | 120 | 2 (2) | No | EEC |
| 20 | 48 | pre | na | LNG-IUD | na | Menorrhagia | Multiple | na | No | Multiple | 211 | na | na | No |
| 21 | 44 | pre | na | na | na | Other | 1 | na | No | Multiple | 229 | na (3) | na | No |
Pre: premenopausal, LNG-IUD: levonorgestrel intrauterine device, COC: combined oral contraceptive, HRT: hormone replacement therapy, OEC: ovarian endometrioid carcinoma, EEC: endometrial endometrioid carcinoma, FTC: fallopian tube carcinoma.
aPelvic pain contains lower abdominal pain and defecation pain.
bOther includes uterine prolapse; and mild or unclear symptoms.
Tissue microarray construction
A tissue microarray (TMA) was constructed with 0.8 mm cores acquired from the representative areas of the FFPE blocks selected by the pathologist (AP). Four cores from each sample were punched into an empty paraffin block using a manual tissue arrayer (MTA-I, Beecher Instruments, Sun Prairie, WI, USA). Normal myometrium tissue from two separate FFPE blocks and from two FH negative HLRCC uterine leiomyoma samples were also included in the TMA.
Immunohistochemistry
Immunostainings were performed on 5 μm sections of TMAs or FFPE blocks. HMGA2 expression levels were assessed using an anti-HMGA2 antibody (59170AP, Biocheck Inc., Foster City, CA, USA). Biallelic FH inactivation was analysed indirectly with 2-succinocysteine (2SC) staining, which is based on the recognition of modified (succinated) proteins formed in FH-deficient cells caused by the accumulation of fumarate, as described previously (Nagai et al., 2007; Bardella et al., 2011). A microwave oven was used for antigen retrieval with citrate buffer (pH 6.0). Endogenous peroxidase blocking was followed by incubating with the primary antibody at 4°C (anti-HMGA2 1:2000, anti-2SC 1:2000) overnight. Immunohistochemical staining for HMGA2 and 2SC was detected by the BrightVision system (Immunologic, Duiven, Netherlands) and DAB Quanto system (Thermo Fisher Scientific, Waltham, MA, USA).
All immunostainings were scored independently by two pathologists (AP and RB). For all the antibodies used, the intensity of the immunoreaction was classified into four groups: −= fully negative, (+) = single cell positivity, + = low positivity, ++ = strong positivity. Samples displaying strong positivity were scored as positive. Only nuclear labelling was evaluated for HMGA2. Previously analysed whole section uterine leiomyoma samples were stained simultaneously and used as negative and positive controls for both HMGA2 and 2SC.
DNA extraction and mutation screening
Genomic DNA was extracted from seven representative 10 μm FFPE tissue sections using a ReliaPrep FFPE gDNA Miniprep System (Promega, Madison, WI, USA). If the area of adenomyoma tissue in the FFPE block was small, DNA was extracted from six 0.8 mm cores acquired from the representative areas. MED12 exons 1 and 2 and the coding region of FH were sequenced as described previously (Kämpjärvi et al., 2014; Kämpjärvi et al., 2016), at the Institute of Molecular Medicine Finland, Helsinki, Finland, using Applied Biosystems ABI3730 Automatic DNA Sequencer. The sequence traces were analysed using Mutation Surveyor software (SoftGenetics, State College, PA, USA) and visual inspection. All mutation positive samples were further independently replicated.
Results
All samples were successfully analysed for MED12 exon 1 and 2 mutations. MED12 mutations were identified in two adenomyoma samples (2/21; 9.5%; Table II). Both mutations were c.131 G > A, p.G44D missense changes, which is the most frequent mutation also in uterine leiomyomas (Fig. 1). The first patient with a MED12 mutation positive tumour (Patient 3) was 34 years old at the time of operation. She had previously been pregnant twice and had one delivery by caesarean section. Her main symptom was pelvic pain. A preoperative transvaginal ultrasonography showed a leiomyoma-like lesion in the uterus as well as two large endometriomas. The endometriomas and the uterine lesion were removed by laparotomy. A pathologist’s examination confirmed the adenomyoma diagnosis. The second patient with a MED12 mutation positive adenomyoma (Patient 2) was 66 years old at the time of operation. She hadn’t had any deliveries and age at menopause was 53 years. She presented with postmenopausal bleeding. Subsequent transvaginal ultrasonography revealed a pelvic tumour, which according to computed tomography and MRI scans seemed to be of ovarian origin. A laparotomy was then performed, including hysterectomy, and removal of both adnexes, appendix and pelvic lymph nodes. Postoperative diagnosis after pathologist’s examination was ovarian endometrioid carcinoma with no metastases. Two adenomyomas and multiple leiomyomas were found in the uterus.
Table II.
MED12 mutation status and 2SC and HMGA2 immunohistochemistry scores.
| Patient ID | MED12 mutation | 2SC Score | HMGA2 Score |
|---|---|---|---|
| 1 | wt | Pos | Neg |
| 2 | c.131 G > A, p.G44D | Neg | Neg |
| 3 | c.131 G > A, p.G44D | Neg | Neg |
| 4 | wt | Neg | Neg |
| 5 | wt | Neg | Neg |
| 6 | wt | Neg | Neg |
| 7 | wt | Neg | Neg |
| 8 | wt | Neg | Neg |
| 9 | wt | Neg | Neg |
| 10 | wt | Neg | Neg |
| 11 | wt | Neg | Neg |
| 12 | wt | Neg | Neg |
| 13 | wt | Neg | Neg |
| 14 | wt | Neg | Neg |
| 15 | wt | Neg | Neg |
| 16 | wt | Neg | Neg |
| 17 | wt | Neg | Neg |
| 18 | wt | Neg | Neg |
| 19 | wt | Neg | Neg |
| 20 | wt | Neg | Neg |
| 21 | wt | Neg | Neg |
Wt: wild type, MED12: mediator complex subunit 12, HMGA2: high mobility group AT-hook, 2SC: a robust indirect method to detect FH inactivation.
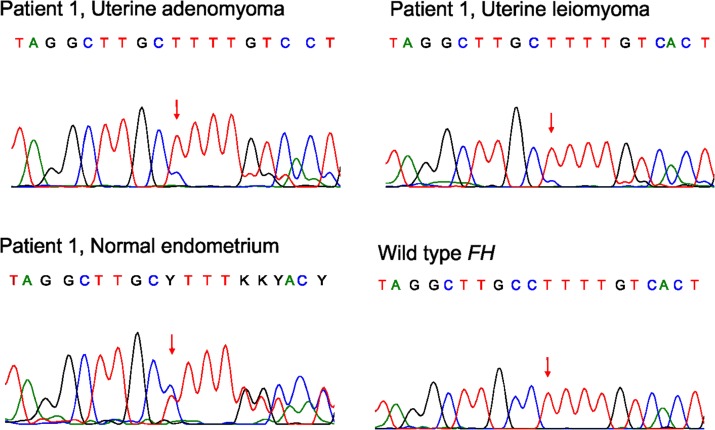
Positive 2SC staining indicated accumulation of succinated proteins and thus biallelic FH inactivation in one adenomyoma sample (Fig. 1). Patient 1 was 38 years old at the time of operation in 2012. She had previously had one pregnancy but no deliveries. Large solitary leiomyomas had been previously removed in abdominal myomectomies in 2000 and 2007. In addition, hysteroscopic resection of a submucotic leiomyoma had been performed three times in 2011. Her main symptom was menorrhagia. Preoperative transvaginal ultrasonography showed two leiomyoma-like lesions in the uterus, which were then removed in abdominal myomectomy. The lesions were clearly separate, one of them in the posterior and the other in the fundal part of the uterus. The pathologist confirmed one lesion as a leiomyoma and the other as an adenomyoma. Direct sequencing revealed an FH mutation c.911delC, p.P304fs in exon 7 leading to a premature stop codon 25 codons later (Fig. 2). The same mutation was present also in the leiomyomyoma sample. Both tumours displayed mostly the mutant allele indicating loss of heterozygosity. Normal endometrium sample was retrieved from the pathology department archive, and sequencing revealed that the mutation was of germline origin. The patient thus has an HLRCC syndrome.
Figure 2.
Germline FH mutation (c.911delC, p.304fs) in a patient with both adenomyoma and leiomyoma. Normal endometrium sample confirms the germline origin of the mutation.
All adenomyoma samples expressed normal levels of HMGA2. Two pathologists agreed on the results of all IHC stainings.
Discussion
Adenomyoma is a common gynaecological condition causing considerable burden to the health care system and affecting the quality of life of a large number of women. As a research topic the disease has, however, been neglected and little is known about its molecular background. Some studies have suggested that the pathogenic mechanisms behind adenomyosis might be similar to endometriosis. According to this concept, adenomyosis and endometriosis would result from auto-traumatization of the uterus during its physiological functions related to the reproductive cycle (Leyendecker et al., 2015). As the most prominent component of adenomyoma is the smooth muscle surrounding the endometrial glands and endometrial-type stroma, we wanted to analyse whether the known uterine leiomyoma driver mutations, namely MED12 mutations, HMGA2 overexpression and FH inactivation, also contribute to the development of these tumours.
A MED12 c.131 G > A, p.G44D mutation was identified in two out of 21 adenomyomas studied (9.5%). The mutation frequency is thus significantly lower in adenomyomas than in uterine leiomyomas, of which ~70% are MED12 mutation positive (Mehine et al., 2014). Similar specific MED12 mutations have been identified at high frequency also in other, predominantly benign female tumours, such as breast fibroadenomas (Lim et al., 2014) and phyllodes tumours (Cani et al., 2015) and in 7–20% of highly malignant uterine leiomyosarcomas (Kämpjärvi et al., 2012; Pérot et al., 2012; Mäkinen et al., 2016). A single APA with a MED12 mutation has been previously reported (Markowski et al., 2012). Histologically, APA closely resembles endometrioid adenocarcinoma and it is still unclear whether APA is a precancerous form of endometrioid adenocarcinoma (Kisu et al., 2011). The mutation in the APA sample is the same c.131 G > A, p. G44D alteration as the mutation identified in the current study. No MED12 mutations were identified in adenomyoma samples from Chinese patients (Wang et al., 2015). MED12 is the component of Mediator complex, which is a large evolutionarily conserved multisubunit protein complex. Mediator complex is the major regulator of transcription of a plethora of genes, many of which are known drivers of tumorigenesis (Clark et al., 2015). The MED12 exon 1 and 2 mutations affect the functions of MED12 by disrupting its interactions with other Mediator kinase module components leading to a diminished Mediator-associated CDK8 kinase activity (Kämpjärvi et al., 2014; Turunen et al., 2014). In a mouse study, Med12 mutations have been reported to promote the formation of leiomyoma-like lesions with a gain-of-function effect (Mittal et al., 2015).
One adenomyoma sample displayed positive 2SC staining indicating loss of FH function. This was confirmed by further analyses, which identified a frameshift mutation in the FH gene. Loss of heterozygosity at the FH locus was evident in the tumour, which mostly displayed the mutant allele. A separate leiomyoma that had been removed during the same operation displayed similar molecular features. The germline origin of the mutation was confirmed in a normal tissue sample and the patient thus has an HLRCC syndrome. HLRCC is a rare hereditary syndrome that predisposes individuals to cutaneous leiomyomas, uterine leiomyomas and renal cell carcinoma, specifically the aggressive type 2 papillary carcinoma (Tomlinson et al., 2002; Patel et al., 2017). Both cutaneous and uterine leiomyomas are typically numerous and appear at a young age (Patel et al., 2017). The possibility of HLRCC should be kept in mind when treating women suffering from multiple and recurrent leiomyomas. If genetic testing confirms a germline mutation in FH, regular ultrasonography or MRI controls are advised to detect potentially developing malignant changes in kidneys. Mutation carriers should also be informed on the potential effect of the syndrome on fertility, and family members should be offered genetic counselling and mutation screening. Rarely, also other tumours such as breast cancer and ovarian cystadenomas have been associated with HLRCC (Patel et al., 2017), but adenomyomas have not been previously reported in the context of HLRCC.
Hysterectomy samples for endometrial adenocarcinoma frequently contain lesions of adenomyosis. The malignant potential of adenomyosis is, however, considered low. In a review article, 44 cases of malignant tumours originating in adenomyosis tissue with an otherwise normal endometrium were reported (Koike et al., 2013). Most patients were postmenopausal, and the symptoms were similar to endometrial cancer. Histologically, the most common subtype was endometrioid carcinoma. Various premalignant changes in the endometrial epithelium of adenomyosis were also recognized, but the overall mechanism and molecular changes behind the possible transformation process are thus far unclear.
The modern trend of postponing pregnancy until later years of reproductive life makes adenomyomas relevant to younger women as well. Increasing evidence shows that adenomyosis impairs fertility, although the underlying mechanisms are unclear (Gordts et al., 2018). Moreover, the risk of uterine rupture in future pregnancies seems to be higher after surgical treatment of adenomyomas compared to myomectomy (Ukita et al., 2011; Nagao et al., 2015). An understanding of the pathogenesis of adenomyomas might facilitate the development of effective conservative treatment options, and hence improve fertility.
Here we show that MED12 mutations, the most common driver event in uterine leiomyomas, can be found in adenomyomas. Our results also show that adenomyomas may develop through biallelic FH inactivation. Overall, leiomyoma driver mutations underlie only a small subset of adenomyomas implying that despite the clinical similarities between these tumours, the pathogenic mechanisms are likely mostly different. Further large-scale studies are warranted to clarify the complete molecular background of uterine adenomyomas and the role they might play in the development of malignant tumours.
Acknowledgements
Annukka Ruokolainen is acknowledged for technical assistance and Biomedicum Imaging Unit for imaging facilities. Dr Norma Frizell (University of South Carolina) is acknowledged for providing the 2SC antibody.
Authors’ roles
All authors have significantly contributed to the present work. T.H., A.Ä., J.H. and T.A. performed the experiments and analysed the data with P.V. R.B. and A.P. provided the patient samples and performed the pathological evaluations. T.H., A.Ä., T.A. and P.V. wrote the manuscript. All authors commented and approved the final manuscript.
Funding
This study was financially supported by the Academy of Finland (#260370 and #292769 for PV, #295640 for T.H.), the Sigrid Jusélius Foundation, and the Cancer Society of Finland.
Conflict of interest
The authors declare no conflict of interest.
References
- Bardella C, El-Bahrawy M, Frizzell N, Adam J, Ternette N, Hatipoglu E, Howarth K, O’Flaherty L, Roberts I, Turner G et al. . Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol 2011;225:4–11. [DOI] [PubMed] [Google Scholar]
- Benagiano G, Brosens I. History of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20:449–463. [DOI] [PubMed] [Google Scholar]
- Cani AK, Hovelson DH, McDaniel AS, Sadis S, Haller MJ, Yadati V, Amin AM, Bratley J, Bandla S, Williams PD et al. . Next-Gen sequencing exposes frequent MED12 mutations and actionable therapeutic targets in phyllodes tumors. Mol Cancer Res 2015;13:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AD, Oldenbroek M, Boyer TG. Mediator kinase module and human tumorigenesis. Crit Rev Biochem Mol Biol 2015;50:393–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril 2018;109:380–388.e1. [DOI] [PubMed] [Google Scholar]
- Kisu I, Banno K, Yanokura M, Kobayashi Y, Ueki A, Ono A, Masuda K, Yamagami W, Nomura H, Hirasawa A et al. . Atypical Polypoid Adenomyoma (APAM) of the uterine: relationship with endometrial cancer. J Cancer Ther 2011;2:458–462. [Google Scholar]
- Koike N, Tsunemi T, Uekuri C, Akasaka J, Ito F, Shigemitsu A, Kobayashi H. Pathogenesis and malignant transformation of adenomyosis (review). Oncol Rep 2013;29:861–867. [DOI] [PubMed] [Google Scholar]
- Kämpjärvi K, Mäkinen N, Kilpivaara O, Arola J, Heinonen HR, Böhm J, Abdel-Wahab O, Lehtonen HJ, Pelttari LM, Mehine M et al. . Somatic MED12 mutations in uterine leiomyosarcoma and colorectal cancer. Br J Cancer 2012;107:1761–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpjärvi K, Mäkinen N, Mehine M, Valipakka S, Uimari O, Pitkanen E, Heinonen HR, Heikkinen T, Tolvanen J, Ahtikoski A et al. . MED12 mutations and FH inactivation are mutually exclusive in uterine leiomyomas. Br J Cancer 2016;114:1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpjärvi K, Park MJ, Mehine M, Kim NH, Clark AD, Bützow R, Böhling T, Böhm J, Mecklin JP, Järvinen H et al. . Mutations in Exon 1 highlight the role of MED12 in uterine leiomyomas. Hum Mutat 2014;35:1136–1141. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G, Bottcher B, Wildt L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet 2015;291:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WK, Ong CK, Tan J, Thike AA, Ng CC, Rajasegaran V, Myint SS, Nagarajan S, Nasir ND, McPherson JR et al. . Exome sequencing identifies highly recurrent MED12 somatic mutations in breast fibroadenoma. Nat Genet 2014;46:877–880. [DOI] [PubMed] [Google Scholar]
- Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids – their relationship to cytogenetic subgroups. Int J Cancer 2012;131:1528–1536. [DOI] [PubMed] [Google Scholar]
- Mehine M, Kaasinen E, Heinonen HR, Mäkinen N, Kämpjärvi K, Sarvilinna N, Aavikko M, Vähärautio A, Pasanen A, Bützow R et al. . Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci USA 2016;113:1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril 2014;102:621–629. [DOI] [PubMed] [Google Scholar]
- Mittal P, Shin YH, Yatsenko SA, Castro CA, Surti U, Rajkovic A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J Clin Invest 2015;125:3280–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen N, Aavikko M, Heikkinen T, Taipale M, Taipale J, Koivisto-Korander R, Bützow R, Vahteristo P. Exome sequencing of uterine leiomyosarcomas identifies frequent mutations in TP53, ATRX, and MED12. PLoS Genet 2016;12:e1005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen N, Kämpjärvi K, Frizzell N, Bützow R, Vahteristo P. Characterization of MED12, HMGA2, and FH alterations reveals molecular variability in uterine smooth muscle tumors. Mol Cancer 2017;16:101–017-0672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai R, Brock JW, Blatnik M, Baatz JE, Bethard J, Walla MD, Thorpe SR, Baynes JW, Frizzell N. Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. J Biol Chem 2007;282:34219–34228. [DOI] [PubMed] [Google Scholar]
- Nagao Y, Osato K, Kubo M, Kawamura T, Ikeda T, Yamawaki T. Spontaneous uterine rupture in the 35th week of gestation after laparoscopic adenomyomectomy. Int Med Case Rep J 2015;9:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemejcova K, Kenny SL, Laco J, Skapa P, Stanek L, Zikan M, Kleiblova P, McCluggage WG, Dundr P. Atypical polypoid adenomyoma of the uterus: an immunohistochemical and molecular study of 21 cases. Am J Surg Pathol 2015;39:1148–1155. [DOI] [PubMed] [Google Scholar]
- Patel VM, Handler MZ, Schwartz RA, Lambert WC. Hereditary leiomyomatosis and renal cell cancer syndrome: an update and review. J Am Acad Dermatol 2017;77:149–158. [DOI] [PubMed] [Google Scholar]
- Pérot G, Croce S, Ribeiro A, Lagarde P, Velasco V, Neuville A, Coindre JM, Stoeckle E, Floquet A, MacGrogan G et al. . MED12 alterations in both human benign and malignant uterine soft tissue tumors. PLoS One 2012;7:e40015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwayder J, Sakhel K. Imaging for uterine myomas and adenomyosis. J Minim Invasive Gynecol 2014;21:362–376. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med 2015;372:1646–1655. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yoshida T, Matsumoto T, Kameda Y, Takano Y, Tazo Y, Inoue H, Saegusa M. Frequent beta-catenin gene mutations in atypical polypoid adenomyoma of the uterus. Hum Pathol 2014;45:33–40. [DOI] [PubMed] [Google Scholar]
- Taran FA, Stewart EA, Brucker S. Adenomyosis: epidemiology, risk factors, clinical phenotype and surgical and interventional alternatives to hysterectomy. Geburtshilfe Frauenheilkd 2013;73:924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S et al. . Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002;30:406–410. [DOI] [PubMed] [Google Scholar]
- Turunen M, Spaeth JM, Keskitalo S, Park MJ, Kivioja T, Clark AD, Mäkinen N, Gao F, Palin K, Nurkkala H et al. . Uterine leiomyoma-linked MED12 mutations disrupt mediator-associated CDK activity. Cell Rep 2014;7:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukita S, Koshiyama M, Yamaguchi A, Ueda M, Ukita M, Hishikawa K, Kakui K, Kim T. Total uterine rupture during pregnancy after an adenomyomectomy. Am J Case Rep 2011;12:106–109. [Google Scholar]
- Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod 2014;29:964–977. [DOI] [PubMed] [Google Scholar]
- Wang H, Ye J, Qian H, Zhou R, Jiang J, Ye L. High-resolution melting analysis of MED12 mutations in uterine leiomyomas in Chinese patients. Genet Test Mol Biomarkers 2015;19:162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CT, Huang WC, Lee CH, Lin MC, Lee CH, Kao YC, Huang HY, Kuo KT, Lee JC. Comprehensive screening for MED12 mutations in gynaecological mesenchymal tumours identified morphologically distinctive mixed epithelial and stromal tumours. Histopathology 2017;70:954–965. [DOI] [PubMed] [Google Scholar]