Abstract
STUDY QUESTION
Are the transient receptor potential cation channels vanilloid 3 (TRPV3) present and able to mediate strontium (Sr2+) induced artificial activation in human oocytes?
SUMMARY ANSWER
Sr2+ did not induce Ca2+ rises or provoke activation in human oocytes, however, mRNA for the TRPV3 channel was present in metaphase II (MII) human oocytes after IVM and TRPV3 agonists induced Ca2+ rises and oocyte activation, demonstrating the channels were functional.
WHAT IS KNOWN ALREADY
Selective activation of TRPV3 by agonists induces Ca2+ entry and promotes mouse oocyte activation, and the absence of TRPV3 channels in mouse oocytes prevents Sr2+ mediated artificial activation. Sr2+ is sometimes used to overcome fertilization failure after ICSI in the clinic, but its efficiency is still controversial and the mechanism(s) of how it mediates the Ca2+ flux has not been studied yet in human.
STUDY DESIGN, SIZE, DURATION
The protein distribution (n = 10) and mRNA expression level (n = 19) of the TRPV3 channels was investigated in human MII oocytes after IVM. The Sr2+ (10 mM) and TRPV3 agonists (200 μM 2-aminoethoxydiphenyl borate [2-APB] and 200 μM carvacrol)-induced Ca2+ response was analyzed in human (n = 15, n = 16 and n = 16, respectively) and mouse oocytes (n = 15, n = 19 and n = 26, respectively). The subsequent embryonic developmental potential following the parthenogenetic activation using these three agents was recorded in human (n = 10, n = 9 and n = 9, respectively) and mouse (n = 20 per agent) oocytes, by determining pronucleus, or 2-cell and blastocyst formation rates.
PARTICIPANTS/MATERIALS, SETTING, METHODS
MII oocytes from B6D2F1 mice (6–10 weeks old) as well as human IVM oocytes and IVO oocytes (from patients aged 25–38 years old) with aggregates of smooth endoplasmic reticulum clusters were used. The expression of TRPV3 channels was determined by immunofluorescence staining with confocal microscopy and RT-PCR, and the temporal evolution of intracellular Ca2+ concentration was measured by time-lapse imaging after exposure to Sr2+ and TRPV3 agonists (2-APB and carvacrol). Artificial activation efficiency was assessed using these agents.
MAIN RESULTS AND THE ROLE OF CHANCE
Sr2+ did not promote Ca2+ oscillations or provoke activation in human oocytes. Transcripts of TRPV3 channels were present in IVM MII human oocytes. TRPV3 protein was expressed and distributed throughout the ooplasm of human oocytes, rather than particularly concentrated in plasma membrane as observed in mouse MII oocytes. Both agonists of TRPV3 (2-APB and carvacrol), promoted a single Ca2+ transient and activated a comparable percentage of more than half of the exposed human oocytes (P > 0.05). The agonist 2-APB was also efficient in activating mouse oocytes, however, significantly fewer mouse oocytes responded to carvacrol than 2-APB in both the Ca2+ analysis and activation test (P < 0.001).
LIMITATIONS REASONS FOR CAUTION
The availability of fresh IVO matured oocytes in human was limited. Data from TRPV3 knockout model are not included.
WIDER IMPLICATIONS OF THE FINDINGS
The benefit of clinical application using Sr2+ to overcome fertilization failure after ICSI requires further validation.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by FWO-Vlaanderen, China Scholarship Council and Special Research Fund from Ghent University (Bijzonder Onderzoeksfonds, BOF). No competing interests are declared.
Keywords: failed fertilization, ICSI, assisted oocyte activation, TRPV3 channels, Ca2+, release, human oocytes, embryo development, strontium, ionomycin
Introduction
Mammalian oocyte activation is triggered by sperm factor phospholipase C zeta (PLCζ), which initiates a series of oscillations of Ca2+ levels within the ooplasm (Saunders et al., 2002; Tesarik, 2002). The PLCζ hydrolyzes the precursor lipid phosphatidylinositol 4,5-bisphosphate to form both diacylglycerol and inositol 1,4,5-trisphosphate (IP3). Further downstream, the IP3 binds to its cognate receptors (IP3Rs) present in the oocyte and thus generates the Ca2+ release from endoplasmic reticulum (ER) (Fedorenko et al., 2014; Mak and Foskett, 2015). To enable the series of oscillations, extracellular Ca2+ influx is further required to restore the Ca2+ concentration in the ER during oocyte activation (Miao et al., 2012; Wakai et al., 2013). As such, the Ca2+ signaling mediates successful fertilization and plays a vital role in supporting further embryonic development (Ramadan et al., 2012; Wakai et al., 2013). Irregularities in Ca2+ oscillation pattern of the oocyte during activation may thus prevent successful fertilization and reduce embryonic developmental potential (Ajduk et al., 2011; Miao and Williams, 2012).
What does this mean for patients?
This study looks at a way of trying to overcome fertilization failure after fertility treatment by helping the processes which occur in the egg at this time. Sometimes a substance called strontium is used in clinics to try to initiate fertilization if ICSI is not working. It is still controversial as it is not known how it might work, although there is some evidence that it works in mice.
In this study the researchers looked at both human and mouse eggs. They looked closely at the possible way that strontium initiates fertilization and development of the eggs, and found that strontium did not seem to work with human eggs and that the processes involved were different. They conclude that more evidence is needed about any possible benefits of using strontium when ICSI is not working.
In IVF centers worldwide, deficiencies during oocyte activation are associated with fertilization failure (FF) or low fertilization rate following treatment involving ICSI (Rawe et al., 2000; Yanagida, 2004; Swain and Pool, 2008; Neri et al., 2014). The oocyte activation potential of sperm from patients who experienced FF can be evaluated in heterologous ICSI models (Araki et al., 2004; Heindryckx et al., 2005). Thereafter, the technology of assisted oocyte activation (AOA) is frequently applied during ICSI, with the principle of inducing a Ca2+ increase within the FF oocytes. Thus far, a number of physical, mechanical and chemical AOA methods have been evaluated, with successful oocyte activation and development to term after applying electrical pulses (Egashira et al., 2009), modified ICSI procedures (Tesarik et al., 2002), Ca2+ ionophores (Heindryckx et al., 2008; Ebner et al., 2012; Vanden Meerschaut et al., 2014) and strontium chloride (SrCl2) (Kim et al., 2012, 2014).
Despite several studies reporting successful pregnancies obtained following AOA treatment with Sr2+ (Yanagida et al., 2006; Kyono et al., 2008; Kim et al., 2012, 2014), the efficiency of Sr2+ as an activating agent for human oocytes is still under debate. In contrast to mice and rats oocytes studies, in which repetitive Ca2+ transients are observed when Sr2+ is used (Whittingham and Siracusa, 1978; Roh et al., 2003), there is a lack of evidence supporting the same for human oocytes (Versieren et al., 2010). Recently in mouse, transient receptor potential cation channels, subfamily V, vanilloid 3 (member 3) (TRPV3) were identified to mediate Sr2+ induced oocyte activation (Carvacho et al., 2013; Lee et al., 2016). The TRPV3 are highly temperature-dependent channels (Peier et al., 2002; Smith et al., 2002) and are modulated by various stimuli and ligands, including natural compounds, such as carvacrol, thymol and eugenol, as well as 2-aminoethoxydiphenyl borate (2-APB) (Ramsey et al., 2006; Xu et al., 2006). These agonists, in particular 2-APB and carvacrol, showed their capacity to activate TRPV3 channels and promote Ca2+ influx, and as consequence, provoked mouse oocyte activation (Carvacho et al., 2013; Lee et al., 2016).
In animal studies, Sr2+ has shown to be a highly efficient activating agent in parthenogenetic activation (Kishikawa et al., 1999; Versieren et al., 2010) and somatic cell nuclear transfer of mouse (Otaegui et al., 1999), bovine (Méo et al., 2004; Yamazaki et al., 2005) and porcine oocytes (Che et al., 2007). Moreover, Sr2+ was reported to be the most effective AOA method in a mouse model with deficient sperm activation capacity (Vanden Meerschaut et al., 2013) and more recently in a knockout mouse model for PLCζ (Hachem et al., 2017), compared to other agents. Since Sr2+ induces Ca2+ rises similar to the peaks provoked by rodent sperm, it is of interest to investigate the Sr2+ triggered Ca2+ release and oocytes activation, as well as the expression and functionality of the TRPV3 channels in human oocytes. In addition, the TRPV3 channel agonists, such as 2-APB and carvacrol, could be used as an alternative AOA method in human.
Materials and Methods
All chemicals were purchased from Sigma-Aldrich (Bornem, Belgium) unless otherwise indicated.
Ethical approval
The study was approved by the local Ethical Committee of the Ghent University Hospital, Belgium (2009/130, 2010/808 and 2010/182). Written informed consents were obtained from all patients. All procedures involving animal handling and sacrifice were approved by the Ghent University Hospital Ethical Committee for Laboratory Animals (ECD no. 11/41).
Source and culture of human oocytes
Patients (25–38 years old) undergoing ICSI treatment at the Ghent University Hospital between October 2014 and June 2015, were included in this study. Patients undergoing a hormone stimulated cycle were administrated a GnRH agonist (Decapeptyl; Ferring, Belgium) or antagonist (Cetrotide; Merck Serono, Germany). Ovarian stimulation was performed by administering hMG (Menopur; Ferring, Belgium) or recombinant (rec) FSH (Gonal-F; Merck Serono, Germany) at a dose of 112.5–300 IU daily and ovulation was induced with 5000 IU hCG (Pregnyl;MSD, Belgium). Oocytes were enzymatically denuded by brief exposure to 80 IU/ml hyaluronidase (Irvine Scientific, Belgium), followed by mechanical denudation prior to ICSI. Nuclear status was assessed and classified as germinal vesicle (GV) (presence of a GV structure), metaphase I (MI) (absence of a both polar body and a GV structure) or metaphase II (MII) stage (presence of a polar body and absence of a GV structure). Donated GV oocytes were further cultured in medium 199, supplemented with 10 ng/ml epidermal growth factor, 1 mg/ml estradiol, 10 mIU/ml recFSH, 0.5 mIU/ml hCG, 1 mM l-glutamine, 0.3 mM sodium pyruvate, 0.8% (v/v) human serum albumin (Red Cross, Belgium), 100 IU/ml penicillin G and 100 mg/ml streptomycin sulfate for 24 h. Immature MI stage oocytes were cultured in Sydney IVF COOK cleavage (CC) medium (Cook Ireland Ltd, Belgium) for 3 h or 24 h based on the first polar body extrusion. All oocytes were cultured at 37°C in 6% CO2 and 5% O2.In vivo matured (IVO) fresh MII oocytes showing visible aggregates of tubular smooth ER clusters (SERs) were collected as well, for this study. Although fertilized SER oocytes can lead to healthy live births, the clinical application of them is still under critical review at our Department, owing to the previously reported malformations and impaired pregnancy outcomes (Otsuki et al., 2004; Sa et al., 2011; Itoi et al., 2016). We included a total of 104 oocytes (37 GV–MII, 46 MI–MII and 21 IVO MII with SERs), allocated to the different groups. The distribution of IVM and IVO MII oocytes across the same sets of experiments was analyzed using the Chi-square test, and no significant difference was observed (Table I, P > 0.05).
Table I.
The allocations of IVM and IVO human oocytes across all groups in the study.
| No. oocytes (n) | GV–MII n (%) | MI–MII n (%) | IVO with SERs n (%) | ||
|---|---|---|---|---|---|
| Ca2+ imaging | Sr2+ | 15 | 6 (40%) | 6 (40%) | 3 (20%) |
| 2-APB | 16 | 6 (38%) | 6 (38%) | 4 (25%) | |
| Carvacrol | 16 | 8 (50%) | 5 (31%) | 3 (19%) | |
| Immunostaining | TRPV3 | 10 | 4 (40%) | 6 (60%) | n/a |
| RT-PCR | Group I | 11 | 3 (27%) | 8 (73%) | n/a |
| Group II | 8 | 2 (25%) | 5 (63%) | 1 (12%) | |
| Activation test | Sr2+ | 10 | 2 (20%) | 4 (40%) | 4 (40%) |
| 2-APB | 9 | 3 (33%) | 3 (33%) | 3 (33%) | |
| Carvacrol | 9 | 3 (33%) | 3 (33%) | 3 (33%) |
n/a, Not applicable.
IVO: in vivo matured.
SER: aggregates of tubular smooth endoplasmic reticulum clusters.
2-APB: 2-aminoethoxydiphenyl borate.
TRPV3: transient receptor potential cation channels vanilloid 3.
MI: metaphase I.
MII: metaphase II.
Source and culture of mouse oocytes
B6D2F1 hybrid female mice (6–10-week-old) were stimulated with 7.5IU pregnant mare’s serum gonadotrophin (PMSG, Folligon®, Intervet, Boxmeer, The Netherlands), followed by 7.5 IU hCG (Chorulon®, Intervet, Boxmeer, The Netherlands) 46–48 h later. MII oocytes were harvested in HEPES-buffered potassium simplex optimized medium (KSOM-HEPES) supplemented with 4 mg/ml bovine serum albumin (BSA, Calbiochem, Belgium) 12–14 h after hCG injection. Cumulus cells surrounding the oocytes were removed by treatment with 200 IU/ml hyaluronidase (0.3 mg/ml, type VIII) in KSOM-HEPES. Oocytes were cultured under paraffin oil at 37°C in 6% CO2 and 5% O2 in KSOM containing 4 mg/ml BSA until further treatments (Lawitts and Biggers, 1991).
Ca2+ imaging in human and mouse oocytes following exposure to Sr2+ and TRPV3 agonists. Human (n = 47) and mouse oocytes (n = 67) were loaded with 7.5 μM of the radiometric Ca2+ sensitive dye Fura-2 acetoxymethyl (AM) ester (Invitrogen, Life Technologies Europe B.V., Belgium) at 37°C in 6% CO2, 5% O2 and for 30 min and then washed extensively. Subsequently, oocytes were placed in individual glass bottom dishes (MatTek, Corporation, Ashland, USA) and Ca2+ imaging was performed on an inverted epi-fluorescence microscope (TH4-200, Olympus Soft Imaging Solutions GmBH, Belgium) with a ×20 objective. Fluorescence was recorded at an emission wavelength of ~510 nm every 5 s. The ratio of both Ca2+ induced signals (340/380 nm) was proportional to the concentration of free intracellular Ca2+ (expressed in arbitrary units, AU).
For measuring the Ca2+ responses of human oocytes following Sr2+ exposure, the fura-2 loaded IVM (n = 12) and SERs (n = 3) oocytes were transferred to a drop of GIBCO®Ca2+/Mg2+ free Earle’s Balanced Salt Solution (EBSS) supplemented with 10 mM SrCl2 (Life Technologies, Leuven, Belgium). The Ca2+ images were recorded every 5 s for a duration of 6 h. Following the first 2 h of exposure, a group of human oocytes (n = 5) that did not respond to the Sr2+ was subsequently exposed to 10 μM ionomycin (cat. no. I9657) dissolved in COOK Cleavage medium for 15 min. The Ca2+ images were acquired to assess their ability to mobilize the intracellular Ca2+. The Sr2+ induced Ca2+ oscillations of mouse oocytes were recorded every 5 s for a duration of 2 h, immediately after transferring the eggs to a drop of Ca2+-free KSOM with 10 mM SrCl2, in the glass bottom dish.
Following the application of TRPV3 agonists, the Ca2+ responses of both human and mouse oocytes were investigated. Ca2+ images were recorded from human oocytes exposed to 200 μM 2-APB (n = 16) or 200 μM carvacrol (n = 16) in 1% polyvinyl alcohol (PVA)-supplemented albumin-free IVF™ medium (Vitrolife, Göteborg, Sweden) for 30 min. The PVA was previously reported to substitute for BSA in mouse embryo culture medium following TRPV3 agonists activation (Carvacho et al., 2013). Mouse oocytes were subjected to the same concentration of the agonists but supplemented in 1% PVA KSOM (BSA-free) medium for 30 min. The stock solutions of TRPV3 agonists were prepared by dissolving them in dimethyl sulphoxide (DMSO), as carried out for ionomycin in our clinic (Heindryckx et al., 2008). The amount of DMSO added to the activation medium was tested and shown not to induce Ca2+ release or activation of mouse oocytes.
All oocytes were distributed randomly across the groups and tested within 2 h after assessing the maturation state. A maximum of three oocytes were measured simultaneously. Baseline drifting was adjusted before retrieving values for amplitude (value at maximum increase in fluorescence intensity per peak) expressed in AU. Relative amplitude (RA, amplitude subtracted from the baseline and expressed in AU) and AUC of the Ca2+ rise (expressed in AU × minutes) was calculated.
Parthenogentetic activation of MII human and mouse oocytes
To test the activation efficiency of 10 mM Sr2+, human oocytes were incubated in Ca2+-free EBSS medium and mouse oocytes were activated in Ca2-free KSOM medium with Sr2+ for a duration of 4 h. For creating diploid parthenogenetic embryos, 2 μg/ml cytochalasin D (CCD) was added to both of the activation media. In the agonist activation tests, human oocytes were treated with 200 μM 2-APB for 30 min or 200 μM carvacrol for 10 min dissolved in IVF medium at 37°C, while mouse oocytes were exposed at the same conditions as the human oocytes but within KSOM medium (albumin-free 0.1% PVA). CCD was added to the subsequent culture medium for 4 h. Following activation, human oocytes were further cultured in CC medium for 16 h, and mouse oocytes were incubated in KSOM for 60–72 h followed by a 24 h extended culture in COOK Blastocyst medium. The activation of human oocytes was evaluated by formation of a single pronucleus (1PN) 16 h after treatment. Mouse embryo development was assessed at 24 (two-cell), 72 (morula/early blastocyst) and 96 h (blastocyst) post-activation time.
Immunofluorescence staining of TRPV3 channels
Oocytes were fixed and stained as previously reported (Carvacho et al., 2013). Briefly, the zona pellucida (ZP) of the oocytes was removed with Tyrode’s solution. The zona-free oocytes were washed intensively in 2% goat serum and 1% BSA-supplemented PBS and fixed in PBS–BSA containing 2% paraformaldehyde for 45 min at room temperature. Oocytes were washed and blocked in PBS containing 0.1 M glycine, 2% goat serum and 0.01% Tween 20 for at least 1 h. Oocytes were then permeabilized with 0.1% Triton X-100 for 15 min at room temperature. Subsequently, samples were washed with PBS supplemented with 2% goat serum and 1% PVA followed by incubation at 4°C with the primary antibody against TRPV3 (1:100, 10 μg/ml, Neuromab, Davis, CA, USA). After washing, oocytes were treated with the secondary antibody Alexa Fluor 488-conjugated goat anti-mouse IgG (H + L) (1:200, Molecular Probes, Eugene, OR, USA) for 1 h at room temperature, followed by extended washings. In addition, chromosomes were stained with ethidium homodimer-2 (1:500, Life Technologies, Carlsbad, CA, USA) for 1 h at room temperature. The negative controls were treated with the secondary antibodies alone. Finally, the oocytes were mounted in Mowiol containing 0.01% phenylenediamine. To validate the immunostaining of TRPV3 protein, a different fixation process and antigen retrieval experiment was performed (Supplementary Data), however, no protocol showed improved immunostaining results (Supplementary Fig. S1). All samples were imaged using a laser scanning microscope, Nikon A1R confocal microscope (Nikon Instruments, Paris, France) with a ×60 Plan Apo VC oil immersion objective. The TRPV3 distribution and chromosome alignments were obtained from Z-stacks (0.5–0.75 μm/Z-step), using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
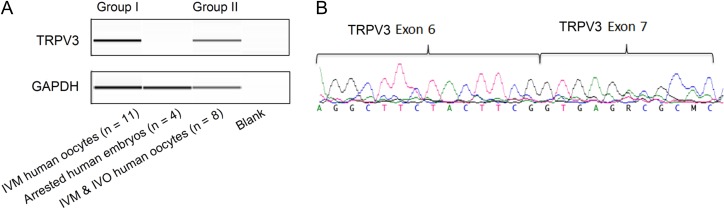
RT-PCR on pooled IVM human oocytes
RNA was extracted from pooled IVM MII and IVO SER human oocytes (Group I, n = 11 and Group II, n = 8, Table I), using a PicoPure RNA isolation kit, as described by the manufacturer (Life Technologies, CA, USA). The following cDNA synthesis was performed using the Superscript VILO cDNA synthesis kit (Invitrogen, USA). Primers (5′–3′) were designed to amplify the fragment spanning exons 6 and 7 of TRPV3 (Forward AGGCTTCTACTTCGGTGAGAC, Reverse AGGGCGTGAAGGATGTTGTTG) using Primer3 program.
The RT-PCR was performed on the cDNA of these two groups of human oocytes and Day 5 arrested human embryos (n = 4), while GAPDH was used as a positive control. Briefly the PCR conditions are as follows: initial denaturation at 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 64°C for 30 s, extension at 72°C for 45 s and a final extension at 72°C for 10 min. The RT-PCR products were subsequently loaded onto the fragment analyzer and analyzed using PROsize program (Advanced Analytical Technologies, Ankeny, IA, USA). Subsequently, the RT-PCR products was purified using Exo-AP and bi-directional Sanger sequencing was performed on the purified RT-PCR products using the Big Dye Terminator kit (ABI, USA). The Sanger sequencing products were further cleaned using magnetic beads with 85% alcohol and analyzed using the ABI 3130 genetic analyzer (ABI, USA).
Statistical analysis
The Statistical Package for the Social Sciences version 21 (SPSS® Statistics, IBM corp, NY, USA) was used for statistical analysis. Proportions were compared by a contingency table analysis followed by a chi-square or Fisher’s exact test. Means (RA and AUC) from multiple groups were compared using ANOVA and Bonferroni’s multiple comparison test. Differences yielding a P value <0.05 were considered as being statistically significant.
Results
Sr2+ failed to induce Ca2+ release and activation in human MII oocytes
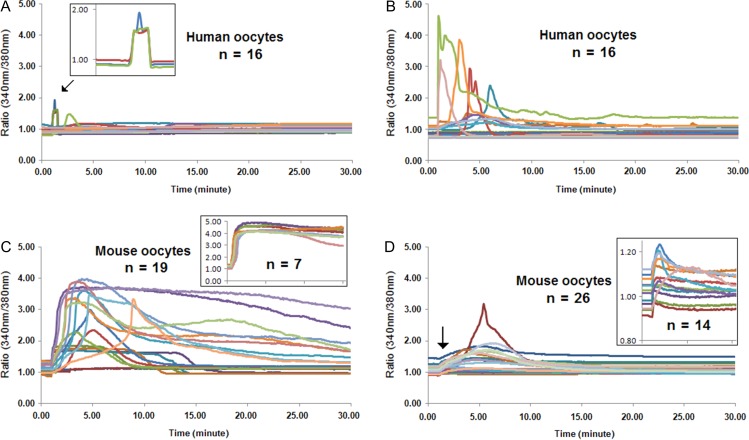
To verify the Ca2+ releasing and activating ability of Sr2+ exposure, IVM and IVO MII human oocytes (n = 25, Table I) were subjected to 10 mM Sr2+. Following the exposure, the changes of intracellular Ca2+ levels of analyzed oocytes were recorded by Ca2+ imaging. However, no increases in the intracellular Ca2+ concentrations were observed in a total number of 15 oocytes within a duration of 6 h (Fig. 1A shows records from the first 2 h). In contrast, all mouse oocytes (n = 15) exposed to the same concentration of Sr2+ showed dynamic intracellular Ca2+ oscillations during the 2 h analysis (five traces are presented in Fig. 1B). To test the reactivity to Ca2+ triggers of the human oocytes, five IVM human oocytes were further subjected to 10 μM ionomycin following a 2 h exposure of Sr2+. All five IVM oocytes exhibited a single Ca2+ rise with a rapid upstroke followed by a slower decline towards the baseline immediately after the ionomycin exposure (Fig. 1C and Table II). Activation capacities of the human oocytes (n = 10) were further evaluated following Sr2+ exposure. None of the human oocytes showed 1PN after 16 h following Sr2+ exposure while, in contrast, all of the mouse oocytes activated and cleaved, 16 h post Sr2+ activation (Table III).
Figure 1.
Sr2+ is not capable of provoking Ca2+ release in human oocytes. The intracellular Ca2+ level of fura-2 acetoxymethyl ester loaded human and mouse oocytes was recorded by Ca2+-imaging, following exposure to 10 mM Sr2+ for 2 h. (A) Sr2+ cannot induce Ca2+ release in human metaphase II (MII) oocytes during 2 h of measurement. (B) Mouse MII oocytes exhibit repetitive Ca2+ transients following exposure to 10 mM Sr2+. (C) Exposure of human oocytes to ionomycin following Sr2+ incubation verifies the reactivity of human oocytes to another artificial oocyte activation trigger.
Table II.
The Ca2+ response of human oocytes following exposure to various activation agents.
| Activation agent | No. oocytes | Survived oocytes, n (%) | Responded oocytes, n (%) | RA of the Ca2+ rise | AUC of the Ca2+ rise |
|---|---|---|---|---|---|
| Sr2+ | 15 | 15 (100%) | 0 | n/a | n/a |
| 2-APB | 16 | 16 (100%) | 4 (25%) | 0.70 ± 0.07a | 2.36 ± 0.90b |
| Carvacrol | 16 | 16 (100%) | 9 (31%) | 2.32 ± 0.73a | 4.98 ± 1.93b |
| Ionomycin post Sr2+ | 5 | 5 (100%) | 5 (100%) | 2.44 ± 0.20c | 13.14 ± 9.32d |
| Ionomycin post 2-APB | 9 | 8 (89%) | 8 (100%) | 1.91 ± 0.74 | 6.45 ± 5.50 |
| Ionomycin post carvacrol | 7 | 7 (100%) | 7 (100%) | 1.14 ± 0.90c | 2.30 ± 0.80d |
Oocytes are either exposed to 10 mM Sr2+ for 2 h or subjected to 200 μM 2-APB or 200 μM carvacrol for 30 min. The subsequent exposure of 10 μM ionomycin is for 15 min. ANOVA and Bonferroni’s multiple comparison test. aP < 0.01, b,c,dP < 0.05, n/a, not applicable. Values with the same superscripts differ significantly. Data are shown as mean ± SD.
2-APB, 2-aminoethoxydiphenyl borate.
RA, relative amplitude.
Table III.
The response of human and mouse oocytes following exposure to Sr2+, 2-APB and carvacrol.
| Oocytes | Activating agent | No. | Survived, n (%) | 1PN, n (%) | 2-cell, n (%) | Blastocyst, n (%) |
|---|---|---|---|---|---|---|
| Human | Sr2+ | 10 | 10 (100) | 0a,b | n/a | n/a |
| 2-APB | 9 | 8 (89) | 5 (63)a | n/a | n/a | |
| Carvacrol | 9 | 7 (78) | 5 (71)b | n/a | n/a | |
| Mouse | Sr2+ | 20 | 19 (95) | n/a | 19 (100)d | 18 (95) |
| 2-APB | 20 | 14 (70)c | n/a | 13 (93)e | 12 (92) | |
| Carvacrol | 20 | 20 (100)c | n/a | 1 (5)d,e | 1 (100) |
Oocytes are exposed to 10 mM Sr2+ for 4 h, or subjected to 200 μM 2-APB for 30 min or 200 μM carvacrol for 10 min. Chi-square and Fisher’s exact test: a,b,d,eP<0.01, cP<0.05, n/a, not applicable. Values with the same superscripts differ significantly.
PN, pronucleus.
TRPV3 channels are expressed in human oocytes
The TRPV3 channels were recently identified as the major channels to conduct Sr2+ influx and induce activation of mouse oocytes (Carvacho et al., 2013; Lee et al., 2016). Since Sr2+ failed to activate human oocytes or elicit Ca2+ transients, we then examined the expression and the function of the channels. Following the fluorescence staining, we observed a non-specific distribution of TRPV3 channels throughout the ooplasma of the analyzed human oocytes (n = 10) (Fig. 2A and B). To validate the staining, we further examined the TRPV3 localization in mouse oocytes (n = 10), which (unlike human) showed a distribution that was particularly concentrated at the plasma membrane, as reported previously (Carvacho et al., 2013; Lee et al., 2016) (Fig. 2C and D). To further confirm this result, TRPV3 mRNA expression levels in human oocytes were investigated at transcriptional level by RT-PCR. The transcripts of TRPV3 were detected in pooled IVM MII and IVO SERs human oocytes (Group I, n = 11 and Group II, n = 8, Table I), as demonstrated by the expected mRNA products in both groups (Fig. 3A). Sanger sequencing of the RT-PCR products further revealed that the sequencing reads were aligned with the TRPV3 cDNA sequence downloaded from the University of California Santa Cruz genome browser with the transcript containing 791 amino acids (NM_001258205) using DNASTAR (Fig. 3B).
Figure 2.
The localization of TRPV3 channels in human MII oocytes. Human and mouse oocytes were stained with Transient receptor potential cation channels vanilloid 3 (TRPV3) antibody and analyzed by confocal microscope. (A) Chromosomes of IVM human oocytes are encompassing two regular rings, observed from the optical axis passing through the spindle poles. (B) TRPV3 protein of human IVM MII oocyte shows a diffused, non-specific pattern. (C) Chromosomes of mouse in vivo matured (IVO) oocytes are aligned at the equatorial plate. (D) Mouse TRPV3 channels are expressed and concentrated at the cytoplasmic membrane.
Figure 3.
TRPV3 mRNA expression levels in IVM MII and IVO human oocytes with aggregates of smooth endoplasmic reticulum clusters. The mRNA expression of TRPV3 was analyzed by RT-PCR and verified by Sanger sequencing in pooled IVM and in vivo matured (IVO) human oocytes. (A) RT-PCR products from the agarose gel show the transcripts of TRPV3 are expressed in a group of pooled IVM human oocytes and a group of pooled IVM and IVO human oocytes. (B) Chromatogram showing the exons 6–7 junction of the TRPV3 gene after Sanger sequencing of the RT-PCR product from pooled IVM human oocytes.
TRPV3 agonists triggered Ca2+ release and provoked oocyte activation of human and mouse oocytes
The Ca2+ releasing and activating ability of the TRPV3 channels were further investigated by subjecting human oocytes to the agonists 2-APB and carvacrol. Following the exposure of 16 MII human oocytes to 200 μM 2-APB, the intracytoplasmic Ca2+ increased in 25% (4 out of 16) of them, displaying a small rise during the first 5 min of measurement (Fig. 4A and Table II). When subjecting 16 human oocytes to the same concentration of carvacrol, a similar proportion (31%, 5/16) of the oocytes responded, and exhibited a significant increase in Ca2+ level compared to the Ca2+ rise triggered by 2-APB (P = 0.0034), during the first 10 min of exposure (Fig. 4B and Table II). Moreover, four more IVM oocytes showed much lower Ca2+ fluctuations (RA 0.28 ± 0.14, AUC 1.54 ± 0.56) which were not defined as responding oocytes (Fig. 4B and Table II). All of the human oocytes survived following these chemical exposures and imaging. The groups of human oocytes which failed to respond to these TRPV3 agonists, were further exposed to ionomycin to evaluate the response of the oocytes to other Ca2+ triggers. All oocytes exhibited a sharp Ca2+ rise following the ionomycin exposure, showing a positive reactivity to the Ca2+ ionophore (Table II).
Figure 4.
The TRPV3 agonists, 2-APB and carvacrol, induced a Ca2+ response in both human and mouse MII oocytes. The intracellular Ca2+ level of fura-2 acetoxymethyl ester loaded human and mouse oocytes was recorded by Ca2+-imaging, following exposure to 200 μM 2-aminoethoxydiphenyl borate (2-APB) and 200 μM carvacrol for 30 min. (A) Increased intracellular Ca2+ was provoked by 2-APB in 4 out of 16 IVM and IVO human MII oocytes, displaying a small peak. The three overlapped Ca2+ rises of the responding oocytes are shown in the inset picture. (B) Five out of 16 human MII oocytes responded to carvacrol, showing a sharp Ca2+ rise. (C) Elevated intracellular Ca2+ in 17 out of 19 alive mouse oocytes following the exposure to 2-APB, displaying a large Ca2+ peak. The inset picture showed the Ca2+ pattern of another seven damaged oocytes during 2-APB stimulation. (D) A lower Ca2+ response was observed in all mouse oocytes (n = 26) in response to carvacrol, 54% of which (14/26) exhibited a minor Ca2+ rise fluctuation (absolute amplitude <0.2) in the first 5 min of exposure (picture inset).
The addition of 200 μM 2-APB to MII mouse oocytes (n = 26) dramatically increased Ca2+ levels and immediately provoked a protracted peak during the 30 min exposure (Fig. 4C). Following the exposure, 73% (19/26) oocytes survived and 89% (17/19) showed intracellular Ca2+ release (Fig. 4C). The seven damaged oocytes showed a specific protracted Ca2+ pattern (Fig. 4C, inset picture). Carvacrol (200 μM) evoked increases of intracellular Ca2+ in all subjected mouse oocytes (n = 26), however, only displaying a small peak during the 10 min of exposure (Fig. 4D). Moreover, the 54% (14/26) that responded to carvacrol exhibited a minor Ca2+ rise fluctuation (absolute amplitude <0.2) in the first 5 min of exposure (Fig. 4D, inset picture).
Furthermore, the activation potential of human and mouse MII oocytes was investigated as well, following the application of the two TRPV3 agonists. We allocated in total 18 IVM and IVO human oocytes (Table I) and 40 mouse oocytes to the 2-APB and carvacrol (each 200 μM) activation tests. Interestingly, more than half of the human oocytes activated and formed 1PN, observed at 16 h post stimulation (63 and 71% for 2-APB and carvacrol, respectively, Table III). When exposing mouse oocytes to 200 μM 2-APB, 70% of them survived following the stimulation, and subsequently, more than 90% of them cleaved and developed to blastocysts (Table III). In contrast, carvacrol induced significantly less (5%) cleavage to the two-cell stage compared to the 2-APB group (P < 0.0001, Table III).
Discussion
To date, Sr2+ has been applied as an AOA method to overcome FF or low fertilization rates (Kyono et al., 2008; Kim et al., 2012, 2014) in several IVF centers. However, the efficiency and the exact mechanism of Sr2+ as an activation agent in human oocytes remains largely unknown. In the present study, we investigated the activation capacity and Ca2+ response of human oocytes after Sr2+ exposure. Since it was recently shown that the TRPV3 channel mediates Sr2+ induced artificial activation in mouse oocytes (Carvacho et al., 2013), we evaluated the presence and functionality of these TRPV3 channels in human oocytes. We demonstrated that Sr2+ failed to mediate Ca2+ release and induce activation in human oocytes, despite the presence and functioning of TRPV3 channels.
In contrast to other artificial activation agents that induce a single Ca2+ transient of mammalian oocytes (Vanden Meerschaut et al., 2014; Nikiforaki et al., 2016), Sr2+ activates rodent eggs by inducing a series of Ca2+ like oscillations (Whittingham and Siracusa, 1978; Roh et al., 2003), which closely mimic the pattern of Ca2+ rises triggered by PLCζ at fertilization. As such, Sr2+ has been applied initially as an AOA agent in the clinic to overcome FF after ICSI. Consequently, improved fertilization rates and embryo qualities were reported for several couples with repetitive FF (Kyono et al., 2008; Chen et al., 2010), frozen–thawed testicular spermatozoa (Kim et al., 2012) and a globozoospermia case (Yang et al., 2012), with resulting healthy live births. Despite these studies showing the potential of Sr2+ to overcome activation failure in human, we demonstrated that Sr2+ was not capable of inducing a Ca2+ increase or provoking activation of human oocytes. These discrepancies could be due to the lack of diagnostic methods used in these studies to show they were real sperm-related activation deficiencies (Kyono et al., 2008; Chen et al., 2010; Kim et al., 2014). Still, one patient with globozoospermia (Yang et al., 2012) was also successfully treated with Sr2+, which could be explained by the fact that some globozospermic patients can achieve successful fertilization even after the application of routine ICSI in the absence of AOA (Huang et al., 2010). Therefore, it is difficult to know whether the patients enrolled in those AOA studies using Sr2+ as the activating agent really required AOA in first instance, as diagnostic evidence (heterologous ICSI, genetic screening of PLCz, Ca2+ pattern analysis) was not obtained to show a lack of activation capacity in their sperm. However, it is still possible that the Sr2+ might act through unknown Ca2+-irrelevant pathways or through the mechanical permeabilization created during ICSI. More studies are required to verify this further.
To enable activation of mouse oocytes, extracellular Sr2+ influxes are transported across its major membrane channel, TRPV3 (Carvacho et al., 2013), promoting downstream oscillations in [Ca2+]i/[Sr2+] of the oocytes, probably by sensitizing IP3Rs and thus facilitating Ca2+ oscillations (Zhang et al., 2005), or substituting for Ca2+ in the potentiation of IP3Rs (Girard and Clapham, 1993; Marshall and Taylor, 1994; Lee, 2016). Thus, the failure to activate human oocytes with Sr2+ alone could be attributed to the absence of membrane TRPV3 channels (which was proven not to be the case in the present study) or, unlikely, the inability of the residues to conduct Sr2+, which localize at position 412 on the loop of the membrane proximal domain on the N terminus responsible for switching the temperature dependence of the channel (Latorre et al., 2007; Liu and Qin, 2017), as well as the insensitivity of IP3R1 in responding to Sr2+ in human oocytes. Further investigations are required to verify these options.
Furthermore, we did confirm that the transcripts of TRPV3 channels were present in IVM human oocytes. The TRPV3 channels were also recently demonstrated to be expressed in pooled fresh IVO human oocytes by a whole transcriptome analysis, albeit at low levels (Kocabas et al., 2006). The diffuse cytoplasmic localization of TRPV3 protein might contribute to the observed inability of conducting Sr2+ influx by TRPV3 or the aberrant Ca2+ release pattern following 2-APB treatment, owing to failure of trafficking functional TRPV3 protein to the membrane during in vitro culture, as the cytoskeleton modulates the function of TRPV3 channels (Kuipers et al., 2012; Smani et al., 2014; Lee et al., 2016). Unfortunately, we could not rule out the possibility of low specificity of the immunostaining TRPV3 antibody that was used in the present setting. Moreover, the TRPV3 protein distribution in human oocytes could be impaired by IVM, as no TRPV3 channels are expressed on the plasma membrane of mouse GV oocytes and the TRPV3 proteins are transferred from the ooplasm to the plasma membrane during oocyte maturation in mouse (Lee et al., 2016). Future analysis, for example tracking the TRPV3 protein distribution in IVM and IVO human oocytes by TRPV3 tagging with ruby fluorescent protein (Lee et al., 2016), is required to verify this.
Although the expressed TRPV3 channels in human oocytes failed to conduct Sr2+ influx or the conducted Sr2+ was not sufficient to promote Ca2+ release from the ER, they were shown to be functional in supporting an agonist triggered Ca2+ rise and oocyte activation in both mouse and human oocytes. In view of the efficiency of activating rodent oocytes, 2-APB was suggested as a potential AOA agent targeting TRPV3 channels (Lee et al., 2016; Lee, 2016). However, in our view, it is too soon to encourage its use at this moment, as both 2-APB and carvacrol activate multiple Ca2+ related channels (Bilmen and Michelangeli, 2002; Bilmen et al., 2002; Colton and Zhu, 2007; Pires et al., 2015) and the exact mechanism remains largely unclear. Moreover, 2-APB mediated Ca2+ influx exclusively from the external culture medium (Xu et al., 2006; Lee et al., 2016), whereas, Ca2+ ionophore promoted Ca2+ increase from both the ER and extracellular Ca2+ influx (Lu et al., 2018). Currently, ionomycin is still the most recommended agent to overcome FF after ICSI (Heindryckx et al., 2005; Vanden Meerschaut et al., 2014; Nikiforaki et al., 2016) due to its high efficiency in provoking a Ca2+ rise and inducing activation, as demonstrated in both mouse and human oocytes (Vanden Meerschaut et al., 2014; Nikiforaki et al., 2016).
Supplementary Material
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Authors’ roles
Y.L., B.H. and P.D.S. conceived and designed the study. Y.L., M.F.B., J.N. and W.H.D. performed experiments reported in Tables I–III and Figs 1–2, 4. R.R. performed the RT-PCR and Sanger sequencing leading to Fig. 3. Y.L., B.H., M.F.B. wrote the article and assembled the figures. M.V.d.J. assisted in English editing. All authors contributed to the interpretation of the results and the editing of the article.
Funding
This study was supported by grants from FWO-Vlaanderen (Research Foundation Flanders, Fonds Wetenschappelijk Onderzoek—Vlaanderen, Grant no. G060615N); China Scholarship Council (no.2010616105); and BOF of Ghent University (Special Research Fund, Bijzonder Onderzoeksfonds, Grant no. 01SC3712).
Conflict of interest
None declared.
References
- Ajduk A, Ilozue T, Windsor S, Yu Y, Seres KB, Bomphrey RJ, Tom BD, Swann K, Thomas A, Graham C et al. Rhythmic actomyosin-driven contractions induced by sperm entry predict mammalian embryo viability. Nat Commun 2011;2:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Yoshizawa M, Abe H, Murase Y. Use of mouse oocytes to evaluate the ability of human sperm to activate oocytes after failure of activation by intracytoplasmic sperm injection. Zygote 2004;12:111–116. [DOI] [PubMed] [Google Scholar]
- Bilmen JG, Michelangeli F. Inhibition of the type 1 inositol 1,4,5-trisphosphate receptor by 2-aminoethoxydiphenylborate. Cell Signal 2002;14:955–960. [DOI] [PubMed] [Google Scholar]
- Bilmen JG, Wootton LL, Godfrey RE, Smart OS, Michelangeli F. Inhibition of SERCA Ca2+ pumps by 2-aminoethoxydiphenyl borate (2-APB). 2-APB reduces both Ca2+ binding and phosphoryl transfer from ATP, by interfering with the pathway leading to the Ca2+-binding sites. Eur J Biochem 2002;269:3678–3687. [DOI] [PubMed] [Google Scholar]
- Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep 2013;5:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che L, Lalonde A, Bordignon V. Chemical activation of parthenogenetic and nuclear transfer porcine oocytes using ionomycin and strontium chloride. Theriogenology 2007;67:1297–1304. [DOI] [PubMed] [Google Scholar]
- Chen J, Qian Y, Tan Y, Mima H. Successful pregnancy following oocyte activation by strontium in normozoospermic patients of unexplained infertility with fertilisation failures during previous intracytoplasmic sperm injection treatment. Reprod Fertil Dev 2010;22:852. [DOI] [PubMed] [Google Scholar]
- Colton CK, Zhu MX. 2-Aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb Exp Pharmacol 2007;179:173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner T, Koster M, Shebl O, Moser M, Ven H, Van der, Tews G, Montag M. Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Fertil Steril 2012;98:1432–1437. [DOI] [PubMed] [Google Scholar]
- Egashira A, Murakami M, Haigo K, Horiuchi T, Kuramoto T. A successful pregnancy and live birth after intracytoplasmic sperm injection with globozoospermic sperm and electrical oocyte activation. Fertil Steril 2009;92:2037.e5–9. [DOI] [PubMed] [Google Scholar]
- Fedorenko OA, Popugaeva E, Enomoto M, Stathopulos PB, Ikura M, Bezprozvanny I. Intracellular calcium channels: inositol-1,4,5-trisphosphate receptors. Eur J Pharmacol 2014;739:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard S, Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science 1993;260:229–232. [DOI] [PubMed] [Google Scholar]
- Hachem A, Godwin J, Ruas M, Lee HC, Buitrago MF, Ardestani G, Bassett A, Fox S, Navarrete F, de Sutter P et al. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but offspring can be conceived in its absence. Development 2017;144:2914–2924. http://www.ncbi.nlm.nih.gov/pubmed/28694258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindryckx B, Van der Elst J, de Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod 2005;20:2237–2241. [DOI] [PubMed] [Google Scholar]
- Heindryckx B, Gheselle S De, Gerris J, Dhont M, de Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod Biomed Online 2008;17:662–668. [DOI] [PubMed] [Google Scholar]
- Huang D, Jiang L, Xu W, Tong X, Zhu H, Li C, Zhou F, Liu L, Lin X, Zhang S. [Fertilizing ability, cleavage potential and inheritance risk of globozoospermia]. Zhonghua Yi Xue Za Zhi 2010;90:2351–2353. [PubMed] [Google Scholar]
- Itoi F, Asano Y, Shimizu M, Honnma H, Murata Y. Embryological outcomes in cycles with human oocytes containing large tubular smooth endoplasmic reticulum clusters after conventional in vitro fertilization. Gynecol Endocrinol 2016;32:315–318. http://www.ncbi.nlm.nih.gov/pubmed/26607857. [DOI] [PubMed] [Google Scholar]
- Kim J-W, Choi J-L, Yang S-H, Yoon S-H, Jung J-H, Lim J-H. Live birth after SrCl(2) oocyte activation in previous repeated failed or low fertilization rates after ICSI of frozen-thawed testicular spermatozoa: case report. J Assist Reprod Genet 2012;29:1393–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kim SD, Yang SH, Yoon SH, Jung JH, Lim JH. Successful pregnancy after SrCl2 oocyte activation in couples with repeated low fertilization rates following calcium ionophore treatment. Syst Biol Reprod Med 2014;60:177–182. [DOI] [PubMed] [Google Scholar]
- Kishikawa H, Wakayama T, Yanagimachi R. Comparison of oocyte-activating agents for mouse cloning. Cloning 1999;1:153–159. [DOI] [PubMed] [Google Scholar]
- Kocabas AM, Crosby J, Ross PJ, Otu HH, Beyhan Z, Can H, Tam WL, Rosa GJM, Halgren RG, Lim B et al. The transcriptome of human oocytes. Proc Natl Acad Sci USA 2006;103:14027–14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers AJ, Middelbeek J, van Leeuwen FN. Mechanoregulation of cytoskeletal dynamics by TRP channels. Eur J Cell Biol 2012;91:834–846. [DOI] [PubMed] [Google Scholar]
- Kyono K, Kumagai S, Nishinaka C, Nakajo Y, Uto H, Toya M, Sugawara J, Araki Y. Birth and follow-up of babies born following ICSI using SrCl2 oocyte activation. Reprod Biomed Online 2008;17:53–58. [DOI] [PubMed] [Google Scholar]
- Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium 2007;42:427–438. [DOI] [PubMed] [Google Scholar]
- Lawitts JA, Biggers JD. Optimization of mouse embryo culture media using simplex methods. J Reprod Fertil 1991;91:543–556. [DOI] [PubMed] [Google Scholar]
- Lee HC. Mammalian Egg Activation: The Roles of TRPV3 Channels and PLCZ1. 2016.
- Lee HC, Yoon S-Y, Lykke-Hartmann K, Fissore RA, Carvacho I. TRPV3 channels mediate Ca2+ influx induced by 2-APB in mouse eggs. Cell Calcium 2016;59:21–31. [DOI] [PubMed] [Google Scholar]
- Liu B, Qin F. Single-residue molecular switch for high-temperature dependence of vanilloid receptor TRPV3. Proc Natl Acad Sci USA 2017;114:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Bonte D, Ferrer-Buitrago M, Popovic M, Neupane J, Jeught M, Van der, Leybaert L, de Sutter P, Heindryckx B. Culture conditions affect Ca2+ release in artificially activated mouse and human oocytes. Reprod Fertil Dev 2018; doi:10.1071/RD17145 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Mak DO, Foskett JK. Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: a single-channel point of view. Cell Calcium 2015;58:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall IC, Taylor CW. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem J 1994;301:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci USA 2012;109:4169–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y-L, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol Reprod Dev 2012;79:742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méo SC, Leal CLV, Garcia JM. Activation and early parthenogenesis of bovine oocytes treated with ethanol and strontium. Anim Reprod Sci 2004;81:35–46. [DOI] [PubMed] [Google Scholar]
- Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium 2014;55:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforaki D, Vanden Meerschaut F, de Roo C, Lu Y, Ferrer-Buitrago M, de Sutter P, Heindryckx B. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril 2016;105:798–806e2. [DOI] [PubMed] [Google Scholar]
- Otaegui PJ, O’Neill GT, Wilmut I. Parthenogenetic activation of mouse oocytes by exposure to strontium as a source of cytoplasts for nuclear transfer. Cloning 1999;1:111–117. [DOI] [PubMed] [Google Scholar]
- Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod 2004;19:1591–1597. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002;296:2046–2049. [DOI] [PubMed] [Google Scholar]
- Pires PW, Sullivan MN, Pritchard HA, Robinson JJ, Earley S. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am J Physiol Heart Circ Physiol 2015;309:H2031–H2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan WM, Kashir J, Jones C, Coward K. Oocyte activation and phospholipase C zeta (PLCzeta): diagnostic and therapeutic implications for assisted reproductive technology. Cell Commun Signal 2012;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 2006;68:619–647. [DOI] [PubMed] [Google Scholar]
- Rawe VY, Olmedo SB, Nodar FN, Doncel GD, Acosta AA, Vitullo AD. Cytoskeletal organization defects and abortive activation in human oocytes after IVF and ICSI failure. Mol Hum Reprod 2000;6:510–516. [DOI] [PubMed] [Google Scholar]
- Roh S, Malakooti N, Morrison JR, Trounson AO, Du ZT. Parthenogenetic activation of rat oocytes and their development (in vitro). Reprod Fertil Dev 2003;15:135–140. [DOI] [PubMed] [Google Scholar]
- Sa R, Cunha M, Silva J, Luis A, Oliveira C, Teixeira da Silva J, Barros A, Sousa M. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril 2011;96:143–149 e7. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 2002;129:3533–3544. [DOI] [PubMed] [Google Scholar]
- Smani T, Dionisio N, Lopez JJ, Berna-Erro A, Rosado JA. Cytoskeletal and scaffolding proteins as structural and functional determinants of TRP channels. Biochim Biophys Acta 2014;1838:658–664. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002;418:186–190. [DOI] [PubMed] [Google Scholar]
- Swain JE, Pool TB. ART failure: oocyte contributions to unsuccessful fertilization. Hum Reprod Update 2008;14:431–446. [DOI] [PubMed] [Google Scholar]
- Tesarik J. Calcium signalling in human oocytes and embryos: two-store model revival. Hum Reprod 2002;17:2948–2949. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril 2002;78:619–624. [DOI] [PubMed] [Google Scholar]
- Vanden Meerschaut F, Nikiforaki D, de Roo C, Lierman S, Qian C, Schmitt-John T, de Sutter P, Heindryckx B. Comparison of pre- and post-implantation development following the application of three artificial activating stimuli in a mouse model with round-headed sperm cells deficient for oocyte activation. Hum Reprod 2013;28:1190–1198. [DOI] [PubMed] [Google Scholar]
- Vanden Meerschaut F, Nikiforaki D, Heindryckx B, de Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod Biomed Online 2014;28:560–571. [DOI] [PubMed] [Google Scholar]
- Versieren K, Heindryckx B, Lierman S, Gerris J, de Sutter P. Developmental competence of parthenogenetic mouse and human embryos after chemical or electrical activation. Reprod Biomed Online 2010;21:769–775. [DOI] [PubMed] [Google Scholar]
- Wakai T, Zhang N, Vangheluwe P, Fissore RA. Regulation of endoplasmic reticulum Ca(2+) oscillations in mammalian eggs. J Cell Sci 2013;126:5714–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingham DG, Siracusa G. The involvement of calcium in the activation of mammalian oocytes. Exp Cell Res 1978;113:311–317. [DOI] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 2006;9:628–635. [DOI] [PubMed] [Google Scholar]
- Yamazaki W, Ferreira CR, Méo SC, Leal CLV, Meirelles FV, Garcia JM. Use of strontium in the activation of bovine oocytes reconstructed by somatic cell nuclear transfer. Zygote 2005;13:295–302. [DOI] [PubMed] [Google Scholar]
- Yanagida K. Complete fertilization failure in ICSI. Hum Cell 2004;17:187–193. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Morozumi K, Katayose H, Hayashi S, Sato A. Successful pregnancy after ICSI with strontium oocyte activation in low rates of fertilization. Reprod Biomed Online 2006;13:801–806. [DOI] [PubMed] [Google Scholar]
- Yang X-Y, Wang J, Liu J-Y, Gao Y, Zhou Z-M, Sha J-H, Zhang W, Cui Y-G, Qian X-Q. Pregnancy outcome after intracytoplasmic sperm injection with strontium oocyte activation in a globozoospermic patient. Asian J Androl 2012;14:341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Pan L, Yang LH, He XK, Huang XY, Sun FZ. Strontium promotes calcium oscillations in mouse meiotic oocytes and early embryos through InsP3 receptors, and requires activation of phospholipase and the synergistic action of InsP3. Hum Reprod 2005;20:3053–3061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.