Abstract
Background
Serum testosterone levels and insulin sensitivity both decrease with age. Severe testosterone deficiency is associated with the development of insulin resistance. However, the effects of long-term testosterone administration on insulin sensitivity in older men with low or low-normal testosterone levels remain unknown.
Methods
The Testosterone Effects on Atherosclerosis in Aging Men Trial was a placebo-controlled, randomized, double-blind trial. The participants were 308 community-dwelling men, ≥60 years old, with total testosterone 100 to 400 ng/dL or free testosterone <50 pg/mL. A subset of 134 nondiabetic men (mean age, 66.7 ± 5.1 years) underwent an octreotide insulin suppression test at baseline and at 3 and 36 months after randomization to measure insulin sensitivity. Insulin sensitivity was estimated as the steady-state plasma glucose (SSPG) concentration at equilibrium during octreotide and insulin administration. Secondary outcomes included total lean mass (TLM) and total fat mass (TFM) by dual energy x-ray absorptiometry.
Results
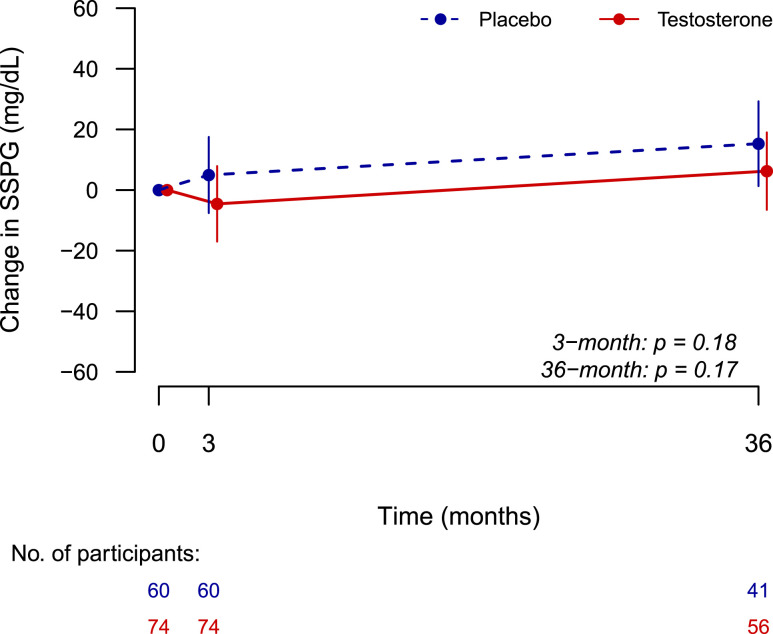
There was a significant (P = 0.003) increase in SSPG in the placebo group, whereas no change was seen in testosterone-treated subjects from baseline to 36 months; however, the between-group differences in change in SSPG over 3 years were not statistically significant (+15.3 ± 6.9 mg/dL in the placebo group vs +6.2 ± 6.4 mg/dL in the testosterone group; mixed-model effect, P = 0.17). Changes in SSPG with testosterone treatment were not associated with changes in serum total or free testosterone concentrations. Changes in TFM but not TLM were associated with increases in SSPG. Stratification by age or baseline total testosterone level did not show significant intervention effects.
Conclusion
Testosterone administration for 36 months in older men with low or low-normal testosterone levels did not improve insulin sensitivity.
Testosterone levels and insulin sensitivity both decrease with age. We found that long-term testosterone therapy for 36 months in nondiabetic older men did not improve insulin sensitivity.
Circulating testosterone levels and insulin sensitivity both decrease with age (1–3). Testosterone levels peak in the second and third decades of life and then decline gradually with advancing age (4). In aging men, this decline in testosterone level is associated with a decrease in skeletal muscle mass and an increase in fat mass (5). Furthermore, in observational studies, low circulating testosterone concentrations in men were associated with obesity, insulin resistance, type 2 diabetes, and metabolic syndrome (6, 7). However, the association of endogenous testosterone concentrations with type 2 diabetes and metabolic syndrome was attenuated substantially after adjustment for sex hormone-binding globulin (SHBG) levels (8). Furthermore, epidemiologic studies found free testosterone to be only weakly associated or not associated at all with diabetes and metabolic syndrome, suggesting that SHBG, rather than testosterone, may be the primary determinant of this apparent association between total testosterone levels and type 2 diabetes (9–11).
Laboratory studies have demonstrated that testosterone promotes the commitment of mesenchymal multipotent progenitor cells to the myogenic lineage while inhibiting their differentiation into adipocytes (12), which would be expected to result in a more favorable metabolic profile. These laboratory studies are consistent with clinical trial data in hypogonadal as well as eugonadal older men demonstrating favorable body composition changes with testosterone administration, including increased total lean body mass and decreased total fat mass (13–16); this suggests that the effects of testosterone on insulin resistance may be mediated by changes in body composition. Data from animal models suggest that testosterone may also have direct effects on insulin sensitivity by increasing the expression of insulin receptors as well as by potentiating insulin signaling, resulting in enhanced glucose uptake into the muscle and adipose tissue (17–20). Furthermore, male mice lacking the androgen receptor demonstrate insulin resistance, suggesting that androgen receptor signaling is important in the regulation of glucose metabolism (21). Thus, a large body of preclinical evidence supports the view that testosterone plays an important role in regulating glucose metabolism and insulin sensitivity.
Several randomized controlled trials of testosterone administration on insulin sensitivity/resistance have been conducted in men with low testosterone levels; these studies have yielded inconsistent results, with some reporting improvement (22–25) and others not showing a benefit (26–29). Previous trials have been limited by their small sample sizes and short intervention durations. No randomized controlled trial has examined the effect of long-term testosterone replacement on insulin sensitivity in middle-aged or older men. In addition, most studies evaluated insulin sensitivity/resistance using surrogate markers (i.e., homeostasis model assessment, oral glucose tolerance test), and only a few studies have used rigorous techniques such as the hyperinsulinemic-euglycemic clamp or the octreotide insulin suppression test (OIST). The hyperinsulinemic-euglycemic clamp is considered the gold standard method for the direct measurement of insulin sensitivity; however, the clamp method is labor-intensive and requires a team of experienced operators and physician supervision to ensure participant safety and obtain valid results (30), limiting its usability in large randomized trials.
The OIST, another method to directly measure insulin sensitivity, uses the somatostatin analogue octreotide to suppress endogenous secretion of insulin and glucagon followed by a constant infusion of insulin and glucose to determine steady-state plasma glucose (SSPG) concentrations (31, 32). Compared with the glucose clamp, the OIST is less labor-intensive and less technically demanding and provides a direct measure of insulin sensitivity that is highly reproducible (30). Because glucose and insulin infusions are kept constant in the OIST, steady-state conditions are achieved consistently. The results of the OIST have been strongly correlated with those of the glucose clamp method in patients with and without diabetes (30, 31, 33).
We conducted a randomized, placebo-controlled, double-blind trial to determine the effects of long-term (36-month) testosterone administration on insulin sensitivity, measured by the OIST, in older men with low or low-normal testosterone levels. This investigation was a substudy of the Testosterone Effects on Atherosclerosis in Aging Men (TEAAM) Trial, a study with the primary aim of determining the effects of long-term testosterone treatment on subclinical atherosclerosis progression and the main findings have been published (34). Here, we describe the results of the substudy, which evaluated the effects of 36 months of testosterone administration on insulin sensitivity.
Methods
Study design
The eligibility criteria and design of the TEAAM Trial have been published (34) and are described here briefly. This parallel-group, placebo-controlled, double-blind, randomized trial was approved by the institutional review boards of Charles Drew University, Los Angeles, California, and Boston University Medical Center, Brigham and Women’s Hospital, Boston, Massachusetts, and by the Western Institutional Review Board, Puyallup, Washington, for the Kronos Longevity Research Institute, Phoenix, Arizona. All participants provided written informed consent.
Eligibility
The participants were community-dwelling men, aged ≥60 years, with a total testosterone level of 100 to 400 ng/dL or a free testosterone level <50 pg/mL. We excluded men who had diseases of the testes, pituitary, or hypothalamus; prostate cancer or cancers other than nonmelanotic skin cancers; a lower urinary tract symptom score >21; a prostate specific antigen value >4 ng/mL; a Mini-Mental Status Examination score <24; untreated major depression or schizophrenia; New York Heart Association class III or IV heart failure; myocardial infarction within 6 months of study entry; uncontrolled hypertension; alanine or aspartate aminotransferase concentrations more than three times the upper limit of normal; untreated thyroid disease; a hemoglobin A1c value >9.0%; a hematocrit level >48%; or body mass index (BMI) >35 kg/m2. Men using testosterone, growth hormone, or any drugs that affect gonadal function were also excluded.
Randomization and study intervention
Eligible participants were randomly assigned to either placebo or testosterone gel using a 1:1 computer-generated randomization table stratified by age (60 to 75 years and >75 years) and site. The participants and all study personnel were blinded to the intervention.
The participants applied gel containing either placebo or 7.5 g of 1% testosterone daily for 3 years. Two weeks after randomization, total testosterone levels were measured 2 to 12 hours after gel application. If the total testosterone concentration was <500 ng/dL (17.3 nmol/L), the testosterone dose was increased to 10 g; if it was >900 ng/dL (31.2 nmol/L), the dose was reduced to 5 g daily. At the same time, the placebo dose was adjusted for another participant in the placebo group by an unblinded observer to maintain blinding.
Hormone measurements
Total testosterone level was measured at Quest Diagnostics (reference range: 239 to 827 ng/dL; San Juan Capistrano, CA), using a Bayer Advia Centaur immunoassay (Siemens Medical Solutions, Malvern, PA) that has been validated against liquid chromatography-mass spectrometry and has a sensitivity of 8.6 ng/dL (34). Free testosterone was calculated using a published law-of-mass-action equation (reference range: 49.9 to 199.9 pg/mL) (35). SHBG levels were measured using an immunofluorometric assay with a sensitivity of 1.0 nmol/L (Delfia; Wallac) (34).
Assessment of insulin sensitivity
A subset of 134 nondiabetic men recruited at the Phoenix, Arizona, trial site underwent baseline and postintervention OISTs (30). Glucose and insulin were infused at constant rates proportional to body surface area, whereas endogenous insulin secretion was inhibited by octreotide administered as an initial bolus followed by constant infusion for 180 minutes. Blood samples were drawn every 30 minutes until 150 minutes to monitor plasma glucose levels, and then every 10 minutes until 180 minutes to measure serum glucose and insulin concentrations. Insulin sensitivity was estimated as concentration of glucose at equilibrium (SSPG) by averaging the values during the last 30 minutes of the infusion at the 160-, 170-, and 180-minute time points. SSPG concentrations are a direct measure of the ability of exogenous insulin to mediate disposal of an infused glucose load under steady-state conditions, during which endogenous insulin secretion has been suppressed by octreotide administration. SSPG values are inversely related to insulin sensitivity; thus, patients who are insulin sensitive would be expected to have lower SSPG values than patients with insulin resistance.
Body composition
Total lean mass and total fat mass were measured by dual energy x-ray absorptiometry using the Hologic QDR4500 (Hologic Inc., Marlborough, MA) or the GE Lunar Prodigy (General Electric Co., Little Chalfont, United Kingdom) instruments. The GE Lunar Prodigy data were corrected using cross-validation measurements in eight men aged 20 to 60 years who were tested on both instruments. All dual energy x-ray absorptiometry instruments were cross-validated using a soft tissue phantom and calibrated daily following manufacturer’s recommendations.
Statistical analysis
The analytic sample consisted of subjects who had a baseline and at least one postrandomization assessment of insulin sensitivity by OIST. A “per-protocol” analysis restricted to participants who completed 3 years of intervention and had both baseline and 36-month insulin sensitivity assessments was also performed. Mean and standard deviation were provided for continuous variables. Change from baseline in insulin sensitivity outcome was compared between intervention arms using linear mixed models with repeated measures, allowing for within-subject correlation of measurements and effects of intervention, visit, and visit-by-intervention interaction, controlling for baseline insulin sensitivity. Estimates and corresponding 95% confidence intervals for differences between intervention groups were assessed at 3 and 36 months. Simple linear regressions were implemented to test the association between changes in insulin sensitivity and changes in on-treatment serum testosterone concentrations, free testosterone concentrations, and lean and fat body mass measures at 36 months. Sensitivity analyses for insulin sensitivity changes were performed, stratified by men aged ≤75 years vs those aged >75 years, as well as by men with baseline total testosterone levels ≤300 ng/dL vs those with levels >300 ng/dL. All tests were two-sided and performed at α = 0.05 level of significance. Statistical analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC) and R software version 2.15.1 (https://www.r-project.org/).
Results
Flow of participants
Of the 1893 who underwent screening, 1098 did not meet eligibility criteria or declined to participate, and 308 were randomly assigned. Two withdrew consent before receiving study medication, leaving 306; 155 were randomly assigned to the testosterone arm and 151 to the placebo arm. Of these 306 men, a subset of 134 nondiabetic men (60 randomly assigned to the placebo group and 74 to the testosterone group) who had baseline and at least one postrandomization assessment of insulin sensitivity by OIST constituted the analytic sample. Of these 134 men, 98 (placebo group, n = 42; testosterone group, n = 56) completed the OIST at baseline and at 36 months of the study and constituted the completer sample.
Baseline characteristics
Baseline characteristics were similar in the two treatment groups (see Table 1). The mean age of men in the analytic sample was 66.7 ± 5.1 years, and the mean BMI was 28.1 ± 3.2 kg/m2. Participants in both dose groups were comparable in terms of age, BMI, fasting glucose level, hemoglobin A1c value, and body composition. Baseline characteristics were also similar between groups in the per-protocol study population (data not shown).
Hormone levels
The mean (standard deviation) on-treatment total testosterone concentrations at 36 months increased from 330.3 ± 54.3 ng/dL at baseline to 477.1 ± 346.9 ng/dL in the testosterone group but did not change significantly in the placebo group. Similarly, mean on-treatment free testosterone concentrations increased from 68.8 ± 14.2 pg/mL at baseline to 94.3 ± 94.8 pg/mL in the testosterone arm. At baseline, 10 participants (7.46%) had total serum testosterone levels below the lower limit of normal (<239 ng/dL; range, 200 to 230 ng/dL).
Insulin sensitivity
Insulin sensitivity, estimated as the concentration of glucose at equilibrium (SSPG), was similar in the two groups at baseline (Table 1). There was a significant increase in SSPG over 36 months in men randomly assigned to placebo (P = 0.03), whereas no significant change over time was seen in men randomly assigned to testosterone (P = 0.33). There were no significant differences in change in SSPG across 3 years in the testosterone group compared with the placebo group after adjustments for baseline SSPG (+15.3 ± 6.9 mg/dL in the placebo group vs +6.2 ± 6.4 mg/dL in the testosterone group; mixed-model effect, P = 0.17) (Fig. 1; Table 2). These results remained unchanged after adjustments for baseline SHBG levels and BMI.
Table 1.
Baseline Characteristics of Participants
| Testosterone (n = 74) | Placebo (n = 60) | |
|---|---|---|
| Age, y | 65.7 ± 5.0 | 67.9 ± 5.1 |
| Weight, kg | 86.1 ± 11.2 | 86.4 ± 9.9 |
| BMI, kg/m2 | 28.0 ± 3.1 | 28.4 ± 3.3 |
| Total lean mass, kg | 55.3 ± 5.7 | 55.4 ± 5.0 |
| Total fat mass, kg | 21.1 ± 5.4 | 21.0 ± 6.3 |
| Total testosterone, ng/dL | 329.3 ± 52.5 | 327.1 ± 51.3 |
| Free testosterone, pg/mL | 67.8 ± 13.4 | 65.5 ± 18.0 |
| SHBG, nmol/L | 32.5 ± 10.9 | 34.5 ± 12.3 |
| Fasting glucose, mg/dL | 90.5 ± 13.6 | 92.2 ± 12.3 |
| Hemoglobin A1c, % | 5.33 ± 0.36 | 5.39 ± 0.45 |
| SSPG, mg/dLa | 106.2 ± 61.1 | 112.3 ± 64.3 |
Data are presented as mean ± standard deviation.
SSPG was assessed from the OIST.
Figure 1.
Change in insulin sensitivity over time measured by the OIST and estimated as the mean concentration of glucose at equilibrium SSPG by averaging the values during the last 30 minutes of the infusion at the 160-, 170-, and 180-minute time points. P values were extracted from mixed-model regression.
Table 2.
Changes From Baseline in Anthropometric and Metabolic Parameters
| Testosterone (n = 74) | Placebo (n = 60) | P Value | |
|---|---|---|---|
| Weight, kg | 0.35 ± 0.60 | −0.82 ± 2.1 | 0.59 |
| BMI, kg/m2 | 0.12 ± 0.19 | −0.29 ± 0.72 | 0.59 |
| Total lean mass, kg | 1.3 ± 0.41 | 0.50 ± 0.38 | 0.18 |
| Total fat mass, kg | 1.4 ± 0.48 | 3.7 ± 0.42 | <0.001 |
| Fasting glucose, mg/dL | −0.41 ± 1.33 | −0.64 ± 3.01 | 0.94 |
| SSPG, mg/dL | 6.2 ± 6.4 | 15.3 ± 6.9 | 0.17 |
P value for SSPG was derived from a mixed model; other P values were derived from the t test.
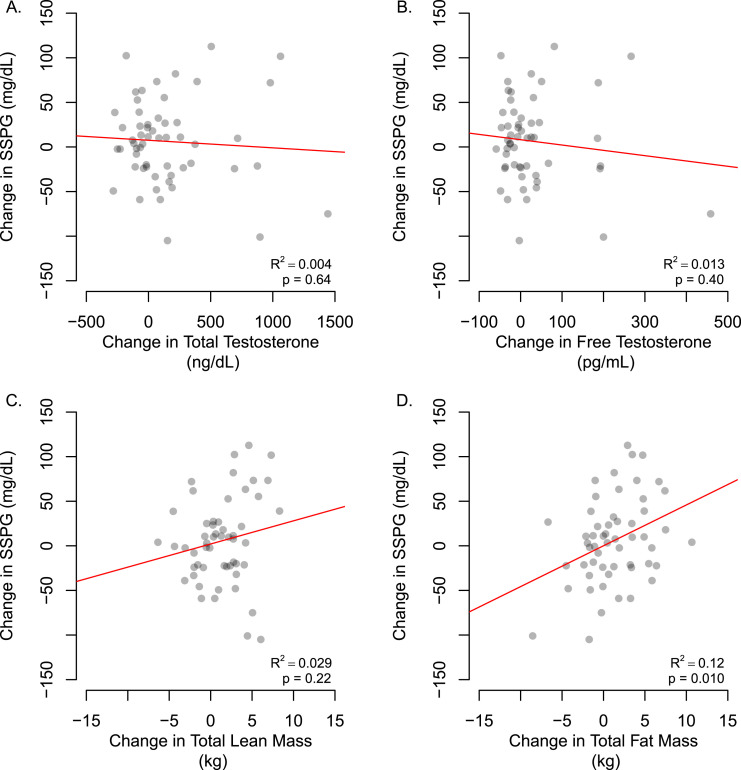
The changes in SSPG from baseline in men assigned to the testosterone arm were not significantly related to changes in total or free testosterone concentrations (Δ total testosterone: R2 = 0.043, P= 0.64; Δ free testosterone: R2 = 0.004, P = 0.40) (Fig. 2).
Figure 2.
Scatter plot of association between change from baseline in insulin sensitivity estimated as SSPG from the OIST, with changes in (A and B) serum testosterone concentrations and (C and D) body composition at 36 months in testosterone-treated men (n = 74). P value and R2 were extracted from a simple linear regression model.
Relationship of body composition with insulin sensitivity
There was a significant increase in total fat mass in the placebo group relative to the testosterone group (P < 0.008). There were no significant differences in changes in body weight or BMI from baseline to 36 months in the testosterone group compared with the placebo group (Table 2). The changes in total lean mass over time were not significantly associated with changes in SSPG from baseline to 36 months postintervention in the testosterone arm, whereas increases in total fat mass over time were significantly associated with increases in SSPG (Δ lean body mass: R2 = 0.029, P = 0.22; Δ total fat mass: R2 =0.12; P = 0.01) (Fig. 2).
Per-protocol analyses restricted to men who completed the 36-month intervention and underwent both the baseline and the 36-month OIST assessments also showed no significant difference in change in SSPG between the testosterone and the placebo arms (Table 3).
Table 3.
Changes From Baseline in Insulin Sensitivity in the Completer Sample
| Variable | Month | Testosterone (n = 56) | Placebo (n = 41) | P Value a |
|---|---|---|---|---|
| SSPG, mg/dL | 3 | −4.6 ± 4.9 | −1.5 ± 5.8 | 0.68 |
| 36 | 6.0 ± 6.1 | 15.6 ± 7.2 | 0.31 | |
| Weight, kg | 36 | 0.39 ± 0.62 | 1.3 ± 0.41 | 0.23 |
| BMI, kg/m2 | 36 | 0.12 ± 0.20 | 0.44 ± 0.15 | 0.20 |
| Total lean mass, kg | 36 | 1.3 ± 0.43 | 0.40 ± 0.41 | 0.14 |
| Total fat mass, kg | 36 | 1.3 ± 0.49 | 3.9 ± 0.46 | <0.001 |
The completer sample included all participants who completed 36 months of study intervention. Data are presented as mean ± standard error.
P value for SSPG was derived from the mixed regression model; other P values were derived from the t test.
Sensitivity analyses
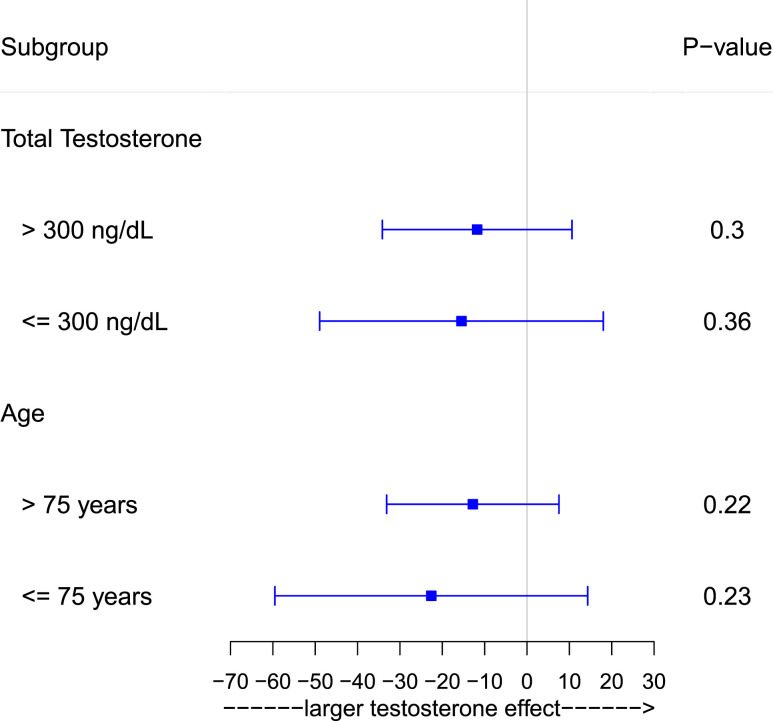
Stratification by men who had baseline total testosterone levels ≤300 ng/dL vs those who had baseline total testosterone levels >300 ng/dL did not show any significant intervention effects in either of the subgroups (total testosterone ≤300 ng/dL, P = 0.44; total testosterone >300 ng/dL, P = 0.46). Stratification by men aged ≤75 years vs those aged >75 years did not show any significant intervention effects in either of the subgroups (Fig. 3).
Figure 3.
Forest plot of estimated changes from baseline at 36 months between testosterone and placebo groups stratified by baseline total testosterone levels ≤300 ng/dL and >300 ng/dL and by age ≤75 years and >75 years. P values were extracted from mixed-model regression.
Discussion
In this trial of older, nondiabetic men with low or low-normal serum testosterone levels, testosterone treatment for 3 years raised levels into a range that was midnormal for healthy young men. Despite mild worsening of insulin sensitivity in placebo-treated men over time and no significant change in insulin sensitivity in men randomly assigned to testosterone replacement, we found no significant difference between treatment groups. Per-protocol analyses of men who completed 3 years of study intervention also did not reveal significant between-group differences. Finally, changes in insulin sensitivity from baseline were not related to changes in serum total or free testosterone concentrations. Therefore, these data do not support the use of testosterone for improving insulin sensitivity in older men with age-related decline in testosterone levels.
A substantial body of epidemiologic literature has shown that low serum total testosterone levels in men are associated with insulin resistance, type 2 diabetes, and metabolic syndrome (2, 4, 7, 36). However, in contrast to these epidemiologic studies, we did not find a significant effect of testosterone replacement therapy on insulin sensitivity in this randomized trial. The men in our study were recruited on the basis of their serum testosterone levels regardless of symptoms. It is important to acknowledge that testosterone treatment is recommended only in men with unequivocally low testosterone levels and symptoms of testosterone deficiency. More than half the men in this trial had screening testosterone levels in the eugonadal range, and even those whose levels were below the normal range had only mildly reduced testosterone levels, typical of the vast majority of middle-aged and older men receiving testosterone therapy in the United States (37). It is possible that testosterone replacement may improve insulin sensitivity only in men with severe testosterone deficiency. This speculation is supported by preclinical data showing that surgical castration of adult male rats impaired insulin sensitivity, whereas testosterone replacement reversed this derangement (38). Furthermore, acute withdrawal of testosterone in men with prostate cancer who receive androgen-deprivation therapy is associated with the development of insulin resistance (39).
As with hormonal therapies for women, differences in the effects of endogenous hormones vs hormonal therapies as well as variable at-risk periods could contribute to the apparent discrepancies between epidemiologic and clinical trial results. It is also conceivable that SHBG, rather than testosterone, is a risk factor for insulin resistance and diabetes and that the apparent association between total testosterone and insulin resistance in epidemiologic studies may reflect the strong relation between total testosterone and SHBG levels.
It is possible that the beneficial effects of testosterone on insulin sensitivity may be limited to high-risk individuals with diabetes/metabolic syndrome. The participants in our study were healthy nondiabetic individuals with normal glycemic control. A number of intervention trials have examined the effects of testosterone on glucose metabolism in men with mildly reduced testosterone levels and type 2 diabetes or metabolic syndrome (22–29). These trials failed to show a consistent and clinically meaningful improvement in insulin resistance measures. Our findings are consistent with those of the majority of trials in showing no significant effect of testosterone treatment in men without diabetes who have either normal or only mildly low testosterone levels.
The inconsistencies between clinical trials may be due to their small sample sizes, their short treatment durations, and differences in the methods used to estimate insulin sensitivity/resistance. The sample size of this trial was larger than that of most previous randomized trials of the effects of testosterone therapy on insulin sensitivity. With an intervention duration of 3 years, our trial also was one of the longest. We used the OIST, a well-validated direct measure of insulin sensitivity that is well correlated with the gold standard hyperinsulinemic-euglycemic clamp method. Even with the use of a direct measure of insulin sensitivity and a sufficiently long intervention duration, we did not find significant changes with testosterone replacement. Our data do not support the hypothesis that testosterone administration improves insulin sensitivity in older men with low-normal or slightly reduced testosterone levels.
Our study has notable strengths and some limitations. The trial had many features of a good trial design: concealed randomization, placebo control, parallel groups, blinding, and oversight by an independent DSMB. To date, this is the longest trial to evaluate the effect of testosterone replacement on insulin sensitivity in older men. The testosterone dose was adjusted in a blinded manner to achieve and maintain testosterone levels in the target range. We measured insulin sensitivity directly using the OIST method, an accurate direct measure of insulin sensitivity that highly correlates with hyperinsulinemic-euglycemic clamp estimates in patients with and without diabetes (31, 33). The OIST also has the added advantage of being less technically demanding than the clamp method because it does not require variable infusions, making it easier to achieve steady-state conditions (30).
Our study also has some limitations. The primary aim of the TEAAM Trial was to determine the effect of testosterone replacement on subclinical atherosclerosis progression; hence, insulin sensitivity was not the primary outcome of the trial. The enrolled men were not hypogonadal; therefore, the results cannot be generalized to men with classic hypogonadism due to known diseases of the testis, pituitary, and hypothalamus. Our study was limited to a nondiabetic population to prevent confounding due to the independent effects of glucose-lowering drugs as well as variability in glycemic control. Future studies should evaluate the effects of testosterone therapy on insulin sensitivity in men with type 2 diabetes and/or metabolic syndrome. Testosterone levels were measured using an immunoassay, given that the liquid chromatography-mass spectrometry assays for testosterone were not available to us at study initiation.
In conclusion, testosterone replacement for 3 years in men with low or low-normal testosterone levels did not improve insulin sensitivity. Thus, our findings do not support the use of testosterone supplementation to improve insulin sensitivity in older men with low-normal or slightly reduced testosterone levels.
Acknowledgments
We thank the staff members of the General Clinical Research Unit of Boston University’s Clinical and Translational Science Institute and the Clinical Research Center of Charles Drew University of Medicine and Science for their help with these studies and the study participants for their commitment and generosity. Data Safety Monitoring Board members were Dr. Thomas Yoshikawa, David Geffen School of Medicine at University of California, Los Angeles, California (Chair); Dr. William French, Division of Cardiology, Harbor-UCLA Medical Center, Torrance, California; and Dr. Nand Datta, Department of Urology, Charles Drew University, Los Angeles, California.
Financial Support: This investigator-initiated study was supported by a grant from Solvay Pharmaceuticals, Inc., and later by AbbVie Pharmaceuticals Inc. (to S. Bhasin) when AbbVie acquired the Androgel brand from Solvay Pharmaceuticals, and by a grant from the Aurora Foundation to the Kronos Longevity Research Institute (to S.M.H.). Additional support was provided by Grants K08 HL132122-02 from the National Institute of Health, 5P30AG031679 from the Boston Claude D. Pepper Older Americans Independence Center (to S. Bhasin), and 1UL1RR025771 from Boston University’s Clinical and Translational Science Institute (to S. Bhasin). Testosterone and placebo gel for the study were provided by Solvay Pharmaceuticals, Inc., and later by AbbVie Pharmaceuticals Inc.
Clinical Trial Infor mation: ClinicalTrials.gov no. NCT00287586 (registered 7 February 2006).
Disclosure Summary: S. Basaria has received grant support from Abbott Pharmaceuticals for investigator-initiated studies unrelated to this study and has previously consulted for Eli Lilly, Inc. S. Bhasin has received research grant support from AbbVie Pharmaceuticals Inc., Transition Therapeutics, and Metro International Biotechnology, LLC, for investigator-initiated research unrelated to this study. S. Bhasin has served as a consultant to AbbVie and Novartis. S. Bhasin has a financial interest in Function Promoting Therapies, LLC, a company aiming to develop innovative solutions that enhance precision and accuracy in clinical decision making and facilitate personalized therapeutic choices in reproductive health. S. Bhasin’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- OIST
octreotide insulin suppression test
- SHBG
sex hormone-binding globulin
- SSPG
steady-state plasma glucose
- TEAAM
Testosterone Effects on Atherosclerosis in Aging Men
References
- 1. Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest. 1983;71(6):1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grossmann M. Testosterone and glucose metabolism in men: current concepts and controversies. J Endocrinol. 2014;220(3):R37–R55. [DOI] [PubMed] [Google Scholar]
- 3. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724–731. [DOI] [PubMed] [Google Scholar]
- 4. Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9(7):414–424. [DOI] [PubMed] [Google Scholar]
- 5. Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999;22(5Suppl):110–116. [PubMed] [Google Scholar]
- 6. Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L;. MRFIT Research Group Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. Am J Epidemiol. 1996;143(9):889–897. [DOI] [PubMed] [Google Scholar]
- 7. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90(5):2618–2623. [DOI] [PubMed] [Google Scholar]
- 8. Hsu B, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ. Associations between circulating reproductive hormones and SHBG and prevalent and incident metabolic syndrome in community-dwelling older men: the Concord Health and Ageing in Men Project. J Clin Endocrinol Metab. 2014;99(12):E2686–E2691. [DOI] [PubMed] [Google Scholar]
- 9. Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. J Gerontol A Biol Sci Med Sci. 2010;65A(5):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhasin S, Jasjua GK, Pencina M, D’Agostino R Sr, Coviello AD, Vasan RS, Travison TG. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: the Framingham heart study. Diabetes Care. 2011;34(11):2464–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holmboe SA, Jensen TK, Linneberg A, Scheike T, Thuesen BH, Skakkebaek NE, Juul A, Andersson AM. Low testosterone: a risk marker rather than a risk factor for type 2 diabetes. J Clin Endocrinol Metab. 2016;101(8):3180–3190. [DOI] [PubMed] [Google Scholar]
- 12. Bhasin S, Taylor WE, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid NF. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci. 2003;58(12):M1103–M1110. [DOI] [PubMed] [Google Scholar]
- 13. Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, Farwell WR, Choong K, Lakshman K, Mazer NA, Coviello AD, Knapp PE, Ulloor J, Zhang A, Brooks B, Nguyen AH, Eder R, LeBrasseur N, Elmi A, Appleman E, Hede-Brierley L, Bhasin G, Bhatia A, Lazzari A, Davis S, Ni P, Collins L, Bhasin S. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66A(10):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Storer TW, Basaria S, Traustadottir T, Harman SM, Pencina K, Li Z, Travison TG, Miciek R, Tsitouras P, Hally K, Huang G, Bhasin S. Effects of testosterone supplementation for 3 years on muscle performance and physical function in older men. J Clin Endocrinol Metab. 2017;102(2):583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab. 2008;93(1):139–146. [DOI] [PubMed] [Google Scholar]
- 16. Tapper J, Arver S, Pencina KM, Martling A, Blomqvist L, Buchli C, Li Z, Gagliano-Jucá T, Travison TG, Huang G, Storer TW, Bhasin S, Basaria S. Muscles of the trunk and pelvis are responsive to testosterone administration: data from testosterone dose-response study in young healthy men. Andrology. 2018;6(1):64–73. [DOI] [PubMed] [Google Scholar]
- 17. Parthasarathy C, Renuka VN, Balasubramanian K. Sex steroids enhance insulin receptors and glucose oxidation in Chang liver cells. Clin Chim Acta. 2009;399(1-2):49–53. [DOI] [PubMed] [Google Scholar]
- 18. Muthusamy T, Murugesan P, Balasubramanian K. Sex steroids deficiency impairs glucose transporter 4 expression and its translocation through defective Akt phosphorylation in target tissues of adult male rat. Metabolism. 2009;58(11):1581–1592. [DOI] [PubMed] [Google Scholar]
- 19. Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294(5):E961–E968. [DOI] [PubMed] [Google Scholar]
- 20. Muthusamy T, Murugesan P, Srinivasan C, Balasubramanian K. Sex steroids influence glucose oxidation through modulation of insulin receptor expression and IRS-1 serine phosphorylation in target tissues of adult male rat. Mol Cell Biochem. 2011;352(1-2):35–45. [DOI] [PubMed] [Google Scholar]
- 21. Lin HY, Yu IC, Wang RS, Chen YT, Liu NC, Altuwaijri S, Hsu CL, Ma WL, Jokinen J, Sparks JD, Yeh S, Chang C. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008;47(6):1924–1935. [DOI] [PubMed] [Google Scholar]
- 22. Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, Green K, Makdissi A, Hejna J, Chaudhuri A, Punyanitya M, Dandona P. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, Morales AM, Volterrani M, Yellowlees A, Howell JD, Channer KS; TIMES2 Investigators . Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care. 2011;34(4):828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6):899–906. [DOI] [PubMed] [Google Scholar]
- 25. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P; BLAST Study Group . Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11(3):840–856. [DOI] [PubMed] [Google Scholar]
- 26. Gianatti EJ, Dupuis P, Hoermann R, Strauss BJ, Wentworth JM, Zajac JD, Grossmann M. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(8):2098–2107. [DOI] [PubMed] [Google Scholar]
- 27. Magnussen LV, Glintborg D, Hermann P, Hougaard DM, Højlund K, Andersen M. Effect of testosterone on insulin sensitivity, oxidative metabolism and body composition in aging men with type 2 diabetes on metformin monotherapy. Diabetes Obes Metab. 2016;18(10):980–989. [DOI] [PubMed] [Google Scholar]
- 28. Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73(5):602–612. [DOI] [PubMed] [Google Scholar]
- 29. Huang G, Bhasin S, Tang ER, Aakil A, Anderson SW, Jara H, Davda M, Travison TG, Basaria S. Effect of testosterone administration on liver fat in older men with mobility limitation: results from a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68(8):954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muniyappa R, Madan R, Quon MJ. Assessing insulin sensitivity and resistance in humans In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. South Dartmouth, MA: Endotext; 2000. [Google Scholar]
- 31. Mimura A, Kageyama S, Maruyama M, Ikeda Y, Isogai Y. Insulin sensitivity test using a somatostatin analogue, octreotide (Sandostatin). Horm Metab Res. 1994;26(4):184–187. [DOI] [PubMed] [Google Scholar]
- 32. Harano Y, Hidaka H, Takatsuki K, Ohgaku S, Haneda M, Motoi S, Kawagoe K, Shigeta Y, Abe H. Glucose, insulin, and somatostatin infusion for the determination of insulin sensitivity in vivo. Metabolism. 1978;27(9Suppl 1):1449–1452. [DOI] [PubMed] [Google Scholar]
- 33. Knowles JW, Assimes TL, Tsao PS, Natali A, Mari A, Quertermous T, Reaven GM, Abbasi F. Measurement of insulin-mediated glucose uptake: direct comparison of the modified insulin suppression test and the euglycemic, hyperinsulinemic clamp. Metabolism. 2013;62(4):548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, Pencina KM, Vita J, Dzekov C, Mazer NA, Coviello AD, Knapp PE, Hally K, Pinjic E, Yan M, Storer TW, Bhasin S. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314(6):570–581. [DOI] [PubMed] [Google Scholar]
- 35. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512–519. [DOI] [PubMed] [Google Scholar]
- 36. Pitteloud N, Hardin M, Dwyer AA, Valassi E, Yialamas M, Elahi D, Hayes FJ. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab. 2005;90(5):2636–2641. [DOI] [PubMed] [Google Scholar]
- 37. Schoenfeld MJ, Shortridge E, Cui Z, Muram D. Medication adherence and treatment patterns for hypogonadal patients treated with topical testosterone therapy: a retrospective medical claims analysis. J Sex Med. 2013;10(5):1401–1409. [DOI] [PubMed] [Google Scholar]
- 38. Holmäng A, Björntorp P. The effects of testosterone on insulin sensitivity in male rats. Acta Physiol Scand. 1992;146(4):505–510. [DOI] [PubMed] [Google Scholar]
- 39. Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29(5):534–539. [DOI] [PubMed] [Google Scholar]