Abstract
Context
Thyroid nodules, adenomas, and goiter have consistently been associated with thyroid cancer risk. Few studies have assessed whether thyroid dysfunction and thyroid autoimmunity influence this risk.
Objective
To examine thyroid cancer risk after diagnoses of a wide range of benign thyroid conditions.
Design
Hospital and cancer registry linkage cohort study for the years 1978 to 2013.
Setting
Nationwide (Denmark).
Participants
Patients diagnosed with hyperthyroidism (n = 85,169), hypothyroidism (n = 63,143), thyroiditis (n = 12,532), nontoxic nodular goiter (n = 65,782), simple goiter (n = 11,582), other/unspecified goiter (n = 21,953), or adenoma (n = 6,481) among 8,258,807 residents of Denmark during the study period.
Main Outcome Measures
We computed standardized incidence ratios (SIRs) for differentiated thyroid cancer, excluding the first 12 months of follow-up after benign thyroid disease diagnosis.
Results
SIRs were significantly elevated for all benign thyroid diseases apart from hypothyroidism. SIRs were higher for men than women and in the earlier follow-up periods. Elevated SIRs were observed for localized and regional/distant thyroid cancer. After excluding the first 10 years of follow-up, hyperthyroidism [n = 27 thyroid cancer cases; SIR = 2.00; 95% confidence interval (CI): 1.32 to 2.92], nontoxic nodular goiter (n = 83; SIR = 4.91; 95% CI: 3.91 to 6.09), simple goiter (n = 8; SIR = 4.33; 95% CI: 1.87 to 8.53), other/unspecified goiter (n = 20; SIR = 3.94; 95% CI: 2.40 to 6.08), and adenoma (n = 9; SIR = 6.02; 95% CI: 2.76 to 11.5) remained positively associated with thyroid cancer risk.
Conclusions
We found an unexpected increased risk of differentiated thyroid cancer, including regional/distant disease, following diagnosis of hyperthyroidism and thyroiditis that could not be solely attributed to increased medical surveillance. Hypothyroidism was less clearly associated with thyroid cancer risk.
This large nationwide study provides strong evidence for an increased risk of differentiated thyroid cancer following diagnosis of hyperthyroidism and thyroiditis.
Thyroid cancer is the most common endocrine malignancy, accounting for 2.1% of all cancer diagnoses worldwide (1). Its incidence has been increasing over the past few decades in several countries (2), including Denmark (3). Although much of the increase in thyroid cancer incidence appears to stem from improved detection and diagnosis, the rising incidence of larger, more advanced-stage tumors suggests that some of the increase is due to etiologic factors (4). However, the recognized risk factors for thyroid cancer (e.g., female sex, white race, high-dose ionizing radiation exposure in childhood, and obesity) are either inherent or difficult to modify (5, 6).
Benign thyroid nodules, adenoma, and goiter have been associated consistently with thyroid cancer risk in epidemiologic studies. The relative risks are ~30 for benign thyroid nodules and adenoma and 5 for goiter, in comparisons of individuals with and without each condition (6–13). The increased risk of thyroid cancer immediately following detection of these conditions likely reflects increased surveillance or diagnostic workup, whereas long-term elevated risks may reflect shared genetic and environmental risk factors. Functional thyroid diseases have been associated less consistently with subsequent risk of thyroid cancer, with some (7, 8, 14), but not all (9–12), studies finding an association for hyperthyroidism and one study (12), but not others (7–11), finding an association for hypothyroidism. Evidence on whether autoimmune thyroiditis influences thyroid cancer risk is limited and inconsistent (8, 12, 15). Of continued debate has been whether Hashimoto thyroiditis predisposes to papillary thyroid cancer; however, early support for this hypothesis was largely based on retrospective studies of patients undergoing thyroidectomy, raising concerns about selection bias and inability of such studies to establish a temporal relationship (16).
More recent observational studies evaluating the relationship between benign thyroid conditions and thyroid cancer risk have been limited by a reliance on self-reported medical history (7–11, 13), with some of the studies collecting this information retrospectively (7, 10, 11, 13), raising concerns about recall bias. Large prospective studies, with long-term follow-up and high-quality medication information, are needed to improve our understanding of the role of thyroid dysfunction and autoimmunity in thyroid cancer etiology and to identify patients at high risk of future malignancy.
We therefore prospectively examined benign thyroid conditions and risk of differentiated thyroid cancer using information for the period 1978 to 2013 from nationwide hospital and cancer registries in Denmark.
Methods
Source population
We constructed a study cohort by linking nationwide registry data on inpatient hospitalizations and hospital outpatient clinic visits, cancer diagnoses, migration status, and vital status in Denmark for the period between 1 January 1978 and 30 November 2013. The Danish National Health Service guarantees tax-supported health care for all residents. Health service utilization is tracked by several nationwide registries, which are linkable using each resident’s unique civil personal registration number, which has been assigned to all Danish residents at birth or upon immigration since 1968 (16). We used the Danish Civil Registration System to identify residents of Denmark and information on migration and vital status (17). Our study’s source population consisted of the 8,258,807 men and women (cumulatively) who resided in Denmark during the study period. Because this analysis was register based, consent from individual participants was not required, in accordance with Danish law.
Benign thyroid conditions
The Danish National Patient Registry contains information on all inpatient hospitalizations at Danish nonpsychiatric hospitals since 1977 and on outpatient and emergency department visits since 1995 (18, 19). Each hospital contact is recorded by physicians with one primary diagnosis and one or more secondary diagnoses classified according to the International Classification of Diseases, Eighth Revision until the end of 1993 and the International Classification of Diseases, Tenth Revision thereafter. We used the Danish National Patient Registry to identify all patients with a first-time diagnosis of a benign thyroid condition during the study period: hyperthyroidism, hypothyroidism, thyroiditis, goiter (nontoxic nodular, simple, or other/unspecified), or thyroid adenoma (see Table 1 for codes). Capture of certain benign thyroid disease subtypes defined only by International Classification of Diseases, Tenth Revision codes were consequently restricted to the 1994 to 2013 period; these included thyrotoxicosis with diffuse goiter (Graves disease), thyrotoxicosis with toxic nodules, and autoimmune, subacute, and acute thyroiditis.
Table 1.
International Classification of Diseases and International Classification of Diseases for Oncology Codes
| Diagnosis | ICD-8 or ICD-10 Codes | ICD-O-3 |
|---|---|---|
| Thyroid cancer | C73 | |
| Papillary | 8050, 8260, 8340–8344, 8350, 8450–8460 | |
| Follicular | 8290, 8330–8335 | |
| Hyperthyroidism | 242; E05 | |
| Thyrotoxicosis with diffuse goiter (Graves) (1994–2013) | E05.0, E05.8, E05.9 | |
| Thyrotoxicosis with toxic nodules (1994–2013) | E05.2, E05.3 | |
| Hypothyroidism | 244; E02, E03.2–E03.9, E89.0 | |
| Thyroiditis (total) | 245; E06 | |
| Autoimmune thyroiditis (1994–2013) | E06.3 | |
| Subacute thyroiditis (1994–2013) | E06.1 | |
| Acute thyroiditis (1994–2013) | E06.0 | |
| Nontoxic nodular goiter | 241; E01.1, E04.1, E04.2 | |
| Simple goiter | 240.0; E01.0, E04.0 | |
| Other/unspecified goiter | 240.9; E01.2, E04.8, E04.9 | |
| Thyroid adenoma | 226; D34 |
Abbreviations: ICD-8, International Classification of Diseases, Eighth Revision; ICD-10, International Classification of Diseases, Tenth Revision; ICD-O-3, International Classification of Diseases for Oncology, Third Edition.
Thyroid cancer
To identify incident thyroid cancer cases, patients with a diagnosis of a benign thyroid disease were linked to the Danish Cancer Registry (DCR). The DCR has recorded all incident cancers in Denmark since 1943, with mandatory reporting since 1987 (20). The DCR also provided data on thyroid cancer histology and stage at diagnosis. Papillary and follicular thyroid cancer were classified according to the International Classification of Diseases for Oncology, Third Edition (Table 1).
Exclusions
Patients with a diagnosis of hypothyroidism and hyperthyroidism on the same date (n = 487) were excluded. We additionally excluded patients with a diagnosis of cancer other than nonmelanoma skin cancer prior to their diagnosis of benign thyroid disease; these exclusions (ranging from 33 individuals in analyses of acute thyroiditis to 8853 individuals in analyses of hypothyroidism) were applied separately for each benign thyroid disease.
Statistical analysis
Follow-up began 1 year after the date of first diagnosis of the benign thyroid disease of interest (to minimize diagnostic workup or surveillance biases) until the date of incident cancer diagnosis (other than nonmelanoma skin cancer), death, emigration, or 30 November 2013, whichever came first. The expected number of thyroid cancer cases was computed using national incidence rates by age (5-year categories), sex, and year of diagnosis (5-year categories) multiplied by person-years of follow-up. Standardized incidence ratios (SIRs) for differentiated thyroid cancer were computed as the ratio of observed to expected cancers. We computed 95% confidence intervals (CIs) for the SIRs assuming that the observed number of cases in a specific category followed a Poisson distribution, using Byar’s approximation. Results were stratified by sex, age at thyroid disease diagnosis, and calendar period at thyroid disease diagnosis. We also stratified the results by time since benign thyroid disease diagnosis (1 to <2 years, 2 to <5 years, 5 to <10 years, or 10+ years) to evaluate the impact of diagnostic workup of the disease, which could have resulted in the detection and diagnosis of prevalent occult thyroid cancers. All analyses were performed separately according to histologic type of thyroid cancer (papillary or follicular) and stage at diagnosis (localized or regional/distant). We additionally stratified the associations for hyperthyroidism and simple goiter according to treatment (vs no treatment) with radioactive iodine (iodine-131) during the period 2004 to 2013, to allow for a 2-year latency following the availability of these data starting in the year 2002.
All statistical analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC). The study was approved by the Danish Data Protection Agency, record number 1-16-02-1-08. Studies based on registry data in Denmark do not require informed consent.
Results
Characteristics of the patients diagnosed with benign thyroid diseases from 1978 to 2013 are shown in Table 2. The most common diagnoses were hyperthyroidism (n = 85,169) and hypothyroidism (n = 63,143); thyroid adenoma was the least common (n = 6481). Most patients in each disease category were women (≥75% for each). The median ages at diagnosis of hyperthyroidism and hypothyroidism (57.3 and 58.9 years, respectively) were higher than those for the other benign thyroid disease types (range: 45.0 to 49.1 years). Follow-up times ranged from 1 to 36 years. By type of benign thyroid disease, median follow-up time ranged from 6.3 years (interquartile range: 3.2 to 11.0 years) for hypothyroidism to 11.6 years (interquartile range: 5.4 to 18.9 years) for nontoxic nodular goiter.
Table 2.
Descriptive Characteristics of Patients Diagnosed With Benign Thyroid Conditions, Denmark, 1978–2013
| Hyperthyroidism | Hypothyroidism | Thyroiditis | Nontoxic Nodular Goiter | Simple Goiter | Other/Unspecified Goiter | Thyroid Adenoma | |
|---|---|---|---|---|---|---|---|
| Total no. of subjects | 85,169 | 63,143 | 12,532 | 65,782 | 11,582 | 21,953 | 6481 |
| Sex | |||||||
| Men | 17% | 15% | 16% | 16% | 15% | 14% | 25% |
| Women | 83% | 85% | 84% | 84% | 85% | 86% | 75% |
| Age at benign thyroid disease diagnosis | |||||||
| Median, y (IQR) | 57.3 (42.7–70.9) | 58.9 (42.2–73.4) | 45.0 (33.8–56.2) | 49.1 (39.2–60.3) | 47.1 (35.9–60.0) | 48.4 (37.7–61.2) | 46.4 (36.0–56.9) |
| Calendar year at benign thyroid disease diagnosis | |||||||
| 1978–1982 | 9% | 5% | 4% | 11% | 1% | 13% | 8% |
| 1983–1987 | 7% | 5% | 4% | 10% | 1% | 9% | 10% |
| 1988–1992 | 8% | 7% | 5% | 9% | 2% | 7% | 11% |
| 1993–1997 | 16% | 11% | 12% | 16% | 23% | 13% | 17% |
| 1998–2002 | 21% | 17% | 17% | 17% | 25% | 19% | 14% |
| 2003–2007 | 21% | 24% | 24% | 16% | 23% | 23% | 15% |
| 2008–2013 | 18% | 32% | 34% | 21% | 24% | 16% | 26% |
| Follow-up | |||||||
| Median, y (IQR) | 9.1 (4.7–14.7) | 6.3 (3.2–11.0) | 7.8 (4.0–13.9) | 11.6 (5.4–18.9) | 9.4 (4.8–14.9) | 10.6 (6.0–17.2) | 10.7 (4.6–18.5) |
| Comorbid thyroid diseases | |||||||
| Hyperthyroidism | — | 12% | 10% | 7% | 12% | 7% | 4% |
| Hypothyroidism | 2% | — | 26% | 2% | 3% | 2% | 1% |
| Thyroiditis | 1% | 6% | — | 1% | 1% | 1% | 1% |
| Nontoxic nodular goiter | 5% | 8% | 6% | — | 23% | 16% | 24% |
| Simple goiter | 1% | 2% | 1% | 3% | — | 3% | 2% |
| Other/unspecified goiter | 3% | 3% | 2% | 8% | 10% | — | 5% |
| Thyroid adenoma | 0.3% | 0.5% | 1% | 2% | 2% | 1% | — |
Abbreviation: IQR, interquartile range.
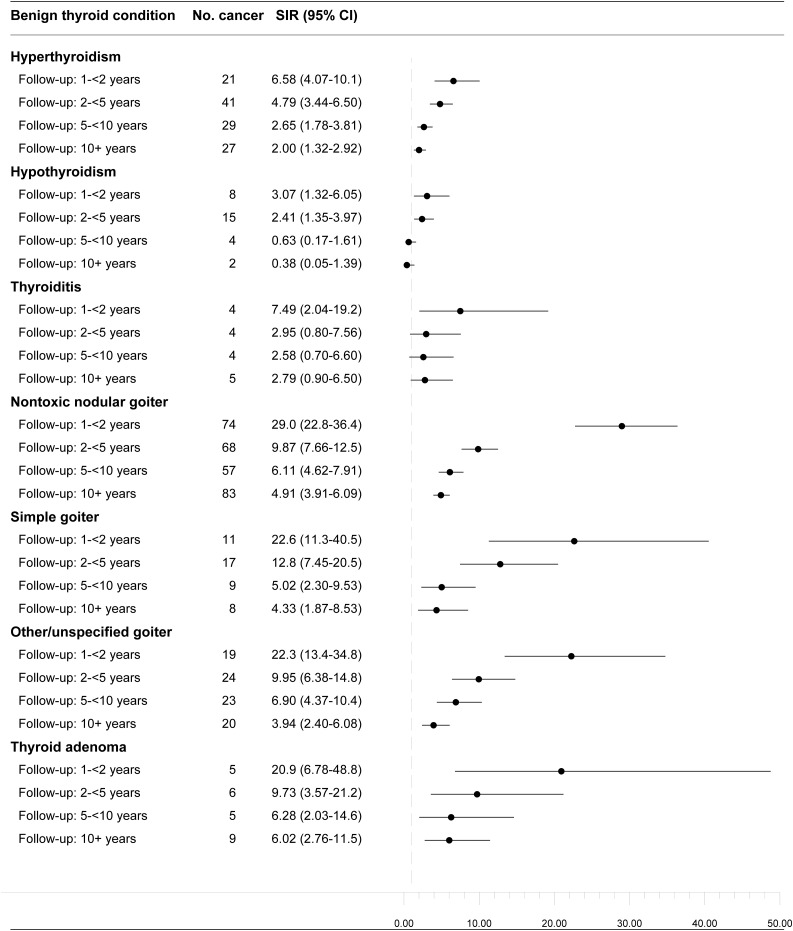
SIRs were significantly elevated for all benign thyroid diseases examined apart from hypothyroidism (Table 3). SIRs were higher for men than women (except for thyroiditis patients), higher for patients diagnosed with hyperthyroidism and thyroiditis at younger vs older ages, and higher for adenoma patients diagnosed at older vs younger ages. Nontoxic nodular goiter, simple goiter, other/unspecified goiter, and thyroid adenoma were more strongly associated with follicular thyroid cancer than papillary thyroid cancer, and thyroiditis was more clearly associated with papillary thyroid cancer; otherwise, the associations for papillary and follicular thyroid cancer were similar in magnitude. Results were generally similar for localized and regional/distant thyroid cancer at diagnosis, though slightly higher SIRs were observed for regional/distant thyroid cancer following thyroiditis, nontoxic nodular goiter, simple goiter, and thyroid adenoma. SIRs were also higher in the earlier follow-up periods (suggesting a role of detection or diagnostic workup bias). After excluding the first 10 years of follow-up, hyperthyroidism (n = 27 thyroid cancer cases, SIR = 2.00, 95% CI: 1.32 to 2.92), nontoxic nodular goiter (n = 83, SIR = 4.91, 95% CI: 3.91 to 6.09), simple goiter (n = 8, SIR = 4.33, 95% CI: 1.87 to 8.53), other/unspecified goiter (n = 20, SIR = 3.94, 95% CI: 2.40 to 6.08), and adenoma (n = 9, SIR = 6.02, 95% CI: 2.76 to 11.5) remained positively associated with thyroid cancer risk; no association was observed for hypothyroidism after excluding the first 5 years of follow-up (Fig. 1). Results were slightly stronger after restricting to benign thyroid diseases diagnosed in 1995 or later, the period when outpatient records were complete (Supplemental Table 1). Among the hyperthyroidism and simple goiter patients diagnosed during 2004 to 2013 and treated with radioactive iodine, none received a subsequent diagnosis of thyroid cancer (data not shown).
Table 3.
SIRs for Differentiated Thyroid Cancer in Patients Diagnosed With Benign Thyroid Conditions, by Patient Characteristics, Denmark, 1978–2013
| Hyperthyroidism | Hypothyroidism | Thyroiditis | Nontoxic Nodular Goiter | Simple Goiter | Other/Unspecified Goiter | Thyroid Adenoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | SIR (95% CI) | Cases | SIR (95% CI) | Cases | SIR (95% CI) | Cases | SIR (95% CI) | Cases | SIR (95% CI) | Cases | SIR (95% CI) | Cases | SIR (95% CI) | |
| Overall | 118 | 3.26 (2.70–3.91) | 29 | 1.42 (0.95–2.04) | 17 | 3.25 (1.89–5.20) | 282 | 7.90 (7.01–8.88) | 45 | 8.25 (6.02–11.0) | 86 | 7.36 (5.89–9.09) | 25 | 7.95 (5.14–11.7) |
| Sex | ||||||||||||||
| Men | 14 | 5.43 (2.96–9.11) | 3 | 2.45 (0.51–7.17) | 1 | 3.09 (0.08–17.2) | 47 | 21.7 (16.0–28.9) | 5 | 17.7 (5.8–41.3) | 11 | 17.6 (8.78–31.5) | 10 | 32.9 (15.8–60.6) |
| Women | 104 | 3.10 (2.53–3.75) | 26 | 1.36 (0.89–1.99) | 16 | 3.26 (1.86–5.29) | 235 | 7.01 (6.14–7.97) | 40 | 7.73 (5.52–10.5) | 75 | 6.78 (5.34–8.50) | 15 | 5.28 (2.95–8.70) |
| Age at benign thyroid disease diagnosis | ||||||||||||||
| <30 y | 23 | 5.31 (3.36–7.97) | 5 | 2.46 (0.80–5.73) | 5 | 6.96 (2.25–16.2) | 50 | 12.5 (9.27–16.5) | 5 | 6.31 (2.04–14.7) | 14 | 7.70 (4.21–12.9) | 3 | 6.03 (1.24–17.6) |
| 30–49 y | 44 | 3.01 (2.19–4.04) | 10 | 1.30 (0.62–2.39) | 10 | 3.51 (1.68–6.45) | 139 | 6.90 (5.80–8.15) | 24 | 7.89 (5.06–11.8) | 49 | 7.74 (5.73–10.2) | 8 | 4.51 (1.94–8.88) |
| 50–69 y | 40 | 3.23 (2.31–4.40) | 9 | 1.26 (0.58–2.40) | 1 | 0.69 (0.02–3.85) | 68 | 6.94 (5.39–8.80) | 8 | 6.07 (2.61–12.0) | 17 | 5.92 (3.45–9.48) | 9 | 11.7 (5.37–22.3) |
| 70+ y | 11 | 2.28 (1.14–4.08) | 5 | 1.40 (0.45–3.27) | 1 | 4.60 (0.12–25.6) | 25 | 14.4 (9.32–21.3) | 8 | 26.3 (11.3–51.8) | 6 | 9.03 (3.31–19.7) | 5 | 46.5 (15.1–108) |
| Calendar year at benign thyroid disease diagnosis | ||||||||||||||
| 1978–1982 | 6 | 1.37 (0.50–2.99) | 3 | 2.40 (0.49–7.01) | 1 | 2.59 (0.07–14.4) | 29 | 4.88 (3.27–7.02) | 0 | — | 7 | 3.39 (1.36–6.99) | 2 | 5.68 (0.69–20.5) |
| 1983–1987 | 9 | 2.65 (1.22–5.04) | 0 | — | 2 | 5.17 (0.63–18.7) | 24 | 4.80 (3.07–7.14) | 0 | — | 6 | 4.05 (1.49–8.83) | 2 | 4.43 (0.54–16.0) |
| 1988–1992 | 5 | 1.35 (0.44–3.13) | 1 | 0.58 (0.01–3.21) | 1 | 2.48 (0.06–13.8) | 29 | 6.54 (4.38–9.40) | 1 | 5.99 (0.15–33.4) | 7 | 6.45 (2.59–13.3) | 3 | 5.87 (1.21–17.1) |
| 1993–1997 | 24 | 3.30 (2.12–4.91) | 3 | 0.90 (0.19–2.62) | 2 | 1.88 (0.23–6.77) | 60 | 8.45 (6.45–10.9) | 13 | 6.85 (3.65–11.7) | 19 | 9.99 (6.01–15.6) | 8 | 11.0 (4.75–21.7) |
| 1998–2002 | 29 | 3.41 (2.28–4.89) | 3 | 0.63 (0.13–1.84) | 4 | 3.34 (0.91–8.54) | 54 | 8.03 (6.03–10.5) | 12 | 7.12 (3.68–12.4) | 22 | 8.71 (5.46–13.2) | 5 | 9.91 (3.21–23.1) |
| 2003–2007 | 28 | 4.19 (2.78–6.05) | 7 | 1.29 (0.52–2.66) | 5 | 4.28 (1.39–9.98) | 49 | 11.0 (8.13–14.5) | 12 | 11.0 (5.66–19.1) | 14 | 6.63 (3.62–11.1) | 3 | 8.23 (1.70–24.0) |
| 2008–2013 | 17 | 7.73 (4.50–12.4) | 12 | 4.38 (2.26–7.65) | 2 | 3.21 (0.39–11.6) | 37 | 18.3 (12.9–25.2) | 7 | 17.3 (6.92–35.5) | 11 | 21.6 (10.8–38.6) | 2 | 8.48 (1.03–30.6) |
| Thyroid cancer histology | ||||||||||||||
| Papillary | 83 | 3.19 (2.54–3.95) | 21 | 1.43 (0.88–2.18) | 14 | 3.51 (1.92–5.89) | 174 | 6.54 (5.61–7.59) | 34 | 8.02 (5.55–11.2) | 59 | 6.69 (5.09–8.63) | 14 | 5.85 (3.20–9.82) |
| Follicular | 35 | 3.47 (2.41–4.82) | 8 | 1.41 (0.61–2.78) | 3 | 2.41 (0.50–7.04) | 108 | 11.9 (9.76–14.4) | 11 | 9.06 (4.51–16.2) | 27 | 9.43 (6.21–13.7) | 11 | 14.6 (7.28–26.1) |
| Thyroid cancer stage | ||||||||||||||
| Localized | 77 | 3.44 (2.72–4.31) | 19 | 1.54 (0.93–2.40) | 9 | 2.64 (1.21–5.01) | 182 | 7.71 (6.63–8.92) | 25 | 6.89 (4.45–10.2) | 56 | 7.23 (5.46–9.38) | 11 | 5.28 (2.63–9.45) |
| Regional/distant | 23 | 2.40 (1.52–3.60) | 5 | 0.92 (0.30–2.14) | 8 | 6.59 (2.84–13.0) | 73 | 8.80 (6.90–11.1) | 13 | 10.6 (5.63–18.1) | 19 | 7.06 (4.25–11.0) | 10 | 13.7 (6.56–25.2) |
Figure 1.
SIRs and 95% CIs for differentiated thyroid cancer in patients diagnosed with benign thyroid disease, by time since benign thyroid disease diagnosis, Denmark, 1978 to 2013. The vertical dotted grey line represents no association (SIR of 1.00).
Significant increased risks of thyroid cancer were observed for all hyperthyroidism subtypes (thyrotoxicosis with diffuse goiter, thyrotoxicosis with toxic nodules) and thyroiditis subtypes (autoimmune, subacute, acute) (Table 4). For thyrotoxicosis with diffuse goiter, thyrotoxicosis with toxic nodules, and autoimmune thyroiditis, thyroid cancer risk was highest among patients diagnosed before age 30. All thyroid cancer diagnoses following autoimmune thyroiditis (n = 7) were in women who had a diagnosis of autoimmune thyroiditis before age 50; thus, no information was available on thyroid cancer risks in men or patients diagnosed with autoimmune thyroiditis at older ages.
Table 4.
SIRs for Differentiated Thyroid Cancer in Patients Diagnosed With Hyperthyroidism or Thyroiditis Subtypes, by Patient Characteristics, Denmark, 1994–2013
| Thyrotoxicosis With Diffuse Goiter (Graves) | Thyrotoxicosis With Toxic Nodules | Autoimmune Thyroiditis | Subacute Thyroiditis | Acute Thyroiditis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | SIR (95% CI) | Cases | SIR (95% CI) | Cases | SIR (95% CI) | Cases | SIR (95% CI) | Cases | SIR (95% CI) | |
| Overall | 71 | 3.77 (2.94–4.75) | 26 | 3.94 (2.57–5.77) | 7 | 3.09 (1.24–6.37) | 4 | 3.76 (1.02–9.64) | 3 | 8.19 (1.69–23.9) |
| Sex | ||||||||||
| Men | 8 | 5.84 (2.52–11.5) | 2 | 4.87 (0.59–17.6) | 0 | — | 0 | — | 1 | 28.8 (0.73–160) |
| Women | 63 | 3.61 (2.77–4.61) | 24 | 3.88 (2.48–5.77) | 7 | 3.24 (1.30–6.67) | 4 | 4.12 (1.12–10.6) | 2 | 6.03 (0.73–21.8) |
| Age at benign thyroid disease diagnosis | ||||||||||
| <30 y | 17 | 7.69 (4.47–12.3) | 2 | 11.2 (1.36–40.5) | 3 | 9.02 (1.86–26.3) | 0 | — | 1 | 19.6 (0.50–109) |
| 30–49 y | 34 | 4.10 (2.84–5.72) | 9 | 4.67 (2.24–8.87) | 4 | 3.40 (0.92–8.69) | 3 | 4.53 (0.93–13.2) | 2 | 8.88 (1.07–32.1) |
| 50–69 y | 16 | 2.73 (1.56–4.43) | 12 | 3.91 (2.02–6.83) | 0 | — | 1 | 3.97 (0.10–22.1) | 0 | — |
| 70+ y | 4 | 1.62 (0.44–4.15) | 3 | 2.10 (0.43–6.14) | 0 | — | 0 | — | 0 | — |
| Calendar year at benign thyroid disease diagnosis | ||||||||||
| 1994–1997 | 11 | 2.26 (1.13–4.04) | 7 | 3.91 (1.57–8.06) | 1 | 2.19 (0.06–12.2) | 1 | 3.40 (0.09–19.0) | 2 | 7.45 (0.19–41.5) |
| 1998–2002 | 24 | 3.55 (2.27–5.28) | 8 | 3.17 (1.37–6.25) | 1 | 1.61 (0.04–8.99) | 1 | 2.93 (0.07–16.3) | 2 | 14.9 (1.80–53.6) |
| 2003–2007 | 23 | 4.25 (2.69–6.37) | 7 | 4.16 (1.67–8.56) | 4 | 5.59 (1.52–14.3) | 1 | 3.15 (0.08–17.6) | 0 | — |
| 2008–2013 | 13 | 7.25 (3.85–12.4) | 4 | 6.61 (1.80–16.9) | 1 | 2.12 (0.05–11.8) | 1 | 9.11 (0.23–50.7) | 0 | — |
| Time since benign thyroid disease diagnosis | ||||||||||
| 1 to <2 y | 11 | 5.29 (2.63–9.46) | 8 | 10.4 (4.50–20.6) | 1 | 3.13 (0.08–17.4) | 2 | 17.8 (2.16–64.4) | 0 | — |
| 2 to <5 y | 30 | 5.42 (3.66–7.74) | 10 | 5.06 (2.42–9.31) | 3 | 3.92 (0.81–11.4) | 0 | — | 0 | — |
| 5 to <10 y | 18 | 2.65 (1.57–4.19) | 3 | 1.27 (0.26–3.72) | 1 | 1.38 (0.03–7.67) | 1 | 2.62 (0.07–14.6) | 3 | 23.5 (4.83–68.5) |
| 10+ y | 12 | 2.70 (1.39–4.72) | 5 | 3.31 (1.07–7.72) | 2 | 4.44 (0.54–16.0) | 1 | 3.72 (0.09–20.7) | 0 | — |
| Thyroid cancer histology | ||||||||||
| Papillary | 56 | 3.97 (3.00–5.15) | 17 | 3.69 (2.15–5.90) | 6 | 3.39 (1.24–7.39) | 3 | 3.57 (0.73–10.4) | 3 | 10.4 (2.14–30.3) |
| Follicular | 15 | 3.17 (1.77–5.23) | 9 | 4.52 (2.07–8.59) | 1 | 2.03 (0.05–11.3) | 1 | 4.52 (0.11–25.2) | 0 | — |
| Thyroid cancer stage | ||||||||||
| Localized | 47 | 3.93 (2.88–5.22) | 19 | 4.71 (2.83–7.36) | 4 | 2.68 (0.73–6.87) | 3 | 4.19 (0.86–12.2) | 1 | 4.08 (0.10–22.7) |
| Regional/distant | 14 | 3.02 (1.65–5.06) | 6 | 3.40 (1.25–7.42) | 3 | 5.89 (1.21–17.2) | 1 | 4.33 (0.11–24.1) | 2 | 24.3 (2.94–87.7) |
Discussion
In this large population-based registry study in Denmark, we found that patients diagnosed with hyperthyroidism, thyroiditis, goiter, and adenoma had significantly elevated risks of differentiated thyroid cancer. These risks were generally greater for men than for women (except for thyroiditis) and closer in time to benign thyroid disease diagnosis (the latter suggesting a role of detection or diagnostic workup bias); however, increased risks were observed for localized and more advanced thyroid cancer at diagnosis. Thyroid cancer risks remained significantly elevated ≥10 years after diagnosis of hyperthyroidism (twofold) and goiter and adenoma (four- to sixfold). Thyroiditis was associated with a nonsignificant, twofold increase in risk ≥10 years after diagnosis, but results were based on small numbers of cases. Risk of differentiated thyroid cancer was not elevated ≥5 years after diagnosis of hypothyroidism.
Although thyroid nodules and goiter have been strongly and consistently associated with increased risk of thyroid cancer, the role of functional thyroid diseases (e.g., hyperthyroidism and hypothyroidism) and thyroid autoimmunity in thyroid cancer development has been less clear. Data from an international pooled analysis of 12 case-control studies (2519 cases and 4176 controls) showed strong associations for self-reported history of benign nodules and goiter, but no association for hypothyroidism (7). Although hyperthyroidism was associated with an increased risk of subsequent thyroid cancer, the association attenuated after excluding thyroid cancers diagnosed within 2 years of a hyperthyroidism diagnosis, and after adjusting for medical history of goiter. A cohort study of 90,713 US radiology technicians yielded similar findings (8), showing positive associations for self-reported baseline medical history of nodules/adenoma, goiter, and hyperthyroidism with thyroid cancer risk. Hypothyroidism was not associated with thyroid cancer risk in either women or men, but the latter result for men was based on only one exposed case. Thyroiditis also was not associated with risk, but results were similarly imprecise. Other studies relying on self-reported history of benign thyroid diseases generally have reported weaker or null associations for hyperthyroidism or hypothyroidism, despite observing relatively high relative risks for nodules and/or goiter (9–11).
The few large cohort studies that have used hospital discharge records provide some of the strongest evidence regarding the association between benign thyroid diseases and thyroid cancer risk. An earlier Danish study based on hospital inpatient data for the 1977 to 1988 period followed 57,326 patients diagnosed with hyperthyroidism, hypothyroidism, or nontoxic goiter and recorded an increased thyroid cancer risk in hyperthyroidism and goiter after exclusion of thyroid cancers detected in the first year after diagnosis of benign thyroid disease (21). We obtained similar results with an added 25 years of follow-up and inclusion of outpatient clinic data starting in 1995. Our finding of an increased risk of thyroid cancer following a diagnosis of thyroiditis is consistent with previous registry-based cohort studies in the United States and Taiwan (12, 15). In our study, however, we found that association between thyroiditis and thyroid cancer risk was elevated following diagnosis of all subtypes of thyroiditis, not just autoimmune thyroiditis, which may indicate a component of surveillance bias. Thyrotoxicosis with diffuse goiter (Graves disease), another autoimmune thyroid disorder and a major underlying cause of hyperthyroidism, was also associated with an increased risk of thyroid cancer that persisted for many years after thyrotoxicosis diagnosis, consistent with findings from Taiwanese and Swedish record-linkage studies (22, 23). A large registry-based cohort study of US male military veterans found a nonsignificant elevated risk of thyroid cancer associated with thyrotoxicosis after excluding the first 5 years of follow-up (relative risk = 2.0, 95% CI: 0.8 to 4.8), but this finding was based on only five thyroid cancer cases, precluding separate evaluation of thyrotoxicosis according to presence or absence of Graves disease (12).
Although not inconsistent with prior studies on the topic, our results showing positive associations for hyperthyroidism, but not hypothyroidism, with differentiated thyroid cancer risk were surprising considering that high thyroid-stimulating hormone (TSH) levels (characteristic of hypothyroidism) have long been hypothesized to promote thyroid cancer development (6). In laboratory settings, TSH stimulates follicular thyroid cell growth, and numerous cross-sectional studies indicate that higher levels of TSH are predictive of malignancy among thyroid nodule patients (24). Consistent with our study, two large prospective studies in the United States and Europe showed unexpected inverse associations between prediagnostic levels of TSH within the euthyroid range and subsequent risk of differentiated thyroid cancer (25, 26). However, these findings may not be directly comparable to ours, because TSH levels in patients with overt thyroid disease are secondary to a primary abnormality of the thyroid gland. One explanation for the increased risk of thyroid cancer following diagnosis of Graves disease is the TSH-mimicking effects of thyroid stimulating antibody (27). Thyroid stimulating antibody has also been shown to be a marker of thyroid cancer aggressiveness in patients with Graves disease (28). Hyperthyroidism is also related to changes in sex steroid–binding globulin and sex steroid hormones, including elevations in estradiol, which may have a direct influence on thyroid carcinogenesis (29, 30). Further investigation is needed to better understand the mechanisms underlying the increased risk of thyroid cancer in hyperthyroid patients.
Radioactive iodine (iodine-131) treatment of overt hyperthyroidism has also been associated with an increased risk of subsequent thyroid cancer in some (31, 32), but not all (33, 34), studies. However, positive findings from those studies were based on small numbers of exposed cases, and none of the thyroid cancer cases diagnosed in our study had a record of radioactive iodine treatment of hyperthyroidism. Taken together, these results suggest that the long-term increased risk of thyroid cancer following hyperthyroidism is more likely attributable to underlying disease as opposed to an effect of treatment.
Our study has several important strengths: the large size of the cohort and its nationwide coverage; prospective, long-term, and complete follow-up for cancer incidence; the availability of highly valid diagnostic data on both thyroid diseases and thyroid cancer; and the wide range of benign thyroid diseases examined. We excluded the first year of follow-up and further evaluated risks by increasing time since benign thyroid disease diagnosis to reduce the potential for diagnostic workup bias or enhanced surveillance. However, considering the slow-growing and indolent nature of most differentiated thyroid cancers, we cannot eliminate the possibility that even long-term elevated risks of thyroid cancer were attributable, in whole or in part, to incidental detection of thyroid cancer following routine diagnostic evaluations. Detection bias is of concern particularly for patients with structural disease, such as nodules, adenoma, or goiter, who might receive ultrasonography of the thyroid region, fine-needle aspiration biopsy, and/or surgery with incidental detection of thyroid cancer, even several years after the initial diagnosis of the benign condition. However, the positive associations observed in this study were not limited to localized thyroid cancer, which is what would be expected if our results were solely attributable to incidental detection. On the contrary, the risks of more advanced (regional/distant) thyroid cancer following diagnoses of thyroiditis, nontoxic nodular goiter, simple goiter, and adenoma were higher than the risks of localized disease. Additional studies that distinguish incidentally detected vs more clinically relevant thyroid cancers (i.e., by separately evaluating risks according to thyroid cancer stage, size, and/or molecular profile) are needed to confirm our findings.
Our study had several additional limitations. Outpatient records were not complete until 1995, which may explain the stronger associations observed for benign thyroid diseases diagnosed in more recent years. Because there are few known risk factors for thyroid cancer, confounding by factors not accounted for or incompletely ascertained in our study is possible, but unlikely to have biased our results substantially. Lack of laboratory measures precluded a biochemical validation of thyroid dysfunction, and lack of prescription data prevented us from determining the disease severity or treatment effects. The Danish population is characterized by mild iodine deficiency, which may limit the generalizability of our results to populations with different susceptibility to thyroid disorders related to iodine status (35). Despite the large size of this study, some of our results were based on small numbers of cases, which limited our statistical power. As our results were not adjusted for multiple comparisons, some findings may be due to chance alone. However, we had strong a priori rationale for expecting increased risks of thyroid cancer following diagnoses of goiter (and goiter subtypes) and adenoma, and indeed our findings showed that these diseases were most strongly related to thyroid cancer risk. We also observed consistent patterns in risk by sex, age at diagnosis, and time since diagnosis.
In conclusion, the results of this large population-based registry study showed an unexpected increased risk of differentiated thyroid cancer, including regional/distant disease, following diagnosis of hyperthyroidism and thyroiditis that could not be solely attributed to greater medical surveillance. Hypothyroidism was less clearly associated with thyroid cancer risk. Whereas our results support a possible role of thyroid dysfunction and autoimmunity in thyroid cancer development, further investigation is needed to better understand the underlying biological mechanisms.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (to C.M.K.), the Danish Program for Clinical Research Infrastructure (to H.T.S.), and the Danish Medical Research Council (DFF-4183-00359 to D.C.-F.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CI
confidence interval
- DCR
Danish Cancer Registry
- SIR
standardized incidence ratio
- TSH
thyroid-stimulating hormone
References
- 1. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2. Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20(5):525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid cancer in Denmark 1943-2008, before and after iodine supplementation. Int J Cancer. 2012;131(10):2360–2366. [DOI] [PubMed] [Google Scholar]
- 4. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitahara CM, Schneider AB, Brenner AV. Thyroid cancer In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, eds. Cancer Epidemiology and Prevention. 4th ed.New York, NY: Oxford University Press; 2016:839–860. [Google Scholar]
- 6. Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20(1):75–86. [DOI] [PubMed] [Google Scholar]
- 7. Franceschi S, Preston-Martin S, Dal Maso L, Negri E, La Vecchia C, Mack WJ, McTiernan A, Kolonel L, Mark SD, Mabuchi K, Jin F, Wingren G, Galanti R, Hallquist A, Glattre E, Lund E, Levi F, Linos D, Ron E. A pooled analysis of case-control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control. 1999;10(6):583–595. [DOI] [PubMed] [Google Scholar]
- 8. Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, Berrington de González A. Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am J Epidemiol. 2010;171(2):242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay Area population. Int J Cancer. 2001;93(5):745–750. [DOI] [PubMed] [Google Scholar]
- 10. Horn-Ross PL, Morris JS, Lee M, West DW, Whittemore AS, McDougall IR, Nowels K, Stewart SL, Spate VL, Shiau AC, Krone MR. Iodine and thyroid cancer risk among women in a multiethnic population: the Bay Area Thyroid Cancer Study. Cancer Epidemiol Biomarkers Prev. 2001;10(9):979–985. [PubMed] [Google Scholar]
- 11. Memon A, Varghese A, Suresh A. Benign thyroid disease and dietary factors in thyroid cancer: a case-control study in Kuwait. Br J Cancer. 2002;86(11):1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balasubramaniam S, Ron E, Gridley G, Schneider AB, Brenner AV. Association between benign thyroid and endocrine disorders and subsequent risk of thyroid cancer among 4.5 million U.S. male veterans. J Clin Endocrinol Metab. 2012;97(8):2661–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Truong T, Orsi L, Dubourdieu D, Rougier Y, Hémon D, Guénel P. Role of goiter and of menstrual and reproductive factors in thyroid cancer: a population-based case-control study in New Caledonia (South Pacific), a very high incidence area. Am J Epidemiol. 2005;161(11):1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeh NC, Chou CW, Weng SF, Yang CY, Yen FC, Lee SY, Wang JJ, Tien KJ. Hyperthyroidism and thyroid cancer risk: a population-based cohort study. Exp Clin Endocrinol Diabetes. 2013;121(7):402–406. [DOI] [PubMed] [Google Scholar]
- 15. Liu CL, Cheng SP, Lin HW, Lai YL. Risk of thyroid cancer in patients with thyroiditis: a population-based cohort study. Ann Surg Oncol. 2014;21(3):843–849. [DOI] [PubMed] [Google Scholar]
- 16. Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98(2):474–482. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. [DOI] [PubMed] [Google Scholar]
- 18. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7):30–33. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7):42–45. [DOI] [PubMed] [Google Scholar]
- 21. Mellemgaard A, From G, Jørgensen T, Johansen C, Olsen JH, Perrild H. Cancer risk in individuals with benign thyroid disorders. Thyroid. 1998;8(9):751–754. [DOI] [PubMed] [Google Scholar]
- 22. Chen YK, Lin CL, Chang YJ, Cheng FT, Peng CL, Sung FC, Cheng YH, Kao CH. Cancer risk in patients with Graves’ disease: a nationwide cohort study. Thyroid. 2013;23(7):879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shu X, Ji J, Li X, Sundquist J, Sundquist K, Hemminki K. Cancer risk in patients hospitalised for Graves’ disease: a population-based cohort study in Sweden. Br J Cancer. 2010;102(9):1397–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab. 2012;97(8):2682–2692. [DOI] [PubMed] [Google Scholar]
- 25. Rinaldi S, Plummer M, Biessy C, Tsilidis KK, Østergaard JN, Overvad K, Tjønneland A, Halkjaer J, Boutron-Ruault MC, Clavel-Chapelon F, Dossus L, Kaaks R, Lukanova A, Boeing H, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Agnoli C, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HB, Peeters PH, Weiderpass E, Lund E, Quirós JR, Agudo A, Molina E, Larrañaga N, Navarro C, Ardanaz E, Manjer J, Almquist M, Sandström M, Hennings J, Khaw KT, Schmidt J, Travis RC, Byrnes G, Scalbert A, Romieu I, Gunter M, Riboli E, Franceschi S. Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. J Natl Cancer Inst. 2014;106(6):dju097. [DOI] [PubMed] [Google Scholar]
- 26. Huang H, Rusiecki J, Zhao N, Chen Y, Ma S, Yu H, Ward MH, Udelsman R, Zhang Y. Thyroid-stimulating hormone, thyroid hormones and risk of papillary thyroid cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rees Smith B, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988;9(1):106–121. [DOI] [PubMed] [Google Scholar]
- 28. Belfiore A, Garofalo MR, Giuffrida D, Runello F, Filetti S, Fiumara A, Ippolito O, Vigneri R. Increased aggressiveness of thyroid cancer in patients with Graves’ disease. J Clin Endocrinol Metab. 1990;70(4):830–835. [DOI] [PubMed] [Google Scholar]
- 29. Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702–755. [DOI] [PubMed] [Google Scholar]
- 30. Kumar A, Klinge CM, Goldstein RE. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors α and β. Int J Oncol. 2010;36(5):1067–1080. [DOI] [PubMed] [Google Scholar]
- 31. Ron E, Doody MM, Becker DV, Brill AB, Curtis RE, Goldman MB, Harris BS III, Hoffman DA, McConahey WM, Maxon HR, Preston-Martin S, Warshauer ME, Wong FL, Boice JD Jr; Cooperative Thyrotoxicosis Therapy Follow-Up Study Group . Cancer mortality following treatment for adult hyperthyroidism. JAMA. 1998;280(4):347–355. [DOI] [PubMed] [Google Scholar]
- 32. Franklyn JA, Maisonneuve P, Sheppard M, Betteridge J, Boyle P. Cancer incidence and mortality after radioiodine treatment for hyperthyroidism: a population-based cohort study. Lancet. 1999;353(9170):2111–2115. [DOI] [PubMed] [Google Scholar]
- 33. Hall P, Berg G, Bjelkengren G, Boice JD Jr, Ericsson UB, Hallquist A, Lidberg M, Lundell G, Tennvall J, Wiklund K, Holm LE. Cancer mortality after iodine-131 therapy for hyperthyroidism. Int J Cancer. 1992;50(6):886–890. [DOI] [PubMed] [Google Scholar]
- 34. Metso S, Auvinen A, Huhtala H, Salmi J, Oksala H, Jaatinen P. Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer. 2007;109(10):1972–1979. [DOI] [PubMed] [Google Scholar]
- 35. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3(4):286–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.