Abstract
Context
Glycogen synthesis is a critical metabolic function of the endometrium to prepare for successful implantation and sustain embryo development. Yet, regulation of endometrial carbohydrate metabolism is poorly characterized. Whereas glycogen synthesis is attributed to progesterone, we previously found that the metabolic B isoform of the insulin receptor is maximally expressed in secretory-phase endometrium, indicating a potential role of insulin in glucose metabolism.
Objective
We sought to determine whether insulin or progesterone regulates glycogen synthesis in human endometrium.
Design, Participants, Outcome Measurements
Endometrial epithelial cells were isolated from 28 healthy women and treated with insulin, medroxyprogesterone (MPA), or vehicle. Intracellular glycogen and the activation of key enzymes were quantified.
Results
In epithelia, insulin induced a 4.4-fold increase in glycogen, whereas MPA did not alter glycogen content. Insulin inactivated glycogen synthase (GS) kinase 3α/β (GSK3α/β), relieving inhibition of GS. In a regulatory mechanism, distinct from liver and muscle, insulin also increased GS by 3.7-fold through increased GS 2 (GYS2) gene expression.
Conclusions
We demonstrate that insulin, not progesterone, directly regulates glycogen synthesis through canonical acute inactivation of GSK3α/β and noncanonical stimulation of GYS2 transcription. Persistently elevated GS enables endometrium to synthesize glycogen constitutively, independent of short-term nutrient flux, during implantation and early pregnancy. This suggests that insulin plays a key, physiological role in endometrial glucose metabolism and underlines the need to delineate the effect of maternal obesity and hyperinsulinemia on fertility and fetal development.
We quantified glycogen in human endometrium and found that synthesis was regulated by insulin, not progesterone, through acute deactivation of GSK and increased glycogen synthase expression.
Endometrial regulation of glycogen synthesis is one of the most essential metabolic processes in preparation for implantation and early pregnancy. The endometrium has sophisticated metabolic capability to sustain nutrient supply to an embryo during development in the first trimester of pregnancy.
Glycogen accumulates 10-fold during peri-implantation, and its release to the developing embryo is crucial for survival when rapid proliferation, organogenesis, and epigenetic programming are occurring (1–3). Impairment of this endometrial function results in implantation failure or early miscarriage (4–7). Understanding of metabolic flux and regulation in the endometrium is critical given the high prevalence of obesity and type 2 diabetes mellitus in reproductive-age women.
The regulation of glycogen synthesis in endometrial epithelial cells must be dynamic to enable a sustained yet cyclical increase in glycogen during the secretory phase, as well as a persistent supply through 10 to 12 weeks of gestation (1–3). Progesterone directs many cellular events in the secretory phase, including differentiation of stromal cells (8–10). In contrast to its regulation in all other tissues, where insulin signaling dominates, the cyclical accumulation of secretory glycogen led early investigators to propose that progesterone regulated glycogenesis. Progesterone treatment for several days increased glycogen content in whole endometrial tissue of spayed cats, suggesting at least a permissive role for progesterone in promoting glycogenesis in vivo (11). However, these findings were not replicated consistently in vitro during progesterone treatment of human whole-tissue extracts (12, 13), indicating that progesterone is unlikely to regulate glycogenesis directly.
Given its centrality to the regulation of glycogen in other tissues, we questioned whether insulin has a direct role in regulating endometrial glycogen, independent of progesterone. Insulin levels are higher during the secretory phase and rise throughout pregnancy (14, 15) as a result of multiple reproductive hormones that increase systemic insulin resistance (15). We previously found that the metabolic insulin receptor isoform infrared radiation B is maximally expressed in secretory-phase endometrium (16). Surprisingly, insulin signaling and downstream events in the endometrium are not well characterized. The role of insulin receptor signaling in the endometrium is essential for the understanding of the normal physiology of fertility. Moreover, it may have profound implications for early fetal development in women with obesity and type 2 diabetes, who are insulin resistant and have high insulin levels.
Here, we show in primary human tissue that insulin, acting through canonical pathways, inactivated glycogen synthase (GS) kinase 3α/β (GSK3α/β) to promote GS activity. In addition, through a regulatory mechanism, distinct from liver and muscle, insulin also increased endometrial GS 2 (GYS2) expression. We anticipate that understanding insulin’s control of endometrial metabolism will shift paradigms for the understanding of infertility and the effect of obesity and diabetes on early human development.
Methods
Study approval
Endometrial tissue for cell culture was obtained from healthy, reproductive-age women (n = 28), of mean age 35.1 ± 1.2 years and mean body mass index 26.8 ± 1.3 kg/m2, undergoing elective gynecological surgery for benign conditions. Three women were on combined estradiol and progestin oral contraception (OC), and their endometrial tissues were analyzed separately from tissues not exposed to exogenous hormones. Endometrium for whole-tissue analysis was obtained from nine women of mean age 34.8 ± 2.5 years and mean body mass index 33.4 ± 3.2 kg/m2 also undergoing elective gynecological surgery for benign conditions. Liver, muscle, and tonsil tissues were obtained from the Tissue Procurement and Distribution Facility of Yale Pathology Tissue Services (New Haven, CT). Placenta tissue was obtained from the Yale University Reproductive Sciences Biobank (New Haven, CT). The use of de-identified tissues was approved by the Yale University Human Investigations Committee.
Isolation and culture of primary epithelial and stromal cells
Endometrial epithelial and stromal cells were isolated from endometrial tissue, as modified from prior studies (17–19). Fresh endometrial tissue was suspended in enzyme digest and minced into small fragments on a petri dish. Enzyme digest was made with 125 mg Collagenase B (Roche Diagnostics, Indianapolis, IN), 12.5 mg DNase I (Roche Diagnostics), 1 mL penicillin/streptomycin, and 1 mL amphotericin B in 150 mL Hank’s Balanced Salt Solution (Thermo Fisher Scientific, Waltham, MA). The tissue suspension underwent mechanical agitation, with serial vortexing and repetitive pipetting every 5 minutes during a 15- to 30-minute incubation in a 37°C water bath. Stromal cells and epithelial glands were separated based on cell size by slowly passing the digested tissue through 40 μm mesh cell strainers (BD Falcon; Becton Dickinson, Franklin Lakes, NJ). The stromal cells, contained in the filtrate, were centrifuged at 3000 rpm for 3 minutes and then resuspended and cultured in 5.5 mM glucose DMEM (Thermo Fisher Scientific) with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% amphotericin B. The epithelial cells and intact glands were rinsed off the strainer and underwent two rounds of serial plating and 15-minute incubations at 37°C to ensure purity. Primary epithelial and stromal cells were cultured separately at 37°C in 5.5 mM glucose DMEM with 10% fetal bovine serum and antibiotics as previously described. Ishikawa cells were obtained from Richard Hochberg (Yale University, New Haven, CT) and cultured in the same media as primary endometrial cells.
Hormone treatment
Once grown to confluency, endometrial cells were starved in serum-free, phenol-free DMEM without glucose for 4 hours at 37°C. Cells were rinsed and treated with insulin 100 nM, medroxyprogesterone (MPA) 100 nM, or vehicle in 5.5 mM glucose serum-free, phenol-free DMEM. As glycogenesis is also under allosteric regulation, we performed all in vitro experiments in physiological 5.5 mM concentrations of glucose. After 30 minutes, 6 hours, 24 hours, or 48 hours of hormone stimulation, the epithelial or stromal cells were lysed and collected for glycogen, protein, or mRNA quantification and analysis.
Glycogen assay
Intracellular glycogen content was assayed without contamination from free glucose in the cell or media by use of a cellulose filter that binds glycogen, as modified from the method of Greenberg et al. (20). Following a 48-hour hormone treatment, endometrial cells were rinsed, trypsinized, and centrifuged at 3000 rpm for 3 minutes at 4°C. The pelleted cells were resuspended in cold PBS on ice and counted using a hemocytometer. The cells were centrifuged, lysed in cell lysis buffer (Cell Signaling Technology, Danvers, MA), and incubated on ice for 10 minutes. The cell lysate was then centrifuged at 13,200 rpm for 10 minutes at 4°C. To precipitate cellular glycogen and eliminate free glucose, the lysate was applied to a Whatman Grade GF/A glass microfiber filter (GE Healthcare, Piscataway, NJ) and placed immediately in cold 70% ethanol. Glycogen standards of 5 to 25 μg were applied to separate filters. The filters were washed with cold 70% ethanol on a shaker at 4°C for 15 minutes and washed twice more with 70% ethanol at room temperature for 10 minutes each. Filters were air dried overnight. Glycogen was enzymatically digested with fresh 0.4 mg/mL amyloglucosidase (MilliporeSigma, St. Louis, MO) in 0.05 M sodium acetate and incubated at 37°C with shaking at 35 rpm for 90 minutes. The glucose concentration of each solution was assayed using the Glucose (GO) Assay Kit (MilliporeSigma) and standard glucose solutions of 0 to 100 μg/ml. The absorbance was measured at 595 nm using an iMark™ Microplate Absorbance Reader (Bio-Rad, Hercules, CA). Glycogen content per cell was calculated using the glycogen standard curve and cell count for each sample.
Protein extraction
Cellular proteins were extracted from cultured endometrial cells after hormone treatment of 30 minutes or 48 hours using 1× cell lysis buffer containing protease and phosphatase inhibitors. After 10 minutes of incubation on ice, cells were lysed by sonication and mechanical agitation using a 23-gauge needle. After centrifugation at 13,200 rpm for 10 minutes at 4°C, the protein concentration in the lysate was quantified. For endometrial and control tissues, protein was isolated from the Trizol® (Thermo Fisher Scientific) chloroform fraction following RNA isolation. Protein was isolated with ethanol, precipitated with isopropanol, and washed with 0.3 M guanidine in 95% ethanol. The protein pellets were redissolved by sonication in 1:1 solution of 1% SDS and 8 M urea containing protease inhibitor cocktail and phenylmethylsulfonyl fluroride. Insoluble material was separated from soluble protein by centrifugation. The protein concentration in each lysate was quantified using the Pierce® Bicinchoninic Acid Protein Assay Kit (Thermo Fisher Scientific) and the iMark Microplate Absorbance Reader (Bio-Rad).
Western blot analysis
Proteins were separated on 4% to 12% SDS-PAGE gels (Bio-Rad), transferred onto Amersham™ Hybond™ polyvinylidene fluoride membranes (GE Healthcare), incubated with antibodies, and developed using Amersham™ ECL Advance (GE Healthcare). Antibodies to phosphorylated and/or total forms of GS, GSK3α/β, protein kinase B (AKT), and glyceraldehyde 3-phosphate dehydrogenase were from Cell Signaling Technology (Supplemental Table 1). AKT phosphorylation was used as an internal control for insulin signaling activity. Densitometry was performed for immunoblots using ImageJ software, version 1.44 (National Institutes of Health, Bethesda, MD).
RNA isolation and quantitative RT-PCR analysis
After 6 or 24 hours of hormone treatment, RNA was extracted from cultured cells using the RNeasy® Mini Kit (Qiagen, Valencia, CA). For whole endometrium and control tissues, tissue was homogenized in Trizol® (Thermo Fisher Scientific). RNA was isolated with chloroform, precipitated with isopropanol, washed twice with 75% ethanol, and dissolved in RNase-free water. RNA was treated with RNase-free DNase I and purified via RNeasy spin columns. The purity and concentration of the isolated RNA were assessed using a Nanodrop™ 2000 spectrophotometer (Thermo Fisher Scientific). RNA was reverse transcribed using the iScript™ cDNA Synthesis Kit (Bio-Rad). For each sample, 12.5 ng cDNA template was amplified in triplicate using iQ™ SYBR® Green Supermix on the MyiQ™ Real-Time PCR Detection System (Bio-Rad). PCR primers were designed using Primer-Blast (National Center for Biotechnology Information, Bethesda, MD; www.ncbi.nlm.nih.gov/tools/primer-blast) and synthesized at the W. M. Keck Foundation Oligo Synthesis Resource (Yale University). Optimal primer concentrations were determined by calculation of primer efficiency using Human XpressRef™ Universal Total RNA (Qiagen), and primer specificity was assessed by melting curve analysis. Supplemental Table 2 summarizes the primer sequences, concentrations, and efficiency for each assay. Each quantitative RT-PCR assay included nonamplification and water controls. Gene expression levels were normalized to β-actin.
Statistical analysis
Significance was defined as P < 0.05 and determined for in vitro glycogen assays by log transformation and one-way ANOVA analysis with Dunnett’s multiple comparisons test using GraphPad Prism (GraphPad Software, La Jolla, CA). Protein densitometry data were normalized to total protein or glyceraldehyde 3-phosphate dehydrogenase. GYS gene expression was normalized to β-actin. Data were log transformed, analyzed by paired two-tailed Student t test, and graphically represented as fold-change per vehicle.
Results
Insulin, not progestin, regulates glycogen synthesis in primary endometrial cells
Endometrial epithelial cells fill with glycogen during the secretory phase (1–3, 21). Due to the timing of this phenomenon and previous findings from in vivo studies, regulation of glycogen synthesis in endometrial tissue has been attributed to progesterone (11–13). We hypothesized that insulin, rather than progesterone, directly regulates glycogenesis in endometrial cells, as it does in liver and muscle cells. To test our hypothesis, we measured the glycogen content initially in an endometrial cell line and then in primary epithelial and stromal cells separately, after a 48-hour treatment with insulin or the progestin MPA under physiological, euglycemic conditions.
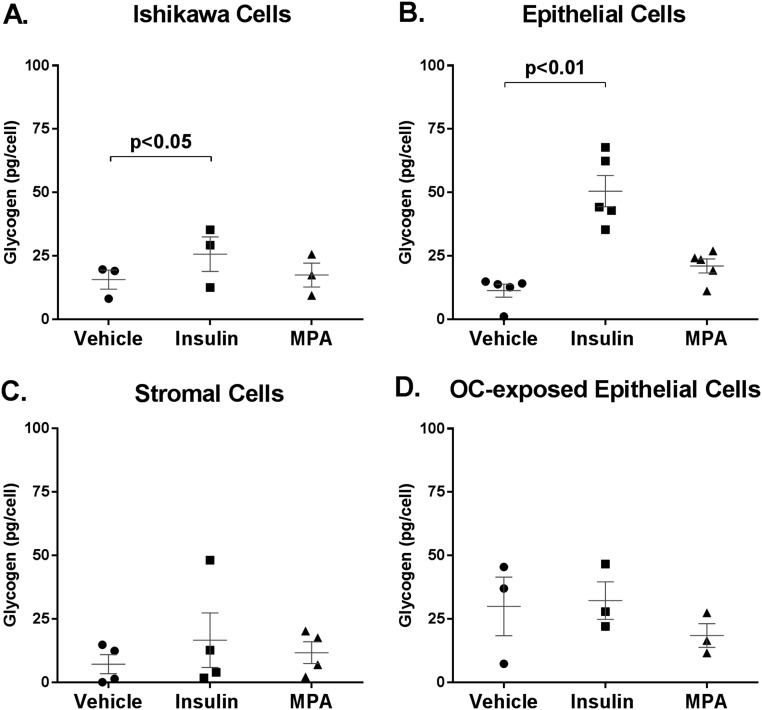
Ishikawa cells are a well-characterized human endometrial adenocarcinoma cell line used to study endometrial biology. In Ishikawa cells, insulin stimulation increased glycogen content by 1.6-fold (25.7 ± 6.8 vs 15.7 ± 3.8 pg/cell, P < 0.05; Fig. 1A). This is a robust finding, because Ishikawa cells proliferate rapidly, using large quantities of fuel, and do not undergo normal differentiation into secretory units. In Ishikawa cells, MPA treatment did not alter glycogen content relative to vehicle [17.5 ± 4.7 vs 15.7 ± 3.8 pg/cell, P = not significant (NS)].
Figure 1.
Insulin, not progestin, promotes glycogen accumulation in human endometrial epithelial cells. Glycogen content (picograms per cell) in (A) Ishikawa epithelial adenocarcinoma cells (n = 3 separate experiments), (B) primary epithelial cells (n = 5), (C) primary stromal cells (n = 4), and (D) primary epithelial cells from women on combined estradiol-progestin OC (n = 3), treated with insulin 100 nM, MPA 100 nM, or vehicle for 48 hours in 5 mM glucose media.
To determine whether the observations in cell lines pertained to primary human tissue, endometrial epithelial cells were obtained from women undergoing elective gynecologic surgery. In primary human epithelial cells, insulin stimulation increased glycogen content by 4.4-fold compared with vehicle (50.6 ± 6.2 vs 11.6 ± 2.4 pg/cell, P < 0.01, n = 5; Fig. 1B). In contrast, MPA treatment did not significantly alter glycogen content in epithelial cells (21.1 ± 2.8 vs 11.6 ± 2.4 pg/cell, P = NS). To verify that this finding was not a consequence of progesterone receptor (PGR) downregulation, we quantified PGR gene and protein expression in primary epithelial cells. MPA did not alter gene or protein expression of PGR (Supplemental Fig. 1). As a control, the primary stromal cells were also assessed. Neither insulin nor MPA treatment significantly altered glycogen content after 48 hours compared with vehicle (16.7 ± 10.7 vs 11.7 ± 4.3 vs 7.2 ± 3.8 pg/cell, P = NS, n = 4; Fig. 1C). Counter to traditional teaching, progestins do not directly promote glycogen accumulation, while insulin-treated cells strongly accumulated glycogen in both a cell line and primary human endometrial epithelial cells.
Combined oral estradiol and progestin contraceptive therapy makes endometrium inhospitable to embryo implantation. To determine whether this therapy impacted glycogen accumulation, epithelial cells were also obtained from women on combined OC therapy (n = 3). In contrast to the potent impact of insulin-mediated glycogen accumulation observed in OC naive tissue, neither insulin nor MPA was capable of increasing glycogen content in the OC-exposed primary epithelial cells (P = NS; Fig. 1D). These findings suggest that part of the action of sustained estrogen and progesterone exposure may be creating a resistant state to insulin-induced glycogen accumulation.
Insulin induces acute deactivation of GSK 3
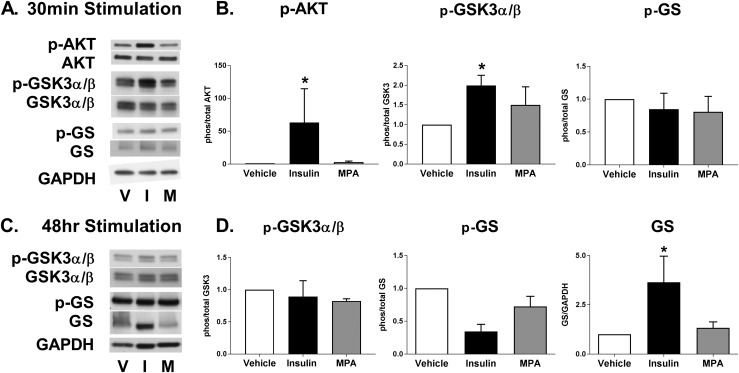
Glycogen accumulation is a balance of glycogenesis vs glycogenolysis. As the primary hormone of energy storage, insulin regulates glycogenesis in liver and muscle by binding to the insulin receptor, initiating phosphorylation cascades through insulin receptor substrate 1 and AKT, and inactivating GSK3α/β through phosphorylation of serines 21 and 9 (22, 23). Inactivated GSK3α/β is unable to catalyze the inhibitory phosphorylation of GS at serine 641. Without this phosphorylation, GS is free to catalyze α-(1 → 4) glycosidic bonds in a growing chain of glycogen in the presence of glucose-6-phosphate (24). To investigate whether insulin stimulation of endometrial cells similarly results in inactivated, phosphorylated GSK3α/β and activated, dephosphorylated GS enzyme forms, acute signaling events were assessed in primary endometrial epithelial cells. Ishikawa cells have persistent AKT activation as a result of a phosphatase and tensin homolog deficiency. Baseline phosphorylated AKT and phosphorylated GSK3α/β are very high in Ishikawa cells, making them a poor model to study insulin-induced signaling events (data not shown).
Acute stimulation of primary human epithelial cells with insulin did not alter expression levels of AKT, GSK3α/β, or GS (Fig. 2A). In contrast, insulin treatment resulted in a robust phosphorylation of AKT (P = 0.03) with a doubling of the inactivating phosphorylation of GSK3α/β (P = 0.01; Fig. 2A and 2B). Insulin did not acutely alter the activation status of GS, although in general, a decrease in phosphorylation is more difficult to demonstrate. Importantly, MPA had no significant effect on AKT, GSK3α/β, and GS phosphorylation in primary epithelial cells (P = NS for all; Fig. 2A and 2B). These data are consistent with a model where insulin-stimulated signal transduction cascades regulate glycogen accumulation, at least in part, via inactivating GSK3α/β.
Figure 2.
Insulin, not progestin, regulates glycogen synthesis by deactivation of GSK3α/β and increase in GS. Primary endometrial epithelial cells were isolated and treated with insulin (I) 100 nM, MPA (M) 100 nM, or vehicle (V) in 5 mM glucose media. Representative (A) Western blot and (B) densitometry analysis of 30-min hormone stimulation, showing changes in phosphorylated (p) AKT at serine 473, total AKT, phosphorylated GSK3α/β at serine 21/9, total GSK3α/β, phosphorylated GS at serine 641, and total GS, n = 3 to 6, from six women. Representative (C) Western blot and (D) densitometry analysis of 48-h hormone stimulation, showing changes in phosphorylated GSK3α/β at serine 21/9, total GSK3α/β, phosphorylated GS at serine 641, and total GS, n = 3 to 6, from six women. Densitometry data presented as fold change ± SEM per vehicle of phosphorylated to total protein or total protein to glyceraldehyde 3-phosphate dehydrogenase. *P < 0.05 vs vehicle.
Insulin increases GS levels
In contrast to the strong diurnal and prandial variations seen in liver and muscle, the endometrium synthesizes glycogen in a continuous manner for days to weeks to support a developing blastocyst. Therefore, signaling events during chronic insulin stimulation of primary epithelial cells were also assessed. Unexpectedly and in contrast to acute stimulation, chronic stimulation with insulin markedly increased GS 3.7-fold (P = 0.02; Fig. 2C and 2D). This mode of regulation has not been shown in either liver or muscle and may be unique to the reproductive tract. Chronic insulin stimulation also increased GS activity, as demonstrated by a decrease in phosphorylated GS (P = 0.06). Alteration of phosphorylated or total GSK3α/β in response to chronic insulin stimulation was not evident relative to vehicle.
Unlike insulin, chronic MPA treatment of primary epithelial cells did not alter the total protein content or activation status of GS (P = NS; Fig. 2C and 2D), confirming that MPA does not, by itself, directly regulate glycogen synthesis in the endometrium. The activation status of GSK3α/β was also unaltered by MPA. As an internal control, we measured signaling and protein expression in primary stromal cells treated with insulin or MPA. Insulin did not induce an increase in total GS protein in stromal cells (data not shown), which is consistent with the finding that insulin did not increase glycogen content in stromal cells. Together, these findings demonstrate that insulin regulates glycogenesis in epithelial cells through acute signaling events, as well as increased GS protein, which is critical for sustained synthesis capacity.
Insulin increases GS via increased GYS2 expression
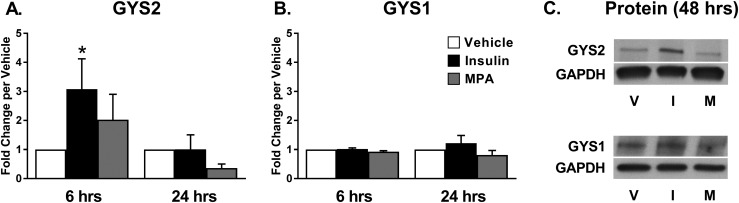
GYS2 is the isoform predominantly found in liver, whereas GYS1 is found in muscle and other tissues. To determine which isoform is responsible for the increase in protein, we examined mRNA from primary epithelial cells at 6 and 24 hours following treatment. Insulin induced a threefold increase in GYS2 expression in primary epithelial cells following 6 hours of treatment (P < 0.05) but not at 24 hours (P = NS; Fig. 3A). In contrast, GYS1 expression was not changed by insulin in primary epithelial cells at either 6 or 24 hours (Fig. 3B). MPA did not increase either GYS1 or GYS2 in either cell group at either time point. In confirmation of regulation through the GYS2 isoform, GYS2 protein was increased with insulin stimulation at 48 hours, whereas no change was seen in GYS1 expression (Fig. 3C). Taken together, these data suggest that insulin-mediated transcriptional regulation of GYS2 is responsible for increased GS activity.
Figure 3.
Insulin, not progestin, regulates GYS2 in endometrial epithelial cells. GS genes GYS2 and GYS1 were quantified in cells treated with insulin 100 nM, MPA 100 nM, or vehicle for 6 or 24 h in 5 mM glucose media. (A) GYS2 and (B) GYS1 in primary epithelial cells (n = 6 for 6 h, n = 3 for 24 h, from nine women). GYS expression was normalized to β-actin and presented as fold change ± SEM per vehicle. (C) GYS2 and GYS1 protein was quantified in cells treated with insulin 100 nM, MPA 100 nM, or vehicle for 48 h in 5 mM glucose media. *P < 0.05 vs vehicle.
Endometrial GYS2 expression is higher during the secretory phase
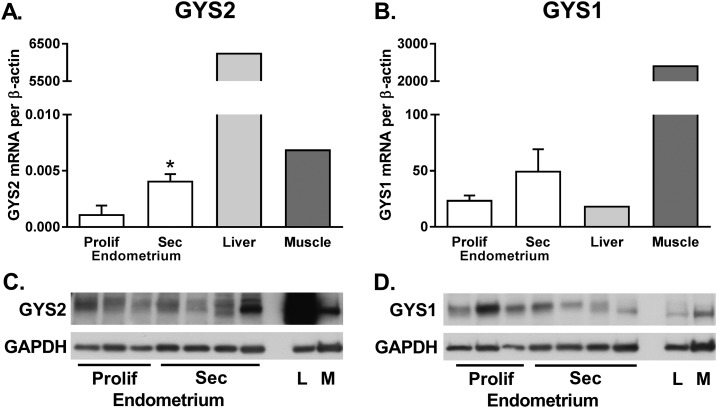
GYS2 is not previously described in the endometrium, so we sought to verify the presence of GYS2 in secretory-phase endometrium. We compared GS isoform mRNA and protein expression in endometrial tissues from both phases of the cycle, alongside tissues with known expression of GYS2 (liver) and GYS1 (skeletal muscle) to demonstrate relative expression levels (Fig. 4). Importantly, GYS2 mRNA was fourfold higher in secretory endometrium than proliferative endometrium (P = 0.01; Fig. 4A and 4C), supporting its role in synthesizing glycogen in secretory endometrium. Endometrial GYS2 expression is in a similar range as skeletal muscle and lower than liver. GYS1 mRNA was similar between proliferative and secretory-phase endometrium (P = NS; Fig. 4B and 4D). As expected, GYS1 expression was robust in skeletal muscle, and lower in liver.
Figure 4.
GYS2 and GYS1 expression in proliferative and secretory-phase endometrium. GS genes (A) GYS2 and (B) GYS1 were quantified in proliferative endometrium (n = 3 to 4), secretory endometrium (n = 4 to 5), and tissues serving as controls. Skeletal muscle (M; n = 1) and liver (L; n = 1) are known to have an abundance of GYS1 and GYS2, respectively, and are shown here to demonstrate relative expression to endometrial tissues. GYS expression was normalized to β-actin and presented as means ± SEM for endometrial tissues. *P < 0.01. Representative Western blot showing protein expression for (C) GYS2 and (D) GYS1 using 40 μg total protein for all samples.
Discussion
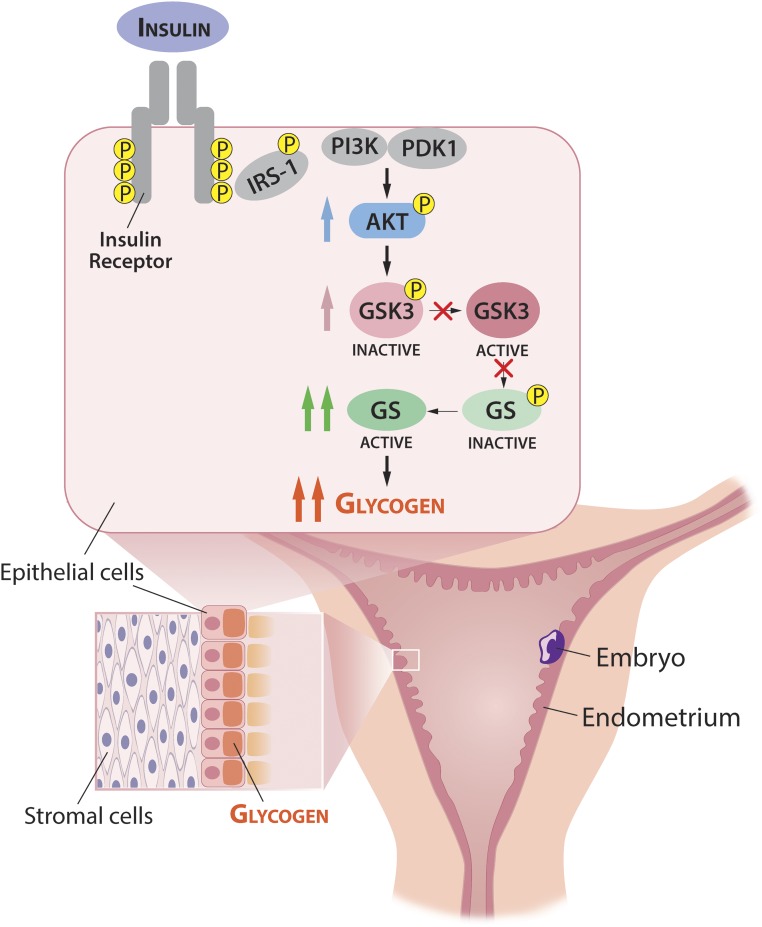
We show that insulin is the primary signal for glycogen deposition in human endometrial epithelium, altering the paradigm of several decades—that progesterone directs glycogen synthesis. Importantly, insulin regulation occurs through a mechanism that increases GS levels, in addition to activation through canonical GSK phosphorylation. Figure 5 shows our proposed model for glycogen regulation in the endometrium.
Figure 5.
Proposed model of glycogen synthesis regulation by insulin in endometrial epithelial cells to provide nutrition to an implanted embryo. In the uterus, endometrial epithelial cells accumulate glycogen over several days during the implantation window and secrete carbohydrate into the uterine lumen. After successful implantation, endometrial epithelium sustains glycogen synthesis for weeks during embryonic development. Maternal insulin promotes glycogen synthesis by binding to the insulin receptor on endometrial epithelial cells, initiating signaling through insulin receptor substrate 1 (IRS-1) and AKT. AKT inactivates GSK3α/β by phosphorylation (P) at serines 9 and 21. When GSK3α/β no longer phosphorylates GS at serine 641, unphosphorylated GS is free to catalyze α (1 → 4) glycosidic bonds in a growing chain of glycogen. Thus, insulin indirectly activates GS by inhibiting GSK3α/β in a classical canonical pathway. In addition, insulin increases total protein levels of GS through increases in GYS2 mRNA, which enables sustained glycogen synthesis over weeks, independent of meal-time nutrient and hormonal fluctuations. PDK1, phosphoinositide-dependent kinase 1; PI3K, phosphatidylinositol 3-kinase.
Based on our findings, we propose that insulin regulation of glycogen synthesis in the endometrium is distinct from regulation in the liver and muscle, as necessitated by their respective physiological roles. Liver and muscle rely on acute allosteric regulation and insulin signaling to promote glycogen synthesis, enabling a rapid response to meal-time boluses of glucose and equally rapid glycogenolysis by glucagon or epinephrine during fasting or exercise (24). Acute signaling changes through GSK and GS are well described in liver and muscle, but insulin did not induce expression of either GYS2 or GYS1 in prior in vitro studies of liver and muscle cells (25, 26). Likewise, insulin did not alter GYS2 mRNA levels in vivo in the livers of streptozosin-treated rats compared with normal rats (25). However, in the uterus, the endometrial epithelium accumulates glycogen over several days during the implantation window and then sustains glycogenesis for weeks during embryonic development. An increase in total protein levels of the key enzyme in glycogen synthesis enables a longer, more durable increase in glycogen content, which is not reliant on meal-time boluses of glucose or insulin release.
These results have critical implications for fertility and fetal development, especially in women with insulin-resistant states, such as obesity and type 2 diabetes. Abnormal endometrial metabolism may, in part, explain why women with obesity have a higher risk of early miscarriage after both spontaneous conception and in vitro fertilization with ova from normal-weight donors (10, 27, 28). Furthermore, altered endometrial metabolism likely results in modified nutrient secretions to an embryo, during a time of rapid development, organogenesis, and epigenetic programming. Maternal obesity is the greatest risk factor for childhood obesity in offspring (29). Several animal models show associations between obesity and epigenetic changes occurring in utero (30). Further work is needed to understand how insulin’s regulation of metabolic flux in the endometrium is altered in the setting of obesity and diabetes.
In conclusion, we demonstrate that insulin plays a key, physiological role in glucose metabolism in human endometrium. Insulin not only activates key enzymes but also induces targeted gene expression, a process that is less well documented for this hormone. An increase in GS enables epithelial cells to synthesize glycogen, independent of meal-time nutrient and hormonal fluctuations, which is critical in reproductive physiology. These findings suggest that successful implantation requires insulin.
Supplementary Material
Acknowledgments
We thank the faculty and staff of the Yale University Reproductive Sciences Biobank and the Yale Pathology Tissue Services for the collection of tissues serving as controls in this study. We thank Dr. Yasuko Iwakiri for the gift of HepG2 cells, which also served as a control in this study.
Financial Support: This project was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K08HD071010 and U54 HD052668) and Yale Diabetes Research Center Pilot Award from the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK45735; to C.A.F.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AKT
protein kinase B
- GS
glycogen synthase
- GSK3α/β
glycogen synthase kinase 3α/β
- GYS1/2
glycogen synthase 1/2
- MPA
medroxyprogesterone
- NS
not significant
- OC
oral contraception
- PGR
progesterone receptor
References
- 1. Milwidsky A, Palti Z, Gutman A. Glycogen metabolism of the human endometrium. J Clin Endocrinol Metab. 1980;51(4):765–770. [DOI] [PubMed] [Google Scholar]
- 2. Taylor SE, Cheung KT, Patel II, Trevisan J, Stringfellow HF, Ashton KM, Wood NJ, Keating PJ, Martin-Hirsch PL, Martin FL. Infrared spectroscopy with multivariate analysis to interrogate endometrial tissue: a novel and objective diagnostic approach. Br J Cancer. 2011;104(5):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002;87(6):2954–2959. [DOI] [PubMed] [Google Scholar]
- 4. Zondek B, Stein L. PGlycogen content of the human uterine mucosa glycopenia uteri. Endocrinology. 1940;27(3):395–399. [Google Scholar]
- 5. Maeyama M, Sudo I, Saito K, Matsuo I, Nakahara K. Glycogen estimation by a rapid enzymic method in very small samples of human endometrium: glycogen content in the endometrium of infertile patients during the menstrual cycle. Fertil Steril. 1977;28(2):159–162. [DOI] [PubMed] [Google Scholar]
- 6. Girish CJ, Kotur NS, Nagarajappa AH, Manjunath ML. A correlative study of endometrial glycogen content and other contributory factors on female infertility. IJBAR. 2012;3(1):30–35. [Google Scholar]
- 7. Gupta A, Mathur SK, Gupta A. Co-orelation of histological dating and glycogen content by histochemical stain during various phases of menstrual cycle in primary infertility. OJPathol. 2013;3(2):65–68. [Google Scholar]
- 8. Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357(1-2):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25(6):445–453. [DOI] [PubMed] [Google Scholar]
- 10. Fox C, Morin S, Jeong J-W, Scott RT Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016;105(4):873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaffe RC, Stevens DM, Verhage HG. The effects of estrogen and progesterone on glycogen and the enzymes involved in its metabolism in the cat uterus. Steroids. 1985;45(5):453–462. [DOI] [PubMed] [Google Scholar]
- 12. Shapiro SS, Dyer SD, Colás AE. Progesterone-induced glycogen accumulation in human endometrium during organ culture. Am J Obstet Gynecol. 1980;136(4):419–425. [DOI] [PubMed] [Google Scholar]
- 13. Ishihara S, Taketani Y, Mizuno M. Stimulatory action of progesterone on the synthesis of glycogen in primary cell culture of human endometrium. Asia Oceania J Obstet Gynaecol. 1988;14(1):117–122. [DOI] [PubMed] [Google Scholar]
- 14. Yeung EH, Zhang C, Mumford SL, Ye A, Trevisan M, Chen L, Browne RW, Wactawski-Wende J, Schisterman EF. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab. 2010;95(12):5435–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–S119. [DOI] [PubMed] [Google Scholar]
- 16. Flannery CA, Saleh FL, Choe GH, Selen DJ, Kodaman PH, Kliman HJ, Wood TL, Taylor HS. Differential expression of IR-A, IR-B and IGF-1R in endometrial physiology and distinct signature in adenocarcinoma. J Clin Endocrinol Metab. 2016;101(7):2883–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94(2):195–204. [DOI] [PubMed] [Google Scholar]
- 19. Lockwood CJ, Nemerson Y, Guller S, Krikun G, Alvarez M, Hausknecht V, Gurpide E, Schatz F. Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab. 1993;76(1):231–236. [DOI] [PubMed] [Google Scholar]
- 20. Greenberg CC, Meredith KN, Yan L, Brady MJ. Protein targeting to glycogen overexpression results in the specific enhancement of glycogen storage in 3T3-L1 adipocytes. J Biol Chem. 2003;278(33):30835–30842. [DOI] [PubMed] [Google Scholar]
- 21. Amso NN, Crow J, Lewin J, Shaw RW. A comparative morphological and ultrastructural study of endometrial gland and fallopian tube epithelia at different stages of the menstrual cycle and the menopause. Hum Reprod. 1994;9(12):2234–2241. [DOI] [PubMed] [Google Scholar]
- 22. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. [DOI] [PubMed] [Google Scholar]
- 23. Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. [DOI] [PubMed] [Google Scholar]
- 24. Roach PJ. Glycogen and its metabolism. Curr Mol Med. 2002;2(2):101–120. [DOI] [PubMed] [Google Scholar]
- 25. Rao PV, Pugazhenthi S, Khandelwal RL. The effects of streptozotocin-induced diabetes and insulin supplementation on expression of the glycogen phosphorylase gene in rat liver. J Biol Chem. 1995;270(42):24955–24960. [DOI] [PubMed] [Google Scholar]
- 26. Fredriksson J, Ridderstråle M, Groop L, Orho-Melander M. Characterization of the human skeletal muscle glycogen synthase gene (GYS1) promoter. Eur J Clin Invest. 2004;34(2):113–121. [DOI] [PubMed] [Google Scholar]
- 27. Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med. 2011;29(6):507–513. [DOI] [PubMed] [Google Scholar]
- 28. Bellver J, Pellicer A, García-Velasco JA, Ballesteros A, Remohí J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril. 2013;100(4):1050–1058. [DOI] [PubMed] [Google Scholar]
- 29. Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29–e36. [DOI] [PubMed] [Google Scholar]
- 30. van Dijk SJ, Tellam RL, Morrison JL, Muhlhausler BS, Molloy PL. Recent developments on the role of epigenetics in obesity and metabolic disease. Clin Epigenetics. 2015;7(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.