Abstract
Context
Uterine leiomyomas are the most common type of gynecologic tumor in women.
Objective
To determine the role of the cytokine receptor activator of nuclear factor κ-Β ligand (RANKL); its receptor, receptor activator of nuclear factor κ-Β (RANK); and the RANKL/RANK pathway inhibitor RANK-Fc in leiomyoma growth.
Design
Messenger RNA (mRNA) or protein levels of RANKL, RANK, and proliferation markers cyclin D1 and Ki67 were assessed in various leiomyoma tissues and cell populations. Human xenograft experiments were performed to determine the effects of RANK-Fc on leiomyoma growth in vivo.
Setting
Research laboratory.
Patients
Twenty-four regularly cycling premenopausal women (age 28 to 49 years) who were not receiving hormone therapy.
Interventions
None.
Main Outcome Measure
Tumor growth in a murine xenograft model following targeting of the RANKL/RANK pathway with RANK-Fc.
Results
RANKL mRNA levels in leiomyoma were significantly higher than those in myometrial tissues. The highest RANK levels were found in the leiomyoma stem cell population, which is deficient in progesterone receptor (PR). Conversely, the highest RANKL levels were found in the PR-rich leiomyoma intermediate cell (LIC) population. R5020, a PR agonist, specifically increased RANKL expression in LICs. RANK-Fc blocked RANKL-induced expression of the proliferative gene cyclin D1. Treatment with RANK-Fc also significantly decreased tumor growth in vivo and diminished the expression of proliferation marker Ki67 in tumors (P < 0.01; n = 4).
Conclusions
Treatment with the RANKL/RANK pathway inhibitor RANK-Fc significantly decreased human leiomyoma cell proliferation and tumor growth. This suggests that the RANKL/RANK pathway could serve as a potential target for the prevention and treatment of uterine leiomyoma.
RANK-Fc impedes leiomyoma growth by inhibiting the RANKL/RANK pathway. RANK-Fc is a potential novel nonsteroidal treatment that blocks a paracrine pathway in uterine fibroids.
Uterine leiomyomas are smooth muscle tumors of the uterine wall and are the most common type of gynecologic tumor. Eighty percent of women will have at least one uterine leiomyoma by age 50 years (1, 2). Women with leiomyoma may experience symptoms such as severe pelvic pain, heavy vaginal bleeding (which can lead to anemia and blood transfusions), miscarriage, and infertility (3, 4). Leiomyomas have been estimated to account for up to $34 billion in costs annually (4).
Currently available medical treatment options for leiomyoma, such as leuprolide, focus on symptom management (3). These treatments can also cause notable adverse effects that limit their long-term use (3). For leiomyomas that fail to respond to medical therapy, procedural options are available, including uterine fibroid embolization and magnetic resonance imaging–guided high-intensity focused ultrasound surgery. Myomectomy, the surgical removal of uterine leiomyoma, is a more invasive option and is associated with complications such as formation of pelvic adhesions, pelvic pain, Asherman syndrome, and weakening of the uterine myometrium with accompanying obstetric concerns (3). Although myomectomy is useful for removing current leiomyoma, it does not prevent the formation of new ones. This is an important distinction because women who have had leiomyoma are more likely to form new tumors and need additional surgery in the future. There is a need for therapeutic options for uterine leiomyoma that not only treat the existing disease but also prevent formation of new tumors.
To provide better therapeutic options for uterine leiomyoma, we need to understand the underlying pathophysiology. Uterine leiomyomas are monoclonal tumors that originate from a single cell (5). Leiomyoma stem cells (LSCs) arise within populations of myometrial stem cells as a result of gene mutations, such as MED12 or HMGA2 mutations (5–8). Our laboratory identified cell surface markers enriched in a leiomyoma side population of cells that has properties characteristic of stem cells (8). On the basis of these markers, we identified three unique cell populations in leiomyoma: CD34+/CD49b+, CD34+/CD49b−, and CD34−/CD49b−. CD34+/CD49b+ cells correspond to the LSC population, CD34+/CD49b− cells make up a leiomyoma intermediate cell (LIC) population with characteristics between those of stem cells and mature leiomyoma cells, and CD34−/CD49b− cells display properties of fully differentiated uterine leiomyoma cells, a leiomyoma differentiated cell (LDC) population (8). We previously showed that CD34+/CD49b+ LSCs are essential for leiomyoma cell colony formation in vitro and tumor growth in vivo (8).
In mammary tissue, the receptor activator of nuclear factor κ-Β ligand (RANKL)/ receptor activator of nuclear factor κ-Β (RANK) pathway has been identified as a key paracrine pathway through which progesterone initiates proliferation and differentiation of mammary stem cells that are progesterone receptor (PR)-negative. Mammary luminal epithelial cells, which are PR-positive, secrete RANKL in response to progesterone action (9). RANKL then acts on its receptor RANK on the mammary stem cells, resulting in their differentiation into mammary epithelial cells and then milk-secreting acini (10, 11). We previously reported that, similar to mammary cells, the LIC population in uterine leiomyoma is PR positive, whereas LSCs lack PR expression (8). In this study, we investigated the role of RANKL and its receptor in leiomyoma tumorigenesis and the potential role of its inhibitor RANK-Fc in preventing uterine fibroid cell proliferation and tumor growth. The ability to inhibit leiomyoma growth and, more importantly, to prevent the growth of new tumors at the stem cell level has immense implications for the treatment of leiomyoma.
Materials and Methods
Tissue collection
The institutional review board at Northwestern University approved this study. Informed consent was obtained before surgery. All patients were premenopausal women undergoing myomectomy or hysterectomy [mean age (± standard deviation), 38 ± 9 years; range, 28 to 49 years]. Women were excluded if they were receiving hormone treatment during the 6 months before surgery. Matched leiomyoma and myometrium tissue fragments from each patient were manually cut into 1- to 2-mm3 pieces and snap frozen in liquid nitrogen for RNA extraction, explant culture, primary cell culture, or fluorescence-activated cell sorting (FACS). All experiments were performed with cells or tissue from at least three independent patients. The exact number of patient samples used for each experiment is reported in the figure legends. The following methods have been previously described by our research group (8).
Cell preparation of human uterine leiomyoma
Leiomyoma fragments were manually cut into small pieces (1 to 2 mm3), followed by incubation for 4 to 6 hours in calcium and magnesium-free Hanks balanced salt solution containing 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO), 1.5 mg/mL collagenase (Sigma-Aldrich), and 8 µg/mL DNase I (Sigma-Aldrich) at 37°C on a shaker. The digested tissue was filtered through a sterile 400-μm polyethylene mesh filter (BD Biosciences, San Jose, CA) to remove undigested tissues. For cell sorting procedures, the digested tissue was filtered again through a 40-μm cell strainer (BD Biosciences) to obtain a single-cell suspension.
Antibody staining for cell sorting procedures
To enrich the LSC population, we stained dissociated leiomyoma cells to identify specific cell surface markers (8). Dissociated leiomyoma cells were resuspended at a concentration of 2 × 106 cells/mL in Hanks balanced salt solution containing 2% fetal bovine serum (staining medium). The cells were washed twice with staining medium and incubated on ice for 30 minutes with fluorescently labeled antibodies against CD45, CD34, or CD49b. All antibodies used are listed in Supplemental Table 1. These antibodies were selected because previous data from our group described the role of these antibodies in enriching the leiomyoma side population and, specifically, LSCs (8). CD45 staining was used to separate out peripheral blood leukocytes, and CD34 and CD49b were used to identify the different subpopulations of leiomyoma cells. After incubation with antibodies against CD45, CD34, and CD49b, the cells were washed three times and then incubated with 1 μg/mL propidium iodide (Sigma-Aldrich) to label nonviable cells. The cells were then subjected to cell sorting by using a FACSAria cell sorter (BD Biosciences). Most cells were selected for analysis on the basis of forward vs side scatter profile. Dead cells (propidium iodide–positive+) and leukocytes (CD45+) were excluded by electronic gating. The remaining cells were analyzed for CD34 and CD49b expression to harvest CD34−/CD49b−, CD34+/CD49b−, and CD34+/CD49b+ populations.
Explant and cell culture studies
Tanos et al. (12) reported that PR signaling induced robust RANKL expression in the ex vivo tissue explant model, but it failed to induce RANKL in PR-positive breast cancer cell lines or in dissociated, cultured breast epithelial cells. This finding suggested that steroid hormone signaling, which relies on paracrine factors, can be studied adequately in the context of tissue samples with intact architecture. They also found that the cell viability (>90%) and hormone responsiveness with active cell proliferation in this model system remain very high after more than 6 days of ex vivo culture (12). Therefore, for experiments designed to investigate the regulation of RANKL expression by progestin, we followed the same protocol with some modifications. Briefly, leiomyoma tissues from fresh myomectomies or hysterectomies were washed with phosphate-buffered saline, cut into small pieces (1 to 2 mm3), and treated with vehicle or R5020 (PR agonist, 1 × 10−7 M) for 24 hours or 48 hours. Then, the explants were harvested for total RNA isolation. The 24-hour–treated explants were also digested and stained with CD34, CD49b, and CD45 for cell sorting, as described above, to determine the regulation RANKL gene expression by R5020 in each cell population. To determine the effect of RANK-Fc on leiomyoma cell function, we isolated leiomyoma smooth muscle cells from fresh fibroid tissues and maintained them in primary culture. The cells were then treated with Dulbecco phosphate-buffered saline (DPBS; Life Technologies, Carlsbad, CA) as vehicle, 50 ng/mL RANKL only (ProSpec, East Brunswick, NJ), 100 ng/mL RANK-Fc only (Amgen Inc., Thousand Oaks, CA), or both RANKL and RANK-Fc for 48 hours. RANK-Fc is a recombinant protein containing the murine extracellular domain of RANK fused to the Fc portion of murine IgG1.
Real-time polymerase chain reaction for RANKL and RANK messenger RNA expression
Total RNA was extracted by using the RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Total RNA was used to generate complementary DNA by using the qSCript complementary DNA synthesis kit (Quanta Biosciences, Beverly, MA). Quantitative real-time (qRT) polymerase chain reaction (PCR) to determine the gene expression of RANKL, RANK, and cyclin D1 was performed by using the ABI7900 with the TaqMan 2x Universal PCR master mix (Applied Biosystems, Foster City, CA). Primers used for PCR are listed in Supplemental Table 2. The qRT-PCR machine was programmed as follows: 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. To determine relative transcript levels, the difference in cycle threshold (ΔCt) was calculated by subtracting the Ct for internal control from the Ct for target genes.
Immunoblotting analysis
Protein was extracted from the total or different leiomyoma cell population by using radioimmunoprecipitation assay buffer, followed by quantification using the bicinchoninic acid protein assay reagent (ThermoFisher Scientific, Waltham, MA) per the manufacturer’s protocols. Then, the protein was diluted with reducing 4X LDS sample buffer (Life Technologies), electrophoresed on a 4% to 12% Novex Bis-Tris polyacrylamide precast gel (Life Technologies), and transferred onto polyvinylidene difluoride membrane. Incubation with primary antibodies (cyclin D1, RANKL, and RANK) (Supplemental Table 1) was performed at 4°C in 5% nonfat milk overnight. Anti-β-actin was used as a loading control. The membranes were then washed and incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for 2 hours at room temperature. Detection was performed by using Luminata Crescendo horseradish peroxidase substrate (Millipore, Billerica, MA). Immunoblot was quantified by using ImageJ software (National Institutes of Health, Bethesda, MD).
Animal studies
Northwestern University's Animal Care and Use Committee approved all procedures involving animals. Dissociated cells from leiomyoma were suspended in rat-tail collagen (type 1) solution (BD Bioscience) at 106 cells/10 µL and cultured for 48 hours in floating cultures. Then, the cell pellets were grafted underneath the kidney capsules of ovariectomized nonobese diabetic-scid IL2Rγnull mouse hosts (NSG, Jackson Laboratory, Bar Harbor, ME) supplemented with subcutaneous implantation of 50 mg progesterone plus 50 µg estradiol 60-day slow-release pellets (Innovative Research of America Inc., Sarasota, FL) as a combination of estradiol and progesterone is indispensable for significant fibroid tumor growth and regeneration in the xenograft mouse model (8, 13). A control group of mice was treated with vehicle (DPBS), whereas the treatment group was treated with RANK-Fc administered subcutaneously at 10 mg/kg twice a week (14, 15). Mice were euthanized after 4 weeks of treatment, and images of regenerated tumors on the kidney surface were taken from the x-, y-, and z-axes by using a dissecting microscope connected to a computer with Leica Application Suite, version 3.8 software (Leica Microsystems Inc., Bannockburn, IL). Tumor volume was estimated by using the following formula: volume (mm3) = 0.52 (derived from π/6) × length × width × height (mm) (16). The fold-change from control to RANK-Fc–treated group was calculated.
Histological analyses
Paraffin-embedded mouse kidneys xenotransplanted with the total leiomyoma cell population were sectioned and immunohistochemistry (IHC) was performed by the Northwestern University Histology and Phenotyping Laboratory to detect proliferation marker Ki67. After deparaffinization, endogenous peroxidase was blocked with 3% hydrogen peroxide in double-distilled water. Endogenous biotin was blocked with avidin, and nonspecific proteins were blocked with 5% normal donkey serum. The tissue sections were then incubated with the primary antibody against Ki67 (Supplemental Table 1), followed by incubation with the biotinylated secondary antibody and 3′-diaminobenzidine. Images were captured with a Leica microscope (Leica Microsystems Inc.). Cells staining positive for Ki67 were counted in 10 high-power fields. The fold-change from control to RANK-Fc treated group was calculated.
Statistical analysis
We analyzed data by using the Student t test when comparing two groups and a one-way analysis of variance, followed by multiple comparisons to compare three or more groups. We used GraphPad software, version 7.0 (La Jolla, CA). Values were considered statistically significant at P < 0.05.
Results
RANKL and RANK are differentially expressed in leiomyoma and myometrial tissue
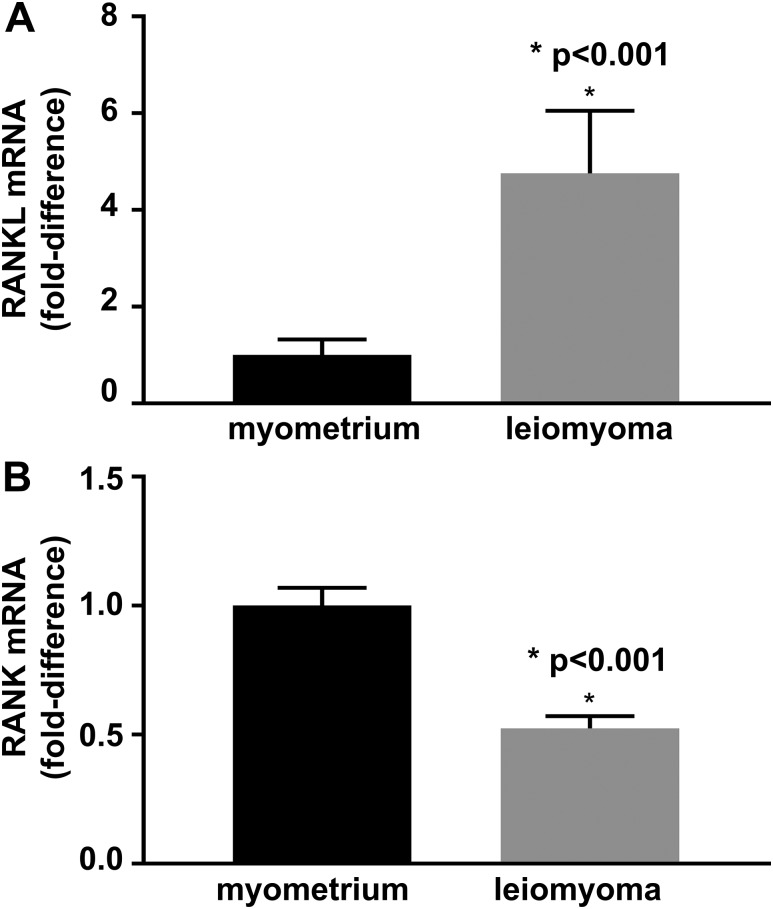
To determine the role of RANKL/RANK pathway in the pathogenesis of leiomyoma, we first compared RANKL and RANK mRNA levels of leiomyoma and normal myometrial tissue. RANKL expression by qRT-PCR was significantly higher in leiomyoma than in myometrium (P < 0.001; n = 12) [Fig. 1(A)]. In contrast, RANK was preferentially expressed in myometrium compared with leiomyoma (P < 0.001; n = 12) [Fig. 1(B)].
Figure 1.
(A) RANKL mRNA fold-change in leiomyoma compared with myometrium; RANKL expression is five times higher in leiomyoma than in myometrium. (B) RANK mRNA fold change in leiomyoma compared with myometrium; RANK expression is 50% lower in leiomyoma than myometrium. n = 12 independent tissue samples. The error bars are the standard error of the mean.
RANK is preferentially expressed in LSCs and RANKL, in LICs
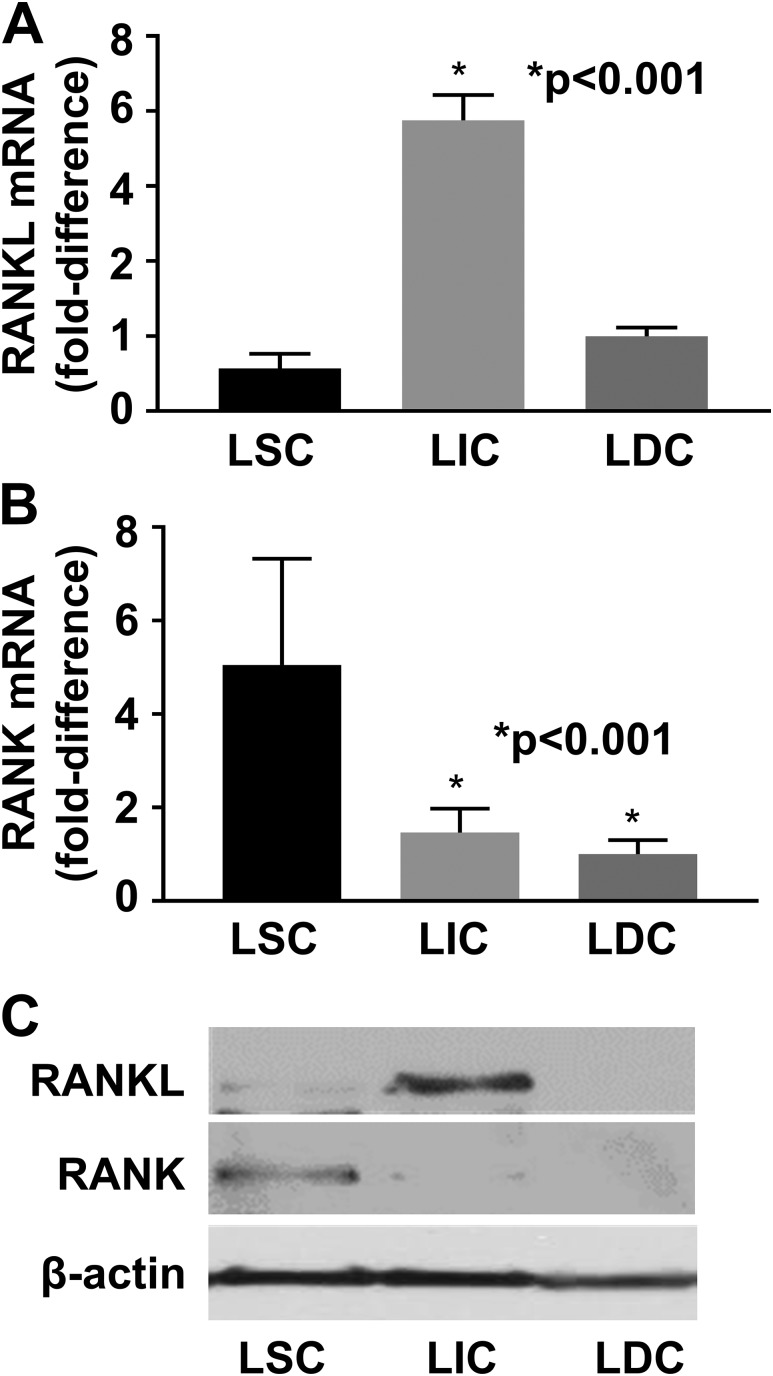
We hypothesized that, similar to mammary progenitor cells, the LIC population in leiomyoma expresses RANKL, which then acts on RANK expressed by LSCs to initiate growth and proliferation. To test this, we FACS-sorted LSCs (CD34+/CD49+), LICs (CD34+/CD49b−), and LDCs (CD34−/CD49b−) and then performed qRT-PCR to compare RANK and RANKL expression in the different cell populations. RANKL expression was significantly higher in LICs than in LSCs and LDCs (P < 0.001; n = 5) [Fig. 2(A)]. Conversely, RANK expression was significantly higher in LSCs than in LICs and LDCs (P < 0.001; n = 5) [Fig. 2(B)]. These differences were recapitulated by immunoblotting analysis: The highest RANKL protein levels were observed in LICs, whereas the highest RANK protein levels were detected in LSCs [Fig. 2(C)].
Figure 2.
(A) RANKL expression is significantly higher in LICs and (B) RANK expression is significantly higher in LSCs on qRT-PCR. (C) Western blot analysis confirmed the highest RANKL and RANK protein levels in LICs and LSCs, respectively. n = 3 (Western blot) to n = 5 (qRT-PCR) independent tissue samples. The error bars are the standard error of the mean.
Progestin induces RANKL but not RANK transcriptional activity
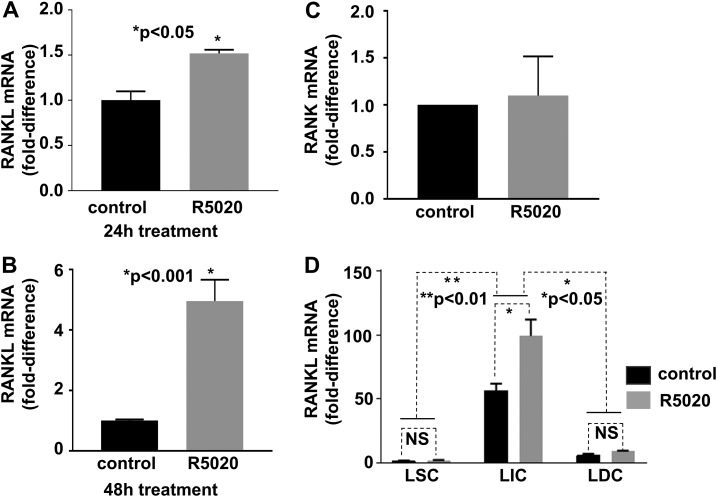
Leiomyomas are hormone-responsive tumors, so we next investigated whether progestin regulated RANKL expression in uterine leiomyoma. We treated tissue explants with the PR agonist R5020 and then extracted RNA to measure RANKL expression by qRT-PCR. Treatment of tissue explants with R5020 for 24 hours and 48 hours significantly increased RANKL expression compared with tissue explants treated with vehicle (24 hours, n = 3, P < 0.05 [Fig. 3(A)]; 48 hours, n = 3, P < 0.001 [Fig. 3(B)]). RANK expression did not differ between R5020- and vehicle-treated groups [Fig. 3(C)]. To determine whether R5020 stimulates RANKL expression preferentially in a particular cell population, we sorted three populations from explants treated with R5020 for 24 hours and measured RANKL expression in each population. We found that RANKL was significantly induced in LICs but not in LSCs or LDCs [Fig. 3(D)].
Figure 3.
The PR agonist R5020 induces RANKL but not RANK gene expression. (A and B) Total RNA was extracted from leiomyoma explants treated with R5020 for 24 hours (A) or 48 hours (B). RANKL mRNA was quantified by using qRT-PCR; n = 3 independent tissue samples. (C) RANK mRNA was quantified in explants treated with R5020 for 48 hours; n = 3. (D) R5020 significantly induced RANKL expression in LICs. LSCs, LICs, and LDCs were sorted from leiomyoma explants treated with R5020 for 24 hours, and then RANKL mRNA level was quantified in each cell population. Data show a representative experiment performed in triplicate from three independent tissue samples. The error bars are the standard error of the mean. NS, not significant.
RANK-Fc inhibits expression of cell proliferative gene in vitro
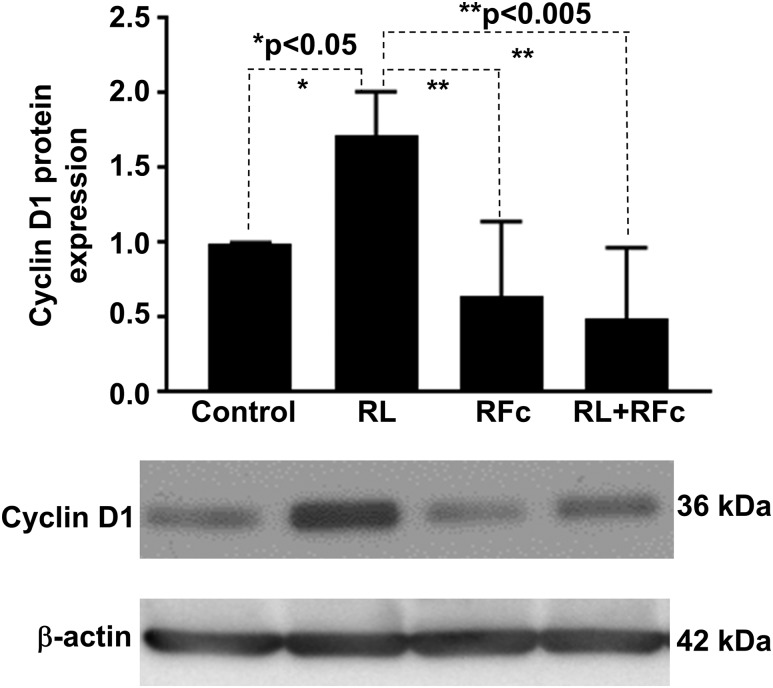
Because RANKL has been shown to increase expression of cell cycle protein cyclin D1 in bone, we investigated whether it had a similar effect in leiomyoma cells (17) and whether this effect could be inhibited by RANK-Fc. Leiomyoma cells (total population) were cultured and separated into four treatment groups: vehicle, RANKL (50 ng/mL), RANK-Fc (100 ng/mL), or both RANKL and RANK-Fc. After 48 hours of treatment, protein was extracted and immunoblotting analysis was performed to quantify cyclin D1 expression. We found that RANKL treatment markedly increased the cyclin D1 protein level, which was blocked by cotreatment with RANK-Fc (Fig. 4). RANK-Fc treatment alone did not significantly affect cyclin D1 expression.
Figure 4.
Treatment of the total leiomyoma cells with RANKL increased cyclin D1 expression that was suppressed with the RANKL/RANK pathway inhibitor RANK-Fc. Upper panel shows immunoblot densities quantified with ImageJ software. Lower panel shows images of representative immunoblots. Experiments were repeated with cells from five patient samples. The error bars are the standard error of the mean. RFc, RANK-Fc; RL, RANKL.
RANK-Fc inhibits estrogen- and progesterone-responsive growth of leiomyoma tumors in vivo
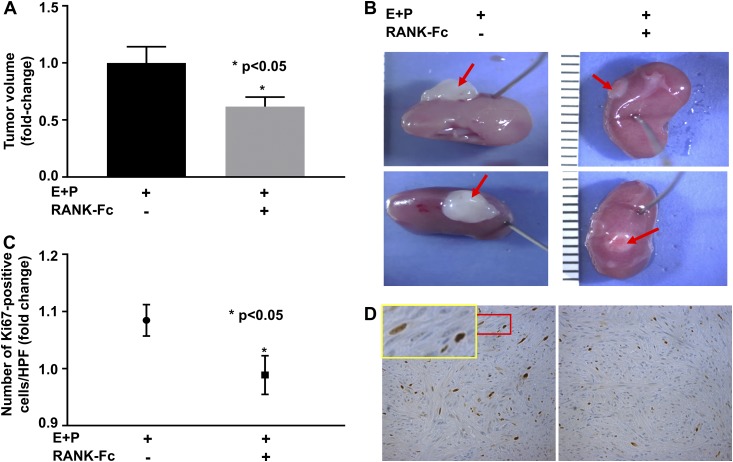
Cell pellets consisting of the total leiomyoma cells were grafted underneath kidney capsules of ovariectomized NSG mouse hosts, and the mice were treated with vehicle or RANK-Fc (10 mg/kg subcutaneously twice a week for 4 weeks). Significantly smaller tumors were excised from mice treated with RANK-Fc compared with mice treated with vehicle (P < 0.05; n = 4 [Fig. 5(A) and 5(B)]). We further performed IHC analysis on the excised tumors to examine the effect of RANK-Fc treatment on expression of Ki67. Ki67 expression was decreased in tumors from mice treated with RANK-Fc compared with controls [Fig. 5(C) and 5(D)]. These findings suggest that RANK-Fc treatment inhibited leiomyoma cell proliferation and tumor growth.
Figure 5.
(A) Xenografted leiomyoma tumors from mice treated with RANK-Fc showed decreased tumor volume compared with mice treated with DPBS. (B) Representative images of regenerated tumors (red arrows) 4 weeks after engrafting. (C) A significantly lower number of cells expressing Ki67 (brown-stained) was seen in xenografted tumors from mice treated with RANK-Fc; n = 4. (D) Representative images of immunohistochemistry staining for Ki67, ×20. The inset in D (yellow rectangle) is the region in the red rectangle at a high magnification, ×2.7. The error bars are the standard error of the mean. E, estradiol; HPF, high-power field; P, progesterone.
Discussion
Despite advances in our understanding of uterine leiomyoma, current treatment options are unable to prevent the formation of new uterine leiomyoma. Our study suggests a potential role for the RANKL/RANK pathway in leiomyoma tumorigenesis. Specifically, we found LSCs preferentially expressed RANK, indicating that these cells are able to respond to RANKL stimulation. We also demonstrated RANKL expression primarily by LIC, providing a potential paracrine mechanism by which LSCs are stimulated by LIC-derived RANKL. Most important, we found that the RANKL/RANK pathway inhibitor RANK-Fc decreased human leiomyoma tumor growth in vivo in the xenograft mouse model. This study evaluated a potential therapeutic option for uterine fibroids that targets the RANKL/RANK pathway in LSCs.
In mammary tissue, the RANKL/RANK pathway provides the key paracrine pathway by which progesterone induces proliferation and differentiation of stem cells into epithelial cells and subsequently milk-secreting acini (10). PR-positive mammary luminal epithelial cells secrete RANKL in response to progesterone. RANKL then acts on its receptor RANK on mammary stem cells to bring about proliferation and differentiation. Similarly, we found that RANKL expression in leiomyoma explants, preferentially in LICs, was increased in response to PR activation by R5020. However, transcription of cyclin D1, a RANKL target gene, was not concomitantly induced by R5020 in these explants (data not shown), which may be due to the absence of key in vivo conditions or cofactors in the in vitro model systems that we used. Treatment of leiomyoma cells directly with RANKL led to a significant increase in cyclin D1 protein expression, which was abolished by the presence of RANK-Fc; this finding suggests that RANKL can specifically regulate cyclin D1 expression in leiomyoma cells. In addition, RANKL is mainly expressed in LICs after depleting immune cells with anti-CD45 antibody [Fig. 2(A) and 2(C)], indicating that RANKL is derived primarily from fibroid cells but not infiltrating immune cells. Thus, our results suggest a potential paracrine interaction between LSCs and LICs, that is, LSCs are activated by LIC-derived RANKL.
The role of the RANKL/RANK pathway in cell proliferation and differentiation was first described in bone. Bone remodeling involves osteoblasts that build bone and osteoclasts that resorb bone (10). Osteoblasts express RANKL, which acts on osteoclast precursor cells to induce their differentiation into mature osteoclasts to break down bone. Osteoprotegerin (OPG) is a decoy receptor for RANKL, and increased levels of OPG are associated with decreased bone resorption. In the hypoestrogenic postmenopausal state, OPG levels are reduced, leading to increased osteoclast differentiation and subsequent bone resorption (18). Denosumab is a highly specific, fully human monoclonal antibody to RANKL and is used in the clinical treatment of osteoporosis (19). However, the product is constrained in pregnancy in consideration of the side effects (20). Our finding that RANK-Fc inhibited estrogen- and progesterone-responsive in vivo leiomyoma regeneration provides a potential alternative therapeutic strategy that may prevent new leiomyoma formation, and this represents a major advance in the clinical care of women affected by uterine leiomyoma. Further studies are needed to determine whether RANK-Fc can effectively reach all target stem cells within a complex whole organ structure to eliminate or inhibit the initiation of additional fibroid lesions.
Some strengths of our study include the use of human tissue and both in vitro and in vivo approaches to describe a potential paracrine pathway driving leiomyoma cell proliferation and tumor growth, and to test a potential therapeutic option for uterine leiomyoma that targets LSCs. Our study is also strengthened by the addition of a murine xenograft model to demonstrate the effects of RANK-Fc on fibroid growth in vivo, confirmed by Ki67 IHC. We performed in vivo experiments using immunodeficient mice, suggesting that the observed antitumor effect of RANK-Fc is likely mediated via direct effects on fibroid cells but not on infiltrating immune cells. The limitation of our study is the use of the total population of leiomyoma cells for the xenograft experiments, as opposed to just the LSC population. One future direction will be to repeat this experiment with specific combinations of the different leiomyoma cell populations and further characterize the RANKL/RANK paracrine pathway in the leiomyoma cell populations.
Conclusion
This study describes the role of the RANKL/RANK pathway in leiomyoma growth and a treatment targeting that pathway in leiomyoma stem cells. Our data show leiomyoma tumor growth suppression in a murine xenograft model after treatment with the RANKL/RANK pathway inhibitor RANK-Fc. These findings suggest that the RANKL/RANK system could serve as a potential target for prevention and treatment of uterine leiomyoma. Medications that can inhibit leiomyoma growth and prevent new leiomyoma would represent a major clinical advance in the management of uterine leiomyoma. Such a treatment could have important implications for women’s reproductive health, future fertility, and quality of life.
Supplementary Material
Acknowledgments
We thank Amgen, Inc., for providing the recombinant RANK-Fc protein used in this study. We acknowledge the Northwestern University Flow Cytometry Facility and Mouse Histology and Phenotyping Laboratory, which are cofunded by National Cancer Institute Cancer Center Grant CA060553.
Financial Support: This study was supported by National Institutes of Health Grant P01 HD057877 (to S.E.B.).
Author Contributions: D.E.I. performed the experiments and wrote the manuscript. S.L. performed experiments. S.K. helped with the animal experiments and reviewed the manuscript. E.E. and J.S.C. performed experiments. J.R. and S.E.B. provided oversight and reviewed the manuscript. P.Y. performed experiments, provided oversight, and reviewed the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- Ct
cycle threshold
- DPBS
Dulbecco phosphate-buffered saline
- FACS
fluorescence-activated cell sorting
- IHC
immunohistochemistry
- LDC
leiomyoma differentiated cell
- LIC
leiomyoma intermediate cell
- LSC
leiomyoma stem cell
- mRNA
messenger RNA
- OPG
osteoprotegerin
- PCR
polymerase chain reaction
- PR
progesterone receptor
- qRT-PCR
quantitative real-time polymerase chain reaction
- RANK
receptor activator of nuclear factor κ-Β
- RANKL
receptor activator of nuclear factor κ-Β ligand
References
- 1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. [DOI] [PubMed] [Google Scholar]
- 2. Marsh EE, Ekpo GE, Cardozo ER, Brocks M, Dune T, Cohen LS. Racial differences in fibroid prevalence and ultrasound findings in asymptomatic young women (18-30 years old): a pilot study. Fertil Steril. 2013;99(7):1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American College of Obstetricians and Gynecologists ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 Pt 1):387–400. [DOI] [PubMed] [Google Scholar]
- 4. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(211):e211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–1355. [DOI] [PubMed] [Google Scholar]
- 6. Ono M, Qiang W, Serna VA, Yin P, Coon JS V, Navarro A, Monsivais D, Kakinuma T, Dyson M, Druschitz S, Unno K, Kurita T, Bulun SE. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7(5):e36935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moravek MB, Yin P, Ono M, Coon JS V, Dyson MT, Navarro A, Marsh EE, Chakravarti D, Kim JJ, Wei JJ, Bulun SE. Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum Reprod Update. 2015;21(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yin P, Ono M, Moravek MB, Coon JS V, Navarro A, Monsivais D, Dyson MT, Druschitz SA, Malpani SS, Serna VA, Qiang W, Chakravarti D, Kim JJ, Bulun SE. Human uterine leiomyoma stem/progenitor cells expressing CD34 and CD49b initiate tumors in vivo. J Clin Endocrinol Metab. 2015;100(4):E601–E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. [DOI] [PubMed] [Google Scholar]
- 10. Sigl V, Jones LP, Penninger JM. RANKL/RANK: from bone loss to the prevention of breast cancer. Open Biol. 2016;6(11):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13(8):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF, Raffoul W, Fiche M, Dougall W, Schneider P, Yalcin-Ozuysal O, Brisken C. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med. 2013;5(182):182ra55. [DOI] [PubMed] [Google Scholar]
- 13. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whang PG, Schwarz EM, Gamradt SC, Dougall WC, Lieberman JR. The effects of RANK blockade and osteoclast depletion in a model of pure osteoblastic prostate cancer metastasis in bone. J Orthop Res. 2005;23(6):1475–1483. [DOI] [PubMed] [Google Scholar]
- 15. Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999;96(7):3540–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hann HW, Stahlhut MW, Rubin R, Maddrey WC. Antitumor effect of deferoxamine on human hepatocellular carcinoma growing in athymic nude mice. Cancer. 1992;70(8):2051–2056. [DOI] [PubMed] [Google Scholar]
- 17. Ogasawara T, Katagiri M, Yamamoto A, Hoshi K, Takato T, Nakamura K, Tanaka S, Okayama H, Kawaguchi H. Osteoclast differentiation by RANKL requires NF-kappaB-mediated downregulation of cyclin-dependent kinase 6 (Cdk6). J Bone Miner Res. 2004;19(7):1128–1136. [DOI] [PubMed] [Google Scholar]
- 18. Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106(10):1203–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–419. [DOI] [PubMed] [Google Scholar]
- 20. AMGEN Prolia® (donosumab). Available at: https://pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/prolia/prolia_pi.pdf. Accessed 28 March 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.