Abstract
Context
Marine long-chain omega-3 fatty acids have been positively related to markers of fecundity in both men and women. However, seafood, their primary food source, can also be a source of toxicants, which could counteract the reproductive benefits.
Objective
To examine the relationship of male and female seafood intake with time to pregnancy (TTP).
Design
Our prospective cohort study included 501 couples planning pregnancy, who participated in the Longitudinal Investigation of Fertility and the Environment study (2005 to 2009) and were followed up for ≤1 year or until pregnancy was detected. Seafood intake was collected daily during follow-up in journals.
Setting
Couples residing in Michigan and Texas were recruited using population-based sampling frameworks.
Main Outcome Measures
The primary outcome was the TTP, determined using an in-home pregnancy test. A secondary outcome was sexual intercourse frequency (SIF) as recorded in the daily journals.
Results
Couples with male and female partners who consumed eight or more seafood servings per cycle had 47% (95% CI, 7% to 103%) and 60% (95% CI, 15% to 122%) greater fecundity (shorter TTP) than couples with male and female partners who consumed one or fewer seafood servings per cycle. Couples with both partners consuming eight or more seafood servings per cycle had 61% (95% CI, 17% to 122%) greater fecundity than couples consuming less. Male and female partners with the highest seafood intake (eight or more servings per cycle) also had 22% greater SIF.
Conclusions
Greater male and female seafood intake was associated with a higher SIF and fecundity among a large prospective cohort of couples attempting pregnancy.
In the present prospective cohort study with preconception enrollment and daily follow-up of couples, seafood intake in both partners was associated with a higher SIF and greater fecundity.
Infertility, the failure to achieve pregnancy after 12 months of unprotected sexual intercourse, affects 15% to 25% of couples (1, 2). Although infertility treatments exist, their costs (3), limited geographic accessibility (4), and modest success (5) justify identifying modifiable factors that increase a couple’s chance of conceiving without medical assistance. Seafood is a recommended component of many healthy eating patterns (6, 7). In the context of fertility, however, seafood has largely been studied as a potential harm, representing a primary source of exposure to reproductive toxicants such as organochlorines, dioxins, and mercury (8–11). In contrast, some studies have found reproductive benefits with higher marine long-chain omega-3 fatty acid intake, such as increased progesterone levels, a shorter time to pregnancy (TTP), and better semen quality (12–14).
For the average US adult, the current recommendation is to eat at least two seafood servings per week (6); however, in January 2017, the US Food and Drug Administration and Environmental Protection Agency recommended that women who are pregnant or might become pregnant should eat no more than three servings per week (15). This guideline was established to limit fetal methyl-mercury exposure, which has been linked to adverse neurocognitive consequences. However, to the best of our knowledge, these guidelines did not consider the potential reproductive benefits of seafood intake. To address this gap, we used data from a prospective cohort of couples attempting to become pregnant, with information on daily seafood intake and sexual intercourse collected in journals, to investigate whether male and female seafood intake was associated with the TTP and whether this association could be due to differences in sexual activity.
Materials and Methods
The Longitudinal Investigation of Fertility and the Environment (LIFE) study is a prospective cohort of 501 couples attempting to conceive in two geographic areas (Texas and Michigan) from 2005 to 2009. Using population-based sampling frameworks, households were contacted to identify eligible couples in a committed relationship. Female partners were required to be aged 18 to 44 years, to have menstrual cycles of 21 to 42 days, and to have had no hormonal birth control injections during the previous year. Male partners were required to be aged ≥18 years. Both partners were required to have the ability to communicate in English or Spanish and to have undergone no sterilization procedures or have physician-diagnosed infertility. The couples were also excluded if they had not been using contraception for >2 months. A complete description of the study’s methods has been previously reported (16). In brief, of the 1188 eligible couples, 501 (42%) were enrolled in the present study and followed up for ≤12 months, with monthly pregnancy tests. The institutional review boards at each institution approved the protocol. All participants provided written informed consent.
Research assistants traveled to the couples’ homes and completed baseline in-person interviews separately with each partner. Both partners were asked how often during the previous 12 months they had eaten canned tuna fish; fish caught in unknown locations; crab, shrimp, or other shellfish caught in an unknown location; fish caught in local waters; and crab, shrimp, or shellfish caught in local waters. The five response options ranged from “never or almost never” to “two or more times per week.” The selected frequency category for each seafood item was then converted to a monthly intake, and all items were summed to find the total baseline seafood intake. In the daily journals, the male and female participants were asked to report the number of 4-oz servings of fish or shellfish consumed. These daily responses were then summed across the cycle to determine their cycle-specific seafood intake. For the analysis, the baseline and daily male and female seafood intake were classified into categories that approximated quartiles.
During the enrollment interview, each partner reported their age, level of education, ethnicity, race, household income, and use of cigarettes. Participants were asked whether they had followed a regular vigorous exercise program in the previous 12 months and, if so, how many days per week. The four-item Cohen perceived stress scale was also administered (17). The men and women reported whether they had consumed ≥12 alcoholic drinks in the previous year, and, if so, how often they had consumed alcoholic beverages. All participants had their weight and height measured using standardized procedures, and the body mass index was calculated as the weight in kilograms divided by the height in square meters.
The primary outcome was fecundity, as measured by TTP. We used daily journal information supplemented with fertility monitors to define the menstrual cycles, defined as the interval (in days) from the onset of bleeding that increased in intensity and lasted ≥2 days to the onset of the next similar bleeding episode. Because couples were allowed to enroll in the LIFE study midcycle, we defined this as cycle 0 to differentiate it from cycle 1, which denoted the first fully observed menstrual cycle. Pregnancy was defined as a positive study-provided home pregnancy test, which was sensitive for 25 mIU/mL human chorionic gonadotropin. A secondary outcome was the frequency of vaginal–penial intercourse, as recorded by the men and women in their daily journals. For each cycle of follow-up, the sexual intercourse frequency (SIF) reports were summed across all days to find the total SIF per cycle. The correlation between the SIF per cycle as reported by the male and female partners was 0.98, and the average difference between the two reports was −0.02 times per month. Because of the slightly lower amount of missing data in the female diaries, the female report of SIF was used as the main outcome variable.
We classified each partner as having high (nine times or more per month; 75th percentile) or low-to-average (less than nine times per month) seafood intake. The male and female demographic data and lifestyle characteristics were then compared using ANOVA for continuous variables or χ2 tests for categorical variables. The correlation within and between male and female seafood intake at baseline and during follow-up was calculated using Spearman correlation coefficients.
Cox proportional odds models for discrete survival data accounting for left truncation (to account for the time without contraception before enrollment) and right censoring (to account for the loss to follow-up or the end of the study) were used to estimate the fecundability ORs (FORs), and their 95% CIs, as a measure of fecundity. FORs represent the relative odds of achieving pregnancy conditional on not becoming pregnant in the previous cycle, such that an FOR <1 indicates diminished fecundity as measured by a longer TTP. Seafood intake was initially considered as quartiles of intake, and in a supplemental analysis, it was modeled continuously using linear and quadratic terms.
To analyze the association between seafood intake and SIF per cycle during the follow-up period, we used generalized linear mixed models with the Poisson distribution. Effect estimates and 95% CIs are presented as the percentage of difference in SIF for a particular group compared with the reference group. We also explored the association between day-level seafood intake and SIF using a generalized linear mixed model with logit link. The results are presented as the ORs and 95% CIs of sexual intercourse in a given day. We imputed the SIF values for cycles with >50% of the days missing information on SIF and any cycle with <14 days of follow-up (n = 159 cycles) using Markov chain Monte Carlo methods (PROC MI in SAS; SAS Institute, Cary, NC) with five multiple imputations based on menstrual cycle length, cycle number of follow-up, female age, the difference between the couple’s ages, female race and education level, and male exercise. Effect estimates from models using multiply imputed values for SIF were estimated using Rubin’s formula for combining estimates across imputations (PROC MIANALYZE in SAS).
Confounding was evaluated using previous knowledge and descriptive statistics from our cohort through the use of directed acyclic graphs. Variables retained in the final multivariable models were female age (in years), the difference between couple’s ages (in years), female race (non-Hispanic white vs other), male exercise (yes vs no), and male and female alcohol intake (one or more time per week vs less than one time per week). Additional models were run further, adjusting for male and female partner seafood intake owing to the high amount of concordance within a couple. The fecundity models were also further adjusted for SIF to evaluate the extent to which this variable explained any observed associations. A P value for trend was calculated across the categories of seafood intake using the median intake level in each category as a continuous variable.
In the main analysis, missing data on seafood intake in the daily journals were considered as no intake, which is common for dietary analyses. Sensitivity analyses were performed in which missing seafood intake was imputed for cycles that were missing 100% and >50% of days of seafood intake data using Markov chain Monte Carlo methods with five multiple imputations and Rubin formula to combine estimates across imputations. To address concerns of residual confounding, we also calculated propensity scores and ran the final model adjusting for this variable and stratified by quintiles of this variable. To quantify the effect of unmeasured confounding, we calculated the e-value, which estimates the minimum strength of an association that an unmeasured confounder would need to have with both the exposure and outcome to fully explain a specific exposure–outcome association (18). SAS, version 9.4 (SAS Institute) was used for all statistical analyses.
Results
Male partners who reported the highest usual seafood intake were less likely to have a non-Hispanic white partner and more likely to exercise regularly and consume alcohol one or more time per week compared with men with lower intake (Table 1). Female partners with the greatest usual seafood intake were, on average, older, had older partners, were less likely to be non-Hispanic white, and were more likely to consume alcohol one or more time per week compared with females with lower intake. Seafood intake was not associated with body mass index, education level of either partner, or household income. Male and female seafood intake within a couple correlated moderately at baseline (r = 0.46) and correlated highly during the follow-up period (r = 0.70; Supplemental Table 1). Within men and women, the baseline seafood intake correlated moderately with the intake during follow-up (r = 0.47 and r = 0.53, respectively).
Table 1.
Demographic and Lifestyle Characteristics Stratified by Seafood Intake at Baseline in the LIFE Study (n = 501 Couples)
| Variable | Male Baseline Seafood Intake | Female Baseline Seafood Intake | ||||
|---|---|---|---|---|---|---|
| Less Than Nine Times per Month (n = 406) | Nine Times or More per Month (n = 95) | P Valuea | Less Than Nine Times per Month (n = 419) | Nine Times or More per Month (n = 82) | P Valuea | |
| Female demographic data | ||||||
| Age, y | 30.1 ± 4.1 | 29.7 ± 4.3 | 0.41 | 29.7 ± 4.0 | 31.2 ± 4.4 | 0.003 |
| Non-Hispanic white | 339 (83.5) | 68 (71.6) | 0.007 | 350 (83.5) | 57 (69.5) | 0.003 |
| College education | 309 (76.1) | 71 (74.7) | 0.78 | 319 (76.1) | 61 (74.4) | 0.74 |
| Male demographic data | ||||||
| Age, y | 31.8 ± 4.8 | 31.7 ± 5.2 | 0.87 | 31.4 ± 4.8 | 33.5 ± 5.2 | 0.004 |
| Non-Hispanic white | 340 (83.7) | 72 (75.8) | 0.07 | 347 (82.8) | 65 (79.3) | 0.44 |
| College education | 256 (63.1) | 55 (57.9) | 0.35 | 264 (63.0) | 47 (57.3) | 0.33 |
| Couple income | 0.14 | 0.29 | ||||
| <$29,999 | 15 (3.8) | 6 (6.3) | 19 (4.6) | 2 (2.4) | ||
| $30,000–$49,999 | 51 (12.8) | 5 (5.3) | 51 (12.4) | 5 (6.1) | ||
| $50,000–$69,999 | 67 (16.8) | 19 (20.0) | 71 (17.3) | 15 (18.3) | ||
| ≥$70,000 | 265 (66.6) | 65 (68.4) | 270 (65.7) | 60 (73.2) | ||
| Female lifestyle factors | ||||||
| BMI, kg/m2 | 27.6 ± 7.2 | 27.0 ± 6.4 | 0.46 | 27.3 ± 7.1 | 28.5 ± 6.9 | 0.15 |
| Current smoker | 45 (11.1) | 11 (11.6) | 0.89 | 46 (11.0) | 10 (12.2) | 0.75 |
| Exercises regularly | 164 (40.4) | 36 (37.9) | 0.65 | 168 (40.1) | 32 (39.0) | 0.86 |
| Seafood intake, times per month | 4.7 ± 4.4 | 7.7 ± 4.4 | <0.001 | 3.7 ± 2.7 | 13.1 ± 4.0 | <0.001 |
| Alcohol intake one or more times per week | 116 (28.6) | 38 (40.4) | 0.13 | 114 (27.3) | 40 (48.8) | <0.001 |
| Stress in previous month | 3.6 ± 2.6 | 3.6 ± 2.4 | 0.90 | 3.5 ± 2.5 | 3.9 ± 2.6 | 0.28 |
| Male lifestyle factors | ||||||
| BMI, kg/m2 | 29.4 ± 4.9 | 29.8 ± 5.2 | 0.52 | 29.5 ± 5.1 | 29.3 ± 4.2 | 0.71 |
| Current smoker | 56 (13.8) | 18 (19.0) | 0.20 | 60 (14.3) | 14 (17.1) | 0.52 |
| Exercises regularly | 162 (39.9) | 49 (51.6) | 0.04 | 170 (40.6) | 41 (50.0) | 0.11 |
| Seafood intake, times per month | 3.9 ± 2.7 | 13.1 ± 4.4 | <0.001 | 5.1 ± 4.3 | 8.7 ± 5.6 | <0.001 |
| Alcohol intake one or more times per week | 212 (52.2) | 65 (68.4) | 0.03 | 221 (52.7) | 56 (68.3) | 0.07 |
| Stress in previous month | 3.0 ± 2.4 | 3.3 ± 2.4 | 0.32 | 3.1 ± 2.3 | 2.8 ± 2.5 | 0.39 |
Data presented as mean ± SD or n (%).
P values presented from χ2 tests for categorical variables and Kruskal-Wallis nonparametric tests for continuous variables.
Higher male (but not female) baseline seafood intake was associated with higher SIF during follow-up after multivariable adjustment (Table 2). Men who usually consumed seafood nine or more times per month had a 22.9% (95% CI, 6.8% to 41.5%) greater SIF compared with men who usually consumed seafood two times or less per month (P for trend = 0.007). A positive association was found between baseline female seafood intake and SIF that became attenuated after adjustment for male partner intake. During follow-up, both male and female seafood intake was independently associated with SIF, with slightly stronger associations observed for male intake. Furthermore, when both partners consumed eight or more servings per cycle, SIF was increased by 21.9% (95% CI, 15.2% to 29.0%) compared with couples consuming less. In the day-level analyses, the odds of sexual intercourse was 39% (95% CI, 29% to 50%) greater if both partners consumed seafood the same day, 3% (95% CI, −5% to 11%) greater if only the woman consumed seafood, and 2% (95% CI, −6% to 10%) greater if only the man consumed seafood compared with couples with neither partner consuming seafood. The associations were identical when the male report of SIF was used (instead of the female report).
Table 2.
Associations Between Male and Female Seafood Intake at Baseline and During Follow-Up and Frequency of Sexual Intercourse (n = 501 Couples; 2372 Follow-Up Cycles)
| Variable | Subjects or Cycles, n (%) | % Difference in SIF (95% CI) | |
|---|---|---|---|
| Model 1a | Model 2b | ||
| Male baseline seafood intake | |||
| Two times or less per month | 153 (31) | Reference | Reference |
| Three to four times per month | 118 (24) | 3.0 (−9.9 to 17.7) | 2.5 (−10.5 to 17.4) |
| Five to eight times per month | 135 (27) | 7.5 (−5.3 to 22.0) | 7.1 (−6.4 to 22.4) |
| Nine times or more per month | 95 (19) | 22.9 (6.8 to 41.5) | 21.7 (4.6 to 41.7) |
| P for trend | 0.007 | 0.02 | |
| Female baseline seafood intake | |||
| Two times or less per month | 177 (35) | Reference | Reference |
| Three to four times per month | 95 (19) | 1.1 (−11.9 to 16.0) | −2.8 (−15.5 to 11.8) |
| Five to eight times per month | 147 (29) | 1.4 (−10.0 to 14.1) | −3.8 (−15.1 to 9.1) |
| Nine times or more per month | 82 (16) | 17.3 (1.2 to 36.0) | 9.7 (−6.3 to 28.4) |
| P for trend | 0.12 | 0.60 | |
| Male daily journal seafood intake | |||
| One serving or less per cycle | 814 (34) | Reference | Reference |
| One to three servings per cycle | 422 (18) | 4.8 (−0.8 to 10.9) | 4.8 (−1.1 to 11.0) |
| Four to seven servings per cycle | 575 (24) | 16.7 (10.8 to 23.0) | 15.2 (8.8 to 22.1) |
| Eight servings or more per cycle | 561 (24) | 32.6 (25.4 to 40.2) | 26.9 (18.7 to 35.6) |
| P for trend | <0.001 | <0.001 | |
| Female daily journal seafood intake | |||
| One serving or less per cycle | 835 (35) | Reference | Reference |
| One to three servings per cycle | 446 (19) | 2.5 (−3.2 to 8.5) | −1.5 (−7.2 to 4.5) |
| Four to seven servings per cycle | 605 (26) | 9.4 (3.7 to 15.4) | 0.5 (-5.2 to 6.6) |
| Eight servings or more per cycle | 486 (20) | 26.4 (19.2 to 34.0) | 10.2 (2.9 to 18.2) |
| P for trend | <0.001 | 0.009 | |
| Couple daily journal seafood intake | |||
| At least one partner consumed fewer than 8 servings per cycle | 2057 (87) | Reference | |
| Both partners consumed eight servings or more per cycle | 315 (13) | 21.9 (15.2 to 29.0) | |
Generalized linear mixed models with Poisson distribution and log link were used to estimate the percentage of difference (95% CIs); cycles with >50% of days with missing information on SIF and cycles with <14 days of follow-up (n = 159 cycles) had their SIF values imputed (using five multiple imputations).
Model 1 adjusted for cycle length, female age, difference in male and female age, female race (non-Hispanic white vs other), male exercise (yes vs no), and male and female alcohol intake (one or more times per week vs less than one time per week).
Model 2 adjusted for variables in model 1 plus male or female partner seafood intake (using the same assessment method).
Baseline seafood intake was not associated with fecundity after multivariable adjustment (Supplemental Table 2). Also, no differences were found in the associations according to whether the seafood was caught in local vs unknown waters or whether the seafood was shellfish vs fish (data not shown). However, the prospectively collected male and female seafood intake from the daily journals was related to increased fecundity (shorter TTP; Table 3). Specifically, men and women who consumed eight or more seafood servings per cycle had 47% (95% CI, 7% to 103%) and 60% (95% CI, 15% to 122%) greater fecundity compared with the men and women who consumed one or fewer seafood servings per cycle after multivariable adjustment. These associations were attenuated with further adjustment for partner seafood intake (model 2), most likely owing to the high correlation between intake during the follow-up period. When modeling intake as a continuous variable, both male and female seafood intake was associated with greater fecundity, plateauing at ~14 to 16 seafood servings per cycle (>90th percentile of intake; Supplemental Fig. 1).
Table 3.
Associations Between Male and Female Seafood Intake During Follow-Up and TTP (n = 501 Couples)
| Variable | Pregnancies/Cycles | FOR (95% CI) | |||
|---|---|---|---|---|---|
| Unadjusted | Model 1a | Model 2b | Model 3c | ||
| Male daily journal seafood intake | |||||
| One serving or less per cycle | 99/814 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| One to three servings per cycle | 58/422 | 1.15 (0.81–1.64) | 1.17 (0.82–1.68) | 1.18 (0.81–1.71) | 1.06 (0.74–1.52) |
| Four to seven servings per cycle | 90/575 | 1.25 (0.91–1.71) | 1.28 (0.93–1.76) | 1.23 (0.87–1.74) | 1.19 (0.86–1.85) |
| Eight servings or more per cycle | 98/561 | 1.37 (1.01–1.87) | 1.47 (1.07–2.03) | 1.24 (0.84–1.83) | 1.33 (0.96–1.85) |
| P for trend | 0.04 | 0.02 | 0.28 | 0.07 | |
| Female daily journal seafood intake | |||||
| One serving or less per cycle | 108/835 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| One to three servings per cycle | 61/446 | 0.99 (0.70–1.39) | 1.04 (0.73–1.47) | 0.98 (0.68–1.40) | 1.00 (0.70–1.42) |
| Four to seven servings per cycle | 83/605 | 1.00 (0.73–1.36) | 1.07 (0.78–1.47) | 0.97 (0.68–1.38) | 1.03 (0.75–1.42) |
| Eight servings or more per cycle | 93/486 | 1.38 (1.02–1.89) | 1.60 (1.15–2.22) | 1.42 (0.96–2.11) | 1.44 (1.04–2.01) |
| P for trend | 0.06 | 0.01 | 0.13 | 0.04 | |
Cox models for discrete survival time accounting for left truncation were used to calculate the FORs and 95% CIs.
Model 1 adjusted for female age, difference in male and female age, female race (non-Hispanic white vs other), male exercise (yes vs no), male and female alcohol intake (one or more times per week vs less than one time per week), and cycle length.
Model 2 adjusted for variables in model 1 plus male or female partner seafood intake during follow-up.
Model 3 adjusted for variables in model 1 plus SIF (modeled with a linear and squared term).
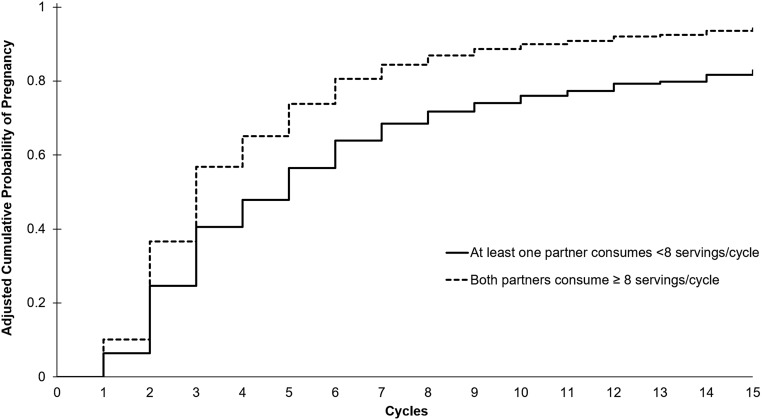
The positive association between seafood intake and fecundity was stronger for couples when both partners consumed high amounts of seafood during follow-up (Fig. 1). On average, the estimated percentages of couples who were pregnant by 6 and 12 months among the couples who consumed eight or more seafood servings per cycle were 81% and 92% compared with 64% and 79% among the couples consuming less. This translated into an adjusted FOR of 1.61 (95% CI, 1.17 to 2.22; Supplemental Table 3) and a 13% lower absolute difference in the incidence of infertility. The positive associations between male, female, and couple seafood intake and fecundity were slightly attenuated after adjustment for SIF; however, the FORs for the highest female and couple seafood consumers remained statistically significant.
Figure 1.
Interaction between male and female partner seafood intake during follow-up on TTP (n = 501 couples). Cox models for discrete survival time accounting for left truncation were used to calculate the adjusted cumulative probabilities of pregnancy at the average value for continuous covariates and the most common value for categorical covariates (female age, 30 years; difference in male and female age, 1.8 years; female non-Hispanic white race; no male exercise; male alcohol intake one or more times per week; female alcohol intake less than one time per week; and cycle length, 30 days).
In sensitivity analyses aimed at testing our assumption that the days with seafood intake data missing in the journals represented days with zero seafood intake, the associations between male, female, and couple daily journal seafood intake and fecundity were attenuated but still positively related to fecundity (Supplemental Table 4). The association between higher seafood intake for both partners and TTP was also robust in the sensitivity analyses with further adjustment and stratification by propensity score (Supplemental Table 5). Finally, the unmeasured confounding analyses showed that the observed FOR of 1.61 could only be explained by an unmeasured confounder that was associated with both the exposure and the outcome by a risk ratio of ≥2.13-fold, above and beyond the measured confounders.
Discussion
In the present prospective cohort study with preconception enrollment and daily follow-up of couples, the seafood intake in both partners was associated with a greater frequency of sexual intercourse and fecundity. Specifically, the daily odds of sexual intercourse were 39% greater when both partners consumed seafood on the same day. Also, for couples in which both partners consumed eight or more seafood servings per cycle had 61% greater fecundity and a 13% lower absolute difference in the incidence of infertility compared with couples consuming less seafood.
The reported data on seafood intake and TTP, although sparse, are conflicting (19, 20). Several reasons exist for this heterogeneity, including differences in study designs, primary sources, types and range of seafood consumed, and outcomes assessments. Both previous studies relied on a retrospective report of seafood intake, which could have introduced a substantial measurement error. Our study has illustrated this point well, because we found no associations between baseline seafood intake, assessed using a typical retrospective questionnaire, and fecundity, despite the moderate positive correlations with the prospectively collected intake. Insufficient power could also be an issue. A retrospective cohort study from Sweden found no differences in the TTP comparing women differentially exposed to fatty fish contaminated with persistent organochlorines. However, within each group, the consumption of locally caught fatty fish had a marginally important, positive relation with fecundity (success OR, 1.27; 95% CI, 0.96 to 1.69; and success OR, 1.36; 95% CI, 0.96 to 1.94) (20).
Only one study found a detrimental effect of female seafood intake on fecundity. That retrospective TTP study of recently pregnant female anglers found that women who consumed one or more fish meal per month from Lake Ontario (a highly contaminated source of fish) had reduced fecundity (fecundability ratio, 0.73; 95% CI, 0.54 to 0.98). No associations were found between male partner intake and TTP (19). A previous report from the LIFE study found inverse relations between select male and female serum organochlorines and dioxins and fecundity (21) and no associations with blood mercury concentrations and TTP (22). These findings suggest that the fertility benefits of seafood consumption might outweigh the potential harms of environmental pollutants carried by these foods. In agreement with our findings, a recent prospective study found that among US women trying to get pregnant who did not use fish oil supplements, intake of omega-3 fatty acids, a primary nutrient found in seafood, was associated with higher fecundity (fecundability ratio, 1.40; 95% CI, 1.13 to 1.73 for quartile 4 vs quartile 1) (12). Similarly, in a prospective cohort study of women undergoing infertility treatment with assisted reproductive technologies, higher serum levels and intake of long-chain omega-3 fatty acids were associated with a higher probability of achieving pregnancy and a live birth (23).
We observed a positive association between seafood intake and SIF, supporting popular beliefs of the aphrodisiac properties of seafood. This association did not completely explain the relationship with fecundity, suggesting that the effects of seafood could result from mechanisms other than increased sexual activity. Several studies have found positive associations between omega fatty acid intake (24, 25), seafood intake (26, 27), and dietary patterns prioritizing seafood intake (28–31) and semen quality parameters, lending support to the idea that higher seafood intake could increase the quantity and quality of sperm. Among women, dietary intake of docosapentaenoic acid was associated with a lower risk of anovulation and dietary intake of total marine omega-3 polyunsaturated fats was associated with increased luteal-phase progesterone concentrations (13), suggesting beneficial effects of seafood on ovulation and menstrual cycle function. Finally, two separate infertility cohort studies have shown that embryo quality measures were improved among women with higher fish (32) and docosahexaenoic acid (33) intake, supporting a favorable role of seafood intake on early embryo development.
Although these previous studies support our findings that greater seafood intake might promote fecundity through various biological mechanisms, it is important to consider alternate explanations. First, individuals with higher seafood intake could have healthier diets overall, which we were unable to account for in the present study. However, the estimated e-value of 2.13 decreases the likelihood of this explanation. Although it is conceivable that unmeasured dietary factors are associated with seafood intake by a risk ratio of >2.13-fold, we are unaware of any studies linking any specific dietary or lifestyle factors to fecundity by a risk ratio (or FOR) >2.13-fold. For context, the FOR comparing women aged <27 years to those aged ≥35 years in this cohort was 1.96. Thus, although residual confounding is possible, it is unlikely to explain the entire association. Second, couples who consume greater amounts of seafood together may share more meals and thus more time together (including nights), which might explain the association between sexual activity and subsequently fecundity. However, after we adjusted for SIF in our models, the association between seafood intake and fecundity remained, suggesting that this behavioral pathway cannot completely explain the association.
Our study had other limitations. First, it consisted solely of couples planning pregnancy without medical assistance. All couples were also given fertility monitors to help time intercourse relative to ovulation and instructed to use them throughout the follow-up period. Because women using fertility monitors are more likely to get pregnant within two cycles than those who do not use a monitor (34), our results might not generalize to all women of reproductive age. However, the use of fertility monitors in our study was also a strength, because it removed any confounding by use of this or similar devices. We were able to assess differences in type (shellfish vs fish) and source (local vs unknown waters) of seafood using the baseline assessment tool and did not find any differences. However, we did not collect these details during follow-up, which limited our ability to distinguish between specific types of seafood and their potential reproductive effects. Finally, although it would have been ideal to include an assessment of daily diet, this was not feasible given the high participant burden of daily 24-hour recalls; therefore, residual confounding by other dietary factors, including dietary supplements, is possible, although unlikely to explain the entire association as discussed.
Our study had multiple strengths, including the reference standard assessment of TTP through the prospective use of fertility monitors and daily journals combined with in-home pregnancy testing. In addition, we had daily, prospective assessment of seafood intake. We were also able to reduce the likelihood of residual confounding by adjusting for many demographic and lifestyle factors. Because our sampling frameworks, which in Texas used the Parks and Wildlife Department's angler database for recruitment and in Michigan used a commercially available marketing database with recruitment filters to identify individuals with fishing interests, we were also able to study a unique population in which seafood intake was not tightly correlated with socioeconomic status. Moreover, despite the overrecruitment of anglers, the average seafood intake of our cohort was very similar to that of men and women from a representative US sample (35). Our population was also recruited because of presumed exposure to persistent environmental chemicals that have been linked to fecundity impairments (36). Thus, it is possible that our results regarding seafood consumption would be even stronger in a population unexposed to sources of seafood contamination. Finally, by including the male partners, we were able to evaluate the separate and joint effects of male and female seafood consumption, which is rare in fecundity studies.
In conclusion, couples in which both partners consumed eight or more seafood servings per cycle, or approximately two or more seafood servings per week, had a significantly greater SIF and higher fecundity. These findings highlight the importance of a couples’ diet for fecundity and the need for appropriate preconception guidance. Future research is needed that specifically evaluates the potential harms associated with predatory fish intake, because such fish tends to contain greater levels of persistent environmental chemicals and mercury.
Supplementary Material
Acknowledgments
We thank the participants and staff of the Longitudinal Investigation of Fertility and the Environment study for their valuable contributions to this research.
Financial Support : The Longitudinal Investigation of Fertility and the Environment study was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grants N01-HD-3-3355, N01-HD-3-3356, and NOH-HD-3-3358 to G.M. Buck Louis). Dr. Gaskins is supported by a career development award from the National Institute of Environmental Health Sciences, National Institutes of Health (grant K99ES026648).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- FOR
fecundability OR
- LIFE
Longitudinal Investigation of Fertility and the Environment
- SIF
sexual intercourse frequency
- TTP
time to pregnancy
References
- 1. Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–1331.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slama R, Hansen OK, Ducot B, Bohet A, Sorensen D, Giorgis Allemand L, Eijkemans MJ, Rosetta L, Thalabard JC, Keiding N, Bouyer J. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum Reprod. 2012;27(5):1489–1498. [DOI] [PubMed] [Google Scholar]
- 3. Katz P, Showstack J, Smith JF, Nachtigall RD, Millstein SG, Wing H, Eisenberg ML, Pasch LA, Croughan MS, Adler N. Costs of infertility treatment: results from an 18-month prospective cohort study. Fertil Steril. 2011;95(3):915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris JA, Menke MN, Haefner JK, Moniz MH, Perumalswami CR. Geographic access to assisted reproductive technology health care in the United States: a population-based cross-sectional study. Fertil Steril. 2017;107(4):1023–1027. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC), American Society for Reproductive Medicine (ASRM), Society of Assisted Reproductive Technology (SART) 2010 Assisted Reproductive Technology Report: National Summary of Fertility Clinic Reports. Atlanta, GA: U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 6.United States Department of Agriculture (USDA). Dietary Guidelines for Americans 2015-2020. Available at: health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf. Accessed 8 August 2017.
- 7. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6, Suppl):1402S–1406S. [DOI] [PubMed] [Google Scholar]
- 8. Vandermeersch G, Lourenço HM, Alvarez-Muñoz D, Cunha S, Diogène J, Cano-Sancho G, Sloth JJ, Kwadijk C, Barcelo D, Allegaert W, Bekaert K, Fernandes JO, Marques A, Robbens J. Environmental contaminants of emerging concern in seafood—European database on contaminant levels. Environ Res. 2015;143(Pt B):29–45. [DOI] [PubMed] [Google Scholar]
- 9. Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol. 2017;233(3):R109–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bloom MS, Parsons PJ, Kim D, Steuerwald AJ, Vaccari S, Cheng G, Fujimoto VY. Toxic trace metals and embryo quality indicators during in vitro fertilization (IVF). Reprod Toxicol. 2011;31(2):164–170. [DOI] [PubMed] [Google Scholar]
- 11. Rignell-Hydbom A, Axmon A, Lundh T, Jönsson BA, Tiido T, Spano M. Dietary exposure to methyl mercury and PCB and the associations with semen parameters among Swedish fishermen. Environ Health. 2007;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wise LA, Wesselink AK, Tucker KL, Saklani S, Mikkelsen EM, Cueto H, Riis AH, Trolle E, McKinnon CJ, Hahn KA, Rothman KJ, Sørensen HT, Hatch EE. Dietary fat intake and fecundability in two preconception cohort studies. Am J Epidemiol. 2018;187(1):60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mumford SL, Chavarro JE, Zhang C, Perkins NJ, Sjaarda LA, Pollack AZ, Schliep KC, Michels KA, Zarek SM, Plowden TC, Radin RG, Messer LC, Frankel RA, Wactawski-Wende J. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr. 2016;103(3):868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esmaeili V, Shahverdi AH, Moghadasian MH, Alizadeh AR. Dietary fatty acids affect semen quality: a review. Andrology. 2015;3(3):450–461. [DOI] [PubMed] [Google Scholar]
- 15.The Food and Drug Administration (FDA) and the Environmental Protection Agency (EPA). FDA and EPA Issue Final Fish Consumption Advice. 2017. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm537362.htm. Accessed 8 August 2017.
- 16. Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd Barr D, Schrader SM, Kim S, Chen Z, Sundaram R. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE study. Paediatr Perinat Epidemiol. 2011;25(5):413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Scamp S, eds. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1998. [Google Scholar]
- 18. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 19. Buck GM, Vena JE, Schisterman EF, Dmochowski J, Mendola P, Sever LE, Fitzgerald E, Kostyniak P, Greizerstein H, Olson J. Parental consumption of contaminated sport fish from Lake Ontario and predicted fecundability. Epidemiology. 2000;11(4):388–393. [DOI] [PubMed] [Google Scholar]
- 20. Axmon A, Rylander L, Strömberg U, Hagmar L. Female fertility in relation to the consumption of fish contaminated with persistent organochlorine compounds. Scand J Work Environ Health. 2002;28(2):124–132. [DOI] [PubMed] [Google Scholar]
- 21. Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Maisog J, Kim S, Chen Z, Barr DB. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121(2):231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Chen Z, Kim S, Caldwell KL, Barr DB. Heavy metals and couple fecundity, the LIFE study. Chemosphere. 2012;87(11):1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiu YH, Karmon AE, Gaskins AJ, Arvizu M, Williams PL, Souter I, Rueda BR, Hauser R, Chavarro JE; EARTH Study Team . Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod. 2018;33(1):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27(5):1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. 2011;43(1):38–47. [DOI] [PubMed] [Google Scholar]
- 26. Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr. 2014;144(7):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod. 2012;27(11):3328–3336. [DOI] [PubMed] [Google Scholar]
- 28. Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, Steegers-Theunissen RP. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod. 2009;24(6):1304–1312. [DOI] [PubMed] [Google Scholar]
- 29. Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27(10):2899–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cutillas-Tolín A, Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Jørgensen N, Navarrete-Muñoz EM, Torres-Cantero AM, Chavarro JE. Mediterranean and western dietary patterns are related to markers of testicular function among healthy men. Hum Reprod. 2015;30(12):2945–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karayiannis D, Kontogianni MD, Mendorou C, Douka L, Mastrominas M, Yiannakouris N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum Reprod. 2017;32(1):215–222. [DOI] [PubMed] [Google Scholar]
- 32. Braga DP, Halpern G, Setti AS, Figueira RC, Iaconelli A Jr, Borges E Jr. The impact of food intake and social habits on embryo quality and the likelihood of blastocyst formation. Reprod Biomed Online. 2015;31(1):30–38. [DOI] [PubMed] [Google Scholar]
- 33. Hammiche F, Vujkovic M, Wijburg W, de Vries JH, Macklon NS, Laven JS, Steegers-Theunissen RP. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil Steril. 2011;95(5):1820–1823. [DOI] [PubMed] [Google Scholar]
- 34. Robinson JE, Wakelin M, Ellis JE. Increased pregnancy rate with use of the Clearblue Easy Fertility Monitor. Fertil Steril. 2007;87(2):329–334. [DOI] [PubMed] [Google Scholar]
- 35. Papanikolaou Y, Brooks J, Reider C, Fulgoni VL III. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003-2008. Nutr J. 2014;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buck Louis GM. Persistent environmental pollutants and couple fecundity: an overview. Reproduction. 2014;147(4):R97–R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.