Abstract
Context
Disturbed circadian rhythms and sleep quality during pregnancy have been related to gestational weight gain and gestational diabetes mellitus (GDM), which affect postpartum glucose metabolism and future risk of type 2 diabetes.
Objective
We assessed whether the circadian rhythm–related melatonin receptor 1B (MTNR1B) genotype was associated with 1 to 5 years of postpartum glycemic changes among women with a history of GDM and whether gestational weight gain modified such associations.
Design, Settings, and Participants
The established circadian rhythm-associated MTNR1B genetic variant (rs10830963) was genotyped in 1025 Chinese women with a history of GDM. Body weight and glycemic traits, during and after pregnancy, were longitudinally collected.
Main Outcome Measures
The main outcome measure was postpartum glycemic changes.
Results
We found that women carrying different MTNR1B genotypes showed distinct postpartum changes in 2-hour oral glucose tolerance test: 0.36, 0.20, and −0.19 mM per additional copy of the shorter sleep duration-related G allele in women with inadequate, adequate, and excessive gestational weight gain, respectively (for interaction, P = 0.028). The corresponding changes in fasting glucose were 0.14, 0.13, and 0.01 mM, although the modification effect of gestational weight gain on the genetic association was marginally significant (for interaction, P = 0.067).
Conclusions
Our findings suggest that gestational weight gain may modify the circadian rhythm–related MTNR1B genetic variant on long-term glycemic changes, highlighting the significance of gestational weight management in diabetes prevention among women with GDM.
Gestational weight gain might modify the effect of the circadian rhythm–related MTNR1B rs10830963 variant on postpartum changes in 2-hour OGTT, and vice versa, among women with a history of GDM.
Gestational diabetes mellitus (GDM), a state of impaired glucose tolerance during pregnancy, is one of the most frequent pregnancy complications associated with postpartum glucose metabolism and future risk of type 2 diabetes (1). Pregnancy may disturb circadian rhythms and therefore, affect sleep quality (2). Emerging data have shown that sleep alterations during pregnancy were associated with greater weight gain (3, 4) and an increased risk for GDM (5, 6).
Circadian rhythms and sleep quality are closely regulated by melatonin, a key hormone determining the sleep-wake cycle in humans (7). Melatonin receptor 1B (MTNR1B) is one of the seven transmembrane G-protein-coupled melatonin receptors expressed in the central nervous system and in the peripheral tissues, including pancreatic β-cells (8). The MTNR1B gene encodes one of the two high-affinity receptors of melatonin (9), which has been implicated in regulation of circadian rhythms. A variant in the MTNR1B gene, rs10830963, was recently identified to be associated with altered melatonin rhythm and melatonin signaling (10, 11). Of note, the MTNR1B genotype has also been associated with obesity, fasting glucose levels, type 2 diabetes, and GDM in genome-wide association studies (12, 13). Thus, we hypothesized that the MTNR1B genotype may affect postpartum glycemic changes in women with GDM.
In this study of a large cohort of women with a history of GDM, we examined the associations of the circadian rhythm–related MTNR1B genotype with postpartum changes in measures of glycemic traits. In addition, we particularly assessed the interactions between the MTNR1B genotype and gestational weight gain on the glycemic changes.
Subjects and Methods
Study population
The current study was a retrospective analysis based on the baseline survey of the Tianjin Gestational Diabetes Mellitus Prevention Program, an ongoing, randomized clinical trial among Han Chinese women with a history of GDM. The study protocol was approved by the Human Subjects Committee of the Tianjin Women’s and Children’s Health Center, and written consent was obtained from all participants at the baseline survey. Details of methodological issues have been described previously (14–16). In brief, all pregnant women in six main areas of Tianjin, China, who had a diagnosis of GDM at 26 to 30 gestational weeks between 2005 and 2009 were invited to participate in a postpartum survey from August 2009 to July 2011 (n = 4644). The World Health Organization criteria for GDM (17) were adopted in the current study. Of the 4644 women who participated in the postpartum survey at 1 to 5 years after delivery, women who could not be contacted, refused, or did not meet the eligibility criteria were excluded (n = 3381). Chronic diseases that could seriously reduce life expectancy were excluded in the baseline survey of the Tianjin GDM Prevention Program because these diseases might affect weight change, and the patients might take medicines that affected the metabolic outcomes of interest. Other criteria for exclusion were the following: age <20 or ≥50 years, taking medicines known to alter 2-hour oral glucose tolerance test (OGTT), pregnant during the follow-up period, or unable to give informed consent. Thus, a total of 1263 women with a history of GDM completed the postpartum survey (participation rate 27%). There were no differences in 2-hour OGTT concentration, fasting glucose concentration, and the prevalence of impaired glucose tolerance and diabetes at 26 to 30 gestational weeks among women who participated in the postpartum survey and those who did not (15). In addition, we further excluded 238 women without genotype information, and these women were not different in clinical characteristics and biochemical markers from those included in the final analysis. After these exclusions, a total of 1025 women were included in the current study.
Measurements of weight change and other variables
Data on medical eligibility and lifestyle measures were collected by self-administered questionnaires. The questionnaire inquired about socio-demographic information, history of GDM, medical history (pregnancy hypertension, diabetes, hypertension, and hypercholesterolemia), family history of diseases (diabetes, hypertension, stroke, and cancer), smoking, alcohol intake, physical activity, sleeping, stress, and use of supplements. Dietary intake data were collected by means of a 3-day, 24-hour food record. The questionnaires and the 3-day, 24-hour food record have been validated in the China National Nutrition and Health Survey (18, 19). Body weight and height had been collected longitudinally based on medical records before the baseline survey of the Tianjin Gestational Diabetes Mellitus Prevention Program. Postpartum weight was measured on the day of delivery, and prepregnancy and current body mass index (BMI; in kilograms per square meter) were calculated (Supplemental Fig. 1). The 2009 Institute of Medicine guideline was used to classify gestational weight gain into inadequate, adequate, and excessive (20). Adequate gestational weight gain was defined by prepregnancy BMI as follows: 12.5 to 18 kg if prepregnancy BMI < 18.5 kg/m2, 11.5 to 16 kg if prepregnancy BMI = 18.5 to 24.9 kg/m2, 7 to 11.5 kg if prepregnancy BMI = 25.0 to 29.9 kg/m2, and 5 to 9 kg if prepregnancy BMI ≥ 30 kg/m2. Gestational weight gain below or above the recommendation was defined as inadequate or excessive, respectively.
Measurements of glycemic traits
Changes in glycemic traits were calculated as difference in glucose and hemoglobin A1c (HbA1c) levels between postpartum 1 to 5 years (at the postpartum survey) and pregnancy. The glycemic traits were measured in plasma both during the pregnancy (26 to 30 gestational weeks, the time point when the women were diagnosed with GDM) and at the postpartum survey (Supplemental Fig. 1). Blood samples were collected from all participants after an overnight fast of at least 12 hours. Fasting glucose and 2 hour OGTT were measured using an automatic analyzer (TBA-120FR; Toshiba, Tokyo, Japan). Glycated HbA1c was measured by using automatic glycohemoglobin analyzer (ADAMS A1c HA-8160; Arkray, Kyoto, Japan).
Genotyping
DNA was extracted from the buffy coat fraction of centrifuged blood using a QIAamp Blood Maxi Kit (Qiagen, Chatsworth, CA). The MTNR1B single nucleotide polymorphism rs10830963 was genotyped by a quantitative real-time TaqMan PCR (Applied Biosystems, Foster City, CA). The success rate of genotyping was >98%. Replicated quality control samples (10%) were conducted with >99% concordance.
Statistical analysis
The Hardy-Weinberg equilibrium of the genotype and comparison of categorical variables were assessed by a χ2 test. Differences in continuous variables by gestational weight gain were tested by using general linear models. Changes in glycemic traits associated with each additional copy of the MTNR1B rs10830963 G allele by gestational weight gain categories were estimated using general linear models. Statistical adjustments were made for age, follow-up time, prepregnancy BMI, dietary fat (percent energy), sitting time, postpartum weight change, the level of corresponding glucose trait (continuous variable), family history of diabetes (yes or no), current smoking (yes or no), current alcohol drinking (yes or no), leisure-time physical activity (0, <30, or ≥30 minutes per day), and GDM therapy (yes or no). The interaction between MTNR1B rs10830963 genotype and gestational weight gain was tested by the introduction of a product term for these variables in the model. We also calculated the multivariable-adjusted mean values of changes in glycemic traits according to gestational weight gain and the MTNR1B rs10830963 genotype by use of general linear models. Two-sided P < 0.05 was considered as statistically significant. Statistical analyses were calculated using SAS version 9.4 (SAS Institute, Cary, NC).
Results
The frequency of the circadian rhythm–related MTNR1B rs10830963 G allele was 45%, and genotype distribution was in accordance with the Hardy-Weinberg equilibrium (P = 0.41). Table 1 shows the characteristics of women with a history of GDM, according to the MTNR1B rs10830963 genotype. The MTNR1B genotype was not associated with any glycemic traits (fasting glucose, 2-hour OGTT, and HbA1c) during pregnancy but had a positive association with postpartum fasting glucose levels (P < 0.03). The frequency of the three categories of gestational weight gain was not different by the MTNR1B genotype.
Table 1.
Characteristics of Participants With a History of GDM by MTNR1B rs10830963 Genotype
| MTNR1B rs10830963 Genotypea | ||||
|---|---|---|---|---|
| CC (n = 313) | CG (n = 495) | GG (n = 217) | P Valueb | |
| Age, y | 32.3 ± 3.7 | 32.4 ± 3.5 | 32.0 ± 3.3 | 0.37 |
| Follow-up time, y | 2.0 ± 0.6 | 2.1 ± 0.7 | 2.0 ± 0.6 | 0.95 |
| Prepregnancy BMI, kg/m2 | 23.2 ± 3.4 | 23.1 ± 3.2 | 23.2 ± 3.5 | 0.91 |
| Weight, kg | ||||
| Prepregnancy | 60.1 ± 9.3 | 59.3 ± 9.1 | 59.5 ± 9.6 | 0.44 |
| Postpartum | 62.9 ± 11.0 | 62.2 ± 10.9 | 62.3 ± 10.7 | 0.54 |
| Gestational weight gain, % | ||||
| Inadequate | 11.8 | 15.2 | 13.8 | 0.31 |
| Adequate | 32.6 | 33.9 | 28.1 | |
| Excessive | 55.6 | 50.9 | 58.1 | |
| Fasting glucose, mM | ||||
| At GDM diagnosis | 5.3 ± 0.8 | 5.3 ± 0.8 | 5.4 ± 0.8 | 0.06 |
| Postpartum | 5.3 ± 1.0 | 5.4 ± 0.9 | 5.5 ± 1.0 | 0.03 |
| 2-hour OGTT, mM | ||||
| At GDM diagnosis | 9.2 ± 1.3 | 9.1 ± 1.2 | 9.1 ± 1.1 | 0.09 |
| Postpartum | 7.1 ± 2.4 | 7.0 ± 2.3 | 7.1 ± 2.8 | 0.94 |
| HbA1c, % | ||||
| At GDM diagnosis | 5.8 ± 0.6 | 5.8 ± 0.6 | 5.8 ± 0.6 | 0.54 |
| Postpartum | 5.6 ± 0.9 | 5.6 ± 0.7 | 5.7 ± 0.7 | 0.80 |
| Family history of diabetes, % | 31.6 | 33.7 | 24.5 | 0.46 |
| Current smoking, % | 1.0 | 2.2 | 2.3 | 0.37 |
| Current alcohol drinking, % | 20.4 | 20.2 | 21.2 | 0.95 |
| Leisure-time physical activity, % | ||||
| 0 min/d | 79.9 | 80.2 | 80.6 | 0.16 |
| <30 min/d | 19.2 | 18.8 | 16.1 | |
| ≥30 min/d | 1.0 | 1.0 | 3.2 | |
| Sitting time, h/d | 3.2 ± 2.2 | 3.3 ± 2.1 | 3.2 ± 2.2 | 0.69 |
| Fat, % energy | 33.6 ± 6.7 | 33.4 ± 6.4 | 33.4 ± 5.6 | 0.26 |
| GDM therapy, % | 15.3 | 13.3 | 14.8 | 0.73 |
Data are presented as means ± standard deviation and percentage for continuous and categorical variables, respectively.
Based on general linear models or χ2 test for continuous and categorical variables, respectively.
When all women were analyzed together, no association was found between the MTNR1B genotype and postpartum glycemic changes (all P ≥ 0.08). We then performed a stratified analysis to examine the associations of the MTNR1B genotype with 1 to 5 years of postpartum glycemic changes, according to gestational weight gain categories (Table 2). Gestational weight gain significantly modified the association of the MTNR1B genotype with postpartum changes in 2-hour OGTT. After multivariable adjustment, changes in 2-hour OGTT, associated with each additional copy of the risk G allele, were 0.36, 0.20, and −0.19 mM in women with inadequate, adequate, and excessive gestational weight gain, respectively (for interaction, P = 0.028). Gestational weight gain had a marginally significant effect modification on the genetic associations of postpartum changes in fasting glucose levels after multivariable adjustment but had no significant effect modification on the genetic associations of postpartum changes in HbA1c. Each additional copy of the risk G allele was associated with changes in fasting glucose of 0.14, 0.13, and 0.01 mM across categories of inadequate, adequate, and excessive gestational weight gain, respectively (for interaction, P = 0.069), and the corresponding changes in HbA1c were −0.02%, 0.12%, and −0.05% (for interaction, P = 0.143).
Table 2.
Changes in Glycemic Traits Associated With Each Additional Copy of the MTNR1B rs10830963 G Allele by Gestational Weight Gain
| Inadequate | Adequate | Excessive | P Interaction | ||||
|---|---|---|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | ||
| Fasting glucose, mM | |||||||
| Age-adjusted | 0.03 (0.16) | 0.87 | 0.14 (0.08) | 0.08 | – 0.04 (0.06) | 0.49 | 0.559 |
| Multivariable adjusteda | 0.14 (0.17) | 0.41 | 0.13 (0.06) | 0.02 | 0.01 (0.05) | 0.83 | 0.069 |
| 2-hour OGTT, mM | |||||||
| Age-adjusted | 0.32 (0.34) | 0.36 | 0.33 (0.20) | 0.10 | – 0.11 (0.13) | 0.40 | 0.046 |
| Multivariable adjusteda | 0.36 (0.33) | 0.28 | 0.20 (0.17) | 0.25 | – 0.19 (0.11) | 0.09 | 0.028 |
| HbA1c, % | |||||||
| Age-adjusted | – 0.11 (0.12) | 0.34 | 0.19 (0.07) | 0.005 | – 0.04 (0.05) | 0.49 | 0.602 |
| Multivariable adjusteda | – 0.02 (0.12) | 0.82 | 0.12 (0.05) | 0.02 | – 0.05 (0.04) | 0.22 | 0.143 |
β represents the change in each glycemic trait per additional copy of the rs10830963 G allele.
Abbreviation: SE, standard error.
Adjustment for age, follow-up time, prepregnancy BMI, dietary fat (percent energy), sitting time, postpartum weight change, the level of corresponding glucose trait (continuous variable), family history of diabetes, current smoking, current alcohol drinking, leisure-time physical activity, and GDM therapy (categorical variables).
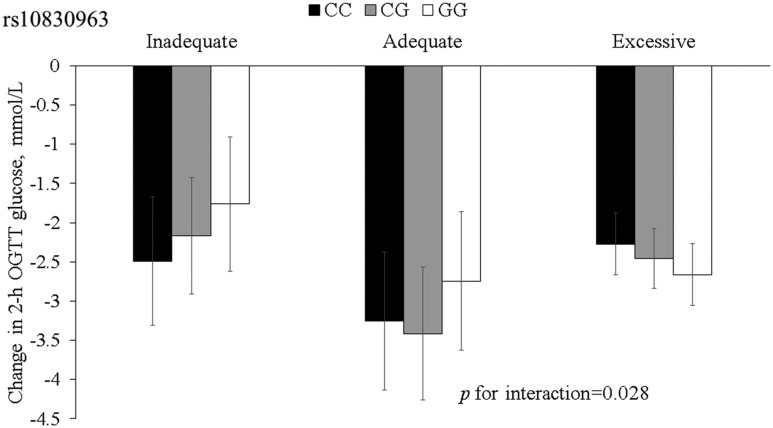
The mean value (standard deviation) of postpartum changes of 2-hour OGTT in all participants was −2.11 (2.44) mM. Figure 1 presents multivariable-adjusted postpartum changes of 2-hour OGTT stratified by gestational weight gain and the MTNR1B genotype. An increasing number of the G alleles were associated with a decrease in 2-hour OGTT levels in women with excessive gestational weight gain, whereas an opposite directional association was found among women with inadequate or adequate gestational weight gain.
Figure 1.
Changes in 2-hour OGTT, according to gestational weight gain and the MTNR1B rs10830963 genotype. Black bars, CC genotype; gray bars, CG genotype; white bars, GG genotype. Data are means ± standard error values, adjusted for age, follow-up time, prepregnancy BMI, dietary fat (percent energy), postpartum weight change, sitting time, level of the corresponding glucose trait during the pregnancy (continuous variables), family history of diabetes, current smoking, current alcohol drinking, leisure-time physical activity, and GDM therapy (categorical variables).
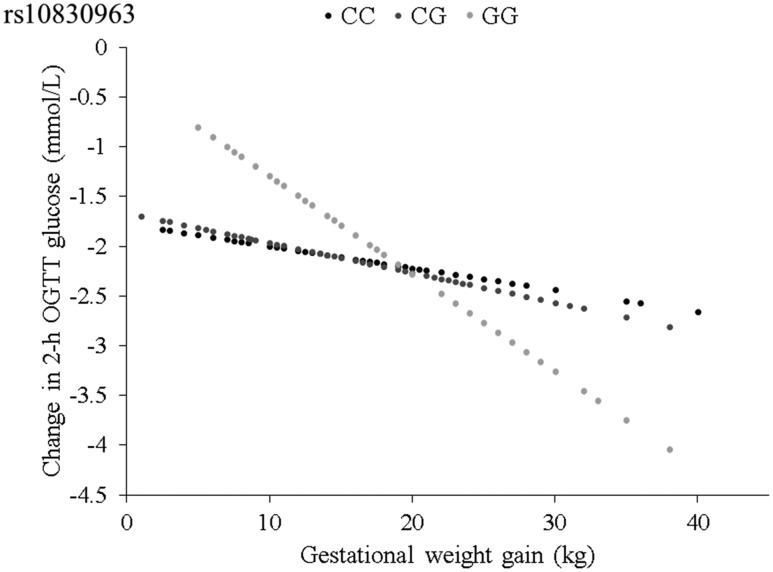
We also analyzed the association between 2-hour OGTT and gestational weight gain, according to the MTNR1B genotype. Figure 2 shows the predicted changes in 2-hour OGTT with gestational weight gain (per 1 kg) by the MTNR1B genotype. Gestational weight gain was associated with a greater postpartum reduction of 2-hour OGTT in women carrying the MTNR1B rs10830963 GG genotype (β = −0.10 mM, P = 0.001) than that in women carrying the CC or CG genotype (β = −0.02 mM, −0.03 mM, respectively; P = 0.34, 0.11, respectively).
Figure 2.
Predicted changes in 2-hour OGTT, according to gestational weight gain by rs10830963. The slope represents the β coefficient.
Discussion
In this study of Chinese women with a history of GDM, we found bidirectional interactions between the circadian rhythm–related MTNR1B genetic variant and gestational weight gain on 1- to 5-year postpartum changes in 2-hour OGTT. The MTNR1B genotype was related to distinct postpartum changes in 2-hour OGTT in women with inadequate, adequate, and excessive gestational weight gain. In addition, women carrying the MTNR1B GG genotype showed a more pronounced relationship between gestational weight gain and improvement in 2-hour OGTT than women carrying the other genotypes.
Numerous studies suggest that gestational weight gain plays an important role in women’s long-term weight trajectory and affects postpartum hyperglycemia and type 2 diabetes in later life, especially among those with a history of GDM (21, 22). This study assesses the effect of gestational weight gain on the association between the circadian rhythm–related MTNR1B genotype and postpartum glycemic changes among women with a history of GDM. A unique finding in the current study is that the relation between gestational weight gain and postpartum changes in 2-hour OGTT significantly differed by the MTNR1B rs10830963 genotype. Insulin plays a central role in regulation of glucose metabolism. Both in vivo and in vitro, insulin secretion by the pancreatic islets is in a circadian manner, as a result of the melatonin action on the melatonin receptors inducing a phase shift in the cells (23). The genetic variant in the MTNR1B gene may directly affect the circadian manner of insulin secretion by the pancreatic islets and subsequently affect glucose metabolism (8, 24). The modification of gestational weight gain on the association between the MTNR1B and the postpartum changes in 2-hour OGTT did not exclude the existence of the potential direct genetic effect; instead, our data suggest that the genetic effect might differ among women with distinct gestational weight gain. Our findings are biologically plausible, as gestational weight gain may also disturb the circadian manner of insulin secretion (3, 4)—the pathway where interaction between gestational weight gain and the genotype may occur.
We found that the risk G allele of the MTNR1B variant was associated with increased 2-hour OGTT levels among women with inadequate or adequate gestational weight gain but was related to the decreased 2-hour OGTT levels among women with excessive gestational weight gain. Such an opposite genetic effect could be fairly explained by the differential-susceptibility hypothesis, a theory suggesting that genes may be conceptualized as plastic because genetic risk can be modified by environmental factors, such as change in body weight (25, 26). Previous studies suggested that the risk G allele was associated with the outcome of an intervention that targeted weight loss (27), whereas only those not carrying the risk G allele benefited from the lifestyle intervention among women at high risk of GDM (28). We assume that the magnitude of gestational weight gain may differently affect expression or activity of the MTNR1B genetic variant and subsequently affect glucose metabolism during the postpartum period.
Intriguingly, we found that the relation between gestational weight gain and postpartum glycemic changes differed according to the MTNR1B genotype—women carrying the GG genotype showed a stronger association of reduction in 2-hour OGTT changes with gestational weight gain than women carrying other genotypes. In a recall by genotype of the MTNR1B study, Tuomi et al. (11) demonstrated that the effect of melatonin on insulin secretion was inhibitory and in a genotype-specific fashion. For example, measures of insulin secretion were lower in the GG carriers of the MTNR1B genotype after 3 months of melatonin treatment, whereas the metabolic consequences of melatonin treatment in CC carriers were modest and restricted to fasting plasma glucose levels (11). On the other hand, Grotenfelt et al. (28) found that women carrying the CC genotype of the MTNR1B had a lower risk of GDM in response to a lifestyle intervention than women carrying other genotypes. Further studies are warranted to explore the potential mechanisms underlying the different effects of the MTNR1B genotypes on glucose metabolism.
A major strength of our study is the repeated measures of weight and glycemic traits during and after pregnancy. Other strengths include the large sample size of women with a prior GDM and longitudinal analysis. However, a number of limitations should be noted. First, the measurement of clock parameters was not done in this study. We used a genetic marker related to circadian rhythm that could be a better marker than biomarkers in causal inference, according to the Mendelian randomization theory (29). Second, even though the current study focused on women with a history of GDM, we acknowledged that it was also of interest to assess the relation between the MTNR1B genotypes and glucose metabolism in women without GDM, which were unfortunately not available in our study samples, and future studies are warranted to verify our findings in women without GDM. Third, we did not collect sufficient reproductive data, such as postpartum depression, which may influence weight change and glucose metabolism. In addition, we acknowledged that exclusions might affect the presence of the association; however, there were no differences between the women with GDM at 26 to 30 gestational weeks who participated and those who did not, with regard to age, fasting glucose, 2-hour OGTT concentrations, and the prevalence of impaired glucose tolerance and diabetes. Although the results of our study might not be generalized to other populations, future studies in different populations are warranted.
In summary, the results of the current study showed that gestational weight gain might modify the effect of the circadian rhythm–related MTNR1B rs10830963 variant on postpartum changes in 2-hour OGTT, and vice versa, among Chinese women with a history of GDM. These findings suggest that women carrying the GG genotype might particularly benefit by controlling gestational weight gain to avoid postpartum hyperglycemia.
Supplementary Material
Acknowledgments
We thank all participants of the study for their dedication and contribution to the research.
Financial Support: The study was supported by grants from the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly Program for Collaborative Research between China and Europe, the National Natural Science Foundation of China (Grant no. 81502821), and the Tianjin Public Health Bureau. L.Q. is supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, and HL126024); National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, and DK078616); and the Boston Obesity Nutrition Research Center (DK46200). G.H. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790) and the National Institute of General Medical Sciences (U54GM104940).
Author Contributions: H.N. conceived of the study and design, performed the statistical analysis, interpreted the data, and drafted and critically revised the manuscript. K.H.T.Q., J.L., T.Z., H.L., W.L., L.W., N.L., and G.H. were involved in the critical revision of the manuscript. G.H. and L.Q. were involved in collection and assembly of data and obtained funding for the study. L.Q. conceived of the study and design, provided statistical advice, interpreted the data, critically revised the manuscript, is the guarantor of the present work, and takes responsibility for the integrity of the contents of the article.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- GDM
gestational diabetes mellitus
- HbA1c
hemoglobin A1c
- MTNR1B
melatonin receptor 1B
- OGTT
oral glucose tolerance test
References
- 1. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. [DOI] [PubMed] [Google Scholar]
- 2. Sedov ID, Cameron EE, Madigan S, Tomfohr-Madsen LM. Sleep quality during pregnancy: a meta-analysis. Sleep Med Rev. 2018;38:168–176. [DOI] [PubMed] [Google Scholar]
- 3. Gunderson EP, Rifas-Shiman SL, Oken E, Rich-Edwards JW, Kleinman KP, Taveras EM, Gillman MW. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol. 2008;167(2):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Gunderson EP, Stuebe AM, Mantzoros CS. Association of maternal short sleep duration with adiposity and cardiometabolic status at 3 years postpartum. Obesity (Silver Spring). 2011;19(1):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Keeffe M, St-Onge MP. Sleep duration and disorders in pregnancy: implications for glucose metabolism and pregnancy outcomes. Int J Obes (Lond). 2013;37(6):765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reutrakul S, Anothaisintawee T, Herring SJ, Balserak BI, Marc I, Thakkinstian A. Short sleep duration and hyperglycemia in pregnancy: aggregate and individual patient data meta-analysis [published online ahead of print October 5, 2017]. Sleep Med Rev. doi: 10.1016/j.smrv.2017.09.003. [DOI] [PubMed]
- 7. Gandhi AV, Mosser EA, Oikonomou G, Prober DA. Melatonin is required for the circadian regulation of sleep. Neuron. 2015;85(6):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44(1):26–40. [DOI] [PubMed] [Google Scholar]
- 9. Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA. 1995;92(19):8734–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lane JM, Chang AM, Bjonnes AC, Aeschbach D, Anderson C, Cade BE, Cain SW, Czeisler CA, Gharib SA, Gooley JJ, Gottlieb DJ, Grant SFA, Klerman EB, Lauderdale DS, Lockley SW, Munch M, Patel S, Punjabi NM, Rajaratnam SMW, Rueger M, St Hilaire MA, Santhi N, Scheuermaier K, Van Reen E, Zee PC, Shea SA, Duffy JF, Buxton OM, Redline S, Scheer FA, Saxena R. Impact of common diabetes risk variant in MTNR1B on sleep, circadian, and melatonin physiology. Diabetes. 2016;65(6):1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tuomi T, Nagorny CLF, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Östman B, Söderström J, Pesonen AK, Martikainen S, Räikkönen K, Forsén T, Hakaste L, Almgren P, Storm P, Asplund O, Shcherbina L, Fex M, Fadista J, Tengholm A, Wierup N, Groop L, Mulder H. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016;23(6):1067–1077. [DOI] [PubMed] [Google Scholar]
- 12. Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJF, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJC, Dehghan A, Deloukas P, Doney ASF, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orrù M, Palmer CNA, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JCM, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, Sparsø T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chèvre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jørgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Lévy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41(1):89–94. [DOI] [PubMed] [Google Scholar]
- 14. Hu G, Tian H, Zhang F, Liu H, Zhang C, Zhang S, Wang L, Liu G, Yu Z, Yang X, Qi L, Zhang C, Wang H, Li M, Leng J, Li Y, Dong L, Tuomilehto J. Tianjin Gestational Diabetes Mellitus Prevention Program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract. 2012;98(3):508–517. [DOI] [PubMed] [Google Scholar]
- 15. Li W, Zhang S, Liu H, Wang L, Zhang C, Leng J, Yu Z, Yang X, Tian H, Hu G. Different associations of diabetes with β-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care. 2014;37(9):2533–2539. [DOI] [PubMed] [Google Scholar]
- 16. Wang L, Liu H, Zhang S, Leng J, Liu G, Zhang C, Li WQ, Li N, Li W, Li Y, Sun S, Yu Z, Yang X, Hu G. Obesity index and the risk of diabetes among Chinese women with prior gestational diabetes. Diabet Med. 2014;31(11):1368–1377. [DOI] [PubMed] [Google Scholar]
- 17. Consultation WHO. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; 1999. [Google Scholar]
- 18. Li YP, He YN, Zhai FY, Yang XG, Hu XQ, Zhao WH, Ma GS. [Comparison of assessment of food intakes by using 3 dietary survey methods]. Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40(4):273–280. [PubMed] [Google Scholar]
- 19. Ma G, Luan D, Li Y, Liu A, Hu X, Cui Z, Zhai F, Yang X. Physical activity level and its association with metabolic syndrome among an employed population in China. Obes Rev. 2008;9(s1, Suppl 1);113–118. [DOI] [PubMed] [Google Scholar]
- 20. Institute of Medicine Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academy Press; 2009. [PubMed] [Google Scholar]
- 21. Liu H, Zhang C, Zhang S, Wang L, Leng J, Liu D, Fang H, Li W, Yu Z, Yang X, Dong L, Hu G. Prepregnancy body mass index and weight change on postpartum diabetes risk among gestational diabetes women. Obesity (Silver Spring). 2014;22(6):1560–1567. [DOI] [PubMed] [Google Scholar]
- 22. Kew S, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, Retnakaran R. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care. 2014;37(7):1998–2006. [DOI] [PubMed] [Google Scholar]
- 23. Sharma S, Singh H, Ahmad N, Mishra P, Tiwari A. The role of melatonin in diabetes: therapeutic implications. Arch Endocrinol Metab. 2015;59(5):391–399. [DOI] [PubMed] [Google Scholar]
- 24. Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14(8):746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885–908. [DOI] [PubMed] [Google Scholar]
- 27. Mirzaei K, Xu M, Qi Q, de Jonge L, Bray GA, Sacks F, Qi L. Variants in glucose- and circadian rhythm-related genes affect the response of energy expenditure to weight-loss diets: the Pounds Lost Trial. Am J Clin Nutr. 2014;99(2):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grotenfelt NE, Wasenius NS, Rönö K, Laivuori H, Stach-Lempinen B, Orho-Melander M, Schulz CA, Kautiainen H, Koivusalo SB, Eriksson JG. Interaction between rs10830963 polymorphism in MTNR1B and lifestyle intervention on occurrence of gestational diabetes. Diabetologia. 2016;59(8):1655–1658. [DOI] [PubMed] [Google Scholar]
- 29. Qi L. Mendelian randomization in nutritional epidemiology. Nutr Rev. 2009;67(8):439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.