Abstract
Purpose
Natriuretic peptides (NPs) negatively feedback on the renin-angiotensin-aldosterone system (RAAS) and play a critical role in preserving cardiac structure and maintaining metabolic homeostasis. Well-treated HIV-infected individuals are at risk for fat redistribution and demonstrate evidence of RAAS dysregulation, which relates to metabolic dysfunction. We investigated circulating NPs in relation to RAAS physiology and metrics of body composition in HIV.
Methods
We assessed atrial natriuretic peptide, brain natriuretic peptide (BNP), and amino terminal pro B-type natriuretic peptide (NT-proBNP) during acute activation of the RAAS using a low-sodium controlled diet among 20 HIV-infected and 10 non–HIV-infected individuals well phenotyped for body composition.
Results
BNP was significantly lower [median, 60 (interquartile range, 44, 152) pg/mL vs 196 (91, 251) pg/mL, respectively; P = 0.04], and serum aldosterone was higher, among HIV-infected than among non–HIV-infected individuals. BNP was significantly and inversely associated with body composition [waist circumference: r = −0.46 (P = 0.04); BMI: r = −0.55 (P = 0.01); body adiposity index: r = −0.49 (P = 0.03)], metabolic indices [total cholesterol: r = −0.44 (P = 0.05), insulin resistance calculated by using homeostatic model assessment: r = −0.44 (P = 0.05); mean arterial pressure: r = −0.44 (P = 0.05)], and serum aldosterone (r = −0.49; P = 0.03) among the HIV-infected group. These relationships were not demonstrated in the non–HIV-infected group. In a four-group comparison stratifying by HIV serostatus and above or below a body mass index (BMI) of 25 kg/m2, BNP decreased significantly across groups; it was highest in non–HIV-infected patients with a BMI <25 kg/m2 and lowest in HIV-infected patients with a BMI ≥25 kg/m2 (overall P = 0.01).
Conclusion
Relatively reduced NP, particularly BNP, among HIV-infected individuals with excess adiposity may contribute to reduced suppression of aldosterone and potentially drive aldosterone-mediated metabolic complications. Strategies that target RAAS blockade and/or augment NPs may be useful to reduce cardiometabolic disease among HIV-infected individuals in whom these systems are perturbed.
After a 6-day low-sodium diet to activate the RAAS, BNP was lower in HIV- vs non–HIV-infected patients and inversely related to increased BMI and other metabolic indices in HIV.
Cardiac natriuretic peptides (NPs) are important neurohormones that serve as negative regulators of the renin-angiotensin-aldosterone system (RAAS). Together, these two hormone systems are integral to maintaining cardiovascular homeostasis. The obese population demonstrates reduced cardiac NPs relative to the nonobese population. This decrease in NPs in the obese population has been attributed to excess adiposity and may provide an underlying mechanism for the demonstrable advanced metabolic risk in this group.
Data suggest that the RAAS has an emerging role in adipose biology and may contribute to insulin resistance, inflammation, and cardiovascular disease (CVD) in states of adipose dysfunction. We have previously reported that HIV-infected individuals with a clinical phenotype of excess adiposity have increased aldosterone during an RAAS-activated state. Furthermore, during conditions of RAAS activation, stimulated with low dietary sodium, increased aldosterone is associated with insulin resistance as well as markers of generalized inflammation and immune activation among the HIV population (1, 2). HIV-infected individuals are at increased risk for fat redistribution and subsequent metabolic consequences, and the exact mechanism linking adipose dysfunction to cardiometabolic disease in HIV remains unclear.
Thus, we hypothesized that, on the basis of known cardiac NP physiology in the obese population, relatively reduced cardiac NPs during a RAAS-activated state would be associated with unfavorable body composition and metabolic indices among the HIV-infected population, a model of acquired fat redistribution (Fig. 1). No studies to date have used rigorous physiologic techniques to comprehensively evaluate RAAS and NP system feedback in HIV. Indeed, these data expand our knowledge of the interaction between these two relevant hormone systems with respect to HIV status, as well as body composition.
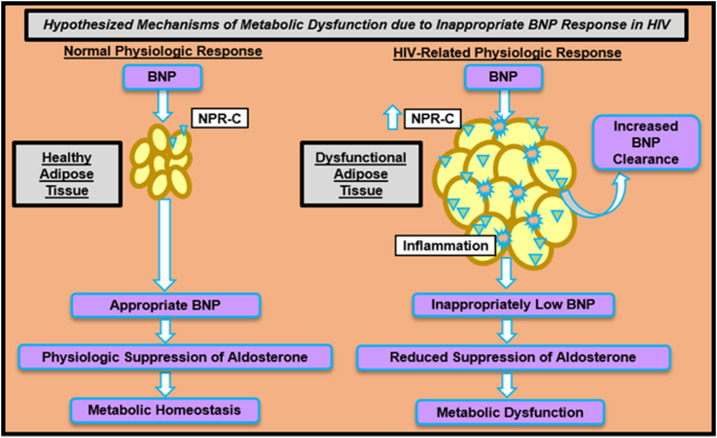
Figure 1.
Hypothesized mechanisms of metabolic dysfunction via inappropriate cardiac natriuretic peptide feedback among HIV-infected individuals. HIV-infected individuals with a clinical phenotype of excessive adiposity demonstrate increased aldosterone during an RAAS-activated state. BNP is a negative regulator of RAAS. Dysfunctional adipose tissue is highly inflamed and may be linked to upregulation of NPR-C. When bound to NPR-C, BNP is degraded and cleared. Relatively lower BNP may permit an exaggerated increase in aldosterone and could be detrimental to preventing metabolic dysfunction. Appropriate BNP response among individuals with healthy adipose tissue may contribute to maintenance of metabolic homeostasis through physiologic suppression of aldosterone. Triangular structures represent NPR-C and star-like structures represent the inflammatory milieu.
Methods
Study participants
Twenty HIV-infected individuals were recruited from Boston-area HIV clinics and community outreach centers and through local advertisements. In addition, 10 non–HIV-infected persons were recruited from the same Boston-area communities. Study criteria for inclusion and exclusion were identical regardless of serostatus (1). Each group was chosen to be of similar age (18 to 65 years), sex, and waist circumference. HIV-infected individuals were required to be receiving stable antiretroviral therapy (ART) regimens for 3 months or longer. Individuals with any known history of CVD (including congestive heart failure), hypertension (blood pressure >140/90 mm Hg), or diabetes mellitus were excluded. Additional exclusion criteria were current antihypertensive or antidiabetic medication use; active tobacco use; steroid, estrogen, progesterone, growth hormone, or growth hormone-releasing hormone use within the past 3 months; and pregnancy. Laboratory criteria for exclusion included urine protein >1 g/d and creatinine >1.5 mg/dL, potassium >5.5 mEq/L, and alanine aminotransferase >2.5 times the upper limit of normal. Institutional review board approval was received by the Partners Human Research Committee to perform this study, and individual informed consent was obtained.
Body composition phenotyping
Body mass index (BMI) was calculated at the time of study enrollment by using height and weight. The iliac crest served as the anatomic reference to standardize waist circumference measurements. Body adiposity index (BAI) was calculated as (hip circumference taken at the broadest location/height1.5) − 18 (3). Magnetic resonance images were acquired by using an axial T1-weighted, fat-suppressed pulse sequence at the L4 vertebral body. Following image acquisition, an offline analysis of tracings was performed by using commercial software (Vitrak; eFilm/Merge, Chicago, IL) to quantitate abdominal visceral adipose tissue and subcutaneous adipose tissue area.
Standardized low-sodium diets to stimulate RAAS activation
After the baseline visit, a standardized 6-day low-sodium diet (10 ± 2 mEq Na+, 100 ± 2 mEq K+, and 1000 ± 50 mg Ca2+) was prepared by the Brigham and Women's Hospital Center for Clinical Investigation Metabolic Phenotyping Core to stimulate RAAS activation. Low sodium balance was achieved if the urine sodium was estimated to be <50 mEq/24 hours during the visit. A formal 24-hour urine sodium collection was obtained at the visit and paired with measurement of the urine creatinine to assess adequacy of the sampling.
Cardiac NP and RAAS characterization
Serum atrial natriuretic peptide (ANP; sensitivity, 1.02 pg/mL; intraassay variability <10%; interassay variability <15%) and brain natriuretic peptide (BNP; sensitivity, 1.66 pg/mL; intraassay variability <10%; interassay variability <15%) were measured via enzyme-linked immunosorbent assay (Ray Biotech, Norcross, GA). Serum amino terminal pro B-type NP (NT-proBNP), (sensitivity, 5 pg/mL) was measured by using the Cobas electrochemiluminescence immunoassay technique (Roche Diagnostics, Indianapolis, IN) through a clinical diagnostic laboratory. Data evaluating RAAS activation in relation to visceral fat among the same participants were reported in a prior publication (1). Data assessing cardiac neurohormones have not previously been analyzed. Serum aldosterone (sensitivity, 2.5 ng/dL; precision, 4% to 10%) was measured by solid-phase radioimmunoassay by the Coat-A-Count method (Diagnostics Products Corp, Los Angeles, CA). Plasma renin activity (PRA) was assayed by using the GammaCoat (125I) radioimmunoassay kit (sensitivity, 0.01 ng/mL/h; precision <10%; DiaSorin, Brea, CA). Blood collection for cardiac neurohormones, RAAS parameters, and all other metabolic indices were obtained after participants underwent a 12-hour fast following the 6-day standardized low-sodium diet. Comparative baseline BNP measures were also obtained during an ad libitum sodium diet. In addition, participants were asked to lie supine overnight to standardize posture effects for all laboratories.
Metabolic and HIV-related parameters
Creatinine, potassium, glucose, and total cholesterol were measured using standard techniques. Insulin was assayed with the Access immunoassay system (Beckman Coulter). Insulin resistance was calculated by using the homeostatic model assessment (HOMA-IR) as follows: [fasting glucose (mg/dL) × fasting insulin (mU/L)]/405. HIV testing was performed by enzyme-linked immunosorbent assay and confirmed by Western blot. HIV viral load was assessed by ultrasensitive reverse transcriptase polymerase chain reaction (COBAS Amplicor; Roche, Burlington, NC). CD4+ T cell counts were determined by flow cytometry.
Statistical analysis
The Shapiro-Wilk test was used to assess the distribution of variables. Data are presented as mean ± standard error of the mean if normally distributed or median [interquartile range (IQR)] if not normally distributed. Categorical variables are reported as proportions. Between-group comparisons (HIV-infected or non–HIV-infected) were made by using the Student t test. Non-normally distributed variables were log-transformed to establish a normal distribution. Pearson correlation coefficient was used to perform linear regression in relation to cardiac NPs by HIV status. A four-group comparison by overweight BMI category (BMI <25 kg/m2 or ≥25 kg/m2) was assessed by using analysis of variance, after appropriate log transformation, to assess independent effects of HIV status and BMI on cardiac NPs. BMI was selected as the body composition parameter for stratification given its well-established clinical categories. To represent the data in a clinically relevant manner, non-normally distributed variables are reported as values before log transformation, and the P value reported is based on the appropriate statistical test applied to the log-transformed values where noted. Determinants of serum aldosterone were evaluated in multivariate regression modeling performed in the HIV group. Statistical significance was defined as P ≤ 0.05. All statistical analyses were performed by using SAS JMP software, version 12.0 (SAS Institute, Cary, NC).
Results
Baseline demographic and clinical characteristics
Both HIV-infected and non–HIV-infected groups were of similar age and sex. Most individuals with HIV had a long-term history of exposure (18 ± 1 years) and ART use (11 ± 1 years) and demonstrated stable immunological control; the mean CD4+ count was 571 ± 73 cells/μL and mean viral load was 1.77 ± 0.19 copies/mL. The most common protease inhibitor and nucleoside/nucleotide reverse transcription inhibitor used were ritonavir and tenofovir, respectively, each by 50% of patients (see Supplemental Table 1 for additional PI and NRTI use). Measures of body composition were overall similar between HIV-infected and non–HIV-infected individuals with respect to iliac waist circumference (94 ± 3 vs 89 ± 4 cm), BMI (26 ± 1 vs 25 ± 1 kg/m2), BAI (48% ± 3% fat vs 44% ± 3% fat), visceral adipose tissue [134 (IQR, 56, 188) vs 140 (IQR, 84, 184) cm2], and subcutaneous adipose tissue [209 (IQR, 107, 342) vs 213 (IQR, 122, 269) cm2] (all P ≥ 0.05). The HIV-infected group appeared to have worse HOMA-IR, but this parameter was not statistically different from that in the non–HIV-infected group. Overall, individuals demonstrated normal renal function and potassium (Supplemental Table 2)
Characterization of cardiac NPs and RAAS among HIV-infected and non–HIV-infected individuals
During the RAAS-activated state, BNP [60 (IQR, 44, 152) vs 196 (IQR, 91, 251) pg/mL; P = 0.04] was significantly lower, and ANP [87 (IQR, 54, 138) vs 145 (IQR, 97, 264) pg/mL; P = 0.07] tended to be lower among HIV-infected vs non–HIV-infected individuals. In contrast to the biologically active BNP, the biologically inactive prohormone NT-proBNP was not significantly different between groups [10 (IQR, 7, 32) vs 13 (IQR, 4, 19) pg/mL; P = 0.36 in HIV-infected vs non–HIV-infected] during RAAS activation. HIV-infected individuals demonstrated increased serum aldosterone [13.8 (IQR, 9.7, 30.9) vs 9.2 (IQR, 7.6, 13.6)ng/dL; P = 0.03] compared with non–HIV-infected individuals. Other related parameters of the RAAS, including PRA, 24-hour urine sodium, and mean arterial pressure (MAP), did not differ between groups on the low-sodium controlled diet (Table 1).
Table 1.
Cardiac NPs During RAAS Activation Among Non–HIV-Infected and HIV-Infected Individuals
| Variable | Non–HIV-Infected (n = 10) | HIV-Infected (n = 20) | P Value a |
|---|---|---|---|
| ANP (pg/mL) | 145 (97, 264) | 87 (54, 138) | 0.07 |
| BNP (pg/mL) | 196 (91, 251) | 60 (44, 152) | 0.04 |
| NT-proBNP (pg/mL) | 13 (4, 19) | 10 (7, 32) | 0.36 |
| PRA (ng/mL/h) | 1.8 (1.1, 3.2) | 2.5 (1.4, 3.5) | 0.98 |
| Serum aldosterone (ng/dL) | 9.2 (7.6, 13.6) | 13.8 (9.7, 30.9) | 0.03 |
| Urine sodium (mmol/24 h) | 18.2 ± 2.3 | 23.8 ± 4.5 | 0.28 |
| MAP (mm Hg) | 85 ± 2 | 84 ± 1 | 0.86 |
Unless otherwise noted, data reported as mean ± standard error of the mean or median (interquartile range).
To represent the data in a clinically relevant manner, non-normally distributed variables are reported as values before log transformation, and the P value reported is based on the appropriate statistical test applied to the log-transformed values.
Because of its significance during RAAS activation, BNP was further characterized during an ad libitum sodium diet to confirm this unique physiology in HIV. BNP [108 (IQR, 88, 134) vs 149 (IQR, 115, 546) pg/mL; P = 0.03] was also significantly reduced during baseline physiologic conditions in HIV-infected vs non–HIV-infected groups. Both groups demonstrated similar RAAS parameters, urine sodium, and MAP on the ad libitum sodium diet.
Relationships with cardiac NPs among HIV-infected and non–HIV-infected individuals
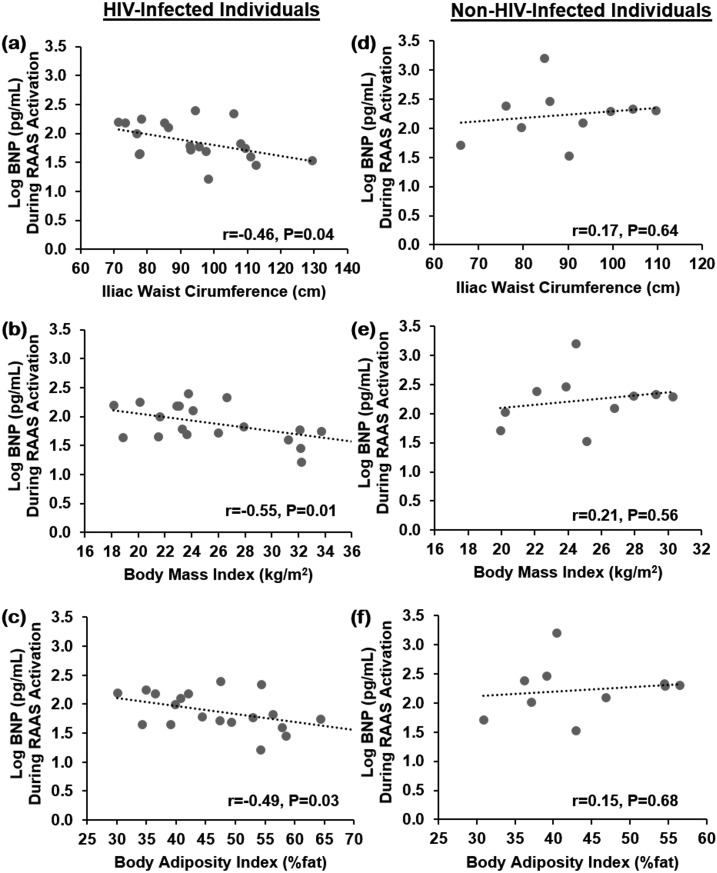
In the HIV-infected group, BNP was significantly and inversely related to multiple indices of body composition [iliac waist circumference: r = −0.46 (P = 0.04); BMI: r = −0.55 (P = 0.01), BAI: r = −0.49 (P = 0.03)], metabolic parameters [total cholesterol: r = −0.44 (P = 0.05), HOMA-IR: r = −0.44 (P = 0.05), MAP: r = −0.44 (P = 0.05)], and RAAS components [serum aldosterone: r = −0.49 (P = 0.03)] [Table 2; Fig. 2(a)–2(c)], whereas no relationships with ANP were demonstrated. In addition, no significant correlations were demonstrated to either cardiac NPs among non–HIV-infected individuals during RAAS-activated conditions [Table 2; Fig. 2(d)–2(f)].
Table 2.
Relationships of Cardiac NPs Among Non–HIV-Infected and HIV-Infected Individuals During RAAS Activation
| Body Composition and Metabolic Parameters | Non–HIV-Infected | HIV-Infected | ||||||
|---|---|---|---|---|---|---|---|---|
| log ANP (pg/dL) | log BNP (pg/dL) | log ANP (pg/dL) | log BNP (pg/dL) | |||||
| r | P Value | r | P Value | r | P Value | r | P Value | |
| Iliac waist circumference, cm | 0.24 | 0.50 | 0.17 | 0.64 | 0.18 | 0.45 | −0.46 | 0.04 |
| BMI, kg/m2 | 0.03 | 0.93 | 0.21 | 0.56 | 0.14 | 0.56 | −0.55 | 0.01 |
| BAI, % fat | 0.15 | 0.67 | 0.15 | 0.68 | 0.12 | 0.61 | −0.49 | 0.03 |
| log VAT, cm2 | −0.19 | 0.59 | 0.04 | 0.91 | 0.16 | 0.50 | −0.26 | 0.26 |
| log SAT, cm2 | 0.24 | 0.51 | 0.38 | 0.28 | 0.14 | 0.56 | −0.36 | 0.12 |
| log TAT, cm2 | 0.09 | 0.81 | 0.26 | 0.46 | 0.18 | 0.45 | −0.42 | 0.06 |
| Total cholesterol, mg/dL | 0.31 | 0.38 | 0.22 | 0.54 | 0.06 | 0.81 | −0.44 | 0.05 |
| log HOMA-IR | 0.07 | 0.85 | 0.14 | 0.69 | 0.14 | 0.57 | −0.44 | 0.05 |
| MAP, mm Hg | 0.07 | 0.85 | 0.50 | 0.14 | 0.06 | 0.80 | −0.44 | 0.05 |
| log Serum aldosterone, ng/dL | −0.41 | 0.24 | −0.01 | 0.98 | 0.28 | 0.23 | −0.49 | 0.03 |
Relationships were assessed by Pearson correlation coefficient.
Abbreviations: SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
Figure 2.
Relationship between BNP (pg/mL) and measures of body composition [iliac waist circumference (cm), BMI (kg/m2), and BAI (%fat)] among HIV-infected individuals (a–c) and non–HIV-infected individuals (d–f) during the RAAS-activated state. Data were analyzed by using Pearson correlation coefficient and are represented here as linear regression.
Effects of HIV status and BMI on BNP
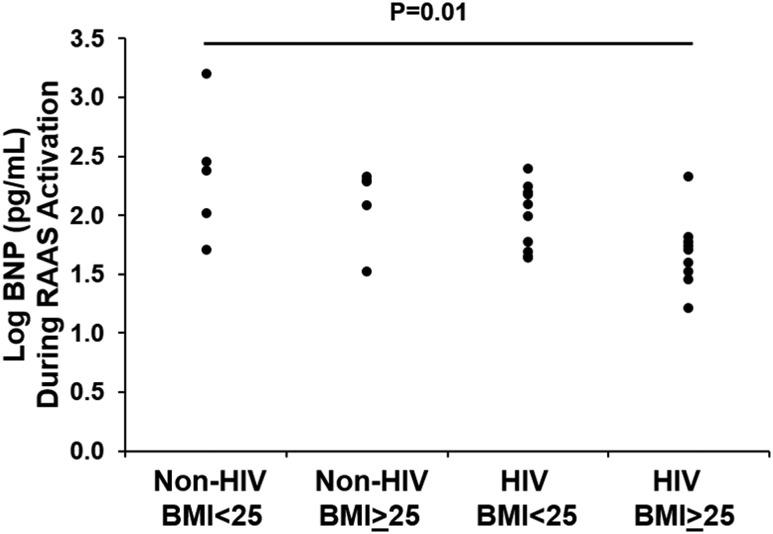
A four-group comparison was made after individuals were stratified by HIV status and above and below BMI 25 kg/m2 (overweight category). BNP decreased significantly across groups, being highest in non–HIV-infected individuals with BMI <25 kg/m2 and lowest in the HIV-infected individuals with BMI ≥25 kg/m2 [238 (IQR, 77, 935), 193 (IQR, 78, 206), 125 (IQR, 49, 157), and 52 (IQR, 31, 63)] pg/dL in non–HIV-infected persons with BMI <25 kg/m2 (n = 5), non–HIV-infected persons with BMI ≥25 kg/m2 (n = 5), HIV-infected persons with BMI <25 kg/m2 (n = 11), and HIV-infected persons with BMI ≥25 kg/m2 (n = 9), respectively (overall P = 0.01) (Fig. 3).
Figure 3.
Comparison of BNP (pg/mL) during the RAAS-activated state among HIV-infected and non–HIV-infected individuals stratified by above and below overweight BMI. P value reported is based on the appropriate statistical test applied to the log-transformed values.
Relationship of BNP to serum aldosterone among HIV-infected individuals during RAAS activation
In assessing for significant mediators of serum aldosterone (overall model r2 = 0.59; P = 0.007) in HIV-infected persons using multivariate modeling, BNP (β estimate = −0.3545; P = 0.04) remained a negative independent predictor, and PRA (β estimate = 0.4824; P = 0.005) a positive independent predictor, of serum aldosterone after additionally controlling for 24-hour urine sodium and MAP (Supplemental Table 3). In additional modeling controlling for HOMA-IR and serostatus (overall model r2 = 0.22; P = 0.03), HIV serostatus was a significant predictor of reduced BNP (β estimate = −0.1681; P = 0.03), and in contrast HOMA-IR was not significant in this model (β estimate = −0.2196; P = 0.29).
Discussion
This study investigated cardiac NPs in HIV by using a well-controlled physiologic protocol to activate the RAAS. Few studies in non–HIV-infected persons have evaluated cardiac NPs under strict physiologic conditions (4, 5) and to our knowledge have not performed a comprehensive evaluation to assess the interaction between NP and aldosterone hormone physiology, controlling for diet and posture.
Under conditions stimulating RAAS activation, we would expect BNP to increase in response to the rise in aldosterone levels, functioning as a negative regulator of RAAS. Indeed, acute infusions of BNP in human physiology studies have suppressed aldosterone (6). This normal physiology was clearly demonstrated among the non–HIV-infected individuals, who overall had increased BNP during the RAAS-activated state. Instead, we demonstrate contrasting physiology among the HIV-infected individuals, such that BNP was relatively decreased among HIV-infected individuals compared with non−HIV-infected individuals during the RAAS-activated state. These data suggest dysfunctional feedback between aldosterone and the cardiac NP system in HIV, leading to an exaggerated rise in aldosterone. This pathophysiology, which reflects an inability of cardiac NPs to counterregulate when perturbed in a state of RAAS activation, may be detrimental to the prevention of long-term cardiac and metabolic complications in HIV (Fig. 1).
Few studies in HIV have evaluated NPs. We measured the mature forms of BNP and ANP, which are the active forms of the hormone, rather than the inactive forms of the hormone specific to the n-terminal fragments. Results from the SMART study demonstrated that HIV-infected individuals with a CVD event had higher levels of NT-proBNP than did HIV-infected individuals without a CVD event (7). Our data differ from findings of the SMART study, in which NPs were not measured under strict controlled RAAS conditions, in comparison with a non−HIV-infected population, or stratified by body composition as in our current study. In addition, we studied only a well-treated population receiving continuous ART. A prior study from our group comparing NT-proBNP among HIV-infected and non−HIV-infected individuals demonstrated no difference between groups when measured during uncontrolled physiologic conditions (8). The SATURN-HIV study did show an increase in NT-proBNP in HIV-infected compared with non−HIV-infected persons, which was attenuated with statin use. Interestingly, the attenuation in NT-proBNP was associated with worsening HOMA-IR and increased peripheral fat (9), similar to our data, and also suggest that relatively lower NT-proBNP could have metabolic detriment in HIV.
ANP and BNP exert their action by primarily binding to NP receptor (NPR)-A and have lower affinity for NP receptor B. In addition, there are NP clearance receptors (NPR-C), which when bound to ANP and BNP result in NP internalization and degradation. In contrast, the biologically inactive prohormone NT-proBNP is not cleared by the NPR-C. NP receptors are well known to be expressed abundantly in the adipose depot (10), and NPR-C may be more widely upregulated in obesity and in the presence of insulin, presumably because of excess adipose depots (11, 12). As such, an imbalance between the ratio of NPR-A and NPR-C in obesity has been described (13) and is specifically localized to the adipose depot and associated with inflammation (14). In support of this, NPR-C is downregulated with weight reduction (15).
We took advantage of the unique interaction between BNP and NT-proBNP with NPR-C to understand whether reduced BNP in HIV might be related to enhanced clearance or reduced hormone secretion. If we were to attribute the mechanism to reduced hormone secretion, we would expect that both BNP and NT-proBNP would be reduced in HIV. In contrast, we demonstrate similar NT-proBNP levels regardless of serostatus, whereas BNP is reduced in HIV during an RAAS-activated state. In this regard, we postulate that the difference in BNP may be due to enhanced clearance in HIV. As NPR-C modulates NP metabolism, greater NPR-C receptor expression in the adipose depot may dampen RAAS suppression through NP reduction, thereby permitting a type of aldosterone escape. By this mechanism, it is conceivable that a maladaptive interplay between the RAAS and NP in states of excess adiposity may contribute to metabolic burden in HIV.
With regard to body composition, an inverse correlation of BNP was demonstrated among the HIV population in this study. We did not see any relationships to ANP. In a large community sampling, aldosterone correlated with central obesity, and aldosterone levels in the highest tertile, although within normal range, were associated with lower cardiac NPs and greater mortality (5). Results from a clinical study investigating peptide physiology following intervention with gastric bypass surgery show that weight loss was related to increased cardiac NPs (4). Although we did not see a relationship specifically between BNP and the visceral depot, other similar measures, such as BMI, waist circumference, and BAI, were inversely related to BNP in the HIV-infected group and serve as clinical surrogates for excess adipose and likely fat redistribution. In contrast, we saw no relationship between BNP and body composition among the non–HIV-infected group.
Cardiac NPs also have a critical role in mediating lipolysis in the adipose depot aside from suppressing aldosterone. In this regard, adipose-tissue specific knockout murine models of the NPR-C show that these mice develop resistance to obesity and ectopic fat accumulation and benefit from greater insulin sensitivity (16, 17). We did see an inverse correlation of BNP to HOMA-IR in the HIV-infected group during the RAAS-activated state. In our study, BNP remained different in HIV-infected and non–HIV-infected persons, after controlling for HOMA-IR. These data suggest that differences in BNP were not due to any differences in insulin sensitivity between the groups.
Our data suggest a mechanism of increased aldosterone in HIV via reduced BNP and prompt the question of whether RAAS blockade may be of use in this population. Studies in non-HIV settings suggest maximal cardiovascular benefits of spironolactone and angiotensin-receptor blockade in populations with the lowest BNP (18, 19). In our study, we stratified non–HIV-infected and HIV-infected individuals across the weight spectrum and demonstrate that BNP is lowest in the HIV-infected/overweight category and increases gradually to the non–HIV-infected/normal-weight category. Taken together, these data suggest that the HIV population, especially those with excessive adiposity, may be a subgroup that could benefit from RAAS blockade.
Limitations of this study include the small sample size. Nonetheless, we demonstrate reduced NP in the context of RAAS activation in HIV. Moreover, we show that several adverse measures of body composition were inversely related to NP. Although on the basis of the current data we can only hypothesize that the adipose depot is dysfunctional, future investigations should evaluate tissue-specific expression of adipocyte NPR-C to reveal a potential mechanism for reduced NP among HIV-infected individuals. Moreover, detailed phenotyping of the subcutaneous and visceral adipose depots in a larger cohort will permit us to discern whether this physiology is distinct to fat redistribution or compounded by generalized obesity, which is increasing in prevalence in the HIV population.
In summary, these data provide further insight into a potential mechanism for RAAS activation in HIV, which could be driven by enhanced NP clearance from the adipose depot. Studies have investigated neprilysin inhibition, which blocks degradation of NPs, along with angiotensin-receptor blockade in heart failure (20–22), and few studies evaluating this therapeutic strategy in obesity have demonstrated improved glucose and lipid metabolism (23). As such, a dual strategy that targets RAAS blockade and augments the cardiac NP system may have synergistic effects to reduce cardiometabolic disease in HIV. In this regard, an ongoing study will begin to evaluate the effects on mineralocorticoid receptor blockade on cardiac NPs (the MIRACLE HIV study, NCT02740179) among HIV-infected individuals with excess visceral adiposity.
Supplementary Material
Acknowledgments
The investigators thank the nursing staff on the Massachusetts General Hospital Clinical Research Center and Brigham and Women's Hospital Center for Clinical Investigation for their dedicated patient care, as well as the volunteers who participated in this study.
Financial Support: Funding was provided by National Institutes of Health (NIH) R01DK49302 (to S.K.G.); Harvard cMeRIT (to S.S.); NIH K23 HL136262 (to S.S.); NIH UL1 TR000170, NIH UL1 RR025758, and NIH UL1 TR001102 from the National Center for Research Resources and National Center for Advancing Translational Sciences (Harvard Catalyst/Harvard Clinical and Translational Science Center); and NIH P30 DK040561 (to S.K.G., Nutrition and Obesity Research Center at Harvard). Funding sources had no role in the design of the study, data analysis, or writing of the manuscript.
Clinical Trial Information: ClinicalTrials.gov no. NCT01407237 (registered 2 August 2011).
Disclosure Statement: G.K.A. has been a consultant for Pfizer; S.K.G. has received research funding from Gilead, KOWA, and Theratechnologies, and served as a consultant for Navidea Inc. and Theratechnologies, all unrelated to this manuscript. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- ANP
atrial natriuretic peptide
- ART
antiretroviral therapy
- BAI
body adiposity index
- BMI
body mass index
- BNP
brain natriuretic peptide
- CVD
cardiovascular disease
- HOMA-IR
insulin resistance calculated using the homeostatic model assessment
- IQR
interquartile range
- MAP
mean arterial pressure
- NP
natriuretic peptide
- NPR
natriuretic peptide receptor
- NPR-C
natriuretic peptide clearance receptor
- NT-proBNP
amino terminal pro B-type natriuretic peptide
- PRA
plasma renin activity
- RAAS
renin-angiotensin-aldosterone system
References
- 1. Srinivasa S, Fitch KV, Wong K, Torriani M, Mayhew C, Stanley T, Lo J, Adler GK, Grinspoon SK. RAAS activation is associated with visceral adiposity and insulin resistance among HIV-infected patients. J Clin Endocrinol Metab. 2015;100(8):2873–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Srinivasa S, Burdo TH, Williams KC, Mitten EK, Wong K, Fitch KV, Stanley T, Adler GK, Grinspoon SK. Effects of sodium restriction on activation of the renin-angiotensin-aldosterone system and immune indices during HIV infection. J Infect Dis. 2016;214(9):1336–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, Xiang AH, Watanabe RM. A better index of body adiposity. Obesity (Silver Spring). 2011;19(5):1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arora P, Reingold J, Baggish A, Guanaga DP, Wu C, Ghorbani A, Song Y, Chen-Tournaux A, Khan AM, Tainsh LT, Buys ES, Williams JS, Heublein DM, Burnett JC, Semigran MJ, Bloch KD, Scherrer-Crosbie M, Newton-Cheh C, Kaplan LM, Wang TJ. Weight loss, saline loading, and the natriuretic peptide system. J Am Heart Assoc. 2015;4(1):e001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buglioni A, Cannone V, Cataliotti A, Sangaralingham SJ, Heublein DM, Scott CG, Bailey KR, Rodeheffer RJ, Dessì-Fulgheri P, Sarzani R, Burnett JC Jr. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension. 2015;65(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richards AM, Crozier IG, Holmes SJ, Espiner EA, Yandle TG, Frampton C. Brain natriuretic peptide: natriuretic and endocrine effects in essential hypertension. J Hypertens. 1993;11(2):163–170. [DOI] [PubMed] [Google Scholar]
- 7. Duprez DA, Neuhaus J, Tracy R, Kuller LH, Deeks SG, Orkin C, Stoehr A, Woolley IJ, Neaton JD; INSIGHT SMART Group . N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS. 2011;25(5):651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fitch KV, DeFilippi C, Christenson R, Srinivasa S, Lee H, Lo J, Lu MT, Wong K, Petrow E, Sanchez L, Looby SE, Hoffmann U, Zanni M, Grinspoon SK. Subclinical myocyte injury, fibrosis and strain in relationship to coronary plaque in asymptomatic HIV-infected individuals. AIDS. 2016;30(14):2205–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dirajlal-Fargo S, Kinley B, Jiang Y, Longenecker CT, Hileman CO, Debanne S, McComsey GA. Statin therapy decreases N-terminal pro-B-type natriuretic peptide in HIV: randomized placebo-controlled trial. AIDS. 2015;29(3):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarzani R, Dessì-Fulgheri P, Paci VM, Espinosa E, Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest. 1996;19(9):581–585. [DOI] [PubMed] [Google Scholar]
- 11. Dessì-Fulgheri P, Sarzani R, Tamburrini P, Moraca A, Espinosa E, Cola G, Giantomassi L, Rappelli A. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens. 1997;15(12 Pt 2):1695–1699. [DOI] [PubMed] [Google Scholar]
- 12. Nakatsuji H, Maeda N, Hibuse T, Hiuge A, Hirata A, Kuroda Y, Kishida K, Kihara S, Funahashi T, Shimomura I. Reciprocal regulation of natriuretic peptide receptors by insulin in adipose cells. Biochem Biophys Res Commun. 2010;392(1):100–105. [DOI] [PubMed] [Google Scholar]
- 13. Kovacova Z, Tharp WG, Liu D, Wei W, Xie H, Collins S, Pratley RE. Adipose tissue natriuretic peptide receptor expression is related to insulin sensitivity in obesity and diabetes. Obesity (Silver Spring). 2016;24(4):820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gentili A, Frangione MR, Albini E, Vacca C, Ricci MA, De Vuono S, Boni M, Rondelli F, Rotelli L, Lupattelli G, Orabona C. Modulation of natriuretic peptide receptors in human adipose tissue: molecular mechanisms behind the “natriuretic handicap” in morbidly obese patients. Transl Res. 2017;186:52–61. [DOI] [PubMed] [Google Scholar]
- 15. Haufe S, Kaminski J, Utz W, Haas V, Mähler A, Daniels MA, Birkenfeld AL, Lichtinghagen R, Luft FC, Schulz-Menger J, Engeli S, Jordan J. Differential response of the natriuretic peptide system to weight loss and exercise in overweight or obese patients. J Hypertens. 2015;33(7):1458–1464. [DOI] [PubMed] [Google Scholar]
- 16. Wu W, Shi F, Liu D, Ceddia RP, Gaffin R, Wei W, Fang H, Lewandowski ED, Collins S. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal. 2017;10(489):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, Desai AS, O’Meara E, Fleg JL, Pfeffer MA, Pitt B, Solomon SD. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail. 2017;5(4):241–252. [DOI] [PubMed] [Google Scholar]
- 19. Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM, Carson PE. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4(5):569–577. [DOI] [PubMed] [Google Scholar]
- 20. Packer M, McMurray JJV. Importance of endogenous compensatory vasoactive peptides in broadening the effects of inhibitors of the renin-angiotensin system for the treatment of heart failure. Lancet. 2017;389(10081):1831–1840. [DOI] [PubMed] [Google Scholar]
- 21. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. [DOI] [PubMed] [Google Scholar]
- 22. Singh JSS, Burrell LM, Cherif M, Squire IB, Clark AL, Lang CC. Sacubitril/valsartan: beyond natriuretic peptides. Heart. 2017;103(20):1569–1577. [DOI] [PubMed] [Google Scholar]
- 23. Jordan J, Stinkens R, Jax T, Engeli S, Blaak EE, May M, Havekes B, Schindler C, Albrecht D, Pal P, Heise T, Goossens GH, Langenickel TH. Improved insulin sensitivity with angiotensin receptor neprilysin inhibition in individuals with obesity and hypertension. Clin Pharmacol Ther. 2017;101(2):254–263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.