Abstract
Context
With the aging of the population and projected increase in osteoporotic fractures coupled with the declining use of osteoporosis medications, there is a compelling need for new approaches to treat osteoporosis. Given that age-related osteoporosis generally coexists with multiple other comorbidities (e.g., atherosclerosis, diabetes, frailty) that share aging as the leading risk factor, there is growing interest in the “Geroscience Hypothesis,” which posits that manipulation of fundamental aging mechanisms will delay the appearance or severity of multiple chronic diseases because these diseases share aging as the underlying risk factor. In this context, one fundamental aging mechanism that has received considerable attention recently as contributing to multiple age-related morbidities is cellular senescence. This mini-review provides an overview on cellular senescence with a focus on its role in mediating age-related bone loss.
Methods
This summary is based on the authors’ knowledge of the field supplemented by a PubMed search using the terms “senescence,” “aging,” and “bone.”
Results
There is compelling evidence from preclinical models and supportive human data demonstrating an increase in senescent cells in the bone microenvironment with aging. These cells produce a proinflammatory secretome that leads to increased bone resorption and decreased bone formation, and approaches that either eliminate senescent cells or impair the production of their proinflammatory secretome have been shown to prevent age-related bone loss in mice.
Conclusions
Targeting cellular senescence represents a novel therapeutic strategy to prevent not only bone loss but potentially multiple age-related diseases simultaneously.
Cellular senescence is an aging mechanism that contributes to age-related osteoporosis and other age-associated diseases. Targeting this pathway could simultaneously treat multiple aging morbidities.
With the aging of the population world-wide, age-related osteoporosis and fractures are enormous, and growing, public health problems. For example, in the year 2000, there were ∼9 million osteoporotic fractures (1.6 million hip, 1.7 million forearm, and 1.4 million clinical vertebral fractures) (1), and by 2050 the worldwide incidence of hip fracture is expected to increase by 240% and 310% in women and men, respectively (2). To put this in further context, the number of women who will experience a fracture in 1 year exceeds the combined number of women who will experience incident breast cancer, myocardial infarction, or stroke (3).
As is the case for many other chronic diseases, there has been tremendous progress over the past 30 years in developing drugs to prevent osteoporotic fractures. Although estrogen replacement therapy is now rarely used for the prevention or treatment of osteoporosis, following the results of the Women’s Health Initiative (4), numerous additional pharmacological options have been developed, including a selective estrogen receptor modulator (raloxifene) (5), four bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) (6), a human monoclonal antibody to receptor activator of nuclear factor κB ligand (denosumab) (7), and the parathyroid hormone/parathyroid hormone–related peptide analogs teriparatide (8) and abaloparatide (9). Despite this substantial progress, many or most patients with osteoporosis are not currently receiving appropriate treatment, even after an event as devastating as hip fracture. For example, a recent study found that among 22,598 patients, the use of bisphosphonates after hip fracture declined from only 15% in 2004 to 3% in 2013 (10).
There are a number of reasons for the lack of appropriate treatment of osteoporosis, including fear of rare bisphosphonate-related side effects such as osteonecrosis of the jaw and atypical femur fractures, misunderstanding of the benefit-to-risk ratio of osteoporosis drugs, poor coordination of health care systems for fracture patients, and inadequate access to appropriate investigation and treatment (11). At a broader level, however, osteoporosis rarely, if ever, exists in isolation in an elderly individual. Rather, it coexists with numerous additional comorbidities, including cardiovascular disease, diabetes, rheumatoid arthritis, osteoarthritis, and other age-associated diseases (12). This leads to two key barriers to osteoporosis treatment: (1) the busy primary care physician is often dealing with perceived more pressing, immediate medical problems (e.g., treating hypertension or diabetes), and (2) the older patient is increasingly burdened with the growing problem of polypharmacy. Indeed, the most recent estimates are that 36% to 39% of older adults take five or more prescription medications; when over-the-counter medication use is included, the prevalence of older adults taking five or more medications increases to 67% (13). Current treatment strategies for all chronic age-related morbidities are disease specific, with each drug targeting a single disease (e.g., a statin for cardiovascular risk reduction, an antihyperglycemic agent for diabetes, a bisphosphonate for osteoporosis, etc.), leading to polypharmacy and the problems related to adverse drug interactions and compliance.
Fundamental Aging Mechanisms and Cellular Senescence
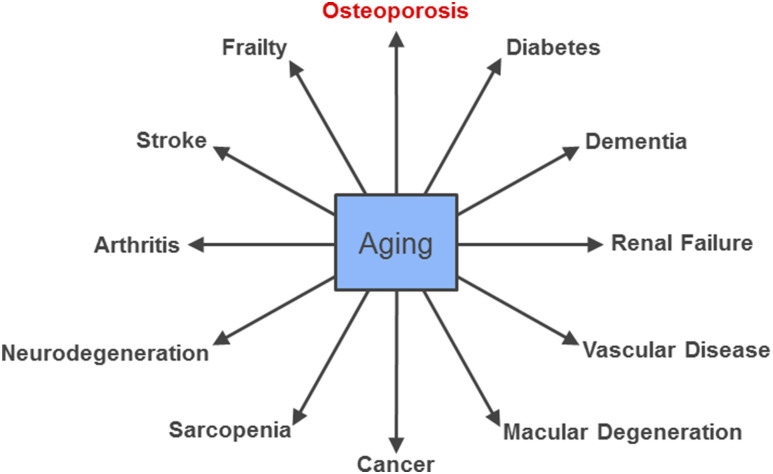
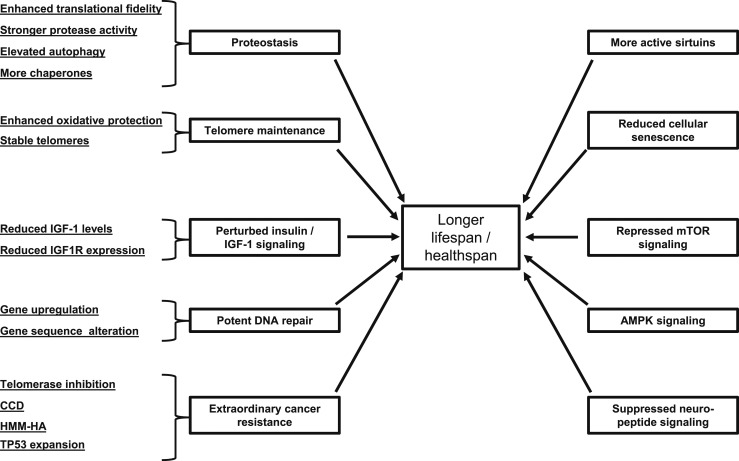
It is in this context of multiple aging comorbidities that the gerontology community has increasingly recognized the concept that aging itself is the greatest risk factor for most age-related chronic diseases, including atherosclerosis, cancers, dementias, diabetes, and osteoporosis (Fig. 1) (14). This has led to the “Geroscience Hypothesis,” which posits that manipulation of fundamental aging mechanisms will delay (in parallel) the appearance or severity of multiple chronic diseases because these diseases share aging as the underlying risk factor–namely (14). Figure 2 summarizes common aging mechanisms that influence lifespan and healthspan that have been identified based on studies across a range of species (15). At least one of these pathways, 5′-adenosine monophosphate–activated protein kinase signaling, which is targeted by the antidiabetes drug metformin, is the focus of a planned clinical trial (Targeting Aging with Metformin) in which 3000 subjects, ages 65 to 79 years, will be randomized to treatment with placebo or metformin (16). Moreover, in the first example of its kind to be considered by the US Food and Drug Administration, the investigators will measure time to a new occurrence of a composite outcome that includes cardiovascular events, cancer, dementia, and mortality, along with important functional and geriatric endpoints, as opposed to the traditional clinical trial approach of testing effects of an intervention on each separate condition.
Figure 1.
The central role of aging in most serious chronic diseases. Adapted from Tchkonia et al. (14).
Figure 2.
Molecular mechanisms determining lifespan and healthspan that have been identified across species. Reproduced from Tian et al. (15).
Of the other fundamental aging mechanisms depicted in Fig. 2, cellular senescence has received considerable attention recently as a potentially druggable target to prevent or treat multiple aging comorbidities. Cellular senescence was originally described by Hayflick (17), who showed over 40 years ago that human fibroblasts initially underwent robust cell division in culture. Over many cell doublings, they lost their ability to divide but remained viable in a senescent state for many weeks. Subsequent work more clearly defined cellular senescence as an essentially irreversible growth arrest that occurs when cells experience potentially oncogenic insults, consistent with its role as an anticancer mechanism [reviewed by Tchkonia et al. (14)]. However, as summarized herein, there is convincing evidence that the accumulation of senescent cells is causally related to multiple aging phenotypes and pathologies.
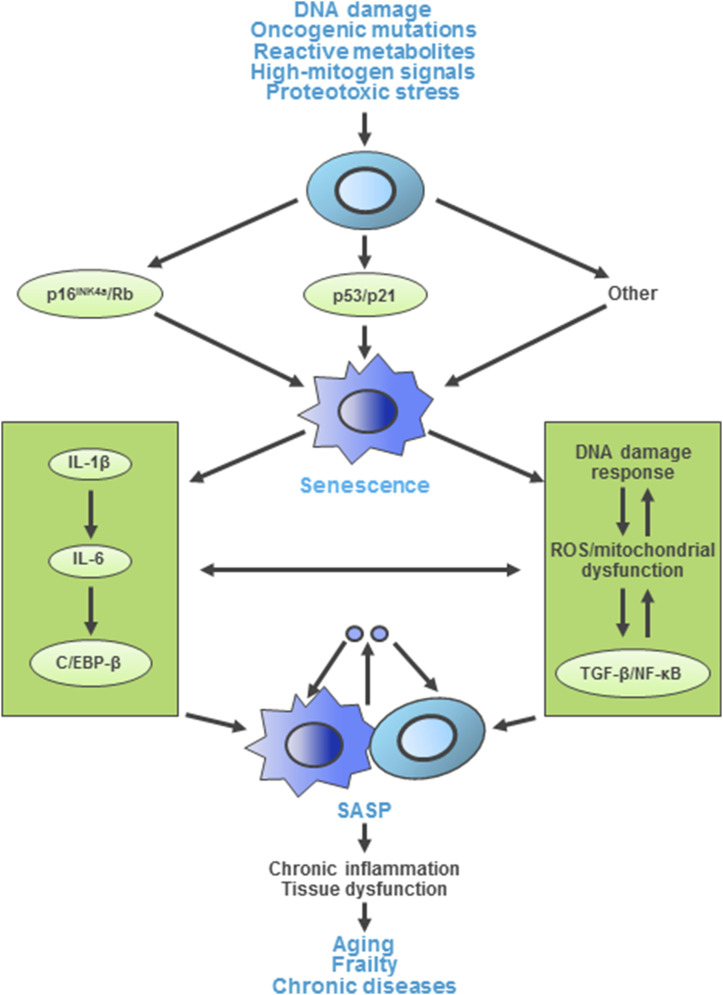
Figure 3 provides an overview of the cellular senescence pathway, whereby repeated cell division (as in the original Hayflick model) as well as other cellular stresses (e.g., telomere shortening, DNA damage, protein aggregation, increased reactive oxygen species) activate the p53/p21 and p16Ink4a/Rb tumor suppressor pathways to initiate senescence. Downstream of these signals, the senescence response is amplified by a number of mediators, including nuclear factor κB, interleukin-1β, interleukin-6, and CCAAT/enhancer binding protein-β. A fully mature senescent cell acquires a number of key characteristics: increased cell size and protein content; resistance to apoptosis; and a series of biomarkers that are now used to identify these cells, including increased p16Ink4a and p21 expression, cytochemical staining for senescence-associated β-galactosidase, and immunofluorescent staining for laminin B1 and γH2AX (an H2A histone family member that is recruited to sites of DNA damage in senescent cells) [for review of these senescence markers, see Zhao et al. (18)]. Senescent cells also develop a fairly striking decondensation of pericentromeric satellite heterochromatin termed “senescence-associated distension of satellites” (SADS) (19). Finally, these cells develop a revved-up machinery to secrete proinflammatory cytokines, chemokines, and proteases, termed the “senescence-associated secretory phenotype” (SASP), which has deleterious paracrine and systemic effects (Fig. 3) (14). Indeed, in part due to the remarkable ability of the SASP to cause “sterile inflammation,” even a relatively low abundance of senescent cells [e.g., ∼10% to 15% in old primates (20)] is sufficient to cause tissue dysfunction.
Figure 3.
Overview of pathways and molecular mechanisms inducing cellular senescence and the SASP. C/EBP-β, CCAAT/enhancer binding protein-β; IL-1β, interleukin 1β; IL-6, interleukin 6; NF-κB, nuclear factor κB; ROS, reactive oxygen species; TGF-β, transforming growth factor β; Adapted from Tchkonia et al. (14).
Although the accumulation of senescent cells and aging pathologies have been associated for some time, the critical proof-of-concept for a causal role for senescent cells in mediating aging morbidities came from Baker et al. (21) in 2011. Using an accelerated aging model (BubR1 progeroid mice), these investigators showed that in progeroid mice transgenic for a “suicide” transgene, INK-ATTAC, that permits inducible elimination of p16Ink4a-expressing senescent cells upon administration of a synthetic drug (AP20187), reduction of the senescent cell burden ameliorated multiple aging phenotypes in adipose tissue, skeletal muscle, and eye. However, because BubR1 mice do not have premature bone loss, the skeletal effects of eliminating senescent cells could not be assessed in this study.
Although clearance of senescent cells in a genetically engineered mouse model provided key evidence for causality, this approach cannot be translated to humans. The crucial step toward translation came from Zhu et al. (22), who described the identification and validation of a class of drugs termed senolytics, which selectively kill senescent cells. The approach used to identify senolytics took advantage of the known resistance of senescent cells to apoptosis; thus, small interfering RNAs were used to silence expression of key nodes in prosurvival networks in senescent cells that were identified using proteome and transcript analysis. Specifically, small interfering RNAs targeting ephrins (EFNB1 or EFNB3), PI3Kδ, p21, BCL-xL, or plasminogen-activated inhibitor-2 killed senescent cells but not proliferating or quiescent differentiated cells. Zhu et al. (22) next tested 46 candidate drugs that targeted these same pathways. Of these, dasatinib [an FDA-approved tyrosine kinase inhibitor (23)] and quercetin [a flavanol present in many fruits and vegetables (24)], when used in combination, showed efficacy in killing senescent cells in vitro. In vivo, periodic administration of the combination of dasatinib and quercetin reduced senescent cell burden in chronologically aged and irradiated mice and in a different accelerated aging model (Ercc1−/∆ progeroid mice). Specifically, in old mice, cardiac function and carotid vascular reactivity improved after treatment; in single limb irradiated mice, exercise capacity improved; and in Ercc1−/∆ progeroid mice, there was improvement in multiple health outcomes (22). These studies established the feasibility of selectively ablating senescent cells using a pharmacological approach that could be translated to humans with demonstrable improvements in multiple health outcomes in aging mice. After this initial description of dasatinib and quercetin, a number of additional senolytic drugs have been identified that target the Bcl-2 family (navitoclax, ABT263; A1331852, A1155463), p53/p21 (FOXO4-related peptide, piperlongumine), HSP-90 (geldanamycin), and PI3K/AKT (fisetin) pathways [for a detailed review of senolytics, see Kirkland and Tchkonia (25)].
Cellular Senescence and Bone
Given the growing evidence for a critical role for cellular senescence in mediating a number of age-related pathologies and the advent of senolytic drugs to potentially treat these pathologies simultaneously, several groups have sought to address the role of cellular senescence in mediating age-related bone loss. To identify the cell type(s) within the bone microenvironment that undergo senescence with aging in vivo, our group measured senescence and SASP markers in highly enriched cell populations rapidly isolated from bone or bone marrow without in vitro culture from young (6 months) vs old (24 months) mice (26). In mice of both sexes, expression of the key senescence marker p16Ink4a (Fig. 3) by real-time quantitative polymerase chain reaction was significantly higher with aging in B cells, T cells, myeloid cells, osteoblast progenitors, osteoblasts, and osteocytes. To further validate these findings, we measured SADS in vivo and found significantly more senescent osteocytes in old as compared with young bone cortices (11% vs 2%; P < 0.001) (26). Evaluation of a panel of 36 established SASP genes in each of the respective cell populations revealed that relatively few SASP factors were altered significantly with aging in osteoblast progenitors and osteoblasts; by contrast, 23 of the 36 SASP genes were significantly elevated in old vs young osteocytes. In the hematopoietic cell populations, relatively few SASP factors were increased with aging in B and T cells, but 26 of the 36 genes analyzed were significantly upregulated in old as compared with young myeloid cells. These findings in mice were validated in humans by obtaining needle biopsies of bone from young (mean age, 27 years) and old (mean age, 78 years) healthy female volunteers (n = 10 per group). Consistent with the data in mice, p16Ink4a and p21 expression was significantly higher in the bone biopsies from the old vs young women (2.2-fold and 1.5-fold, respectively; P < 0.01), and 12 of the SASP factors were significantly upregulated in the biopsies from old women. Collectively, these findings demonstrated that, with aging in mice and in humans, a subset of cells of various lineages in the bone microenvironment become senescent, although upregulation of the SASP occurs predominantly in osteocytes and myeloid cells.
The above findings on increased osteocyte senescence with aging were confirmed by Piemontese et al. (27), who demonstrated increased protein expression of the senescence marker γH2AX in osteocytes from old (21-month-old) as compared with young (7-month-old) mice as well as increased osteocyte expression of p16Ink4a and several SASP markers. These alterations were also associated with increased osteocyte receptor activator of nuclear factor κ-B ligand expression and cortical porosity, suggesting that osteocyte senescence may drive an increase in endocortical and intracortical bone resorption. Additional studies from the same group extended these findings to osteoprogenitors (28), showing that osterix-expressing osteoprogenitor cells decreased with age in mice and that this decrease was associated with increased expression of γH2AX and other senescence markers.
To establish a causal relationship between cellular senescence and age-related bone loss, our group used three different strategies to target senescent cells and assessed their impact on the skeleton in old mice (29): reducing senescent cell burden using either a genetic approach [i.e., the INK-ATTAC suicide transgene described previously (21)] or senolytic compounds [dasatinib and quercetin (23)] or inhibiting the production of the proinflammatory SASP of senescent cells using a JAK 1/2 inhibitor (30, 31). The skeletal effects of all three interventions were remarkably similar in that, in old (20- to 22-month-old) mice with established bone loss, treatment for 2 to 4 months markedly improved bone mass and microarchitecture in trabecular and cortical bone. Using either the genetic approach or senolytics, there was an ∼50% reduction in senescent osteocytes as assessed by the SADS assay, indicating that complete elimination of senescent cells was not required for beneficial skeletal effects. Moreover, the combination of dasatinib and quercetin only needed to be administered once monthly to achieve skeletal benefits, highlighting a key potential advantage of the senolytic approach: Because senescent cells take time to accumulate in various tissues, intermittent administration of senolytic drugs appears to be adequate to achieve therapeutic effects, thereby minimizing the risk of off-target effects. The specificity of these interventions for aging was demonstrated by the lack of effects of these treatments on bone mass, microarchitecture, or turnover in young mice with a low burden of senescent cells.
Histomorphometric analyses revealed important insights regarding the cellular mechanisms that have beneficial effects of targeting senescent cells on bone and how these mechanisms differ from current osteoporosis drugs. The improvements in trabecular bone mass and microarchitecture were associated with decreased bone resorption and with no change in bone formation on trabecular bone surfaces. In cortical bone, increased cortical thickness was associated with decreased bone resorption and increased bone formation. In vitro studies extended the in vivo findings by demonstrating that a senescent cell–conditioned medium impaired osteoblast mineralization and enhanced osteoclast-progenitor survival, leading to increased osteoclastogenesis. This inhibition of bone resorption without a concomitant reduction (trabecular bone) and a stimulation (cortical bone) in bone formation as a result of targeting senescent cells contrasts with the effects of all currently available antiresorptive drugs for osteoporosis, where a reduction in bone resorption is consistently associated with a reduction in bone formation due to the coupling between bone resorption and bone formation (32), as illustrated in Fig. 4a. By contrast, as shown in Fig. 4b, senolytics reduce senescent cell burden, which leads to a suppression of bone resorption with either an increase (cortical bone) or maintenance (trabecular bone) in bone formation, thereby effectively “uncoupling” bone resorption and formation. Because of this mixed antiresorptive/anabolic effect, senolytic treatment may have more sustained beneficial effects on bone as compared with conventional antiresorptive therapies, although further studies are needed to test this.
Figure 4.
Comparison of the effects of antiresorptive vs senolytic therapies on bone metabolism. (a) (1) Senescent cells (SCs) increase in the bone microenvironment with aging, where they (2) increase bone resorption by osteoclasts (OCs) and (3) reduce bone formation by osteoblasts (OBs). (4) Antiresorptive drugs inhibit or eliminate OCs and decrease bone resorption; (5) because of coupling between OCs and OBs, bone resorption is also reduced. (b) (1) Senolytic therapy reduces the burden of (2) SCs, which leads to (3) a reduction in bone resorption with (4) either an increase (cortical bone) or maintained (trabecular bone) bone formation, resulting in (5) a beneficial “uncoupling” between bone resorption and bone formation. Adapted from Farr et al. (29).
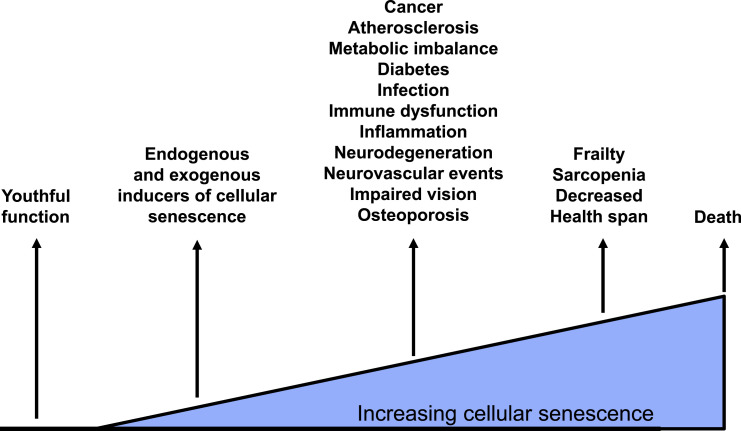
In the context of the Geroscience Hypothesis (i.e., manipulation of fundamental aging mechanisms will delay, in parallel, the appearance or severity of multiple chronic diseases), it is important to place the skeletal effects of targeting senescent cells in the context of other aging comorbidities. From parallel studies by our group and others, there is clear evidence that eliminating senescent cells or inhibiting the production of their SASP improves cardiac (22) and vascular function (33), reduces aortic calcification (33) and plaque instability (34), improves insulin sensitivity (30), and reduces frailty (31) in old mice. Thus, as depicted in Fig. 5, because senescent cell burden increases with age and is causally associated with multiple aging diseases (14), targeting senescent cells through a variety of approaches may provide the most viable opportunity to modulate a fundamental aging mechanism through a single drug (or a limited number of drugs) and alter the course of many age-associated diseases (14). For the osteoporosis field, particularly after the failure of the cathepsin K inhibitor odanacatib due to an increase in stroke risk (35) and questions regarding the future of the sclerostin inhibitor romosozumab due to a potential increase in cardiovascular events (36), there is declining interest by the pharmaceutical industry in developing new osteoporosis drugs (37). However, placing osteoporosis in the context of treating multiple aging conditions offers perhaps renewed hope for new drug development for this important age-associated disease.
Figure 5.
The accumulation of senescent cells with aging in multiple tissues leads to a number of age-related diseases. Targeting these cells holds the prospect of simultaneously preventing or ameliorating these age-associated morbidities. Adapted from Tchkonia et al. (14).
Unresolved Issues and Future Directions
Despite accumulating evidence that reducing the burden of senescent cells or inhibiting their SASP may prevent or reverse aging pathologies, there are remaining questions regarding the pros and cons of these approaches. As noted previously, senescence likely evolved as an anticancer mechanism, whereby cells that had accumulated oncogenic insults were redirected toward a senescent, growth-arrested phenotype rather than uncontrolled proliferation and cancer (14). As such, there is concern that preventing cells from becoming senescent may increase tumorigenesis. However, because it appears that senolytic drugs are effective when given intermittently, this concern may be mitigated by the fact that these drugs periodically reduce the burden of senescent cells rather than prevent them from forming. Senescent cells may also promote cancer growth through the proinflammatory SASP (14), and reducing the numbers of these cells and/or their SASP may lead to decreased tumor development. Furthermore, senescent cells that can harbor oncogenic mutations are eliminated by senolytics, several of which (e.g., dasatinib) are currently used or being studied for preventing or treating cancers.
There is increasing evidence that cell senescence may play an important role during embryogenesis and growth. Indeed, developmentally programmed cell senescence has been identified at multiple locations during mammalian embryonic development, including the mesonephros and the endolymphatic sac of the inner ear (38) as well as the apical ectodermal ridge and the neural roof plate (39). Postnatally, programmed cell senescence has been described in the primary spongiosa of long bones in mice at late puberty, when bone growth and accrual decelerate (40). Perhaps most relevant to the use of senotherapeutic approaches in aging, cellular senescence has been shown to play a role in would healing (41), during which senescent fibroblasts and endothelial cells appear early in response to a cutaneous wound and accelerate healing through the secretion of platelet-derived growth factor AA. Again, whether the intermittent reduction in senescent cell burden through senolytic drugs would have a negative effect on wound healing or other forms of tissue repair warrants careful study.
Although in the animal models (e.g., transgenic INK-ATTAC mice) senescent cells specifically expressing high levels of p16Ink4a were eliminated, leading to a potential concern that targeting this or other critical regulatory genes may have untoward effects, this is not the approach proposed for application in humans. Rather, the senolytic drugs being developed for humans target multiple pathways that senescent cells rely on for their survival and not specifically p16Ink4a or other single regulatory genes.
Although issues related to tumorigenesis, tissue repair, and targeting specific pathways in senescent cells need to be carefully evaluated as senolytic therapy is developed, early-phase clinical trials using senolytic drugs have been initiated, and, given the growing list of age-related diseases in which cellular senescence may play a role, the number of these trials is likely to grow in the coming years. As an example, because chemotherapy can induce cell senescence and a proinflammatory state, our group has initiated a pilot study to evaluate the efficacy of dasatinib and quercetin in clearing senescent cells and to assess other health outcomes in patients after hematopoietic stem cell transplantation (ClinicalTrials.gov ID NCT02652052). As such, the preclinical findings in mouse models are very much in the translation phase to humans, and studies assessing the effects of senolytics on skeletal metabolism in aged individuals will soon be underway. However, there are a number of unresolved issues related to using senolytic drugs for age-related osteoporosis. A major concern is that, given the growing reluctance of patients to accept current options for osteoporosis, these patients may not be willing to consider senolytic therapies. It seems plausible that the likelihood of patient acceptance will be greater if it could be shown that a given senolytic drug or drug combination helps treat not just osteoporosis but also other aging comorbidities. Although more than one senolytic drug may be needed to target different tissues, there is evidence for a single drug combination (dasatinib + quercetin) in preventing age-related bone loss (29) and in improving vascular function and reducing aortic calcification (33) in mice, indicating that it may be possible to identify a limited combination of senolytic drugs targeting senescent cells in multiple tissues, which may lead to better acceptance of this approach.
Summary and Conclusions
Targeting cellular senescence represents a new therapeutic paradigm for preventing or even reversing age-related osteoporosis and simultaneously treating multiple aging comorbidities. This approach does not focus specifically on bone but rather on a fundamental aging mechanism operative across tissues. If the remarkable promise of preclinical models is realized in human studies, we may truly have a novel approach to enhance healthspan (and perhaps lifespan) in the rapidly growing aging population in the Unites States and throughout the world.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants P01 AG004875 (S.K.), R01 AG048792 (S.K.), AR027065 (S.K.), K01 AR070241 (J.N.F.), R37 AG013925 (J.L.K.), and AG and R21 049182 (J.L.K.); the Connor Group (J.L.K.); the Noaber and the Ted Nash Foundations (J.L.K.); Career Development Awards from the Mayo Clinic Robert and Arlene Kogod Center on Aging; and a Richard F. Emslander Career Development Award in Endocrinology (J.N.F.).
Disclosure Summary: S.K. and J.N.F. have no conflicts to disclose. J.L.K. has patents related to this research.
Glossary
Abbreviations:
- SADS
senescence-associated distension of satellites
- SASP
senescence-associated secretory phenotype
References
- 1. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. [DOI] [PubMed] [Google Scholar]
- 2. Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407–413. [DOI] [PubMed] [Google Scholar]
- 3. Cauley JA, Wampler NS, Barnhart JM, Wu L, Allison M, Chen Z, Hendrix S, Robbins J, Jackson RD; Women’s Health Initiative Observational Study . Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19(12):1717–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. [DOI] [PubMed] [Google Scholar]
- 5. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators: mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629. [DOI] [PubMed] [Google Scholar]
- 6. Khosla S, Bilezikian JP, Dempster DW, Lewiecki EM, Miller PD, Neer RM, Recker RR, Shane E, Shoback D, Potts JT. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab. 2012;97(7):2272–2282. [DOI] [PubMed] [Google Scholar]
- 7. Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29(2):155–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. [DOI] [PubMed] [Google Scholar]
- 9. Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG, Fitzpatrick LA, Cosman F, Christiansen C, Investigators AS; ACTIVE Study Investigators . Effect of abaloparatide vs. placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722–733. [DOI] [PubMed] [Google Scholar]
- 10. Kim SC, Kim DH, Mogun H, Eddings W, Polinski JM, Franklin JM, Solomon DH. Impact of the U.S. Food and Drug Administration’s safety-related announcements on the use of bisphosphonates after hip fracture. J Bone Miner Res. 2016;31(8):1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khosla S, Cauley JA, Compston J, Kiel DP, Rosen C, Saag KG, Shane E. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res. 2017;32(3):424–430. [DOI] [PubMed] [Google Scholar]
- 12. Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH, Adachi JD, Boonen S, Chapurlat R, Díez-Pérez A, Anderson FAJ Jr, Hooven FH, LaCroix AZ, Lindsay R, Netelenbos JC, Pfeilschifter J, Rossini M, Roux C, Saag KG, Sambrook P, Silverman S, Watts NB, Greenspan SL, Premaor M, Cooper C; GLOW Investigators . Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone. 2012;50(6):1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy HB. Polypharmacy reduction strategies: tips on incorporating American Geriatrics Society Beers and Screening Tool of older people’s prescriptions criteria. Clin Geriatr Med. 2017;33(2):177–187. [DOI] [PubMed] [Google Scholar]
- 14. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian X, Seluanov A, Gorbunova V. Molecular mechanisms determining lifespan in short- and long-lived species. Trends Endocrinol Metab. 2017;28(10):722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23(6):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37(3):614–636. [DOI] [PubMed] [Google Scholar]
- 18. Zhao J, Fuhrmann-Stroissnigg H, Gurkar AU, Flores RR, Dorronsoro A, Stolz DB, St Croix CM, Niedernhofer LJ, Robbins PD. Quantitative analysis of cellular senescence in culture and in vivo. Curr Protoc Cytom. 2017;79:9.51.1–9.51.25. [DOI] [PubMed] [Google Scholar]
- 19. Swanson EC, Manning B, Zhang H, Lawrence JB. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J Cell Biol. 2013;203(6):929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311(5765):1257. [DOI] [PubMed] [Google Scholar]
- 21. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yi J-S, Huang Y, Kwaczala AT, Kuo IY, Ehrlich BE, Campbell SG, Giordano FJ, Bennett AM. Low-dose dasatinib rescues cardiac function in Noonan syndrome. JCI Insight. 2016;1(20):e90220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. [DOI] [PubMed] [Google Scholar]
- 25. Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S. Identification of senescent cells in the bone microenvironment. J Bone Miner Res. 2016;31(11):1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piemontese M, Almeida M, Robling AG, Kim HN, Xiong J, Thostenson JD, Weinstein RS, Manolagas SC, O’Brien CA, Jilka RL. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight. 2017;2(17):93771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC, O’Brien CA, Jilka RL, Zhou D, Almeida M. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell. 2017;16(4):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. 2015;4:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA. 2015;112(46):E6301–E6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khosla S. Odanacatib: location and timing are everything. J Bone Miner Res. 2012;27(3):506–508. [DOI] [PubMed] [Google Scholar]
- 33. Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mullard A. Merck & Co. drops osteoporosis drug odanacatib. Nat Rev Drug Discov. 2016;15(10):669. [DOI] [PubMed] [Google Scholar]
- 36. Khosla S. Bone diseases: Romosozumab - on track or derailed? Nat Rev Endocrinol. 2017;13(12):697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155(5):1104–1118. [DOI] [PubMed] [Google Scholar]
- 39. Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155(5):1119–1130. [DOI] [PubMed] [Google Scholar]
- 40. Li C, Chai Y, Wang L, Gao B, Chen H, Gao P, Zhou FQ, Luo X, Crane JL, Yu B, Cao X, Wan M. Programmed cell senescence in skeleton during late puberty. Nat Commun. 2017;8(1):1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]