Abstract
Context
In healthy adults with detectable cold-induced brown adipose tissue activation (CIBA), the relationships between sympathetic nervous system (SNS) or thyroid activity during energy balance (EBL) with CIBA and body composition change are undetermined.
Objective
To investigate the relationships between CIBA and thermoneutral catecholamines and thyroid hormones measured during EBL and to determine if CIBA, catecholamines, or thyroid hormones predict body composition changes.
Design, Setting, Participants, and Interventions
Twelve healthy volunteers (seven male and five female) with positive CIBA [>2 standardized uptake value (g/mL)] had 24-hour energy expenditure (24hEE) assessed during EBL via whole-room indirect calorimetry while residing on a clinical research unit. Positron emission tomography/computed tomography scans were performed after exposure to 16°C for 2 hours to quantify CIBA.
Main Outcome Measures
CIBA, 24hEE during EBL, and thermoneutrality with concomitant measurement of urinary catecholamines and plasma free T3 and free T4. Body composition at baseline and 6 months by dual-energy X-ray absorptiometry.
Results
Lower urinary norepinephrine and free T4 were associated with higher CIBA (r = −0.65, P = 0.03; and r = −0.75, P < 0.01, respectively), but CIBA was not associated with 24hEE at thermoneutrality (P = 0.77). Lower CIBA (β = −3.5 kg/standardized uptake value; P < 0.01) predicted fat mass gain, whereas higher urinary norepinephrine and free T4 predicted future fat mass gain at 6 months (β = 3.0 kg per twofold difference in norepinephrine, P = 0.03; and β = 1.2 kg per 0.1-ng/dL difference in free T4, P = 0.03, respectively).
Conclusion
Lower SNS and free thyroid measurements at baseline indicate a greater capacity for CIBA, which may be predictive against fat mass gain.
Lower sympathetic and thyroid tone are associated with higher cold-induced BAT activity (CIBA) in humans. Lower CIBA and greater norepinephrine and thyroxine concentrations predict fat mass gain.
Obesity is the result of sustained energy intake exceeding energy expenditure (EE). Brown adipose tissue (BAT), a thermogenic tissue that dissipates energy (1), may be a contributor of daily EE and may play a role in regulating weight or body composition changes. The sympathetic nervous system (SNS) is a primary regulator of BAT function via promotion of brown preadipocyte proliferation and activation of beta-adrenergic receptors on mature brown adipocytes (1, 2). Sympathetic activation of BAT results in nonshivering thermogenesis in response to cold exposure, a powerful stimulus of BAT, in both rodents and humans (3–5).
Cold-induced BAT activation (CIBA) can be quantified through positron emission tomography (PET) scans in adult humans and is associated with increased EE (6–8). The thermogenic properties of BAT make it an intriguing target for tackling obesity (9); however, the relationship of CIBA to change in weight or body composition in humans is not well understood. CIBA is inversely associated with measures of adiposity such as body mass index and body fat percentage (3, 4, 10). We have reported that CIBA, measured in standardized uptake value (SUV), is negatively associated with fat-free mass (FFM) and that subjects with CIBA (SUV ≥2 g/mL) who have lower activation of BAT are more likely to have an increase in fat mass (FM) after 6 months in free-living conditions (11). Further, the treatment of 6 weeks with cold exposure in adults with previously undetectable CIBA (<2 g/mL SUV) activates BAT and increases FM loss in those with the greatest increase in CIBA after therapy (12).
In humans, SNS activity can be assessed by urine or plasma catecholamine measurements (13). Studies investigating the association between catecholamines and BAT have predominantly examined individuals diagnosed with pheochromocytoma, a tumor in the adrenal medulla that oversecretes catecholamines and causes BAT hypertrophy (14–16). In these studies, those with higher plasma catecholamine concentrations have higher BAT activity (17). However, among healthy individuals, there are no differences in plasma norepinephrine concentrations in those with detectable vs undetectable CIBA (18). Whether SNS activity measured during thermoneutrality and energy balance (EBL), as a static marker of SNS activity, is associated with CIBA has not been examined in healthy individuals.
Free T3 and free T4 are reduced in individuals with positive BAT activity compared with BAT-negative individuals (19). A case report has shown the presence of substantial BAT volume in an adolescent with severe primary hypothyroidism, a low thyroid hormone state (20), that subsequently decreased in volume after 2 months of treatment with levothyroxine. However, this study used infrared thermal imaging and MRI techniques rather than gold-standard PET/CT to quantify BAT activity within the supraclavicular region. Conversely, in a hyperthyroid state, excess thyroid hormone concentrations increase BAT activity in rodents and humans (21, 22). These studies indicate that thyroid hormones are a potential regulator of BAT in humans, and additional investigation in healthy subjects with normal thyroid function is warranted.
To further understand the role of the SNS and thyroid hormones in humans with cold-induced activation of BAT, we evaluated the relationships between CIBA and the SNS and thyroid function by assessing 24-hour urinary catecholamines and plasma thyroid hormones (free T3 and free T4) measured during EBL and thermoneutrality in healthy individuals with CIBA >2 SUV (11). In so doing, we aimed to establish a means to identify subjects who have greater potential for cold-induced BAT that might allow for detection of those who might benefit from interventions that act via BAT. We also examined whether CIBA, urinary catecholamines, or thyroid hormones predicted weight or body composition changes after 6 months in free-living conditions.
Methods
Participants
Fifty-two volunteers between the ages of 18 and 50 years recruited from the Phoenix, AZ metropolitan area between 2009 and 2012, deemed healthy by medical history, physical examination, and laboratory measures, were admitted to the clinical research unit as part of a larger ongoing study (NCT00523627). Thirty-six volunteers were excluded based on PET/CT substudy criteria, which included an age <18 or >40 years, radiation exposure to the torso within the past 12 months, or declining participation. Of the 16 volunteers who participated in this substudy, 12 participants had measurements of CIBA and thyroid hormones, and 11 had urinary catecholamines analyzed. On admission, volunteers were placed on a weight-maintaining diet (WMD) consisting of 50% carbohydrates, 30% fat, and 20% protein. A 75-g oral glucose tolerance test was performed after 3 days on the WMD, and only volunteers with normal glucose regulation (23) continued the study. Body composition was measured by a dual-energy X-ray absorptiometry scan (DPX-1; Lunar Corp, Madison, WI). Volunteers were invited to return to our unit for follow-up at 6 months to remeasure weight and body composition. All participants provided written and informed consent prior to beginning the study. This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
EE Measurements
Following 4 days of a WMD, a whole-room indirect calorimeter was used to measure 24-hour EE (24hEE) by an initial eucaloric acclimation session followed by a second eucaloric session for the precise determination of 24hEE, as previously described (24), in thermoneutrality (mean ± SD; ambient temperature 23.9 ± 1.4°C). Participants were allowed to move freely inside the metabolic chamber, and this activity was measured using radar sensors, expressed as a percentage of time when activity was detected. As part of this study, participants also had 24hEE measured during fasting conditions (participants allowed only water and noncaloric beverages). Diet-induced thermogenesis (DIT) was calculated in two manners, the first as the difference between 24hEE during the eucaloric session and 24hEE during fasting and a second measurement of DIT as previously described (25).
PET/CT Imaging
Study participants underwent an 18F-fluorodeoxglucose (18F-FDG) PET/CT scan after a day on a WMD and following an overnight fast. Prior to the scan, volunteers were exposed to mild cold (16°C) for 2 hours while wearing standardized clothing of ∼0.3 clo. All possible measures were taken to avoid shivering, and if shivering occurred, the volunteer was removed from the cold room for 5 minutes and then returned into the cold room.
The images were collected 1 hour after injection of 18F-FDG into the antecubital vein (mean dose 14.7 ± 0.3 mCi). PET and CT images by the Reveal 16 High Rez (CTI Molecular Imaging, Knoxville, TN) were taken from the diaphragm to the brain, and the images were coregistered with BAT quantified using a statistical parametric mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) package in MATLAB (The Math Works, Inc.). CIBA was defined as the average SUV activity of the collection of voxels with an SUV of ≥2.0 in the PET image, coinciding with areas in the CT image with Hounsfield units between −250 and −10, in which the mean BAT SUV and volume were calculated. PET images were reconstructed into image voxels of 1.95 mm × 1.95 mm × 4.00 mm and CT image voxels of 0.98 mm × 0.98 mm. CIBA SUV (g/mL) was calculated as the activity of BAT (Becquerel) per milliliter of tissue divided by the dose (Becquerels) per gram of body weight. CIBA SUV was further adjusted for FFM by multiplying CIBA SUV to FFM/weight ratio (26).
Catecholamines and Thyroid Hormones
Urinary catecholamines were collected during the 24hEE measurement, whereas volunteers were in EBL and thermoneutrality as an index of SNS tone activity. Mayo Medical Laboratories (27) measured catecholamines using the HPLC method for epinephrine and norepinephrine and liquid chromatography-tandem mass spectrometry (stable isotope dilution analysis) for metanephrine and normetanephrine. Fasting plasma free T3 and free T4 were collected the morning after EBL and stored in a freezer at −70°C. The batched samples were measured using the EIA kit from Phoenix Pharmaceuticals (Burlingame, CA) by the National Institute of Diabetes and Digestive and Kidney Diseases Laboratory Core in Bethesda, MD. The intra-assay and interassay CV were 3.0% and 4.5% for free T3 and 2.8% and 4.0% free T4, respectively.
Statistical Analyses
Alpha was set at 0.05. Analyses were performed using SAS software (version 9.4; Cary, NC). Data are presented as mean ± SD, unless otherwise stated. Catecholamines values were log10-transformed to meet normal distribution requirements for parametric analysis. Differences between sexes were assessed using Student t test. Pearson correlations were used to assess relationships between continuous variables. Linear models with 24hEE as a dependent variable and FFM as independent variable were used to calculate the residual 24hEE. Similar linear models were used with 24-h mean respiratory quotient (RQ) as the dependent variable and body fat percentage as an independent variable. Due to the small sample size, serial individual partial adjustments were used to verify if associations between CIBA SUV and volume and catecholamine levels were independent of age, sex, body fat percentage, FM, or FFM. To assess weight and body composition changes, linear models were used with weight or FM change as dependent variable and CIBA SUV, catecholamines, or thyroid hormones as independent variables.
Results
CIBA Measurements
Baseline and metabolic characteristics during EBL are shown in Table 1. Twelve volunteers had positive CIBA 18F-FDG PET/CT scans with a mean CIBA SUV of 3.25 ± 0.70 g/mL (range 2.35–4.57) and mean BAT volume of 120.17 ± 91.82 cm3 (range 3.00–289.00 cm3) with no differences by sex (P = 0.23 and P = 0.15, respectively).
Table 1.
Baseline and Metabolic Characteristics of the Cohort
| Variable | Total (N = 12) | Male (n = 7) | Female (n = 5) |
|---|---|---|---|
| Race | 5 AA/2 H/3 NA/2 W | 1 AA/2 H/2 NA/2 W | 4 AA/1 NA |
| Age, y | 31.10 ± 9.78 (19.35, 50.64) | 28.89 ± 11.10 (19.35, 50.64) | 34.19 ± 7.60 (21.26, 39.55) |
| Weight, kg | 78.08 ± 14.22 (56.40, 103.50) | 75.40 ± 14.91 (56.40, 98.10) | 75.40 ± 14.91 (60.60, 103.50) |
| BMI, kg/m2 | 26.03 ± 4.81(18.29, 34.43) | 25.71 ± 5.07 (18.29, 33.41) | 26.48 ± 2.22 (20.74, 34.43) |
| FM, kg | 22.35 ± 14.27(4.93, 52.78) | 15.90 ± 11.29 (4.93, 33.02) | 31.37 ± 13.95 (13.65, 52.78) |
| FFM, kg | 55.73 ± 12.45(42.75, 79.41) | 64.81 ± 10.02 (49.45, 79.41)a | 44.04 ± 1.31 (42.75, 45.55) |
| Body fat, % | 27.68 ± 14.82(6.90, 53.80) | 18.87 ± 9.07 (6.90, 33.0)a | 39.98 ± 10.51 (24.20, 53.80) |
| Fasting glucose, mg/dL | 89.50 ± 4.77 (80.00, 95.50) | 88.50 ± 6.03 (80.00, 95.00) | 90.70 ± 2.84 (80.00, 95.50) |
| Two-hour glucose, mg/dL | 99.27 ± 17.57 (69.00, 124.00) | 101.20 ± 21.55 (69.00, 124.00) | 97.00 ± 13.38 (80.00, 116.00) |
| 24hEE, kcal/d | 2020 ± 315 (1568, 2461) | 2201 ± 277 (1788, 2461)a | 1767 ± 141 (1568, 1918) |
| Intake, kcal/d | 2032 ± 330 (1529, 2575) | 2230 ± 275 (1779, 2575)a | 1756 ± 152 (1529, 1910) |
| EBL, kcal/d | 12 ± 61 (−59, 135) | 29 ± 74 (−59, 135) | −11 ± 30 (−42, 31) |
| Twenty-four–hour RQ, ratio | 0.87 ± 0.02 (0.83, 0.91) | 0.87 ± 0.02 (0.83, 0.91) | 0.87 ± 0.03 (0.83, 0.91) |
| CIBA SUV, g/mL | 3.25 ± 0.70 (2.35, 4.57) | 3.04 ± 0.56 (2.35, 3.80) | 3.55 ± 0.80 (2.75, 4.57) |
| BAT volume, cm3 | 120.17 ± 91.82 (3.00, 289.00) | 27.14 ± 68.80 (3.00, 185.00) | 166.40 ± 107.20 (52.00, 289.00) |
| Norepinephrine, µg/24 hb | 26.39 (20.25–36.42) | 30.33 (25.44–36.42) | 20.25 (14.39–28.95) |
| Normetanephrine, µg/24 hb | 230.56 (193.14–315.29) | 247.56 (227.17–315.29) | 227.74 (159.26–240.88) |
| Epinephrine, µg/24 hb | 5.88 (4.42–7.39) | 6.07 (5.88–7.39) | 4.42 (2.50–5.01) |
| Metanephrine, µg/24 hb | 129.94 (123.38–160.44) | 138.28 (129.22–160.42 | 128.44 (63.43–167.34) |
| Free T3, pg/mL | 2.86 ± 0.92 (1.32, 4.92) | 3.37 ± 0.77 (2.68, 4.92)a | 2.16 ± 0.63 (1.32, 2.80) |
| Free T4, ng/dL | 1.21 ± 0.18 (0.92, 1.47) | 1.31 ± 0.15 (1.08, 1.47)a | 1.07 ± 0.14 (0.92, 1.24) |
| Weight change, kgc | 1.80 ± 4.81 (−5.20, 8.40) | 3.37 ± 1.44 (−1.60, 7.30) | −0.40 ± 5.62 (−5.20, 8.40) |
| FM change, kgc | 1.18 ± 3.27 (−4.45, 5.43) | 2.76 ± 1.13 (−1.96, 5.43) | −0.22 ± 3.56 (−4.45, 4.73) |
| FFM change, kgc | 0.16 ± 2.16 (−2.65, 3.93) | −0.45 ± 2.18 (−1.94, 3.93) | 2.33 ± 1.04 (−2.65, 3.67) |
Data are mean ± SD (lowest and highest values), except for catecholamines, which are median (interquartile range).
Abbreviations: AA, African American; BMI, body mass index; H, Hispanic; NA, Native American; W, white.
P < 0.05 vs females by Student t test.
n = 11 with urinary catecholamine measurements.
n = 11 with 6-month follow-up body composition measurements.
Relationship of CIBA to 24hEE and body composition
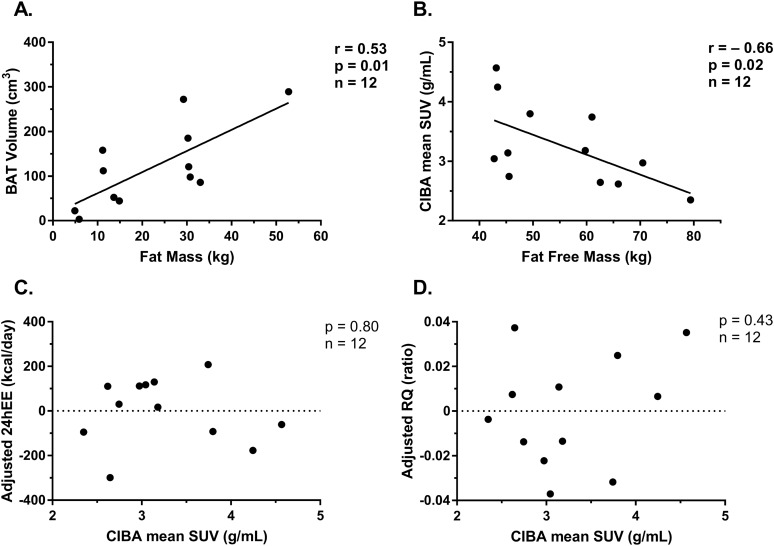
As previously described (11), CIBA was negatively correlated with FFM (r = −0.66; P = 0.02), but not with other baseline body composition measurements. BAT volume was positively associated with body fat percentage and FM (r = 0.76, P < 0.01; and r = 0.74, P < 0.01, respectively; Fig. 1). BAT measurements were not associated with 24hEE measured during EBL after adjustment for FFM (CIBA: r = −0.08, P = 0.80; BAT volume: r = 0.19, P = 0.56) or 24-h mean RQ after adjustment for body fat percentage (CIBA: r = 0.25, P = 0.43; BAT volume: r = 0.29, P = 0.36). CIBA was also not associated with either measurement of DIT (both P > 0.21).
Figure 1.
Correlation of (A) BAT volume (g) and FM (kg), (B) correlation of CIBA mean SUV (g/mL) and FFM (kg), (C) 24hEE measured during EBL and thermoneutrality, after adjustment for FFM, and (D) 24-h mean RQ measured during EBL and thermoneutrality, after adjustment for body fat percentage.
Relationship of CIBA measurements to urinary catecholamine concentrations and thyroid hormone measurements
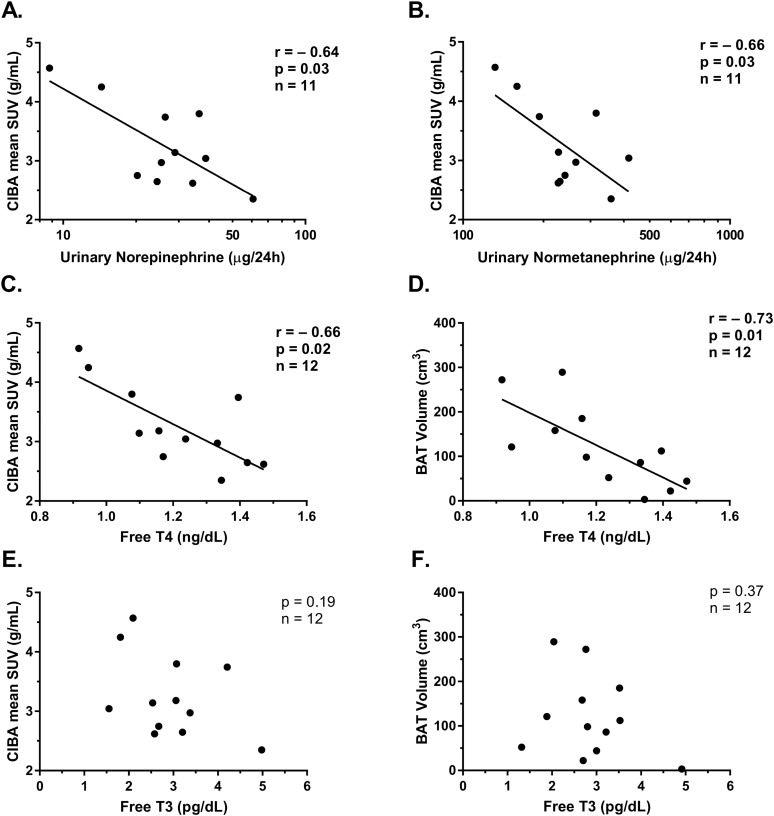
Urinary norepinephrine was not associated with baseline characteristics including age, FFM, FM, body fat percentage, or 24hEE adjusted for FFM (all P > 0.05). CIBA mean SUV was negatively associated with 24-h urinary norepinephrine and normetanephrine concentrations (r = −0.64, P = 0.03; and r = −0.66, P = 0.03, respectively; Fig. 2). After adjustment for FFM, the relationship was attenuated, but the directionality remained (r = −0.58, P = 0.07; and r = −0.62, P = 0.05). BAT volume was associated with epinephrine and metanephrine concentrations (r = −0.70, P = 0.02; and r = −0.71, P = 0.02, respectively); however, upon removal of outliers, these results were no longer significant (P > 0.70).
Figure 2.
Correlation between (A) CIBA mean SUV (g/mL) and urinary norepinephrine (µg/24 h) measured during EBL and thermoneutrality and (B) urinary normetanephrine (µg/24 h) measured by HPLC during EBL and thermoneutrality. Relationship between free T4 (ng/dL) and both (C) CIBA and (D) BAT volume. Lack of correlation between plasma free T3 (pg/dL) and both (E) CIBA and (F) BAT volume. Both free T4 and free T3 were measured fasting the morning after EBL diet and during thermoneutrality. Results remained similar after serial adjustment for age, sex, FM, or FFM.
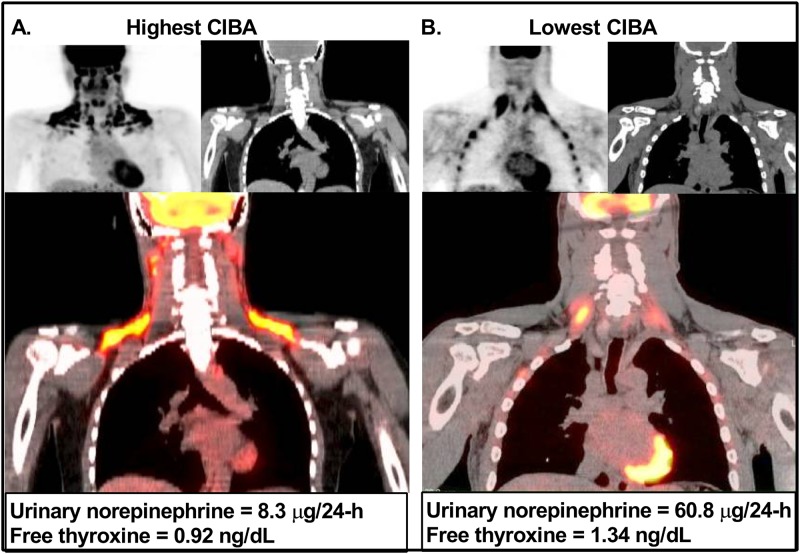
Free T3 was associated with FFM (r = 0.83; P < 0.01), but not with other baseline measurements, including age, weight, and body fat percentage (all P > 0.05). Free T4 was associated with FFM (r = 0.75; P < 0.01) and inversely with body fat percentage (r = −0.69, P = 0.01), but not with other baseline measurements including age (P = 0.56), weight (P = 0.73), and FM (P = 0.07). Both CIBA mean SUV and BAT volume were negatively associated with free T4 (r = −0.75, P = 0.005; and r = −0.73, P = 0.007, respectively), but not free T3 (P = 0.27 and P = 0.37, respectively), such that individuals with higher T4 had lower CIBA. After partial adjustment for FFM, CIBA and BAT volume were still associated with free T4 (r = −0.61, P = 0.05; and r = −0.67, P = 0.02). In a model with both free T4 and urinary norepinephrine, neither were independently associated with CIBA (data not shown), but these results should be interpreted with caution given the small sample size. Pictorial comparisons of the PET/CT scans of the individual with the highest CIBA and the lowest urinary norepinephrine and free T4 vs the individual with the lowest CIBA and the highest free T4 and urinary norepinephrine are shown in Fig. 3. Sensitivity analyses using nonweighted SUV scores were used, and results were similar (data not shown).
Figure 3.
Comparison of the 18F-FDG PET/CT images of the subjects with highest and lowest CIBA SUV values. Volunteer with the lowest urinary norepinephrine levels and lowest plasma free T4 images in the left panel, in which PET, CT, and fused coronal PET/CT images (A) show a large area of CIBA, with the highest SUV in our cohort of 4.57 SUV (g/mL).This series of images can be compared with the PET and CT and fused coronal PET/CT images (B) of the volunteer with the highest urinary norepinephrine and free T4 levels in the right panel, in which no visualization of CIBA is seen along with a mean SUV of 2.51 (g/mL), the lowest SUV in our cohort.
Relationship of 6-month body composition changes to CIBA and urinary catecholamine concentrations
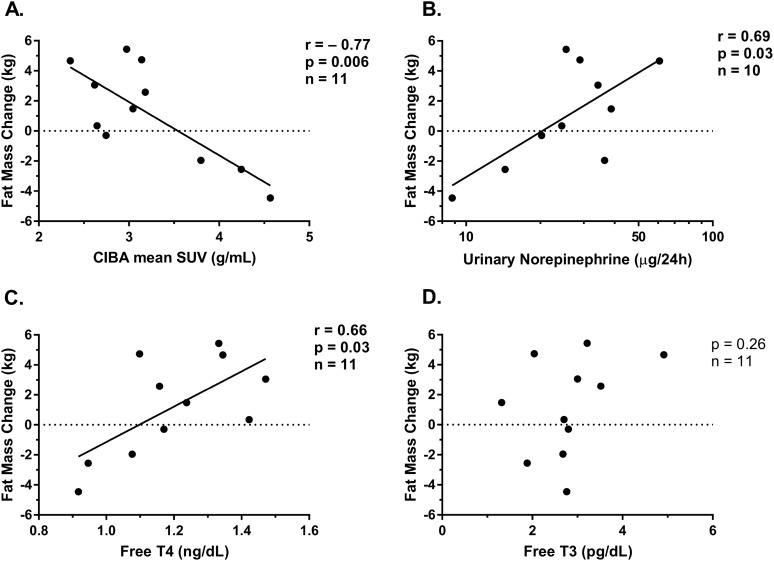
Eleven out of the initial 12 volunteers (92%) returned for follow-up assessment at 6 months. The mean weight change observed at 6 months was 1.80 ± 4.81 kg (P = 0.22 vs zero), with a mean FM change of 1.18 ± 3.27 kg (P = 0.25) and a mean FFM change of 0.16 ± 2.16 kg (P = 0.81). As previously reported (11), CIBA was associated with FM change (kg) at 6 months [r = −0.77, β = −3.5 (95% CI −5.8 to −1.3) kg per 1 SUV; P = 0.006], but not with overall weight change (P = 0.15) or FFM change (P = 0.79). BAT volume was not associated with weight (P = 0.91), FFM (P = 0.09), or FM (P = 0.40) changes at 6 months.
Baseline 24-hour urinary norepinephrine concentration (r = 0.69; P = 0.03; n = 10) and free T4 (r = 0.66; P = 0.03) measured during thermoneutrality were correlated with the change in FM at 6 months (Fig. 4), such that a twofold increase in norepinephrine predicted a 3.0-kg (0.4 to 5.5) increase in FM and 0.1-µg/dL increase in free T4 predicted a 1.18-kg (0.17 to 2.19) increase in FM at 6 months. Normetanephrine (P = 0.10), metanephrine (P = 0.88), epinephrine (P = 0.55), and free T3 (P = 0.26) concentrations were not associated with FM change at 6 months.
Figure 4.
Relationship between FM gain at 6 months and CIBA, urinary norepinephrine, free T4, and free T3. FM change was inversely associated with (A) CIBA and positively associated with both (B) 24-h urinary norepinephrine concentration and (C) free T4, whereas it was not associated with (D) free T3. Results by Pearson correlation coefficient.
Discussion
In 12 volunteers with CIBA, CIBA was negatively associated with markers of SNS activity and thyroid function measured during EBL and thermoneutrality, but not with 24hEE. As we previously reported, CIBA was negatively associated with baseline FFM and FM change at 6 months (11) (e.g., those with the highest CIBA had the lowest baseline FFM and lost FM at 6 months follow-up). In these same individuals, urinary catecholamine concentrations were positively associated with FM change at 6 months. Our data indicate a link between lower norepinephrine concentration and CIBA, and both were predictors of FM gain. We hypothesize that a lower baseline sympathetic activity, as reflected by 24-hour urinary norepinephrine measures, indicates a greater capacity for both increasing sympathetic, and thus BAT, activation. This increased ability to activate CIBA then drives the protection against FM gain that we observed at 6 months. We suspect the observed associations among free T4, CIBA, and FM gain indicate similar physiology.
Contrary to previous studies, we observed CIBA to be negatively associated with FFM and did not find CIBA to be associated with age or adiposity measurements (3, 4, 10), possibly due to the young age of our small cohort and its ethnic diversity (3, 12). Because FFM is the most substantial determinant of EE (28), our results indicate that CIBA may not be an important predictor of EE during EBL and thermoneutrality after controlling for this confounder. Although prior studies have shown a positive association between BAT and EE during cold exposure (4, 6), these studies used a hood system that allows for the measurement of EE during a brief period of cold exposure, as short as 20 minutes, and does not capture the physiologic conditions of meals and sleep. Our methodology using an initial acclimation chamber, measured during thermoneutrality, and the gold standard whole-room indirect calorimeter allowed for very precise measurement of 24hEE with the subject in EBL (EBL 12 ± 61 kcal/d; P = 0.51 vs zero) prior to cold exposure to determine whether the baseline conditions influence BAT activation upon cold exposure. Although we did not find CIBA to be a determinant of thermoneutral and EBL EE, prior studies have assessed the contributions of BAT to overall EE. BAT has been reported to increase EE by 20% to 30% (4, 5) and involved in DIT at thermoneutrality (25); however, other studies have found minimal contribution of activated BAT to overall EE (29–31). Therefore, further larger studies are warranted to definitely determine the contribution of BAT in potentially combating obesity (32).
Studies assessing catecholamine concentrations in settings in which BAT is not purposefully activated in humans have primarily been done in patients with pheochromocytomas, a medullary adrenal tumor that oversecretes catecholamines. PET/CTs performed in these patients to stage the disease have shown widespread BAT activation (17, 33, 34). However, in a study with healthy subjects, plasma norepinephrine and its metabolite dihydroxyphenylglycol increased with cold exposure, but there were no differences in those catecholamines measured before and after cold stimulation in volunteers with positive vs negative CIBA (18). In contrast, using 24-hour urinary concentrations instead of plasma measures, we demonstrated a negative association between CIBA and urinary norepinephrine measured during EBL and thermoneutrality in healthy individuals. Our results may suggest that lower sympathetic tone during EBL and thermoneutrality signify an increased ability to both activate SNS to some maximal threshold and recruit BAT upon cold exposure. One potential mechanism may be via an undersaturation of available beta-adrenergic receptors in BAT in individuals with lower sympathetic tone. Thus, those with lower SNS tone during thermoneutrality might have a greater potential to activate BAT during cold, requiring a lesser increase in norepinephrine from the SNS to do so.
In this cohort of individuals with positive CIBA, we also found a negative association between free T4 and CIBA such that individuals with higher BAT activity in response to cold had lower baseline free T4 measured during EBL and thermoneutrality. This association was in the same direction as that for urinary norepinephrine, which may be not surprising given the complex synergistic interactions of thyroid hormones and the adrenergic system (35). The correlation with CIBA was observed only with free T4 and not with free T3; this could possibly be explained due to the relatively higher secretion of T4 from the thyroid and the lesser free T3 production by deiodinases during states of thermoneutrality and EBL (36). In addition, free T3 conversion is tissue specific, and peripheral measurements may not be as reflective of the local free T3 action (37) that may be observed in BAT. Higher free T4 has been reported in BAT-negative subjects (19, 38) with normal thyroid function, in line with our cohort of positive CIBA individuals, in which those individuals with the lowest activation had the highest free T4. Therefore, in our cohort of BAT-positive subjects, our free T4 results may be due to our methodology in measuring thyroid function during thermoneutrality and EBL. The similar, yet independent, results between free T4 and urinary catecholamine concentrations with CIBA and future FM change seem to reflect a similar physiology.
The fact that the subjects at 6 months follow-up did not gain weight is not surprising, because our subjects were weight stable prior to admission to our unit. However, the variation in the change in FM is intriguing, and identifying intrinsic factors that predispose to fat gain in a small period of time may provide insight for identification of those prone to longer-term weight gain. Given our previous finding that greater CIBA predicted less FM gain at 6 months, we tested if body composition changes were also associated with urinary catecholamines or thyroid function measures. We found that both urinary norepinephrine and plasma free T4 measured in thermoneutrality and during EBL were positively associated with FM change at 6 months. As we noted earlier, it is possible that greater ability to activate brown fat leads to more fat utilization and therefore less fat storage. Thus, a lower urinary norepinephrine and free T4 state elicits a greater BAT response, and this could drive FM change. How BAT might affect FM change is unclear, as we did not find an association with baseline EE. However, diets with different macronutrient composition, specifically those with high carbohydrates, which are known to stimulate SNS, may activate BAT (39). Because EE was not associated with CIBA in our study, it is also possible that CIBA might be evoking an effect on food intake.
Our study has several limitations. First, we have relatively small sample size; however, this cohort was carefully studied in a controlled inpatient clinical research unit, and our sample size provided enough power to assess for relationships between CIBA and both weight change and hormone measures. However, a larger sample of subjects is needed to verify if these are a conditional effect or an independent effect on future FM gain. We also did not have catecholamines or thyroid function tests collected during or after cold exposure, but these thermoneutral measurements may offer evidence of who has the potential to activate BAT and thus may have clinical and research utility. Lastly, there was no assessment of EE or dietary intake while subjects were in free-living condition, and therefore, we cannot directly assess why some individuals gained FM.
In conclusion, we found in individuals with CIBA, urinary norepinephrine concentrations and plasma free T4 measured during EBL and thermoneutrality were negatively associated with CIBA. Further, lower CIBA and higher baseline urinary norepinephrine and plasma free T4 concentrations predicted FM gain at 6 months. We propose that a higher SNS tone during EBL and thermoneutrality may signify a reduced ability to recruit BAT during cold exposure, thereby limiting any potential energy dissipating properties of BAT in these individuals. The use of catecholamines and thyroid hormones as a marker for potential BAT activation may help identify those who may derive benefit from the obesity opposing functions of BAT.
Acknowledgments
The authors thank the nursing and dietary kitchen staff of the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Obesity and Diabetes Clinical Research Section in Phoenix, AZ for care of the participants. Most importantly, the authors thank the volunteers for participating in this study.
Financial Support: This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Clinical Trial Information: ClinicalTrials.gov no. NCT00523627 (registered 31 August 2007).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 18F-FDG
18F-fluorodeoxyglucose
- 24hEE
24-hour energy expenditure
- BAT
brown adipose tissue
- CIBA
cold-induced brown adipose tissue activation
- DIT
diet-induced thermogenesis
- EBL
energy balance
- EE
energy expenditure
- FFM
fat-free mass
- FM
fat mass
- PET
positron emission tomography
- RQ
respiratory quotient
- SNS
sympathetic nervous system
- SUV
standardized uptake value
- WMD
weight-maintaining diet
References
- 1. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 2. Virtue S, Vidal-Puig A. Assessment of brown adipose tissue function. Front Physiol. 2013;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–1508. [DOI] [PubMed] [Google Scholar]
- 5. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–1525. [DOI] [PubMed] [Google Scholar]
- 6. Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, Saito M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring). 2011;19(1):13–16. [DOI] [PubMed] [Google Scholar]
- 7. Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123(8):3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zafrir B. Brown adipose tissue: research milestones of a potential player in human energy balance and obesity. Horm Metab Res. 2013;45(11):774–785. [DOI] [PubMed] [Google Scholar]
- 10. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schlögl M, Piaggi P, Thiyyagura P, Reiman EM, Chen K, Lutrin C, Krakoff J, Thearle MS. Overfeeding over 24 hours does not activate brown adipose tissue in humans. J Clin Endocrinol Metab. 2013;98(12):E1956–E1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123(8):3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17(6):719–734. [DOI] [PubMed] [Google Scholar]
- 14. Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue in patients with phaeochromocytoma. Int J Obes. 1986;10(3):219–227. [PubMed] [Google Scholar]
- 15. Melicow MM. One hundred cases of pheochromocytoma (107 tumors) at the Columbia-Presbyterian Medical Center, 1926-1976: a clinicopathological analysis. Cancer. 1977;40(5):1987–2004. [DOI] [PubMed] [Google Scholar]
- 16. Ricquier D, Nechad M, Mory G. Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. J Clin Endocrinol Metab. 1982;54(4):803–807. [DOI] [PubMed] [Google Scholar]
- 17. Wang Q, Zhang M, Ning G, Gu W, Su T, Xu M, Li B, Wang W. Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity. PLoS One. 2011;6(6):e21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14(2):272–279. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Q, Miao Q, Ye H, Zhang Z, Zuo C, Hua F, Guan Y, Li Y. The effects of thyroid hormones on brown adipose tissue in humans: a PET-CT study. Diabetes Metab Res Rev. 2014;30(6):513–520. [DOI] [PubMed] [Google Scholar]
- 20. Kim MS, Hu HH, Aggabao PC, Geffner ME, Gilsanz V. Presence of brown adipose tissue in an adolescent with severe primary hypothyroidism. J Clin Endocrinol Metab. 2014;99(9):E1686–E1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lahesmaa M, Orava J, Schalin-Jäntti C, Soinio M, Hannukainen JC, Noponen T, Kirjavainen A, Iida H, Kudomi N, Enerbäck S, Virtanen KA, Nuutila P. Hyperthyroidism increases brown fat metabolism in humans. J Clin Endocrinol Metab. 2014;99(1):E28–E35. [DOI] [PubMed] [Google Scholar]
- 22. Martínez-Sánchez N, Moreno-Navarrete JM, Contreras C, Rial-Pensado E, Fernø J, Nogueiras R, Diéguez C, Fernández-Real JM, López M. Thyroid hormones induce browning of white fat. J Endocrinol. 2017;232(2):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 24. Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab. 2013;98(7):2791–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hibi M, Oishi S, Matsushita M, Yoneshiro T, Yamaguchi T, Usui C, Yasunaga K, Katsuragi Y, Kubota K, Tanaka S, Saito M. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes. 2016;40(11):1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, Tal I, Dieckmann W, Gupta G, Kolodny GM, Pacak K, Herscovitch P, Cypess AM, Chen KY. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA. 2017;114(32):8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moyer TP, Jiang NS, Tyce GM, Sheps SG. Analysis for urinary catecholamines by liquid chromatography with amperometric detection: methodology and clinical interpretation of results. Clin Chem. 1979;25(2):256–263. [PubMed] [Google Scholar]
- 28. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respira–tory chamber. J Clin Invest. 1986;78(6):1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med. 2013;54(4):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muzik O, Mangner TJ, Leonard WR, Kumar A, Granneman JG. Sympathetic innervation of cold-activated brown and white fat in lean young adults. J Nucl Med. 2017;58(5):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterson CM, Orooji M, Johnson DN, Naraghi-Pour M, Ravussin E. Brown adipose tissue does not seem to mediate metabolic adaptation to overfeeding in men. Obesity (Silver Spring). 2017;25(3):502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marlatt KL, Ravussin E. Brown adipose tissue: an update on recent findings. Curr Obes Rep. 2017;6(4):389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banzo J, Ubieto MA, Berisa MF, Andrés A, Mateo ML, Tardín L, Parra A, Razola P, Prats E. Extensive hypermetabolic pattern of brown adipose tissue activation on 18F-FDG PET/CT in a patient diagnosed of catecholamine-secreting para-vesical paraganglioma. Rev Esp Med Nucl Imagen Mol. 2013;32(6):397–399. [DOI] [PubMed] [Google Scholar]
- 34. Yamaga LY, Thom AF, Wagner J, Baroni RH, Hidal JT, Funari MG. The effect of catecholamines on the glucose uptake in brown adipose tissue demonstrated by (18)F-FDG PET/CT in a patient with adrenal pheochromocytoma. Eur J Nucl Med Mol Imaging. 2008;35(2):446–447. [DOI] [PubMed] [Google Scholar]
- 35. Silva JE, Bianco SD. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid. 2008;18(2):157–165. [DOI] [PubMed] [Google Scholar]
- 36. Bianco AC, McAninch EA. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol. 2013;1(3):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116(10):2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lapa C, Maya Y, Wagner M, Arias-Loza P, Werner RA, Herrmann K, Higuchi T. Activation of brown adipose tissue in hypothyroidism. Ann Med. 2015;47(7):538–545. [DOI] [PubMed] [Google Scholar]
- 39. Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr. 2013;98(1):57–64. [DOI] [PubMed] [Google Scholar]