Abstract
Purpose
To review and highlight important practical aspects of deep anterior lamellar keratoplasty (DALK) surgery and provide some useful tips for surgeons wishing to convert to this procedure from the conventional penetrating keratoplasty (PK) technique.
Methods
In this narrative review, the procedure of DALK is described in detail. Important pre, intra, and postoperative considerations are discussed with illustrative examples for better understanding. A comprehensive literature review was conducted in PubMed/Medline from January 1995 to July 2017 to identify original studies in English language regarding DALK. The primary endpoint of this review was the narrative description of surgical steps for DALK, its pitfalls, and management of common intraoperative complications.
Results
A standard DALK procedure can be successfully performed taking into consideration factors such as age, ophthalmic co-morbidities, status of the crystalline lens, retina, and intraocular pressure. Careful trephination and dissection of the host cornea employing appropriate technique (such as big bubble technique, manual dissection, visco-dissection, etc.) suitable for the specific case is important to achieve good postoperative outcomes. Prompt identification of intraoperative complications such as double bubble, micro and macroperforations, etc. are vital to change the management strategies.
Conclusion
Although there is a steep learning curve for DALK procedure, considering details and having insight into the management of intraoperative issues facilitates learning and reduces complication rates.
Keywords: DALK, Lamellar corneal transplant, Keratoplasty
Introduction
Penetrating keratoplasty (PK) has been performed for management of keratoconus for over 7 decades.1, 2 Literature shows that keratoconus is one of the most common indications for PK, and the recipients have higher graft survival in keratoconus irrespective of whether the graft was the same size or oversized.3 However, PKs can lead to mediated endothelial rejection, endothelial cell loss, and complications including expulsive hemorrhage, traumatic wound dehiscence, and endophthalmitis.4, 5
Deep anterior lamellar keratoplasty (DALK) has become an alternative procedure and gained popularity in the past one and a half decades.6, 7, 8, 9, 10, 11, 12 In DALKs, the majority of the corneal tissue is removed, leaving behind Descemet's membrane (DM)8 and the corneal endothelium, before suturing a donor corneal graft, which is devoid of DM. Although DALK is generally a longer procedure, it has attracted a lot of interest from surgeons due to its claims on fewer allogenic endothelium rejection episodes13; so much so that it has been reported to have good outcomes even after hydrops (which was previously considered a contraindication of DALK).14 It is also thought that DALK allows endothelial cell counts to be maintained for a longer period.12, 15, 16, 17, 18
In comparison to PK procedures, several advantages and disadvantages have been reported for DALK.19, 20 Some studies have shown that the best corrected visual acuity, refractive results, and complication rates are similar after DALK and PK.6, 8, 12, 20, 21, 22, 23 while others have reported superior visual outcomes after PK.24, 25, 26 Coster et al. in an Australian registry study observed a decrease in PK procedures and a rise in DALK procedures for keratoconus from 1996 to 2012 and that graft survival and visual outcomes were significantly better with PK.2
Studies comparing the outcomes of PK versus DALK have varying inclusion and follow-up criteria. Moreover, the number of randomized studies is sparse and underpowered to detect any meaningful difference.22 There is some evidence that a surgeon's learning curve may affect the outcomes of DALK procedures.27, 28 The difference may be attributed to the different surgical procedures and technical skills, resulting in irregularity at the host–donor interface, which can reduce vision after DALK.20
Over the past decade, numerous surgical strategies have been developed. DALK can be performed using various methods to create a good optical graft-host interface.29 In the literature, DALK has been broadly classified as pre-Descemetic, where a small amount of posterior stroma is left intact along with the DM, and Descemetic DALK where the dissection is achieved up to the DM.11 Several techniques have been employed to achieved these dissections including big bubble technique,8, 30 intrastromal air injection,31, 32 Melles technique,33, 34 hydrodelamination,12 viscodissection,35, 36 femtosecond laser assisted,37, 38 etc.
This review will give a detailed insight into the pre-assessment, the surgical technique, and management of a few frequently encountered issues with DALK procedures for beginners.
Methods
A comprehensive literature review was conducted in PubMed/Medline from January 1995 to July 2017 to identify original studies in the English language regarding DALK. The following MeSH terms were used: “DALK” OR “Deep Anterior Lamellar Keratoplasty” OR “Anterior Lamellar Keratoplasty”. Moreover, the following text words were used as well: “Preoperative DALK”, “Age and DALK”, “Comorbidities and DALK”, “Cataract and DALK”, “Intraocular Pressure and DALK”, “Retina and DALK”, “Anesthesia and DALK”, “Donor Cornea and DALK”, “Techniques and DALK”, “Big Bubble and DALK”, “Manual Dissection and DALK”, “Hydrodelamination and DALK”, “Viscodissection and DALK” and “Complications and DALK”. In each text words, the words “DALK” or “Deep Anterior Lamellar Keratoplasty” were used instead of each other. In addition, the citations from the above searches were also included. The title and abstract of the identified articles were screened for relevancy. Of these, full-texts of the relevant articles were retrieved for eligibility, and relevant studies reporting data required for this narrative review were included in this review. The primary endpoint of this review was the narrative description of surgical steps for DALK, its pitfalls, and management of common intraoperative complications.
Results
Preoperative considerations
Selection of appropriate cases for DALK is vital for successful outcomes.
Age
In general, older patients are less likely to have frequent rejection episodes, as their immune systems are not as strong as young patients.39, 40, 41 The most common indication for DALK is keratoconus,42 and these patients tend to be younger. Hence, in comparison to PK, DALK offers an advantage of reduced chances of rejection.
In younger age groups (age <5 years), DALK shows a high rate of graft failure, which is not statistically different from PK. But in the age group >5 years, the rate of graft failure is less with DALK compared to PK. Therefore, in pediatric patients >5 years, DALK has a distinct advantage over PK.43
Co-morbidities
Active inflammation or infection increases the risk of graft failure, and this should be treated before any corneal transplantation.44 Combining ocular surface procedures with DALK may facilitate visual rehabilitation once the ocular surface is white and quiet for a few months. Such eyes are at a risk of frequent ocular surface inflammation or infections, which can trigger immunological corneal graft rejection. However, performing DALK compared to a PK, in such morbid eyes, offers several advantages including: 1) a reduced risk of immunological rejection; 2) a decreased frequency of topical medications after a few months, hence reducing the surface toxicity; and; 3) a relative surgical ease of repeat DALK, as the tissue can be peeled off the DM after several years.
Ocular surface disease, such as limbal stem cell failure, has been noted to be a ‘relative’ contraindication to DALK in the past. However, when DALK is combined with ocular surface reconstruction, it offers better chances of graft survival than PK.8, 45, 46 There are reports that performing DALK in comparison to PK reduces the risk of secondary glaucoma and cataract formation.47
Big bubble DALK is effective in patients with keratoconus with stromal scars. According to one publication, scar depth/minimal corneal thickness ratio seems to predict the DM perforation.48 DALK provides good long-term visual outcome for the patients with herpetic keratitis, keratoconus, stromal scars and corneal dystrophies.49
Often patients with compromised mental abilities, e.g. Down's syndrome, Turner's syndrome, etc., have ectatic corneal disease. In such patients, performing DALK has an advantage over PK, as it offers reduced risks of rejection and minimizes severe ocular damage secondary to self-trauma or eye rubbing.
Status of the crystalline lens
It is important to assess whether there is a coexisting cataract. If the patient has a cataract, which is contributing to the reduced vision, and if the corneal pathology allows sufficient surgical view for a safe cataract surgery, then the option of cataract surgery with simple monofocal intraocular lens (IOL) can be considered before DALK. If the corneal pathology does not allow adequate surgical view for safe cataract surgery, then DALK should be offered before cataract surgery.50 This will reduce the risk of performing cataract surgery through a hazy cornea, which increases the risk of uncontrolled capsulorhexis, posterior capsule tear, and vitreous loss.51 In addition, the unpredictability in keratometric data, and as a consequence refractive outcome, will be increased if cataract surgery is performed prior DALK. On the other hand, there will be a risk of graft failure and damage to the graft with phacoemulsification. Use of dispersive viscoelastics during cataract surgery will reduce these risks.
Cataract surgery can be planned, once the DALK settles (at least 1 year postoperatively). At this stage, there will be an opportunity to correct any regular astigmatism with a toric IOL following cataract surgery.52 If DALK is performed in a pseudophakic eye, then there is an opportunity to correct residual regular astigmatism with piggyback posterior chamber IOLs.52 Patients with preexisting multifocal IOLs should be counseled about the possible worsening of multifocality following DALK, due to induced irregular astigmatism, and the need for IOL exchange, piggyback IOL or excimer laser surgery following DALK. However, Meyer et al. reported that in eyes with keratoconus, piggyback IOLs are at a risk of rotation.53
Cataract surgery can also be performed at a triple procedure along with DALK. When a type-1 big bubble (described later in the review) is achieved, simultaneous DALK and phacoemulsification can be safely accomplished.51 Dua's layer54 allows a clear view for performing phacoemulsification with the added benefit of its toughness, which can maintain a stable anterior chamber for cataract surgery. This should not be attempted when a type-2 big bubble is achieved.51
Intraocular pressure
Optimal intraocular pressure (IOP) control before any keratoplasty is vital for successful outcomes. DALK should not be offered until the IOP is controlled medically or surgically. Eyes with extensive ocular co-morbidity will need a glaucoma tube device (Ahmed, Baerveldt, etc.) inserted for adequate IOP control before DALK surgery. Preoperative use of intravenous mannitol may be necessary in some patients to reduce the IOP intraoperatively. In cases where peribulbar or retrobulbar anesthesia is used, application of Honan's balloon or digital message for a few minutes before surgery can be helpful.55
In the ex-vivo model of DALK, bubbles of optimal diameters are obtainable only at physiologic pressures (10–20 mmHg). Extremely high IOPs (40 mmHg) resulted in big bubbles of significantly smaller diameter than those obtained at physiologic and low (5 mmHg) IOPs.56
Status of retina
It is vital to establish the visual potential by assessing the status of the macula and retina with detailed indirect ophthalmoscopy, where the view is permitted through the diseased cornea. Where adequate retina view is not available, an ocular coherence tomography (OCT) scan, ultrasound B-scan, etc. can be used to assess the integrity of the macula and retina. Potential acuity meter testing can also be useful in estimating the postoperative visual potential.57
Preoperative counseling
A detailed discussion with the patient and relatives is necessary before planning DALK surgery to ensure that they have realistic expectations. Visual rehabilitation may take over 1 year; and patients may need further interventions (e.g. spectacle prescription, contact lens, suture removal/adjustments, further keratorefractive surgery to treat irregular astigmatism, intraocular surgery to manage cataract and regular astigmatism, glaucoma surgery, etc.). Patients' compliance for regular clinic follow-ups should also be assessed. Patients should be warned about the possibility of rejections years after surgery. Late stromal rejections are related to the possible persistence of donor keratocytes in lamellar grafts.58
The surgery for DALK
Anesthesia
For most patients, DALK can be performed safely as a day procedure under monitored anesthesia care. Retrobulbar or peribulbar anesthesia and a lid block, using a relatively long-lasting drug such as bupivacaine, a combination of bupivacaine and mepivacaine, or lidocaine, can usually suffice.59 General anesthesia may be indicated for young patients or other circumstances such as mental impairment, deafness, aphasia, or language barriers. General anesthesia is also suitable for repeat surgery, or difficult or prolonged procedures. Local anesthesia (blocks) provides an alternative when general anesthesia and ophthalmic regional blocks are less desirable, but overall, its use is limited.60
Preparing the donor corneal button
A donor who is suitable for PK should ideally be chosen for DALK procedure.
The donor cornea quality in DALK is not critical for the clinical outcome. In effect, elderly age of donor (age ≤ 88 years), endothelial cell densities of ≥1000 cells/mm2, overall preservation time of less than 2 weeks for cold storage or ≤35 days in cases of organ culture, and preservation time after split prior to grafting ≤96 h can be considered as safe corneas for DALK.61 Donor tissue quality harvested by corneoscleral disc excision technique is comparable to donor tissue quality after whole globe enucleation.61 Organ culture method for preservation is suitable as well as 4 °C storage techniques of donor tissues used for DALK.61
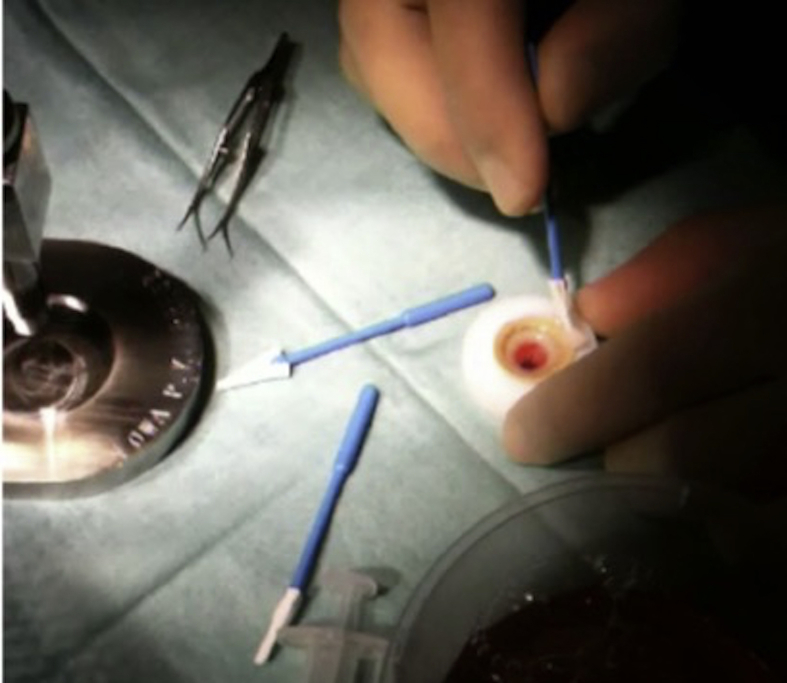
Trephination can be performed with any trephine of the surgeon's choice. The most crucial aspect during this procedure is the centration of the transplant on the trephine block and to ensure that there is no fluid under the tissue (Fig. 1) which may make it slide during the trephination process, leading to an oblique trephination and an oval donor button with high postoperative astigmatism. The donor is stored into the medium until the recipient bed is prepared. The DM should not be peeled until the recipient cornea is dissected barring DM successfully. In the event of macroperforation of the DM (tears larger than 1 quadrant of the cornea), the surgeon may need to convert the procedure to a conventional PK.
Fig. 1.
Dehydration of the Teflon block – corneal tissue interface using a sponge to prevent sliding of the tissue during trephination.
Insertion of eyelid speculum
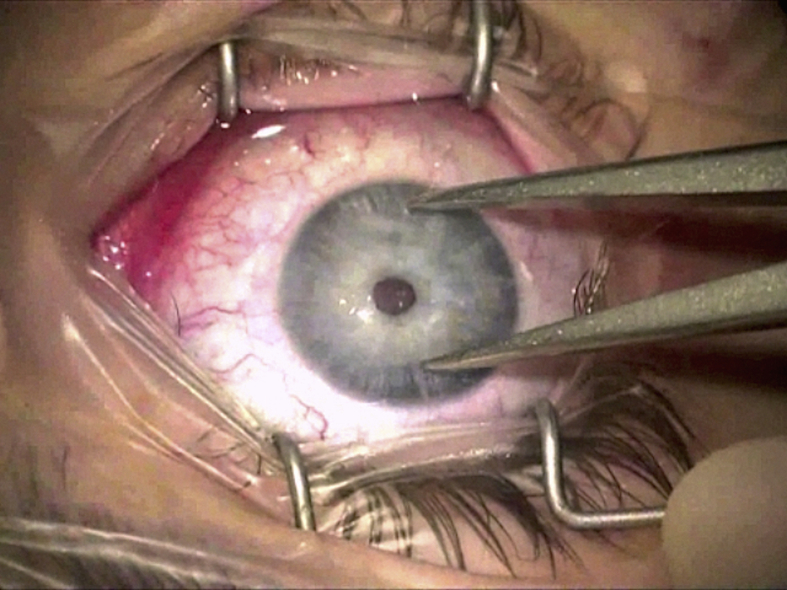
Speculums with adjustable blades/loops (Fig. 2) that do not exert any undue pressure on the eyeball are recommended for DALK procedures compared to non-adjustable wire loop speculums. This ensures decreased positive pressure from the intraocular contents, and hence reduced risks of inducing astigmatism with tight suturing postoperatively.
Fig. 2.
Adjustable speculum, which does not apply under pressure on the eyeball.
Trephination of the host cornea
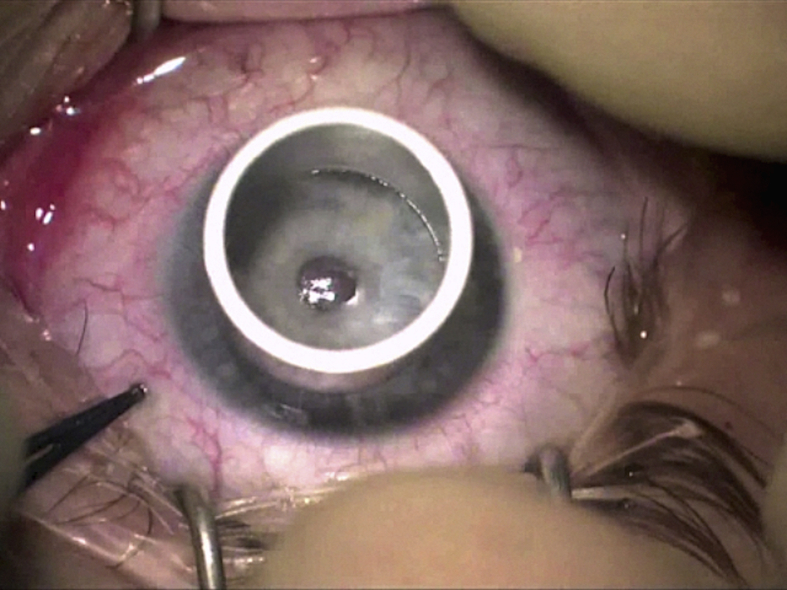
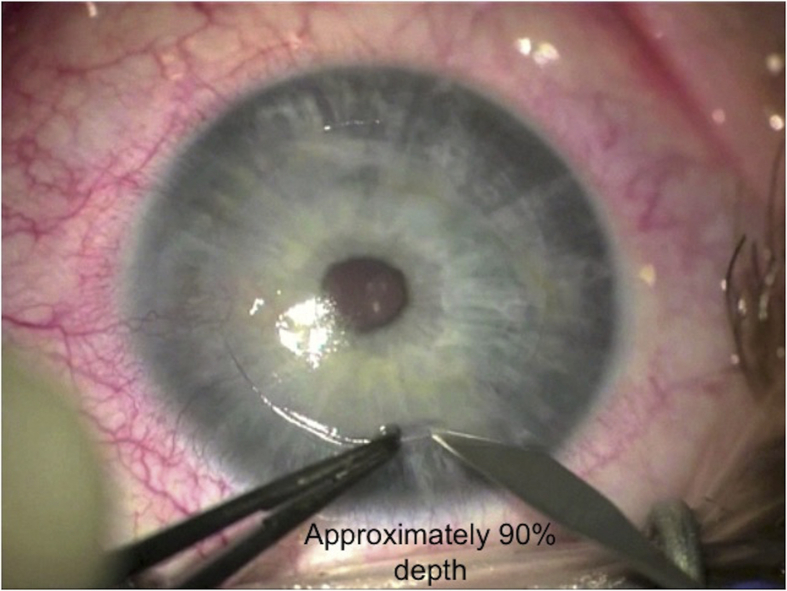
The size of trephine should be chosen to adequately encompass the corneal pathology, but to avoid too large trephination. Recent studies have reported that DALK with same-size donor grafts results in relatively low myopia,9, 24, 62 and the optical outcomes of DALK with same-size grafts for keratoconus are comparable to those of PK.63 The surgeons have a wide choice of trephines available including handheld trephine models and vacuum trephines. Marking the center of the host cornea will ensure adequate centration of trephination. Once the appropriate trephine size is selected and placed on the cornea (Fig. 3), it is rotated for 3 quarter turns with moderate pressure. This is performed after considering the preoperative corneal pachymetry of the patient (assessed on a tomography device, e.g. a Pentacam®) as each quarter turn will trephine the cornea approximately 60 μm deep. This should be taken in consideration of the thinnest pachymetry using a device such as a Scheimpflug tomography machine (Pentacam, Oculus, Germany). According to one study, Pentacam-based big bubble DALK using 90% intended depth of initial lamellar trephination allows for safe, reliable, and successful bubble formation.64 The depth of the trephination is checked with a paracentesis blade to ensure appropriate depth is achieved (Fig. 4). Care should be taken to avoid excessive pressure to avoid full thickness trephination.
Fig. 3.
Appropriately-sized trephine that encompasses the corneal pathology and leaves desired amount of peripheral host corneal tissue.
Fig. 4.
Depth of the trephination checked using a paracentesis blade.
Removing the superficial corneal layers
Manual dissection techniques are recommended when the corneal scar or corneal pathology is deeper, involving the DM. Trying to achieve a Descemet's barring big bubble in such cases can lead to macroperforation of the DM (larger than 1 quadrant of the cornea) necessitating conversion to conventional full thickness penetrating corneal transplant.
In cases of keratoconus, removing the outer 2/3rd of the corneal lamella is recommended by many surgeons to facilitate more accurate placement of the 27 gauge needle or the cannula for air injection/viscodissection in the most posterior layers of the stroma.
Descemet's barring big bubble technique for DALK
In 2013, Dua et al.54 published a paper classifying the types of big bubbles obtained during air injection in posterior corneal layers. They concluded that a novel, well-defined, acellular, strong layer in the pre-Descemet's cornea (Dua's layer) creates two types of bubbles during DALK procedure.65 Type-1: (plane of cleavage between stroma and Dua's layer) is well-circumscribed, up to 8.5 mm in diameter, surrounded by white edges and spreads from the center to periphery. Type-2: (plane of cleavage between Dua's layer and DM) is a thin-walled, large big bubble of maximum 10.5 mm diameter, with a clear edge, which always starts at the periphery and enlarges centrally to form a large big bubble. This type of bubble has thin fragile walls after corneal stroma delamination. The incidence of DM rupture is higher in this type of big bubble.66
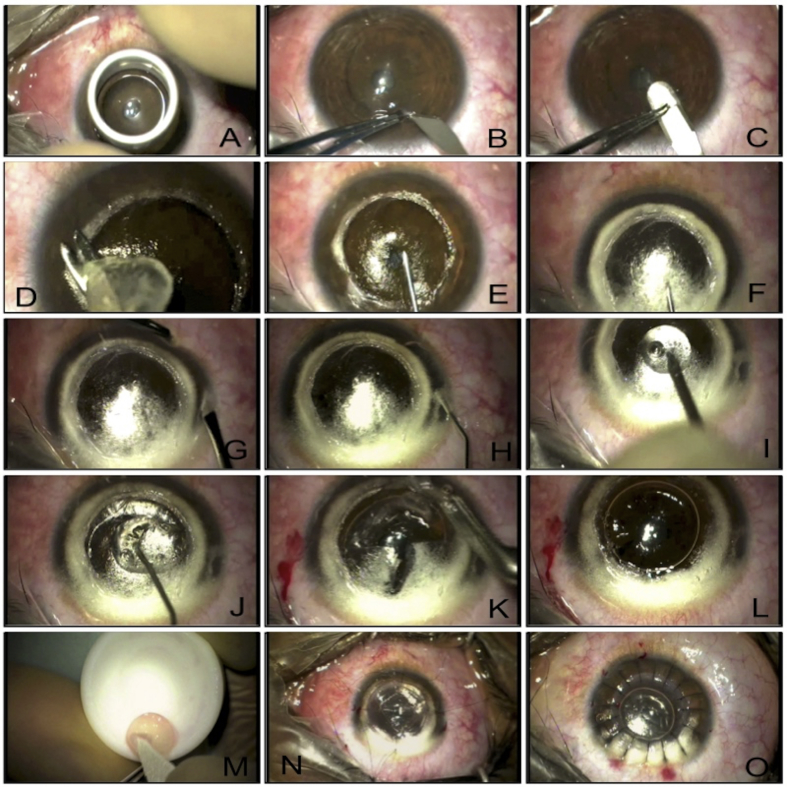
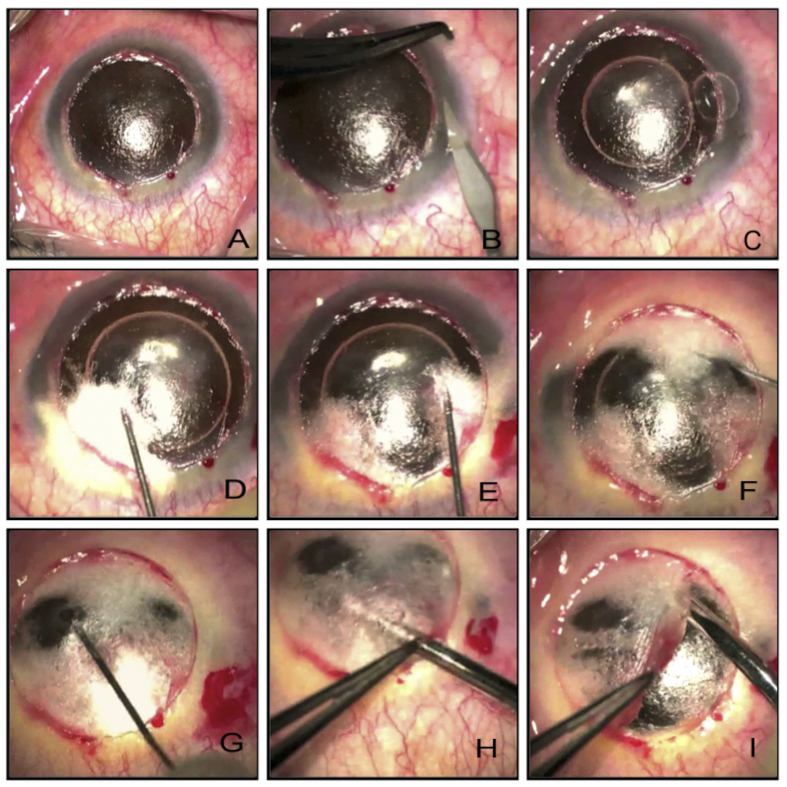
Fig. 5 illustrates a typical example of the “big bubble” technique. After trephination of the host cornea to approximately 2/3rd of the thickness (Fig. 5A), the depth of the trephined cornea is assessed using a tooth pair of forceps and a paracentesis blade (Fig. 5B). The advantage of using a paracentesis blade is that it can be used to deepen the trephination groove if the depth is found inadequate. The superficial lamella of the cornea is dissected off using a simple crescent blade and corneal scissors (Fig. 5C, D). A bent 27-gauge needle (some surgeons use a trocar and cannula) is inserted into the cornea with the bevel facing towards the DM (Fig. 5E). Air is injected gently and big bubble formation is observed. Typically, a type 1 bubble (Fig. 5F) would have a white thick border and will not extend up to the periphery. On achieving this, a vertical paracentesis is created in the peripheral cornea (Fig. 5G), and air is injected inside the anterior chamber. The presence of a big bubble is confirmed as the air injected in the anterior chamber takes a sausage shape (Fig. 5H). Creating a paracentesis will also reduce the positive pressure in the anterior chamber. Performing a large stab on the anterior lamella of the bubble can lead to a rapid deflation of the bubble and subsequently to a break in the DM, as the membrane swiftly bounces towards the sharp blade. In order to have a controlled deflation of the big bubble, placement of a dispersive viscoelastic in the center of the bubble before performing a large stab with a paracentesis blade helps, as the air does not escape suddenly, and the bubble does not deflate rapidly (Fig. 5I). A cohesive viscoelastic is injected through the stab to replace the air inside the air bubble (Fig. 5J). After inflating the big bubble with the cohesive viscoelastic, flaps of the anterior lamella are created for easy removal before they can be excised individually (Fig. 5K, L). The DM is peeled off the donor tissue and is sutured on to the host bed (Fig. 5M, N, O).
Fig. 5.
A. Trephining superficial corneal tissue using appropriately-sized manual trephine. B. Paracentesis blade used to check the depth of the trephined cornea. C. Crescent blade used to dissect superficial corneal lamella. D. Superficial corneal lamella removed. E. 30-gauge needle attached to air filled 2 ml syringe passed in the posterior corneal stroma with bevel facing downwards. F. Air injected and type 1 big bubble formed with thick white rim. G. A vertical paracentesis created carefully to avoid puncturing the big bubble in the anterior chamber. H. Injected air in the anterior chamber takes the shape of a sausage due to the presence of big Descemet's bubble. I. Big bubble punctures with a large cut using a paracentesis blade through a blob of dispersive viscoelastic to prevent collapse of Descemet's membrane (DM). J. Cohesive viscoelastic injected into the big bubble. K. Flaps of the posterior corneal stroma created. L. Flaps removed and DM fully bared. M. DM removed from the donor tissue. N. Four cardinal sutures placed. O. Corneal suturing complete.
Manual dissection technique for DALK
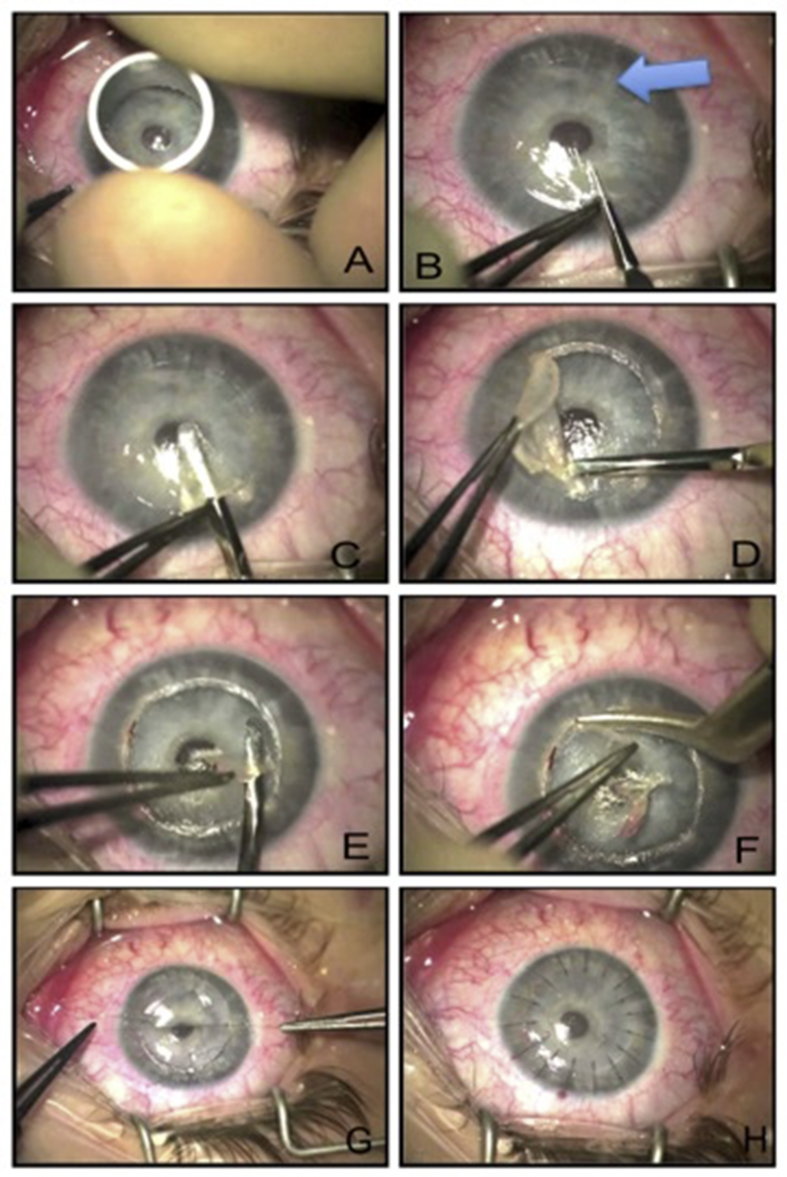
The manual dissection technique described by Melles et al.,33 used ‘air-endothelium interface’ by injecting air bubble into the anterior chamber to facilitate adequate visualization of posterior corneal stroma. The manual dissection technique is employed when there is a thick corneal scar, involving the DM, e.g. post-infection keratitis scars, hydrops,14 dense lipid keratopathy, etc. Fig. 6 illustrates an example of a very wide and deep diffuse scar from recurrent herpetic keratitis. Appropriate trephine size is chosen to encompass as much scar tissue as possible, and to leave adequate distance from the limbus (Fig. 6A). Appropriate depth of the corneal lamella to be dissected is judged using a small, blunt-tipped instrument (Fig. 6B). The superficial lamella of the cornea is taken off using a crescent blade of the surgeon's choice (Fig. 6C, D). Using a blunt-tipped instrument of surgeon's choice, further deeper layers are identified and dissected out (Fig. 6E, F) until the pre-Descemetic layers are reached. Donor corneal button is sutured on the recipient bed, as described above (Fig. 6G, H).
Fig. 6.
A. Trephining superficial corneal tissue using appropriately-sized manual trephine. B. Blunt-tipped instrument insertion to identify the deepest plane for corneal dissection. Arrow highlights diffuse scarring. C. Superficial corneal lamella dissected using crescent blade of surgeon's choice. D. Superficial corneal lamella taken off. E. Deeper corneal planes identified and carefully dissected. F. Deeper corneal planes removed layer by layer until pre-Descemetic layer reached. G. Cardinal sutures placed on donor button. H. Corneal suturing complete with interrupted sutures.
Manual DALK with layer-by-layer dissection is also a safe procedure for pediatric age groups with stromal opacities. Microperforation can happen, but conversion to PK is rarely needed. Though successful structural rehabilitation may not translate to visual and functional recovery due to issues related to amblyopia.67
Air-assisted manual dissection DALK has also been employed in corneal scarring secondary to prior inflammation and fibrosis. Stroma is filled with air and then debulking technique was performed to expose DM. Thus, big bubble technique, though commonly fails to separate predescemtic plane, effectively creates air-filled stroma, which is easier to remove.68 Manual DALK by intrastromal air injection had also been described for postherpetic leukoma opacity. It had a safe intraoperative profile and resulted in significant visual recovery.69
Serial evaluation with confocal microscopy also supports that DALK by intracorneal dissection provides visual and clinical results that are comparable to other DALK techniques.70 Confocal microscopy enables precise evaluation of corneal features, interface morphologic features and reflectivity. Progressive recovery of interface transparency correlated with an increase in visual acuity after 6 months.70
Hydrodelineation for manual dissection during DALK
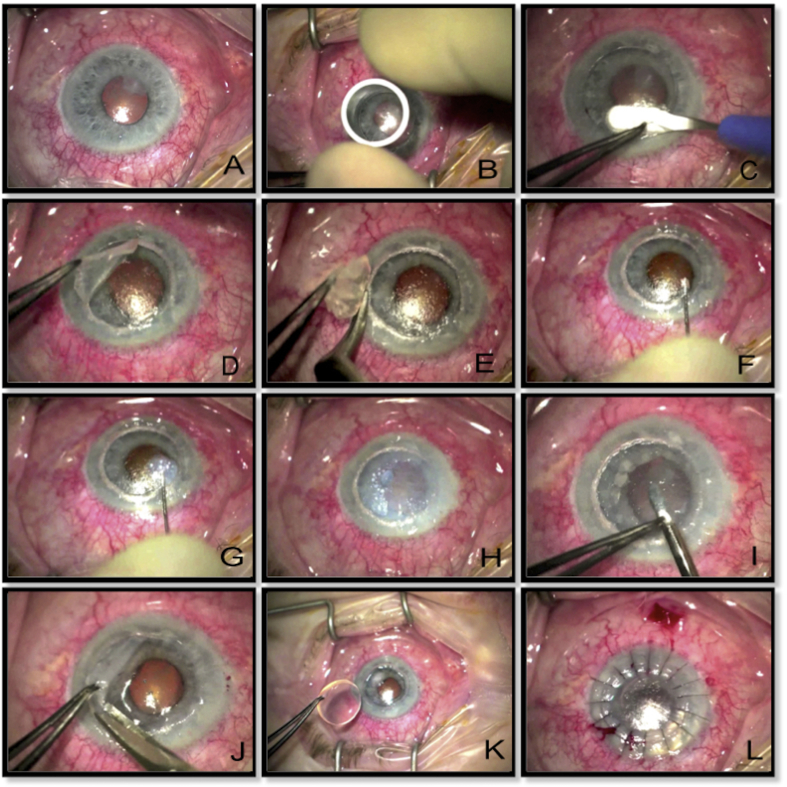
Injecting balanced salt solution (BSS) in the corneal stroma causes localized thickening of the corneal stroma due to hydration. This technique is commonly used to seal the paracentesis incisions during cataract surgery. It can also be employed during DALK where there is a deep corneal scar (potentially involving DM) and where big bubble technique is contraindicated. Fig. 7 shows an example of an eye with chronic ocular surface disease and a thick scar following microbial keratitis (Fig. 7A). After appropriately sized partial thickness trephination of the host cornea, the superficial lamellar is dissected off using a crescent blade (Fig. 7B, C, D, E). A 27-gauge needle attached to a 2 ml BSS filled syringe is inserted in the posterior corneal lamella with bevel facing downwards (Fig. 7F). BSS is injected into the posterior stroma until the desired inflation of the posterior stroma is achieved (Fig. 7G, H). Further layer-by-layer posterior corneal lamellar dissection is continued until a pre-Descemetic layer is reached (Fig. 7I, J). The donor tissue is prepared by removing the Descemetic layer and is sutured to the recipient bed (Fig. 7K, L). Wet-peeling DALK using sterile hypotonic water and blunt dissection has been described as successful in cases with corneal scarring secondary to healed corneal hydrop.71
Fig. 7.
A. Scar following microbial keratitis in a patient with chronic ocular surface disease. B. Corneal trephining to cover the scar tissue. C. Dissecting the superficial corneal lamella. D, E. Taking off the superficial corneal lamella. F. Placement of 27-gauge needle attached to 2 ml syringe filled with balanced salt solution (BSS) with bevel facing towards the Descemet's membrane (DM). G. Injection of BSS into the stroma. H. Complete hydration of corneal stroma achieved. I,J. Further corneal layers dissected and taken off until clear Descemet's reached. K. Donor tissue (without DM) placed on the host bed. L. Suturing completed.
Viscodissection technique for manual dissection in DALK
There are reported techniques of using viscodissection to detach DM. They involve creating a deep dissection with a sharp or blunt instrument, a posterior stromal nick with a sharp blade, or hooking the stroma with a forceps before Ophthalmic Visco-surgical Device (OVD) injection using a blunt cannula.35, 72, 73, 74 However, using sharp instruments for deep dissections may increase the risk of inadvertent DM perforations. On the other hand, blunt dissections may not be deep enough to create a big bubble, which may result in pre-DM plane.72, 73 Moftuoglu et al.75 described a technique involving injection of OVD using a blunt cannula. Therefore, no deep dissection with either sharp or blunt instruments is needed, and with injection, OVD leaks through the gaps in between the deep stroma and fills the spaces and microdetachments that are formed by the preceding air injection.
Femtosecond laser assisted DALK
The use of the femtosecond laser in DALK avoids manual trephination and allows precise identification of tissue depth and insertion of the air needle by following the plane between the lamellar and posterior laser side cuts. Injection of air at this precisely predefined pre-Descemet plane facilitates the big bubble formation with full baring of DM and a smaller diameter area in which the bubble or manual dissection needs to be created.37, 38, 76, 77 In addition to its advantage in facilitating the DALK procedure, using the femtosecond laser to create corneal shaped wound configurations offers the advantages of better donor–recipient fit with increased surface area contact, resulting in faster wound healing that promotes earlier suture removal and reduced astigmatism.78, 79 Thus, some authors80 feel that performing femtosecond laser assisted DALK is technically easier and more successful than manual DALK.
The first reports to describe the utilization of the femtosecond laser to create shaped wound configurations in DALK used the zigzag incision. Farid and Steinert37 described a case in which DALK was performed with the femtosecond laser zigzag incision for the treatment of keratoconus.
Price et al.81 also used femtosecond zigzag incisions on 16 planned DALK cases. In this study, the recipient stroma was removed with the big bubble technique in 7 cases and with deep hand dissection in 9 cases. Two of the big bubble cases were converted to PK as a result of rupture in DM.
Suturing technique for DALK
Surgeons can use any preferred style of suturing for DALK, (e.g. interrupted, continuous, interrupted and continuous, etc.)82; but unlike PKs, the depth of the suturing is slightly different in DALK to ensure firmer and faster apposition of the host DM to the donor corneal button.
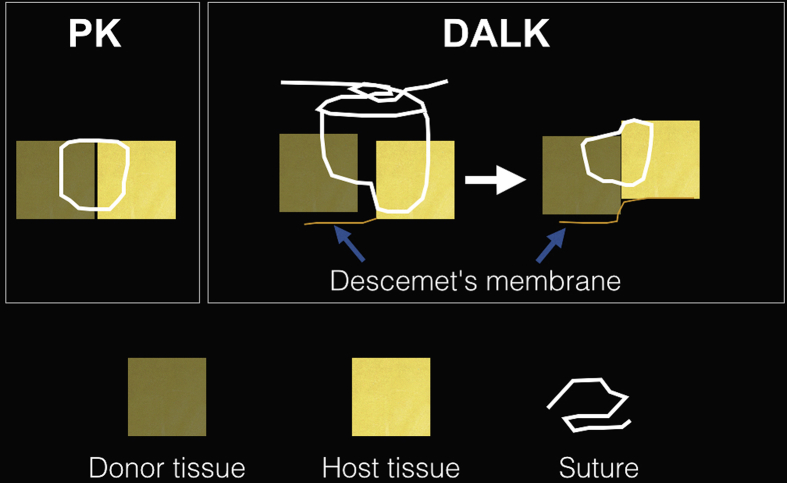
Fig. 8 illustrates a recommended suturing pattern for DALK in comparison to conventional PK (our technique). In DALK surgery, the needle is passed at approximated 50% depth in the donor tissue and 90% depth in the recipient tissue. This will lead to a final picture, where the graft-host junction is slightly elevated towards the host side compared to the central donor tissue (a ‘doughnut’ configuration). We believe that this suturing technique promotes better and faster apposition of the donor with the DM and also will squeeze out any residual viscoelastic in the interface (if viscoelastic used to inflate the big bubble). Suture removal should be performed at least after 12–15 months (slightly longer than conventional PK).83, 84 As early suture removal in DALK may lead to wound gaping,85 which many mandate re-suturing. It can also lead to unpredictable refraction and substantial increase in keratometry. The desirable outcome of DALK can be significantly undermined.86
Fig. 8.
In conventional penetrating keratoplasty (PK), sutures are passed approximately 90% deep into the donor and host cornea whereas in deep anterior lamellar keratoplasty (DALK), it is preferable to pass sutures at approximately 50% depth in the donor tissue and 90% depth in the host tissue for better apposition of the DM to the donor tissue.
DALK in patients with advanced keratoconus using different suturing techniques, e.g. interrupted running, combined interrupted and running, have no significant difference in postkeratoplasty astigmatism after complete suture removal.82 Selective suture removal is possible with interrupted sutures whereas it is more time consuming than single continuous suturing. A combined suturing techniques is also adopted by many where the single continuous suture is removed earlier, and the interrupted sutures are removed based on the topography at a late date. If the continuous suture becomes loose or infected, the entire continuous suture needs to be removed and repeated if necessary whereas if an interrupted suture is infected or loose this can be removed with or without the need for taking the suture again.
Discussion
Our review gives a review of the standard DALK procedure with variations in the techniques. However, there are instances where the surgery does not go as per plan during the DALK.
The frequently encountered issues during DALK surgery are listed below and discussed in detail:
-
a)
Extensive emphysema of the posterior corneal lamella and inability to achieve big bubble
-
b)
Achieving double bubble (presence of type 1 and 2 bubble) simultaneously during air injection
-
c)
Microperforation (less than one quarter of the cornea) and macroperforation (greater than one quarter of the cornea)
-
d)
Double anterior chamber immediately following the surgery
Extensive emphysema of the posterior corneal lamella and inability to achieve big bubble
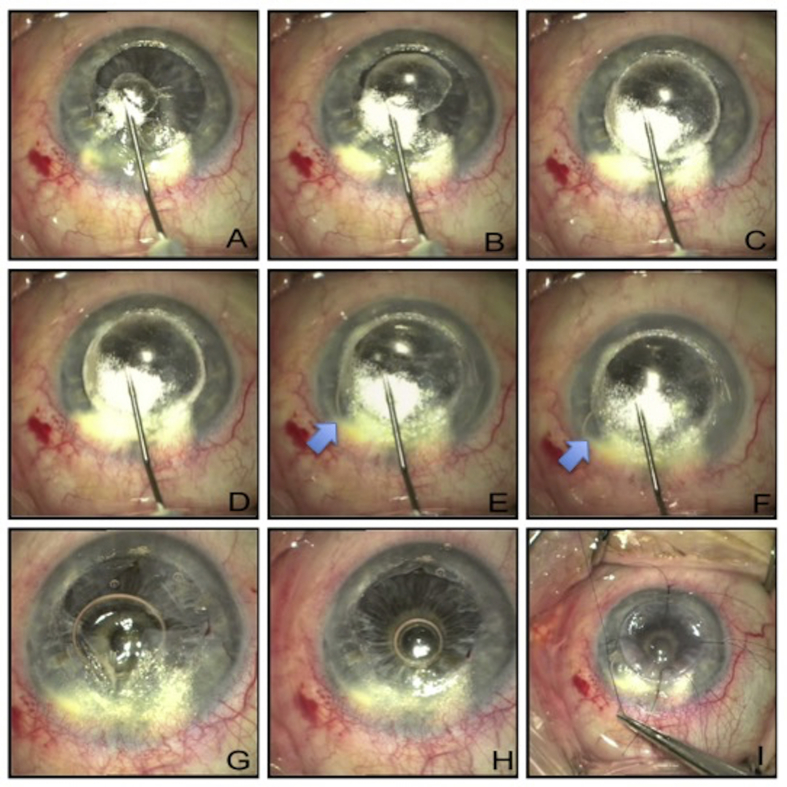
The surgeons end up in this situation when the placement of the air injection needle/cannula is not deep enough. Fig. 9 shows an example of such a situation. After removing the superficial lamella, a paracentesis was made, and air was injected into the anterior chamber (Fig. 9A, B, C). Air injection in the posterior corneal lamella was attempted several times (Fig. 9D, E, F, G) until majority of the corneal tissue was emphysematous and did not allow adequate view of the anterior chamber. It is still possible to achieve a successful DALK at this stage by converting into manual dissection technique (with or without the aid of hydration) (Fig. 9H, I). Microbubble incision can also be employed as a rescue technique for big bubble DALK patients, allowing for a safer dissection down to DM.36, 87
Fig. 9.
A. Superficial corneal lamella already removed. B. Vertical paracentesis created. C. Small air bubble injected into the anterior chamber. D. Big bubble creation attempted. E. Big bubble creation attempted at another site. F, G. Big bubble creation attempted at clear corneal sites. H. Near total stromal emphysema dissected to find a deeper plane. I. Corneal tissue removed in layers by manual dissection.
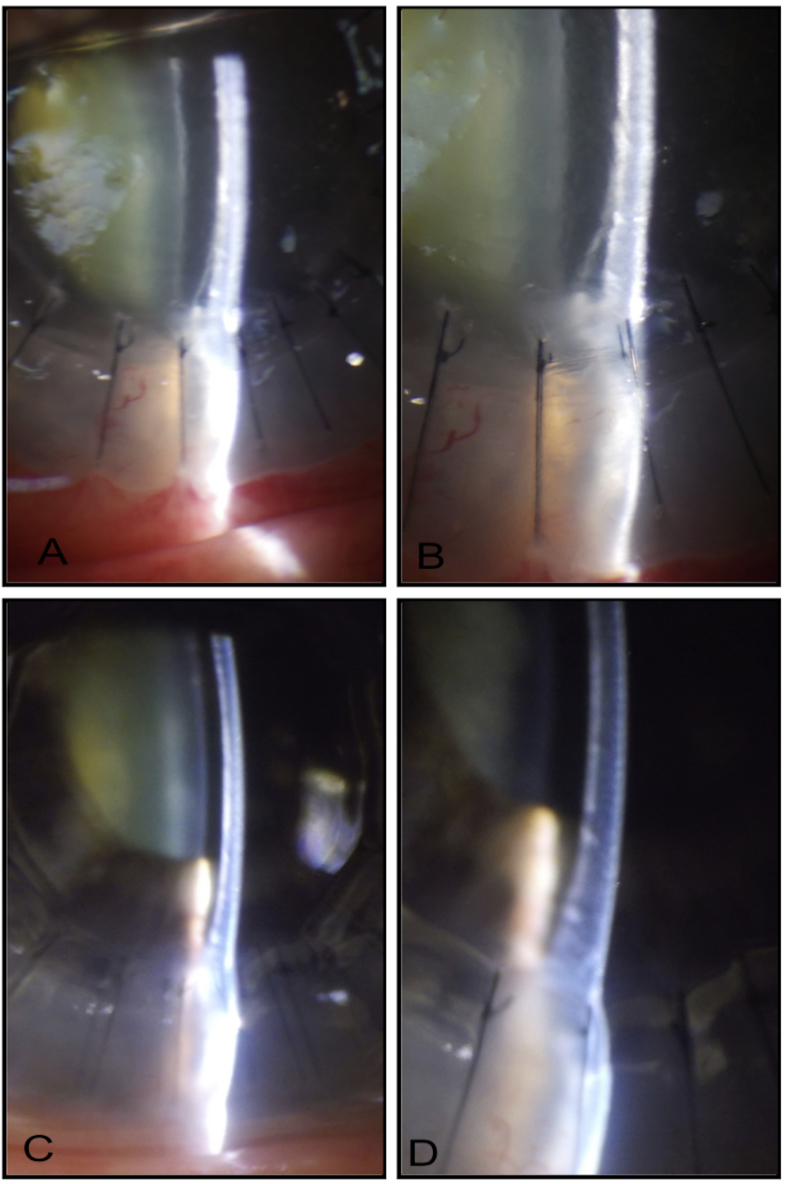
Achieving double bubble (presence of type 1 and 2 bubble) simultaneously during air injection
The surgeons encounter such situation presumably due to awkward placement of the air injection needle/cannula very flush to or into the DM. Fig. 10 illustrates one such example. Fig. 10A–D shows the formation of a classic type 1 big bubble with thick white distinct edges. When type 2 bubble forms and suddenly bursts, the surgeon may feel and hear a ‘pop’. This is followed by an appearance of an air bubble in the anterior chamber (Fig. 10E–I). This indicates a sudden blast of a type 2 bubble into the anterior chamber, leading to some air escape into the anterior chamber. However, the sausage-shaped appearance of the anterior chamber bubble (Fig. 10F) confirms the integrity of the original type 1 bubble. The surgery can still be completed in the usual manner by creating flaps of the posterior lamella (Fig. 10G) and removing each flap individually (Fig. 10H) before suturing the donor tissue (Fig. 10I).
Fig. 10.
A. Initiation of big bubble of Descemet's membrane (DM). B. Gradual enlargement of big bubble. C. Enlarging big bubble with white border (type 1). D. Fully distended type 1 bubble. E. With continued air injection, there is a sudden appearance of small bubble in the anterior chamber (blue arrow) and a second small type 2 bubble creation that perforates. Small type 2 bubble noticed slightly distal to the needle tip. F. The air bubble in the anterior chamber takes a ‘sausage’ shape suggesting full integrity of the original type 1 bubble. G. Posterior stromal flaps created. H. Posterior stromal flaps removed. I. Donor tissue (without DM) sutured.
Microperforation (less than one quarter of the cornea) and macroperforation (greater than one quarter of the cornea)
Most DALK surgeons are now in agreement that DALK can still be successfully completed with almost all microperforations (less than one quarter of the cornea) using air/gas tamponade for Descemet's apposition to the donor cornea. Perforations happen in some cases at the time of removing the flaps of posterior corneal lamellar after achieving a successful big bubble or during manual dissection due to stretch of the DM.
A successful DALK can still be performed by continuous air injection into the anterior chamber while finishing the dissection or flap removal from the region away from the microperforation. Care should be taken at all times to dissect in the microperforation region only at the end, to avoid any extension of the Descemet's tears.8 The donor tissue can be sutured as usual. The anterior chamber is inflated with air/gas to allow the partially torn DM to adhere to the donor corneal button. This part of the procedure is similar to the air inflation of the anterior chamber during endothelial keratoplasty procedures. The pupil is fully dilated, and the patient is asked to lie supine for 2–3 days postoperatively during the majority of the day. Use of fibrin glue to seal microperforations in DM during DALK had also been described.88
After 1 h of the procedure, air is released through a limbal paracentesis at 4'o clock using the slit-lamp in order to reduce the IOP. Oral acetazolamide is recommended for 3 days to control the IOP. In eyes with small pupils where dilation is not fully achieved, there is a risk of posterior pupil block from the air bubble in the anterior chamber. A small inferior peripheral iridotomy is recommended immediately after the procedure using a Neodymium-doped Yttrium Aluminum Garnet (Nd:YAG) laser in eyes with small pupils.
In cases where the surgeon encounters macroperforations (greater than one quadrant of the cornea), it may not be easy to complete the dissection of the posterior lamella fully up to the Descemet's or pre-Descemet's region. In such cases, better visual and refractive outcomes are achieved by converting the procedure to a full thickness PK.
Double anterior chamber immediately following the surgery
Double anterior chamber is encountered immediately following DALK surgery if there was a microperforation.6 This can be noticed on slit-lamp examination showing a distinct space between the donor stroma and host DM (Fig. 11). Very small, peripheral, double chamber (Fig. 11A, B) may resolve spontaneously after 1–2 weeks (Fig. 11C, D). Larger double chambers require air injection into the anterior chamber as discussed above.
Fig. 11.
A, B. Small double anterior chamber on day 1 postoperatively. C, D. Spontaneous resolution of double anterior chamber after 1 week postoperatively.
Retained host DM may result in DM detachment after DALK. The DM detachment without roll rim can spontaneously reattach in several months post-surgery.89 Failed attempt at air tamponade for a DM detachment and a double anterior chamber due to DM perforation following a DALK procedure can resolve spontaneously with good visual outcome after several weeks.90
Intra-DM air bubble can be slid and displaced toward the peripheral cornea using 27-gauge cannula and punctured, taking care that underlying DM was not ruptured. DALK needs completion after baring DM.91
In summary, although performing a DALK procedure is not similar to a conventional full thickness PK procedure and may have a steep learning curve for beginners, the procedure has distinct advantages over PK. The procedure is simple to learn once the surgeon has gained insight into the surgical techniques and management of frequently encountered scenarios as discussed in this review. DALK is more technically challenging, but allows the risk of endothelial rejection to be avoided and may reduce the risk of late endothelial failure.
Footnotes
Financial interests: The author does not have any financial or proprietary interest in any product or procedure mentioned in this chapter.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Castroviejo R. Keratoplasty in treatment of keratoconus. Arch Ophthalmol. 1949;42(6):776–800. doi: 10.1001/archopht.1949.00900050787007. [DOI] [PubMed] [Google Scholar]
- 2.Coster D.J., Lowe M.T., Keane M.C. A comparison of lamellar and penetrating keratoplasty outcomes: a registry study. Ophthalmology. 2014;121(5):979–987. doi: 10.1016/j.ophtha.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Jaycock P.D., Jones M.N., Males J. Outcomes of same-sizing versus oversizing donor trephines in keratoconic patients undergoing first penetrating keratoplasty. Ophthalmology. 2008;115(2):268–275. doi: 10.1016/j.ophtha.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 4.Watson S.L., Tuft S.J., Dart J.K. Patterns of rejection after deep lamellar keratoplasty. Ophthalmology. 2006;113(4):556–560. doi: 10.1016/j.ophtha.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Thompson R.W., Jr., Price M.O., Bowers P.J., Price F.W., Jr. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110(7):1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 6.Coombes A.G., Kirwan J.F., Rostron C.K. Deep lamellar keratoplasty with lyophilised tissue in the management of keratoconus. Br J Ophthalmol. 2001;85(7):788–791. doi: 10.1136/bjo.85.7.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feizi S., Javadi M.A., Kanavi M.R. Cellular changes of donor corneal tissue after deep anterior lamellar keratoplasty versus penetrating keratoplasty in eyes with keratoconus: a confocal study. Cornea. 2010;29(8):866–870. doi: 10.1097/ICO.0b013e3181ca2ed6. [DOI] [PubMed] [Google Scholar]
- 8.Fogla R., Padmanabhan P. Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2006;141(2):254–259. doi: 10.1016/j.ajo.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 9.Fontana L., Parente G., Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143(1):117–124. doi: 10.1016/j.ajo.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Han D.C., Mehta J.S., Por Y.M. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009;148(5):744e1–751e1. doi: 10.1016/j.ajo.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Sarnicola V., Toro P., Gentile D., Hannush S.B. Descemetic DALK and predescemetic DALK: outcomes in 236 cases of keratoconus. Cornea. 2010;29(1):53–59. doi: 10.1097/ICO.0b013e3181a31aea. [DOI] [PubMed] [Google Scholar]
- 12.Sugita J., Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81(3):184–188. doi: 10.1136/bjo.81.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henein C., Nanavaty M.A. Systematic review comparing penetrating keratoplasty and deep anterior lamellar keratoplasty for management of keratoconus. Cont Lens Anterior Eye. 2017;40(1):3–14. doi: 10.1016/j.clae.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Nanavaty M.A., Daya S.M. Outcomes of deep anterior lamellar keratoplasty in keratoconic eyes with previous hydrops. Br J Ophthalmol. 2012;96(10):1304–1309. doi: 10.1136/bjophthalmol-2012-302110. [DOI] [PubMed] [Google Scholar]
- 15.Panda A., Bageshwar L.M., Ray M. Deep lamellar keratoplasty versus penetrating keratoplasty for corneal lesions. Cornea. 1999;18(2):172–175. doi: 10.1097/00003226-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Morris E., Kirwan J.F., Sujatha S., Rostron C.K. Corneal endothelial specular microscopy following deep lamellar keratoplasty with lyophilised tissue. Eye (Lond) 1998;12(Pt 4):619–622. doi: 10.1038/eye.1998.155. [DOI] [PubMed] [Google Scholar]
- 17.van Dooren B.T., Mulder P.G., Nieuwendaal C.P., Beekhuis W.H., Melles G.R. Endothelial cell density after deep anterior lamellar keratoplasty (Melles technique) Am J Ophthalmol. 2004;137(3):397–400. doi: 10.1016/j.ajo.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 18.Ku B.I., Hsieh Y.T., Hu F.R., Wan I.J., Chen W.L., Hou Y.C. Endothelial cell loss in penetrating keratoplasty, endothelial keratoplasty, and deep anterior lamellar keratoplasty. Taiwan J Ophthalmol. 2017;7(4):199–204. doi: 10.4103/tjo.tjo_55_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terry M.A. The evolution of lamellar grafting techniques over twenty-five years. Cornea. 2000;19(5):611–616. doi: 10.1097/00003226-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Liu H., Chen Y., Wang P. Efficacy and safety of deep anterior lamellar keratoplasty vs. Penetrating keratoplasty for keratoconus: a Meta-analysis. PLoS One. 2015;10(1):e0113332. doi: 10.1371/journal.pone.0113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anwar M., Teichmann K.D. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet's membrane. Cornea. 2002;21(4):374–383. doi: 10.1097/00003226-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Reinhart W.J., Musch D.C., Jacobs D.S., Lee W.B., Kaufman S.C., Shtein R.M. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology. 2011;118(1):209–218. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Keane M., Coster D., Ziaei M., Williams K. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for treating keratoconus. Cochrane Database Syst Rev. 2014;(7):CD009700. doi: 10.1002/14651858.CD009700.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funnell C.L., Ball J., Noble B.A. Comparative cohort study of the outcomes of deep lamellar keratoplasty and penetrating keratoplasty for keratoconus. Eye (Lond) 2006;20(5):527–532. doi: 10.1038/sj.eye.6701903. [DOI] [PubMed] [Google Scholar]
- 25.Watson S.L., Ramsay A., Dart J.K. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology. 2004;111(9):1676–1682. doi: 10.1016/j.ophtha.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Jones M.N., Armitage W.J., Ayliffe W., Larkin D.F., Kaye S.B., NHSBT Ocular Tissue Advisory Group and Contributing Ophthalmologists (OTAG Audit Study 5) Penetrating and deep anterior lamellar keratoplasty for keratoconus: a comparison of graft outcomes in the United Kingdom. Investig Ophthalmol Vis Sci. 2009;50(12):5625–5629. doi: 10.1167/iovs.09-3994. [DOI] [PubMed] [Google Scholar]
- 27.Smadja D., Colin J., Krueger R.R. Outcomes of deep anterior lamellar keratoplasty for keratoconus: learning curve and advantages of the big bubble technique. Cornea. 2012;31(8):859–863. doi: 10.1097/ICO.0b013e318242fdae. [DOI] [PubMed] [Google Scholar]
- 28.Javadi M.-A., Feizi S., Jamali H., Mirbabaee F. Deep anterior lamellar keratoplasty using the big-bubble technique in keratoconus. J Ophthalmic Vis Res. 2009;4(1):8–13. [PMC free article] [PubMed] [Google Scholar]
- 29.Karimian F., Feizi S. Deep anterior lamellar keratoplasty: indications, surgical techniques and complications. Middle East Afr J Ophthalmol. 2010;17(1):28–37. doi: 10.4103/0974-9233.61214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anwar M., Teichmann K.D. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28(3):398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 31.Archila E.A. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1984;3(3):217–218. [PubMed] [Google Scholar]
- 32.Price F.W., Jr. Air lamellar keratoplasty. Refract Corneal Surg. 1989;5(4):240–243. [PubMed] [Google Scholar]
- 33.Melles G.R., Lander F., Rietveld F.J., Remeijer L., Beekhuis W.H., Binder P.S. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999;83(3):327–333. doi: 10.1136/bjo.83.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baradaran-Rafii A., Eslani M., Sadoughi M.M., Esfandiari H., Karimian F. Anwar versus Melles deep anterior lamellar keratoplasty for keratoconus: a prospective randomized clinical trial. Ophthalmology. 2013;120(2):252–259. doi: 10.1016/j.ophtha.2012.07.090. [DOI] [PubMed] [Google Scholar]
- 35.Manche E.E., Holland G.N., Maloney R.K. Deep lamellar keratoplasty using viscoelastic dissection. Arch Ophthalmol. 1999;117(11):1561–1565. doi: 10.1001/archopht.117.11.1561. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee S., Li H.J., Tsaousis K.T., Tabin G.C. Salvaging deep anterior lamellar keratoplasty with microbubble incision technique in failed “big bubble” cases: an update study. Eur J Ophthalmol. 2016;26(6):643–645. doi: 10.5301/ejo.5000816. [DOI] [PubMed] [Google Scholar]
- 37.Farid M., Steinert R.F. Deep anterior lamellar keratoplasty performed with the femtosecond laser zigzag incision for the treatment of stromal corneal pathology and ectatic disease. J Cataract Refract Surg. 2009;35(5):809–813. doi: 10.1016/j.jcrs.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Alio J.L., Abdelghany A.A., Barraquer R., Hammouda L.M., Sabry A.M. Femtosecond laser assisted deep anterior lamellar keratoplasty outcomes and healing patterns compared to manual technique. BioMed Res Int. 2015;2015:397891. doi: 10.1155/2015/397891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alldredge O.C., Krachmer J.H. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981;99(4):599–604. doi: 10.1001/archopht.1981.03930010599002. [DOI] [PubMed] [Google Scholar]
- 40.Boisjoly H.M., Bernard P.M., Dube I., Laughrea P.A., Bazin R., Bernier J. Effect of factors unrelated to tissue matching on corneal transplant endothelial rejection. Am J Ophthalmol. 1989;107(6):647–654. doi: 10.1016/0002-9394(89)90262-6. [DOI] [PubMed] [Google Scholar]
- 41.Maguire M.G., Stark W.J., Gottsch J.D. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101(9):1536–1547. doi: 10.1016/s0161-6420(94)31138-9. [DOI] [PubMed] [Google Scholar]
- 42.Le R., Yucel N., Khattak S., Yucel Y.H., Prud'homme G.J., Gupta N. Current indications and surgical approaches to corneal transplants at the University of Toronto: a clinical-pathological study. Can J Ophthalmol. 2017;52(1):74–79. doi: 10.1016/j.jcjo.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Buzzonetti L., Ardia R., Petroni S. Four years of corneal keratoplasty in Italian paediatric patients: indications and clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2239–2245. doi: 10.1007/s00417-016-3447-2. [DOI] [PubMed] [Google Scholar]
- 44.Yu A.L., Kaiser M., Schaumberger M. Perioperative and postoperative risk factors for corneal graft failure. Clin Ophthalmol. 2014;8:1641–1647. doi: 10.2147/OPTH.S65412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omoto M., Shimmura S., Hatou S., Ichihashi Y., Kawakita T., Tsubota K. Simultaneous deep anterior lamellar keratoplasty and limbal allograft in bilateral limbal stem cell deficiency. Jpn J Ophthalmol. 2010;54(6):537–543. doi: 10.1007/s10384-010-0879-9. [DOI] [PubMed] [Google Scholar]
- 46.Joe A.W., Yeung S.N. Concise review: identifying limbal stem cells: classical concepts and new challenges. Stem Cells Transl Med. 2014;3(3):318–322. doi: 10.5966/sctm.2013-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y.M., Wu S.Q., Yao Y.F. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ – Sci B. 2013;14(5):438–450. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozmen M.C., Yesilirmak N., Aydin B., Ceylanoglu K.S., Atalay H.T., Akata F. Prediction of Descemet membrane perforation during deep anterior lamellar keratoplasty in patients with keratoconus with stromal scar. Eye Contact Lens. 2017 doi: 10.1097/ICL.0000000000000434. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Ogawa A., Yamaguchi T., Mitamura H. Aetiology-specific comparison of long-term outcome of deep anterior lamellar keratoplasty for corneal diseases. Br J Ophthalmol. 2016;100(9):1176–1182. doi: 10.1136/bjophthalmol-2015-307427. [DOI] [PubMed] [Google Scholar]
- 50.Den S., Shimmura S., Shimazaki J. Cataract surgery after deep anterior lamellar keratoplasty and penetrating keratoplasty in age- and disease-matched eyes. J Cataract Refract Surg. 2018;44(4):496–503. doi: 10.1016/j.jcrs.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Zaki A.A., Elalfy M.S., Said D.G., Dua H.S. Deep anterior lamellar keratoplasty—triple procedure: a useful clinical application of the pre-Descemet's layer (Dua's layer) Eye. 2015;29(3):323–326. doi: 10.1038/eye.2014.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan S., Ting D.S., Lyall D.A. Implantation of a customized toric intraocular lens for correction of post-keratoplasty astigmatism. Eye (Lond) 2013;27(4):531–537. doi: 10.1038/eye.2012.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer J.J., Kim B.Z., Ziaei M., McGhee C.N. Postoperative rotation of supplementary sulcus-supported toric intraocular lenses. J Cataract Refract Surg. 2017;43(2):285–288. doi: 10.1016/j.jcrs.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Dua H.S., Faraj L.A., Said D.G., Gray T., Lowe J. Human corneal anatomy redefined: a novel pre-Descemet's layer (Dua's layer) Ophthalmology. 2013;120(9):1778–1785. doi: 10.1016/j.ophtha.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Bowman R., Liu C., Sarkies N. Intraocular pressure changes after peribulbar injections with and without ocular compression. Br J Ophthalmol. 1996;80(5):394–397. doi: 10.1136/bjo.80.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhullar P.K., Carrasco-Zevallos O.M., Dandridge A. Intraocular pressure and big bubble diameter in deep anterior lamellar keratoplasty: an ex-vivo microscope-integrated OCT with heads-up display study. Asia Pac J Ophthalmol (Phila) 2017;6(5):412–417. doi: 10.22608/APO.2017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinert R.F., Minkowski J.S., Boruchoff S.A. Pre-keratoplasty potential acuity evaluation. Laser interferometer and potential acuity meter. Ophthalmology. 1984;91(10):1217–1221. doi: 10.1016/s0161-6420(84)34172-0. [DOI] [PubMed] [Google Scholar]
- 58.Roberts H.W., Maycock N.J., O'Brart D.P. Late stromal rejection in deep anterior lamellar keratoplasty: a case series. Cornea. 2016;35(9):1179–1181. doi: 10.1097/ICO.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 59.Muraine M., Calenda E., Watt L. Peribulbar anaesthesia during keratoplasty: a prospective study of 100 cases. Br J Ophthalmol. 1999;83(1):104–109. doi: 10.1136/bjo.83.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chua A., Chua M.J., Kam P. Recent advances and anaesthetic considerations in corneal transplantation. Anaesth Intensive Care. 2018;46(2):162–170. doi: 10.1177/0310057X1804600204. [DOI] [PubMed] [Google Scholar]
- 61.Schaub F., Enders P., Adler W., Bachmann B.O., Cursiefen C., Heindl L.M. Impact of donor graft quality on deep anterior lamellar Keratoplasty (DALK) BMC Ophthalmol. 2017;17(1):204. doi: 10.1186/s12886-017-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noble B.A., Agrawal A., Collins C., Saldana M., Brogden P.R., Zuberbuhler B. Deep Anterior Lamellar Keratoplasty (DALK): visual outcome and complications for a heterogeneous group of corneal pathologies. Cornea. 2007;26(1):59–64. doi: 10.1097/01.ico.0000240080.99832.f3. [DOI] [PubMed] [Google Scholar]
- 63.Oh B.L., Kim M.K., Wee W.R. Comparison of clinical outcomes of same-size grafting between deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus. Korean J Ophthalmol. 2013;27(5):322–330. doi: 10.3341/kjo.2013.27.5.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riss S., Heindl L.M., Bachmann B.O., Kruse F.E., Cursiefen C. Pentacam-based big bubble deep anterior lamellar keratoplasty in patients with keratoconus. Cornea. 2012;31(6):627–632. doi: 10.1097/ICO.0b013e31823f8c85. [DOI] [PubMed] [Google Scholar]
- 65.Dua H.S., Faraj L.A., Kenawy M.B. Dynamics of big bubble formation in deep anterior lamellar keratoplasty by the big bubble technique: in vitro studies. Acta Ophthalmol. 2018;96(1):69–76. doi: 10.1111/aos.13460. [DOI] [PubMed] [Google Scholar]
- 66.Dua H.S., Katamish T., Said D.G., Faraj L.A. Differentiating type 1 from type 2 big bubbles in deep anterior lamellar keratoplasty. Clin Ophthalmol (Auckland, NZ) 2015;9:1155–1157. doi: 10.2147/OPTH.S81089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elbaz U., Kirwan C., Shen C., Ali A. Avoiding big bubble complications: outcomes of layer-by-layer deep anterior lamellar keratoplasty in children. Br J Ophthalmol. 2018:0007–1161. doi: 10.1136/bjophthalmol-2017-310962. [DOI] [PubMed] [Google Scholar]
- 68.Ho Y.J., Wu C.H., Chen H.C., Hsiao C.S., Hsueh Y.J., Ma D.H. Surgical outcome of deep anterior lamellar keratoplasty with air-assisted manual dissection for corneas with previous inflammation or fibrosis. Taiwan J Ophthalmol. 2017;7(4):191–198. doi: 10.4103/tjo.tjo_13_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leccisotti A. Air-assisted manual deep anterior lamellar keratoplasty for treatment of herpetic corneal scars. Cornea. 2009;28(7):728–731. doi: 10.1097/ICO.0b013e3181930a7e. [DOI] [PubMed] [Google Scholar]
- 70.Marchini G., Mastropasqua L., Pedrotti E., Nubile M., Ciancaglini M., Sbabo A. Deep lamellar keratoplasty by intracorneal dissection: a prospective clinical and confocal microscopic study. Ophthalmology. 2006;113(8):1289–1300. doi: 10.1016/j.ophtha.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Z., Li J., Zheng Q., Lin W., Jhanji V., Chen W. Wet-peeling technique of deep anterior lamellar keratoplasty with hypotonic water and blunt dissection for healed hydrops. Cornea. 2017;36(3):386–389. doi: 10.1097/ICO.0000000000001110. [DOI] [PubMed] [Google Scholar]
- 72.Fournie P., Malecaze F., Coullet J., Arne J.L. Variant of the big bubble technique in deep anterior lamellar keratoplasty. J Cataract Refract Surg. 2007;33(3):371–375. doi: 10.1016/j.jcrs.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 73.Shimmura S., Shimazaki J., Omoto M., Teruya A., Ishioka M., Tsubota K. Deep lamellar keratoplasty (DLKP) in keratoconus patients using viscoadaptive viscoelastics. Cornea. 2005;24(2):178–181. doi: 10.1097/01.ico.0000138843.83044.7d. [DOI] [PubMed] [Google Scholar]
- 74.Yao Y.F. A novel technique for performing full-bed deep lamellar keratoplasty. Cornea. 2008;(27 Suppl 1):S19–S24. doi: 10.1097/ICO.0b013e31817f445f. [DOI] [PubMed] [Google Scholar]
- 75.Muftuoglu O., Toro P., Hogan R.N. Sarnicola air-visco bubble technique in deep anterior lamellar keratoplasty. Cornea. 2013;32(4):527–532. doi: 10.1097/ICO.0b013e31826cbe99. [DOI] [PubMed] [Google Scholar]
- 76.Buzzonetti L., Laborante A., Petrocelli G. Standardized big-bubble technique in deep anterior lamellar keratoplasty assisted by the femtosecond laser. J Cataract Refract Surg. 2010;36(10):1631–1636. doi: 10.1016/j.jcrs.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 77.Farid M., Steinert R.F. Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol. 2010;21(4):288–292. doi: 10.1097/ICU.0b013e32833a8dbc. [DOI] [PubMed] [Google Scholar]
- 78.Jonas J.B., Vossmerbaeumer U. Femtosecond laser penetrating keratoplasty with conical incisions and positional spikes. J Refract Surg. 2004;20(4):397. doi: 10.3928/1081-597X-20040701-15. [DOI] [PubMed] [Google Scholar]
- 79.Mian S.I., Shtein R.M. Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol. 2007;18(4):295–299. doi: 10.1097/ICU.0b013e3281a4776c. [DOI] [PubMed] [Google Scholar]
- 80.Shehadeh-Mashor R., Chan C., Yeung S.N., Lichtinger A., Amiran M., Rootman D.S. Long-term outcomes of femtosecond laser-assisted mushroom configuration deep anterior lamellar keratoplasty. Cornea. 2013;32(4):390–395. doi: 10.1097/ICO.0b013e318254a4e4. [DOI] [PubMed] [Google Scholar]
- 81.Price F.W., Jr., Price M.O., Grandin J.C., Kwon R. Deep anterior lamellar keratoplasty with femtosecond-laser zigzag incisions. J Cataract Refract Surg. 2009;35(5):804–808. doi: 10.1016/j.jcrs.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 82.Acar B.T., Vural E.T., Acar S. Does the type of suturing technique used affect astigmatism after deep anterior lamellar keratoplasty in keratoconus patients? Clin Ophthalmol (Auckland, NZ) 2011;5:425–428. doi: 10.2147/OPTH.S18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mannan R., Jhanji V., Sharma N., Pruthi A., Vajpayee R.B. Spontaneous wound dehiscence after early suture removal after deep anterior lamellar keratoplasty. Eye Contact Lens. 2011;37(2):109–111. doi: 10.1097/ICL.0b013e31820c7014. [DOI] [PubMed] [Google Scholar]
- 84.Padrón-Pérez N., Filloy A., Martí-Huguet T. Complete dehiscence of a DALK graft after early suture removal. Eye. 2014;28(7):909–911. doi: 10.1038/eye.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feizi S., Javadi M.A. Factors predicting refractive outcomes after deep anterior lamellar keratoplasty in keratoconus. Am J Ophthalmol. 2015;160(4):648–653 e2. doi: 10.1016/j.ajo.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Feizi S., Javadi M.A., Behnaz N., Fani-Hanife S., Jafarinasab M.R. Effect of suture removal on refraction and graft Curvature after deep anterior lamellar keratoplasty in patients with keratoconus. Cornea. 2018;37(1):39–44. doi: 10.1097/ICO.0000000000001443. [DOI] [PubMed] [Google Scholar]
- 87.Riss S., Heindl L.M., Bachmann B.O., Kruse F.E., Cursiefen C. Microbubble incision as a new rescue technique for big-bubble deep anterior lamellar keratoplasty with failed bubble formation. Cornea. 2013;32(2):125–129. doi: 10.1097/ICO.0b013e31824a226f. [DOI] [PubMed] [Google Scholar]
- 88.Anwar H.M., El-Danasoury A., Hashem A.N. The use of fibrin glue to seal Descemet membrane microperforations occurring during deep anterior lamellar keratoplasty. Cornea. 2012;31(10):1193–1196. doi: 10.1097/ICO.0b013e318242fd94. [DOI] [PubMed] [Google Scholar]
- 89.Lin X., Wu Y., Fu Y., Dai Q. Spontaneous reattachment of Descemet membrane detachment after deep anterior lamellar keratoplasty: a case report. Medicine (Baltimore) 2018;97(8):e0032. doi: 10.1097/MD.0000000000010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venkatraman A. Spontaneous resolution of double anterior chamber with perforation of Descemet's membrane in deep anterior lamellar keratoplasty. Oman J Ophthalmol. 2012;5(2):112–114. doi: 10.4103/0974-620X.99376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma N., Swarup R., Bali S.J., Maharana P., Titiyal J.S., Vajpayee R.B. Management of intra-Descemet membrane air bubble in big-bubble deep anterior lamellar keratoplasty. Cornea. 2013;32(9):1193–1195. doi: 10.1097/ICO.0b013e3182912fa7. [DOI] [PubMed] [Google Scholar]