Abstract
Multikinase inhibitors (MKIs) are a novel target therapy that offers promising long-term survival for patients with advanced-stage cancer. However, they cause a wide range of adverse reactions, skin and skin appendage being the most prevalent. Photosensitivity reactions are well-recognized effects from certain MKIs such as sunitinib and vandetanib. However, phototoxic reaction induced by pazopanib has never been reported. We present here the first case of pazopanib-induced phototoxic drug reaction in a patient with renal cell carcinoma.
Keywords: Pazopanib, Multikinase inhibitors, Phototoxic drug reaction, Photosensitivity, Renal cell carcinoma
Introduction
Novel target therapy has proved to be life-changing for many cancer patients, but not without a wide range of adverse reactions. Cutaneous toxicities are among the most dominant adverse reactions. Photosensitivity reactions have been reported in several multikinase inhibitors (MKIs), namely sunitinib and vandetanib [1]. We present here a case of pazopanib-induced phototoxic drug reaction in a patient with renal cell carcinoma. To the best of our knowledge, this is the first case report on phototoxicity to pazopanib.
Case Presentation
A 78-year-old Thai man diagnosed with renal cell carcinoma stage T2aN0M0 was successfully treated with radical nephrectomy. Three years later, he had local recurrence and computed tomography showed metastases in the lung. Pazopanib was started as first-line therapy (400 mg oral daily dose). Grade 1 skin rash was documented a few weeks after commencing the drug. Subsequent follow-ups revealed a stable disease with no increase in size of lung metastases and no report of rash.
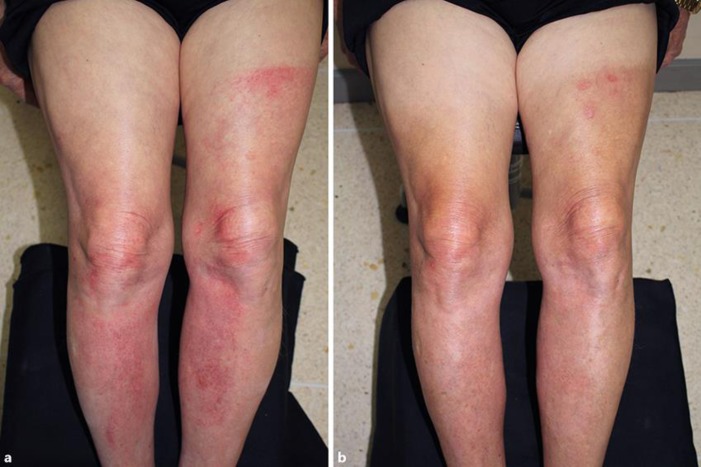
After 2 years of pazopanib therapy, the patient engaged in a prolonged outdoor activity when he sat outside in the sun for 4 h without using any means of sun protection. He denied previous sun exposure in the last 2 years. Within less than 24 h later, he developed several burning and stinging lesions on sun-exposed sites. On physical examination, multiple erythematous patches with pinpoint bleeding, consistent with an exaggerated sunburn response, were observed on the forehead, neck, legs, and arms, with clear demarcation between exposed and unexposed areas (Fig. 1a). His underlying conditions included chronic arterial disease, hypertension, and neurogenic claudication. The patient's current medications consisted of atenolol, simvastatin, aspirin, omeprazole, gabapentin, and pazopanib.
Fig. 1.
Erythematous patches with pinpoint bleeding over the forehead, neck, and legs. a Note the clear demarcation line from the patient's shorts. b Resolution with postinflammatory hyperpigmentation.
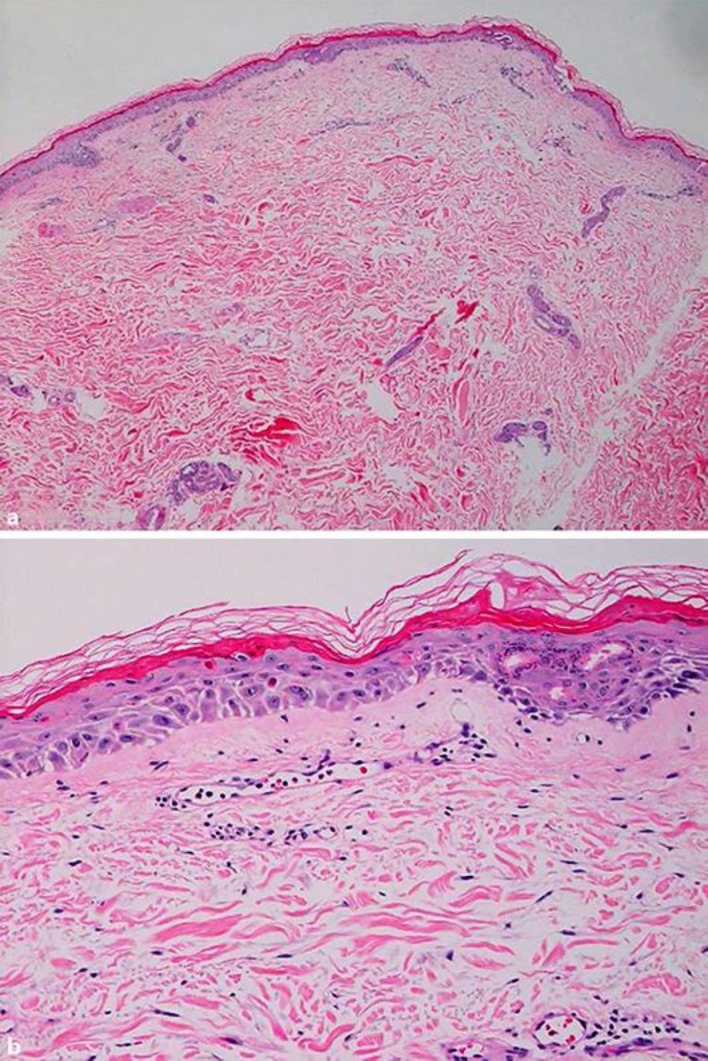
After obtaining inform consent, a skin biopsy specimen from his right thigh was performed and revealed parakeratosis, spongiosis, as well as few scattered necrotic and vacuolated keratinocytes. Atypical mitotic figures were observed in the epidermal basal cell layer. The dermis showed sparse superficial and deep lymphocytic infiltration (Fig. 2a, b).
Fig. 2.
a Mild superficial and deep infiltration of lymphocytes with scattered necrotic keratinocytes in the epidermis. b An atypical mitotic figure was observed in the epidermal basal cell layer.
Given the clinical features of photosensitivity and the dermatopathological finding of phototoxic reaction, phototoxic drug reaction was the final diagnosis. Interestingly, histopathology demonstrated numerous mitotic activity of the basal cell layer, a finding that occurs from drugs that interfere with cell cycle activity, such as chemotherapy. Therefore, the diagnosis of pazopanib-induced phototoxic reaction was made.
The patient was sent for a phototest, which showed normal minimal erythema dose for ultraviolet A, ultraviolet B, and visible light. After evaluation by an oncologist, the patient was advised to withhold pazopanib. Topical betamethasone valerate and moisturizing cream were given as well as advice on strict sun protection and application of sunscreen. A month later, the rash had resolved nicely with residual postinflammatory hyperpigmentation (Fig. 1b), and the patient resumed pazopanib without any further problem.
Discussion
Pazopanib is an MKI that selectively targets vascular endothelial growth factor 1, 2, and 3, platelet-derived growth factor receptor α and β, and c-KIT mast/stem cell growth factor. It is approved by the Food and Drug Administration for treatment of advanced renal cell carcinoma and advanced soft tissue sarcoma. In a randomized controlled phase III trial, pazopanib significantly increased the progression-free survival in patients with advanced or metastatic renal cell carcinoma [2]. The most common adverse events were diarrhea, hypertension, hair color changes, nausea, anorexia, and vomiting.
Few studies have been published regarding cutaneous reactions induced by pazopanib. To the best of our knowledge, phototoxic reaction has never been reported in the English language literature. Although rash was reported in 16% of patients on pazopanib, the nature of the rash was not specified [3]. A recent review on cutaneous adverse effects of target therapy concluded that skin eruptions of varying morphology have been described in the early weeks after initiation of pazopanib (all grades, 6–8%) [4]. Other reported cutaneous reactions include hand-foot skin reaction, hair hypopigmentation, and alopecia [3]. The incidence of hand-foot skin reaction in pazopanib was unexpectedly lower than in sorafenib and sunitinib, despite the drugs sharing the same receptor targets [5]. Another review speculated that the differences in the incidence and severity of side effects may be due to different binding affinity to a particular target [6]. Overall, there were no serious cutaneous adverse reactions with pazopanib [7].
Rare cutaneous eruptions with pazopanib were profound hair and skin hypopigmentation. The mechanism was believed to be the combination of c-kit and platelet-derived growth factor inhibition [8]. Interestingly, a case of pazopanib-induced subacute cutaneous lupus erythematosus has been reported, but the mechanism was unclear [9]. From our recent finding, it is possible that photosensitivity plays a role.
Previous photosensitivity reactions have been found in MKIs that share the same receptors as pazopanib, namely sunitinib and vandetanib. Phototoxicity from sunitinib has been documented in retrospective studies and numerous clinical trials [1]. Vandetanib has also clearly been associated with photosensitivity [10, 11]. Up to 37% of patients on vandetanib experienced a photosensitivity reaction with skin eruptions ranging from exaggerated sunburn to severe photodistributed erythematous eruption [4]. The authors believe that the mechanism may be ABCG2 membrane transporter inhibition. A previous study showed that imatinib inhibits ABCG2 transporter and results in increased phototoxicity in vitro [12]. Furthermore, inhibition of ABCG2 can lead to the disruption of porphyrin homeostasis, and cells lacking ABCG2 accumulate porphyrin [13]. Since the interaction between ABCG2 and MKIs also exists with pazopanib, a similar explanation may be applicable.
Phototoxic reaction from pazopanib is the most likely diagnosis as the patient's other medications are not known to cause phototoxicity. Although simvastatin is a well-recognized culprit for photoallergic reactions, phototoxicity has never been reported. Moreover, the patient's histological finding clearly displayed atypical mitotic basal keratinocytes. As the basal cell layer, consisting primary of keratinocyte stem cells, provides active cell division, drugs that interfere with the cell cycle, such as chemotherapy, could cause cell mitosis in this area. Cell cycle interruption leading to apoptosis has been shown in vitro in the presence of vandetanib [14]. Surprisingly, the phototest results were normal. Since photosensitivity from pazopanib has never been reported, the minimal erythema dose profile for photosensitivity induced by this agent is unknown, although ultraviolet A light is the most prevalent cause of photosensitivity. In fact, phototest results for drug-induced phototoxic reaction are limited, as a recent systematic review concluded that only 23.3% of the studies included phototesting and merely 10% had a challenge-rechallenge design [15].
Our patient developed the cutaneous reaction after 2 years of pazopanib administration. This may be explained partly by the fact that he had not been exposed to the sun in the past 2 years. Interestingly, he also developed a lighter skin color, a finding that is consistent with previous literature. This may have predisposed him to a sunburn reaction. However, epidermal spongiosis and the deep infiltrating nature of our biopsy result confirm that it was indeed a phototoxic reaction, not just a superficial sunburn.
A severity grading system and a corresponding management for MKI-induced photosensitivity have not been established due to its rarity. In our case, the rash improved remarkably after strict sun avoidance, application of sunscreen, and topical steroid.
Conclusion
We report a case of phototoxicity induced by the pazopanib, although photosensitivity has been established in other MKIs that share the same target receptors. In an era where administration of targeted therapeutic agents, particularly MKIs, is increasingly popular, it is important for general physicians, dermatologists, and oncologists to recognize and be aware of their potential photosensitive reactions.
Statement of Ethics
We state that our patient gave inform consent. The research complied with all ethical guidelines for human studies.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Rosenbaum SE, Wu S, Newman MA, West DP, Kuzel T, Lacouture ME. Dermatological reactions to the multitargeted tyrosine kinase inhibitor sunitinib. Support Care Cancer. 2008 Jun;16((6)):557–66. doi: 10.1007/s00520-008-0409-1. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010 Feb;28((6)):1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 3.Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010 Jan;28((3)):475–80. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015 Feb;72((2)):203–18. doi: 10.1016/j.jaad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Balagula Y, Wu S, Su X, Feldman DR, Lacouture ME. The risk of hand foot skin reaction to pazopanib, a novel multikinase inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs. 2012 Aug;30((4)):1773–81. doi: 10.1007/s10637-011-9652-2. [DOI] [PubMed] [Google Scholar]
- 6.Schmidinger M, Bellmunt J. Plethora of agents, plethora of targets, plethora of side effects in metastatic renal cell carcinoma. Cancer Treat Rev. 2010 Aug;36((5)):416–24. doi: 10.1016/j.ctrv.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Faye E, Bondon-Guitton E, Olivier-Abbal P, Montastruc JL, French Network of Regional Pharmacovigilance Centers Spontaneous reporting of serious cutaneous reactions with protein kinase inhibitors. Eur J Clin Pharmacol. 2013 Oct;69((10)):1819–26. doi: 10.1007/s00228-013-1532-6. [DOI] [PubMed] [Google Scholar]
- 8.Sideras K, Menefee ME, Burton JK, Erlichman C, Bible KC, Ivy SP. Profound hair and skin hypopigmentation in an African American woman treated with the multi-targeted tyrosine kinase inhibitor pazopanib. J Clin Oncol. 2010 Jul;28((19)):e312–3. doi: 10.1200/JCO.2009.26.4432. [DOI] [PubMed] [Google Scholar]
- 9.Casado-Verrier B, Pérez-Santos S, Delgado-Mucientes C, Beato-Merino M. Subacute cutaneous lupus erythematosus induced by the new multikinase inhibitor pazopanib. Br J Dermatol. 2014 Dec;171((6)):1559–61. doi: 10.1111/bjd.13175. [DOI] [PubMed] [Google Scholar]
- 10.Chang CH, Chang JW, Hui CY, Yang CH. Severe photosensitivity reaction to vandetanib. J Clin Oncol. 2009 Sep;27((27)):e114–5. doi: 10.1200/JCO.2009.21.8479. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein J, Patel AB, Curry JL, Subbiah V, Piha-Paul S. Photoallergic reaction in a patient receiving vandetanib for metastatic follicular thyroid carcinoma: a case report. BMC Dermatol. 2015 Feb;15((1)):2. doi: 10.1186/s12895-015-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, et al. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res. 2007 Apr;13((8)):2463–70. doi: 10.1158/1078-0432.CCR-06-1599. [DOI] [PubMed] [Google Scholar]
- 13.Tamura A, An R, Hagiya Y, Hoshijima K, Yoshida T, Mikuriya K, et al. Drug-induced phototoxicity evoked by inhibition of human ABC transporter ABCG2: development of in vitro high-speed screening systems. Expert Opin Drug Metab Toxicol. 2008 Mar;4((3)):255–72. doi: 10.1517/17425255.4.3.255. [DOI] [PubMed] [Google Scholar]
- 14.Salvador A, Vedaldi D, Brun P, Dall'Acqua S. Vandetanib-induced phototoxicity in human keratinocytes NCTC-2544. Toxicol In Vitro. 2014 Aug;28((5)):803–11. doi: 10.1016/j.tiv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Kim WB, Shelley AJ, Novice K, Joo J, Lim HW, Glassman SJ. Drug-Induced Phototoxicity: A Systematic Review. J Am Acad Dermatol. 2018 Jul; doi: 10.1016/j.jaad.2018.06.061. S0190-9622(18)32220-5; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]