Abstract
Background
Obesity is increasing worldwide and has become a nontraditional risk factor in chronic kidney disease (CKD).
Summary
Obesity-related nephropathy may aggravate renal complications of the metabolic syndrome and progress to advanced CKD stages, while obesity in early stages of CKD is clearly related to the development of kidney disease. A high body mass index (BMI) in advanced CKD stages and dialysis is an advantage for survival (so called “obesity paradox”). A high lean body to fat mass index indicates a beneficial state of body composition. In contrast, loss of muscle mass with increasing fat mass causes “sarcopenia obesity,” which is related to unfavorable outcomes in renal replacement therapy. Obesity (BMI > 30–35) in renal transplant recipients is associated with a higher risk of complications such as delayed graft function, increased rates of rejection, and graft loss. While conservative management of morbid obesity is failing in most cases, bariatric surgery seems to be an option in some cases to improve renal complications in the early stage of CKD or in transplant candidates.
Key Message
In conclusion, obesity is increasingly prevalent among CKD patients. Adequate management with respect to the specific role of obesity in different stages of CKD should be integrated in routine renal care.
Keywords: Obesity, Nephropathy, Chronic kidney disease, Dialysis, Kidney transplantation
Introduction
The rising obesity pandemic has received major attention in general medicine and nephrology in the last few years [1, 2]. Globally, a high body mass index (BMI) accounted for 4.0 million deaths and more than two thirds of deaths related to a high BMI were due to cardiovascular disease [1]. The prevalence of obesity among children and adults has doubled since 1980 and has shown a continuous increase in many countries including the United States, Africa, and Asia, with highest numbers of obese children in China and India [1].
In view of chronic kidney disease (CKD) as a global public health concern, actions were taken to reduce major traditional and nontraditional risk factors, like obesity, as key determinants of poor health outcomes [3]. CKD is an important risk amplifier within diabetes, hypertension, and cardiovascular disease, conditions which are closely linked to obesity. The hazard ratio (HR) for end-stage kidney disease in obese adolescents is estimated to be between 7 in nondiabetic and almost 20 in diabetic people [3]. Thus, reduction of lifestyle-related risks is a cornerstone of mitigating the public health impact of diabetes, hypertension, and obesity. Primary intervention of obesity includes education, lifestyle, diet, exercise, weight management, and stress reduction.
Because of the limited effects of conservative and medical treatment of morbid obesity [4, 5] bariatric surgery has raised increasing interests in the renal community [6, 7]. Long-term results in patients after bariatric intervention are promising in ameliorating the prevalence of CKD, diabetes, and hypertension [8, 9, 10, 11]. Kidney-related complications of bariatric surgery itself as acute kidney insufficiency or stone formation in specific procedures (Roux-en-Y gastric bypass and biliopancreatic diversion with duodenal switch), which affect oxalate and citrate secretion, may be considered [6].
The aim of this paper is to give a brief overview of the ambiguous role of obesity (so-called “obesity paradox”) in CKD, which is different in early stages with the origin of a specific obese nephropathy, in advanced CKD and in dialysis, and in the course of kidney transplantation (Table 1).
Table 1.
Effect of overweight (BMI >30) in different stages of CKD (for details, see text)
| Condition | CKD 1–4 | CKD 5 and CKD 5D | Kidney transplantation |
|---|---|---|---|
| Renal function | Origin of specific obesity-associated nephropathy Decrease of GFR by hyperfiltration, inflammation, albuminuria ? Contributes to GFR decline in nonobese nephropathies |
? Modifies decline of renal residual function | ? Impaired graft function |
| Risk modification | Depending on metabolic aspects (diabetes control, hypertension, lipid profile) Endocrine status (fertility, adrenal function, hypothyroidism, pituarity functioning) Genetic factors |
Improved survival independent of comorbidity ? Benefits may be different in the age groups and within dialysis modalities |
Increased risk of surgical complications Decreased graft survival ? Increased mortality risks due to metabolic complications |
| Preventive measurement | Diet modification Exercise Behavior changes Psychological counseling Social support |
Adequate dialysis Sufficient nutritional intake (calories, protein) Regular assessment of body weight and conditions which may cause weight loss |
Aggressive treatment of comorbidity risks (glucose control, lipids, smoking, hypertension) Diet modification Exercise/counseling |
| Therapeutic aspects | Medication Bariatric surgery |
Standard nutritional recommendation according to treatment modality Nutritional intervention to avoid weight loss (<25 BMI) |
? Adaption of immunosuppressive regimen (steroids) ? Bariatric surgery |
| Further aspects | Definition of benefits and risks of complications by bariatric surgical intervention | Normalization of body weight in transplant candidates (BMI <30–35) Definition of the role of bariatric surgery |
Special donor care to reduce overweight and prevent OAN |
| ?, denotes limited evidence; OAN, obesity-associated nephropathy. | |||
Obesity in the Early Stage of CKD
Obesity (BMI > 30) can cause or worsen CKD. Genetic (mono-, polygenetic) and epigenetic aspects contribute to the development of obesity [2, 12, 13] but the exact risk estimates of these aspects for the development of kidney disease remain unclear. Metabolic effects of different adipokines (leptin, resistin, visfatin) and downregulation of adiponectin may contribute to hemodynamic and structural renal lesions via insulin resistance, increased insulin blood level, RAAS activation, oxidative stress, and microinflammation [2, 7, 14]. Risks of obesity-associated CKD seems to be different in metabolically healthy subjects and in those with metabolic syndrome [2]. A recent 8-year study in Japanese people (BMI > 25) revealed a crude incidence proportion of CKD in metabolically healthy obese phenotype (i.e., high levels of insulin sensitivity, low prevalence of hypertension, favorable fasting glucose, lipid, and inflammation profile) of 6.7 versus 10.9% (odds ratio 1.44 [95% CI 0.8–2.77] vs. 2.80 [95% CI 1.45–5.35]) in metabolically abnormal subjects [15].
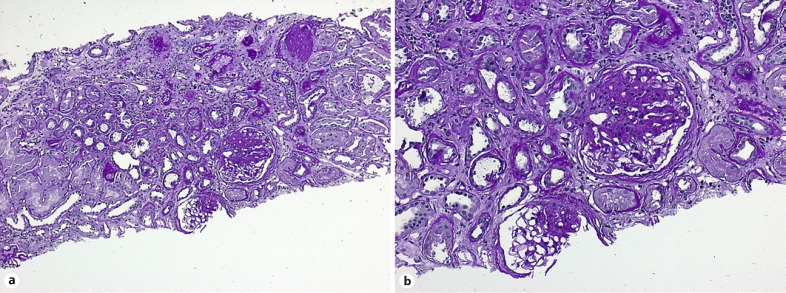
Morphology of obesity-associated nephropathy (OAN) has been derived from autopsy and biopsy studies. Increased kidney weight and hypertrophy of individual nephrons are common findings in OAN. Consistently, a 3-fold increased glomerular size compared to nonobese subjects is found in several studies while glomerular density in the cortex is reduced. The number of glomerular capillaries appears to be increased suggesting de novo formation of microvessels (review by Tsuboi et al. [16]). Onset of albuminuria is associated with focal segmental glomerulosclerosis (FSGS). The extent of FSGS seems to be different in different stages of obesity [16]. Finally, FSGS is accompanied by progressive tubule interstitial fibrosis (Fig. 1a, b) in accordance with loss of kidney function. Conditions of reduced nephron mass as low birth weight, congenital anomalies of the kidney and urinary tract, or nephrectomy contribute significantly to the progression of CKD in obese individuals.
Fig. 1.
Histological features of obesity-related nephropathy (percutaneous renal biopsy of a 48-year-old woman, courtesy Prof. Kerstin Amann, Erlangen, Germany). a Prominent global and focal segmental glomerulosclerosis (FSGS). PAS. ×10. b FSGS and severe tubulointerstitial fibrosis. PAS. ×20.
Indication for renal biopsy may result from rapid progressive loss of kidney function, active urinary sediment findings, and extrarenal features suggesting non-OAN as found in single cases with glomerular or systemic disease. While regular biopsy technique may fail in obese individuals, transjugular access is an option [17].
In the general population, obesity (BMI > 30) increases the risk of CKD, onset of albuminuria in CKD stages 1–2 as well as progression to CKD stages 3 and higher in the general population [18]. The course of CKD may be modulated by different causes of reduced kidney mass, genetic aspects, and metabolic phenotype. Medical RAAS blockade to influence endothelial dysfunction and associated tubulointerstitial fibrosis may be an option to prevent obese nephropathy [14]. Interestingly, decrease of proteinuria has been observed in a single-center study in patients with biopsy-proven obese nephropathy after losing weight by a medical supervised standard weight loss program [19].
Obesity in Advanced CKD and Dialysis: A Paradox
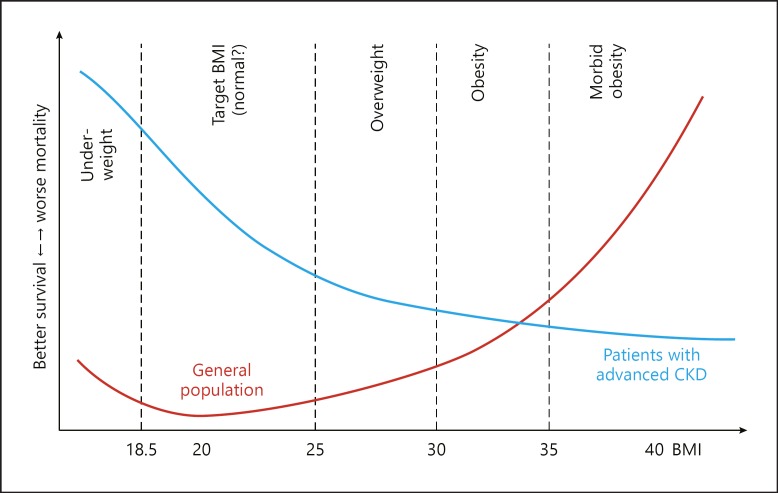
The paradoxical finding that even morbid obesity predicts better outcome in dialysis is well-known since 1999 when first reports from large US cohort and single-center studies were published [20, 21]. Meanwhile, several studies have shown that any gain in body weight is associated with better survival in advanced CKD and dialysis (review by Kalantar-Zadeh et al. 22) (Fig. 2). Analysis of death rates in advanced CKD stages suggest that BMI > 40 was not associated with higher risks of death after excluding diabetes and hypertension from the risk model [22]. While larger body size by fluid retention is associated with poorer outcomes gaining body weight with increased fat and muscle mass (fat-free lean body mass) is favorable [2, 22]. Anthropometry such as mid-arm muscle circumference can be used to estimate lean body mass. Dual energy X-ray absorptiometry is routinely used for the assessment of fat mass in dialysis patients. Findings from different studies suggest that survival advantages from gaining fat mass is lower than from gaining lean body mass [22]. Using waist circumference as surrogate of intra-abdominal or visceral fat shows that each 10-cm increase in waist circumference was associated with higher all-cause and cardiovascular death [23]. The superiority of survival advantages of (gaining) muscle compared to fat mass was confirmed by calculating the relation of lean to fat mass index which results in more favorable outcomes in patients with advanced CKD stages with a high lean/fat tissue index [24].
Fig. 2.
The obesity paradox: reverse association of BMI and survival in CKD patients as compared to the general population (reprint from Kalantar-Zadeh et al. [22]).
New insights of the meaning of muscle mass compared to fat mass in body weight gain comes from the “sarcopenia obesity” concept [25]. Increasing BMI in dialysis patients does not exclude concurrent muscle wasting. Sarcopenia and its individual criteria (hand grip strength, slow gait speed) are associated with mortality in hemodialysis patients [26]. Otherwise, intervention by structured exercise programs, which increase muscle mass in these patients, is proven to ameliorate functional parameters, body composition, quality of life, and survival [27, 28, 29].
The impact of BMI variations may vary by different dialysis populations and age. Most studies are done in hemodialysis patients. While increase in muscle weight in dialysis seems to be a defined advantage in both modalities, peritoneal dialysis and hemodialysis, the role of gaining body weight by fat mass in peritoneal dialysis patients is less well defined [21]. Furthermore, intentional weight loss may differ from unintentional wasting in these populations. In older age groups, hemodialysis patients with stable weight had a longer survival than elderly who lose or gain weight [30]. Death rates of obese younger dialysis patients (< 65 years) are 1.7-fold higher than for the older group (> 65 years) after adjustment for comorbidity and treatment modality [31].
Future studies are needed that advance our understanding of the existence of the obesity paradox. Results from these studies may create more efforts to define a more elaborated therapeutic management of obese patients along with functional improvement, quality of life, and survival.
The Role of Obesity in Kidney Transplantation
The impact of obesity (BMI > 30) in kidney transplant recipients has been recognized for more than 30 years [2]. Cumulate evidence from numerous observational studies and registries including more than 138,000 transplant patients revealed (a) a higher proportion of delayed graft function (odds ratio 1.68, 95% CI 1.39–2.03), (b) an increased risk of death-censored graft loss (HR 1.06, 95% CI 1.01–1.12), but (c) no significant long-term survival risk (HR 1.24, 95% CI 0.9–1.70) [32]. A second meta-analysis including more than 240,000 patients shows similar results for delayed graft function and a slightly increased risk of patient death (HR 1.19, 95% CI 1.10–1.31), increased risks for the presence of biopsy-proven acute rejection (HR 1.51, 95% CI 1.24–1.78), and allograft loss (HR 1.54, 95% CI 1.38–1.68) [33]. Thus, recently published calculators for kidney graft survival include BMI in the risk model [34].
As a consequence, loss of body weight is recommended in renal transplant candidates [34, 35]. Obviously, many patients are unable to achieve significant weight loss from conventional measurements. Bariatric surgery might become an option for this group of patients (summary in Hossain et al. [35]). While gastric banding is no longer the method of choice to reduce body weight and metabolic complications in the long term, newer techniques like sleeve gastrectomy and intestinal bypass procedures are increasingly performed [36, 37]. In view of the limited data, it seems to be premature to advocate routine bariatric surgery for obese kidney recipients. Nevertheless, supervised loss of body weight to achieve a BMI < 30–35 before transplantation is recommended to improve kidney graft survival.
The role of donor to recipient size mismatching is poorly defined today. A recently published single-center study estimates the impact of donor body weight to recipient body weight. Donor to recipient mismatching in the highest group of donor body weight showed no increased risk for 1 and 5 years of allograft survival [38].
Summary and Conclusion
Obesity has emerged as a new risk factor in the development of CKD. Obesity can cause a specific renal nephropathy or contribute to renal complications in metabolic syndrome. Weight reduction by conservative management or metabolic (bariatric) surgery ameliorates renal complications. In advanced CKD as well as in end-stage kidney disease, a high BMI is associated with better survival. Losing weight in the stage of dialysis treatment must be avoided. Maintenance of body weight by adequate nutrition and physical activity is mandatory to avoid weight loss or development of sarcopenia. The phenomenon of “sarcopenia obesity” must be recognized by assessing body composition together with functional testing (handgrip strength, gait speed). Structured exercise programs - mainly at the time of dialysis treatment - are proven to increase muscle mass and functional outcomes. Because high BMI (> 30–35) is associated with higher risks of transplant complications, reduction of body weight should be achieved in transplant candidates. Results of bariatric surgery in this group are limited but promising. Further research needs to focus on strategies to reduce the burden of obesity in early CKD stages, maintaining adequate body weight and body composition in advanced stages of CKD and dialysis, and the development of strategies to reduce transplantation risks in obese recipients. Thus, nephrologists have become players in a multidisciplinary team approach to fight against obesity as a pandemic health problem. Distinct knowledge of the different role of obesity in the trajectory of CKD is crucial for the adequate management of obese patients.
Disclosure Statement
The author has no conflicts of interest to disclose.
References
- 1.The GBD 2015 Obesity Collaborators Health effects of overweight and obesity in 195 countries over 25 years. N Engl J of Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenvinkel P, Zoccali C, Ikizler TA. Obesity in CKD - What should nephrologists know? J Am Soc Nephrol. 2013;24:1727–1736. doi: 10.1681/ASN.2013040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luyckx V, Tuttle KR, Garcia-Garcia G, Gharbi MB, Heerspink HJL, Johnson DW, Zhi-Hong L, Massy ZA, Me O, Nelson RG, Sola L, Wheeler D, White SL. Reducing major risk factors for chronic kidney disease. Kidney Int (Suppl) 2017;7:71–87. doi: 10.1016/j.kisu.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Look AHEAD Research Group Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2014;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal V, Navaneethan SD. Bariatric surgery for obesity-associated decline in kidney function: filling the knowledge gap? Kidney Int. 2016;90:28–30. doi: 10.1016/j.kint.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Furth SL, Zoccali C, for the World Kidney Day Steering Committee Obesity and kidney disease: hidden consequences of the epidemic. Kidney Int. 2017;91:260–262. doi: 10.1016/j.kint.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, Kramer H, Hartle JE, Carey D, Appel LJ, Grams ME. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90:164–171. doi: 10.1016/j.kint.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nehus Ej, Khoury JC, Inge TH, Xiao N, Jenkins TM, Moxey-Mims MM, Mitsnefer MM. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int. 2016;91:451–458. doi: 10.1016/j.kint.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RI, Nanjee N, Gutierrez JM, Frogley SJ, Ibele AR, Brinton EA, Hopkins PN, McKinley R, Simper SC, Hunt SC. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377:1143–1155. doi: 10.1056/NEJMoa1700459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes MV, Pulit SL, Lindgren CM. Genetic and epigenetic studies of adiposity and cardiometabolic disease. Genome Med. 2017;9:82. doi: 10.1186/s13073-017-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92:313–323. doi: 10.1016/j.kint.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto Y, Tanaka M, Okada H, Senmaru T, Hamaguchi M, Asano M, Yamazaki M, Oda Y, Hasegawa G, Toda H, Nakamura N, Fukui M. Metabolically healthy obesity and risk of incident CKD. Clin J Am Soc Nephrol. 2015;10:578–583. doi: 10.2215/CJN.08980914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The renal pathology of obesity. Kidney Int Rep. 2017;2:251–260. doi: 10.1016/j.ekir.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandari J, Fuller TW, Turner RM, D´Agostino LA. Renal biopsy for medical renal disease: indications and contraindications. Can J Urol. 2016;23:8121–8126. [PubMed] [Google Scholar]
- 18.Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91:1224–1235. doi: 10.1016/j.kint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Wen-wen S, Hui-mei C, Hao C, Feng X. Lei-shi L, Zhi-hong L. Obesity-related glomerulopathy: body mass index and proteinuria. Clin J Am Soc Nephrol. 2010;5:1401–1409. doi: 10.2215/CJN.01370210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1,346 hemodialysis patients. Kidney Int. 1999;55:1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple J. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Rhee CM, Chou J, Ahmadi SF, Park J, Chen JLT, Armin AN. The obesity paradox in kidney disease: How to reconcile it with obesity management. Kidney Int Rep. 2017;2:271–281. doi: 10.1016/j.ekir.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postorino M, Marino C, Tripepi MC, Zoccali C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009;53:1265–1272. doi: 10.1016/j.jacc.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Lin TY, Peng CH, Hung SC, Tarng DC. Body composition is associated with clinical outcomes in patients with non-dialysis dependent chronic kidney disease. Kidney Int. 2018;93:733–740. doi: 10.1016/j.kint.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Johansen KL, Lee C. Body composition in chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24:268–275. doi: 10.1097/MNH.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kittiskulnam P, Chertow GM, Carrero JJ, Delgado C, Kaysen GA, Johansen KL. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017;92:238–247. doi: 10.1016/j.kint.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhamb M, Weiner DE. Exercise to improve physical function and quality of life in CKD. Clin J Am Soc Nephrol. 2014;9:2023–2024. doi: 10.2215/CJN.10411014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anding K, Bär T, Trojniak-Hennig J, Kuchinke S, Krause R, Rost JM, Halle M. A structured exercise programme during haemodialysis for patients with chronic kidney disease: clinical benefit and long-term adherence. BMJ Open. 2015;5:e008709. doi: 10.1136/bmjopen-2015-008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J. 2015;8:753–765. doi: 10.1093/ckj/sfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villain C, Ecochard R, Genet L, Jean G, Kuentz F, Lataillade D, Legrand E, Moureau-Gaudry X, Fouque D. Impact of BMI variations on survival in elderly hemodialysis patients. J Ren Nutr. 2015;25:488–493. doi: 10.1053/j.jrn.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Hoogeveen EK, Halbesma N, Rothman KJ, Stijnen T, van Dijk S, Dekker FW, Boeschoten EW, de Mutsert R. Obesity and mortality risk among younger dialysis patients. Clin J Am Soc Nephrol. 2012;7:280–288. doi: 10.2215/CJN.05700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill CJ, Courtney AE, Cardwell CR, Maxwell AP, Lucarelli G, Veroux M, Furriel F, Cannon RM, Hoogeven EK, Doshi M, McCaughan JA. Recipient obesity and outcomes after kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. 2015;30:1403–1411. doi: 10.1093/ndt/gfv214. [DOI] [PubMed] [Google Scholar]
- 33.Sood A, Hakim DN, Hakim NS. Consequences of recipient obesity on postoperative outcomes in a renal transplant: a systematic review and meta-analysis. Exp Clin Tranplant. 2016;14:121–128. [PubMed] [Google Scholar]
- 34.Ashby VB, Leichtman AB, Rees MA, Song PX, Bray M, Wang W, Kalbfleisch JD. A kidney graft survival calculator that accounts for mismatches in age, sex, HLA, and body size. Clin J Am Soc Nephrol. 2017;12:1148–1160. doi: 10.2215/CJN.09330916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hossain M, Woywandt A, Augustine T, Sharma V. Obesity and listing for renal transplantation: weighing the evidence for a growing problem. Clin Kidney J. 2017;10:703–708. doi: 10.1093/ckj/sfx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman AN, Wolfe B. Is bariatric surgery effective treatment for type II diabetic kidney disease? Clin J Am Soc Nephrol. 2016;11:528–535. doi: 10.2215/CJN.07670715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas IA, Gaynor JJ, Joseph T, De la Cruz-Munoz N, Sageshima J, Kupin W, Chin LJ, Ciancio G, Burke GW, Mattiazzi AD, Roth D, Guerra G. Roux-en-Y gastric bypass is an effective bridge to kidney transplantation: results from a single center. Clin Transplant. 2018;32:e13232. doi: 10.1111/ctr.13232. [DOI] [PubMed] [Google Scholar]
- 38.Wong L, Counihan A, O'Kelly P, Sexton DJ, O'Seaghdha CM, Magee C, Little D, Conlon PJ. The impact of donor and recipient weight incompatibility on renal outcomes. Int Urol Nephrol. 2018;50:551–558. doi: 10.1007/s11255-017-1745-1. [DOI] [PubMed] [Google Scholar]