Abstract
Objective
This study assessed the relationship between serum albumin (ALB) at start of peritoneal dialysis (PD) and long-term outcomes of continuous ambulatory PD (CAPD) in Anhui Han patients.
Methods
A total of 149 Anhui Han CAPD patients were enrolled in this study and followed up for 3 years. They were initially diagnosed with the end-stage renal disease and underwent surgical PD catheter placement from January 2009 to December 2013. According to serum ALB at start of PD, the patients were divided into two groups: low ALB group (ALB < 35 g/L) and high ALB group (ALB ≥35 g/L). Demographic, hematologic, biochemical, and dialysis-related data were collected. Kaplan-Meier survival analysis and log-rank test were conducted to compare patient mortality, cardiovascular mortality and technique failure between the low ALB group and the high ALB group. Cox regression analysis was performed to analyze the risk factors, calculate the hazard ratio (HR), adjusted HR (AHR) and 95% confidence interval (CI).
Results
The low ALB group showed a greater number of diabetes mellitus compared with the high ALB group. Patient mortality, cardiovascular mortality, and technique failure in the high ALB group were significantly lower than those in the low ALB group. In Cox regression analysis, serum ALB < 35 g/L was an independent predictor of patient mortality (AHR 3.043, 95% CI 1.085–8.536, p = 0.034), cardiovascular mortality (AHR 11.587, 95% CI 1.466–91.574, p = 0.020), and technique failure (AHR 3.148, 95% CI 1.603–6.182, p = 0.001) in CAPD patients after adjustment for sex, age, estimated glomerular filtration rate, primary renal disease, diabetes mellitus, and cardiovascular disease.
Conclusions
In Anhui Han patients on CAPD, the levels of serum ALB at start of PD are inversely correlated with patient mortality, cardiovascular mortality, and technique failure, and the long-term outcomes of patients with hypoalbuminemia at start of PD are poor. To improve the long-term outcomes of Anhui Han CAPD patients, patients with hypoalbuminemia at start of PD should be closely monitored.
Keywords: Serum albumin, Patient mortality, Cardiovascular mortality, Technique failure, Peritoneal dialysis
Introduction
Peritoneal dialysis (PD) is an effective treatment for end-stage renal disease (ESRD). The prognosis of patients on PD and its influencing factors especially at start of PD which can preliminarily evaluate the prognosis of patients on PD before dialysis, have been a major concern for clinical staff. Previous studies have shown that the serum albumin (ALB) levels of PD patients are closely related to all-cause mortality [1, 2, 3, 4, 5, 6, 7, 8, 9], cardiovascular mortality [1, 2, 3], and technique failure [4, 5, 6]. However, there are few cohort studies that simultaneously analyze the relationship between serum ALB levels and all-cause mortality, cardiovascular mortality and technique failure. In this research, patient survival, time and cause of death, or transfer to hemodialysis (HD) were recorded to obtain the long-term outcomes of patients on continuous ambulatory PD (CAPD), and a retrospective cohort study was designed to simultaneously explore the relationship between serum ALB at start of PD and all-cause mortality, cardiovascular mortality, and technique failure in Anhui Han CAPD patients.
Study Patients and Methods
Study Patients and Design
We conducted a single-center retrospective cohort study with the aim of exploring the relationship between serum ALB at start of PD and patient mortality, cardiovascular mortality, and technique failure in CAPD patients. A total of 175 patients were recruited, who were initially diagnosed with the ESRD and underwent surgical PD catheter placement at the Department of Nephrology in the First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Hospital) from January 2009 to December 2013. Twenty-six patients were excluded for the following reasons: age under 18 years old, survived less than 3 months following the initiation of PD, recovered renal function, no longer required dialysis, malignancy before dialysis, or kidney transplantation after PD. Eventually, 149 Anhui Han patients were in the final data set. According to serum ALB levels at start of PD, the patients were divided into two groups: low ALB group (ALB < 35 g/L) and high ALB group (ALB ≥35 g/L). All patients were followed up for 3 years. The patients who underwent CAPD within 3 years and transferred to HD were still followed up to death or for 3 years. Patient survival, time and cause of death, or transfer to HD were recorded to obtain the long-term outcomes of CAPD patients through outpatient review, letter, or telephone follow-up. None of the patients were lost to follow-up.
Data Collection
Patients were given conventional glucose-based solution and used CAPD on initial PD. The first peritoneal equilibrium test and dialysis adequacy were done within 1–3 months of CAPD initiation, and the dialysate/plasma creatinine and weekly Kt/v urea were evaluated. We retrospectively collected demographic information, and clinical data including cause of ESRD, diabetes mellitus (DM), cardiovascular disease, and laboratory measurements at start of PD such as hemoglobin, calcium, phosphate, ALB, uric acid, and estimated glomerular filtration rate (eGFR).
In this research, the cardiovascular disease was defined as follows [10]: (1) congestive heart failure or atrial fibrillation; (2) coronary artery disease; and (3) cerebrovascular disease. The residual renal function at start of PD was evaluated by eGFR; eGFR was estimated using the simplified modification of diet in renal disease equation: eGFR (mL/min/1.73 m2) = 186 × serum creatinine (mg/dL)–1.154 × age (years)–0.203 × 0.742 (if female).
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics 20.0 version. Continuous variables were summarized as means ± standard deviation, and analyzed using the Student's t test. The number (n) and percentage (%) in each category were calculated for categorical variables, which were analyzed using χ2 test.
Clinical outcomes in this study included patient mortality, cardiovascular mortality, and technique failure. Only death was used as the final event in the patient survival analysis, and patients who were still alive at the end of the study were considered censored data. Only death attributed to cardiovascular disease was used as the final event in the patient cardiovascular mortality analysis, and patients who with death attributed to other reasons or still alive at the end of the study were considered censored data. While analyzing the technique survival, dropout attributed to death or transferred to HD was used as the end point, and patients who remained alive and on PD therapy were considered censored data [11].
The Kaplan-Meier survival analysis and log-rank test were conducted to compare patient mortality, cardiovascular mortality, and technique failure between the low ALB group and the high ALB group. The Cox regression analysis was also performed to analyze the risk factors, calculate the hazard ratio (HR), adjusted HR (AHR), and 95% confidence interval (CI). A p value < 0.05 was considered statistically significant.
Results
Baseline Clinical Parameters of Patients on CAPD
The baseline clinical data of the 149 enrolled Anhui Han patients are shown in Table 1. The causes of ESRD were as follows: glomerulonephritis (n = 105, 70.5%), hypertension (n = 20, 13.4%), DM (n = 17, 11.4%), other (n = 7, 4.7%).
Table 1.
Clinical parameters of patients on continuous ambulatory peritoneal dialysis
| Parameters | Total (n = 149) | Low ALB group (ALB <35 g/L) (n = 88) | High ALB group (ALB ≥35 g/L) (n = 61) | p value |
|---|---|---|---|---|
| Sex, male/female | 91/58 | 58/30 | 33/28 | 0.15 |
| Age, years | 48.5±16.3 | 49.6±15.8 | 47.0±17.1 | 0.34 |
| Primary renal disease | 0.15 | |||
| Diabetes mellitus | 17 (11.4) | 14 (15.9) | 3 (4.9) | |
| Hypertension | 20 (13.4) | 11 (12.5) | 9 (14.8) | |
| Glomerulonephritis | 105 (70.5) | 59 (67.1) | 46 (75.4) | |
| Other | 7 (4.7) | 4 (4.5) | 3 (4.9) | |
| eGFR, mL/min/1.73 m2 | 5.4±2.1 | 5.5±2.1 | 5.2±2.1 | 0.55 |
| Diabetes mellitus | 22 (14.8) | 19 (21.6) | 3 (4.9) | 0.01 |
| Cardiovascular disease | 32 (21.5) | 23 (26.1) | 9 (14.8) | 0.09 |
| Hemoglobin, g/L | 74.9±15.3 | 73.7±13.9 | 76.8±17.1 | 0.22 |
| UA, µmol/L | 516.9±146.9 | 508.8±130.7 | 528.6±168.1 | 0.43 |
| Ca, µmol/L | 1.9±0.3 | 1.9±0.2 | 1.9±0.3 | 0.34 |
| P, µmol/L | 2.1±0.8 | 2.0±0.8 | 2.2±0.8 | 0.16 |
| ALB, g/L | 34.2±5.7 | 30.7±3.3 | 39.2±4.5 | <0.001 |
| Weekly Kt/v urea | 2.1±0.3 | 2.2±0.4 | 2.1±0.3 | 0.13 |
| D/P Cr | 0.03 | |||
| High | 20 (13.4) | 8 (9.1) | 12 (19.7) | |
| High-average | 58 (38.9) | 40 (45.5) | 18 (29.5) | |
| Low-average | 57 (38.3) | 29 (32.9) | 28 (45.9) | |
| Low | 14 (9.4) | 11 (12.5) | 3 (4.9) |
Data are presented as n (%) or mean ± standard deviation). ALB, albumin; eGFR, estimated glomerular filtration rate; UA, uric acid; Ca, calcium; P, phosphate; D/P Cr, dialysate/plasma creatinine.
According to serum ALB at start of PD, the patients were divided into low ALB group (n = 88, 59.1%) and high ALB group (n = 61, 40.9%). Table 1 shows the general characteristics of the low ALB group and the high ALB group. There were no significant differences between two groups in the gender, average age, the cause of ESRD, eGFR, hemoglobin levels, uric acid levels, cardiovascular disease, and weekly Kt/v urea. Compared with the high ALB group, the low ALB group had a greater number of DM cases.
The Influence of Serum ALB at Start of PD on Patient Survival in CAPD Patients
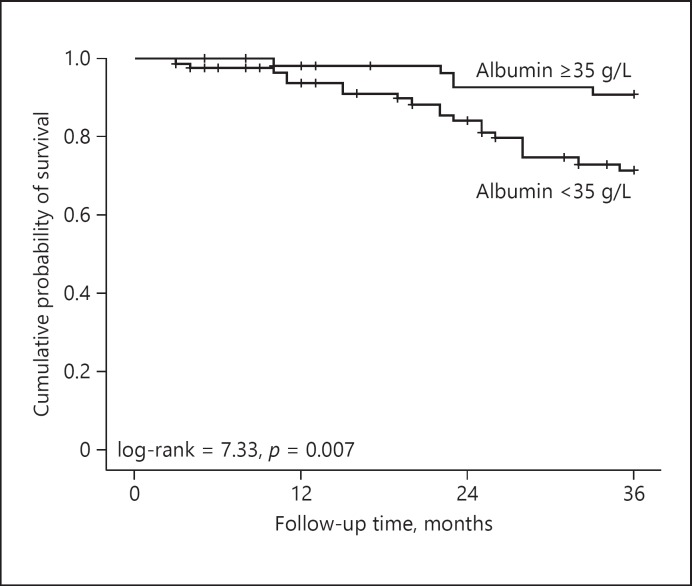
During the 3-year follow-up, 25 of 149 CAPD patients died, the causes of death were cardiovascular disease (15, 60%) and sepsis (10, 40%). Kaplan-Meier survival analysis was used to analyze the cumulative survival rate of the patients on CAPD. The cumulative survival rates at 1, 2, and 3 years were 95.7, 87.9, and 79.4%, respectively.
Kaplan-Meier survival analysis was used to compare the patient survival between the low ALB group and the high ALB group. Figure 1 shows that the cumulative survival rates in the high ALB group at 1, 2 and 3 years were 98.3, 92.8, and 89.2%, respectively, while the cumulative survival rates in the low ALB group at 1, 2, and 3 years were 93.9, 84.3, and 71.4%, respectively. The 3-year cumulative mortality in the low ALB group was significantly higher than that in the high ALB group (log-rank = 7.33, p = 0.007).
Fig. 1.
Kaplan-Meier analysis of survival in 149 continuous ambulatory peritoneal dialysis patients (low ALB group vs. high ALB group).
The Cox regression analysis showed that the low ALB group had an increased risk for patient mortality with an HR of 3.544 (95% CI 1.327–9.459, p = 0.012) in CAPD patients compared with the high ALB group. After adjustment for sex, age, eGFR, primary renal disease, DM, and cardiovascular disease, the low ALB group still had an increased risk for death with an AHR of 3.043 (95% CI 1.085–8.536, p = 0.034) in CAPD patients compared with the high ALB group (Table 2).
Table 2.
Multivariate Cox regression analysis of patient mortality
| Variable | AHR (95% CI) | p |
|---|---|---|
| Albumin <35 g/L | 3.043 (1.085–8.536) | 0.034 |
| Sex (male) | 2.510 (0.939–6.714) | 0.067 |
| Age (years) | 1.054 (l.019–1.090) | 0.002 |
| eGFR | 0.857 (0.679–1.082) | 0.194 |
| Primary renal disease | 1.180 (0.585–2.381) | 0.643 |
| Diabetes mellitus | 0.575 (0.155–2.137) | 0.408 |
| Cardiovascular disease | 2.007 (0.861–4.680) | 0.107 |
eGFR, estimated glomerular filtration rate; AHR, adjusted hazard ratio; CI = confidence interval.
Serum ALB at Start of PD Is a Predictor of Cardiovascular Mortality in CAPD Patients
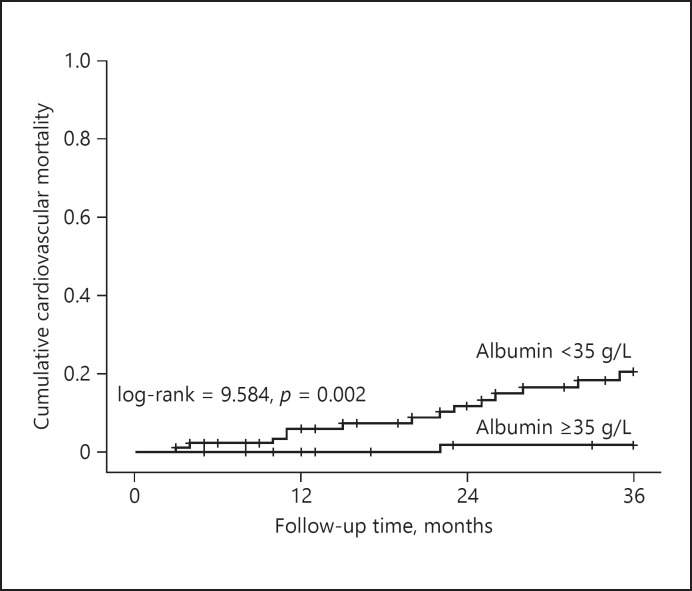
During the 3-year follow-up, 15 of 149 CAPD patients had cardiovascular mortality. Kaplan-Meier survival analysis was used to analyze the cumulative cardiovascular mortality of the patients on CAPD. The cumulative cardiovascular mortality at 3 years was 12.1%.
Kaplan-Meier survival analysis was used to compare the cardiovascular mortality between the low ALB group and the high ALB group. Figure 2 shows that the cumulative cardiovascular mortality in the high ALB group at 3 years is 1.9%, while the cumulative cardiovascular mortality in the low ALB group at 3 years is 20.5%. The 3-year cumulative cardiovascular mortality in the low ALB group was significantly higher than that in the high ALB group (log-rank = 9.584, p = 0.002).
Fig. 2.
Kaplan-Meier analysis of cardiovascular mortality in 149 continuous ambulatory peritoneal dialysis patients (low ALB group vs. high ALB group).
The Cox regression analysis showed that the low ALB group had an increased risk for cardiovascular mortality with an HR of 12.227 (95% CI 1.605–93.142, p = 0.016) in CAPD patients compared with the high ALB group. After adjustment for sex, age, eGFR, primary renal disease, DM, and cardiovascular disease, the low ALB group still had an increased risk for cardiovascular mortality with an AHR of 11.587 (95% CI 1.466–91.574, p = 0.020) in CAPD patients compared with the high ALB group (Table 3).
Table 3.
Multivariate Cox regression analysis of cardiovascular mortality
| Variable | AHR (95% CI) | P |
|---|---|---|
| Albumin <35 g/L | 11.587 (1.466–91.574) | 0.020 |
| Sex (male) | 2.834 (0.752–10.686) | 0.124 |
| Age (years) | 1.045 (0.999–1.093) | 0.054 |
| eGFR | 0.756 (0.549–1.039) | 0.085 |
| Primary renal disease | 0.929 (0.385–2.240) | 0.869 |
| Diabetes mellitus | 0.583 (0.114–2.975) | 0.517 |
| Cardiovascular disease | 2.589 (0.902–7.433) | 0.077 |
eGFR, estimated glomerular filtration rate; AHR, adjusted hazard ratio; CI, confidence interval.
Serum ALB at Start of PD and Technique Survival in CAPD Patients
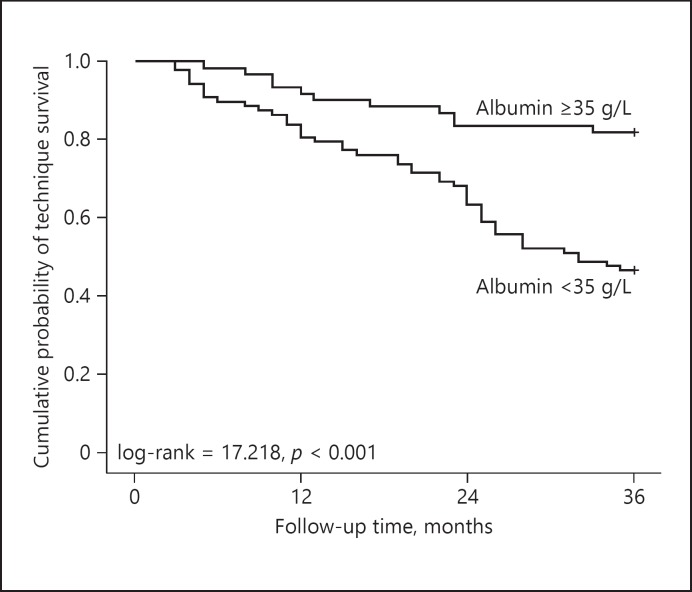
During the 3-year of follow-up, 58 of 149 PD patients had technique failure, the causes of technique failure were as follows: cardiovascular disease (28, 48.3%), peritonitis (12, 20.7%), sepsis except peritonitis (10, 17.2%), and other (8, 13.8%). Kaplan-Meier survival analysis was used to analyze the cumulative technique survival rates of the patients on CAPD. The cumulative technique survival rates at 1, 2, and 3 years were 87.9, 71.8, and 60.4% respectively.
Kaplan-Meier survival analysis was used to compare the technique survival between the low ALB group and the high ALB group. Figure 3 shows that the cumulative technique survival rates in the high ALB group at 1, 2 and 3 years were 91.8, 83.6, and 80.3% respectively, while the cumulative technique survival rates in the low ALB group at 1, 2, and 3 years are 80.7, 63.6, and 46.6%, respectively. The 3-year technique survival in the low ALB group was significantly lower than that in the high ALB group (log-rank = 17.218, p < 0.001).
Fig. 3.
Kaplan-Meier analysis of technique survival rates in 149 continuous ambulatory peritoneal dialysis patients (low ALB group vs. high ALB group).
Cox regression analysis showed that the low ALB group had an increased risk for technique failure with an HR of 3.639 (95% CI 1.885–7.023, p < 0.001) in CAPD patients compared with the high ALB group. After adjustment for sex, age, eGFR, primary renal disease, DM, and cardiovascular disease, the low ALB group still had an increased risk for technique failure with an AHR of 3.148 (95% CI 1.603–6.182, p = 0.001) in CAPD patients compared with the high ALB group (Table 4).
Table 4.
Multivariate Cox regression analysis of technique failure
| Variable | AHR (95% CI) | p |
|---|---|---|
| Albumin <35 g/L | 3.148 (1.603–6.182) | 0.001 |
| Sex (male) | 2.045 (1.116–3.747) | 0.021 |
| Age (years) | 1.008 (0.989–1.028) | 0.388 |
| eGFR | 0.924 (0.806–1.060) | 0.258 |
| Primary renal disease | 1.008 (0.635–1.600) | 0.974 |
| Diabetes mellitus | 0.983 (0.414–2.333) | 0.969 |
| Cardiovascular disease | 2.013 (1.153–3.514) | 0.014 |
eGFR, estimated glomerular filtration rate; AHR, adjusted hazard ratio; CI, confidence interval.
Discussion
This research used a retrospective cohort study to explore the relationship between serum ALB at start of PD and patient mortality, cardiovascular mortality, and technique survival in Anhui Han CAPD patients. The results showed that after adjustment for sex, age, eGFR, primary renal disease, DM, and cardiovascular disease, low serum ALB at start of PD increased the risk of patient mortality, cardiovascular mortality, and technique failure, which was an independent risk factor for the long-term outcomes in Anhui Han CAPD patients.
Previous studies have shown that serum ALB level was an independent risk factor of patient mortality [1, 2, 3, 4, 5, 6, 7, 8, 9, 12] and cardiovascular mortality [1, 2, 3] in PD patients. Serum ALB was also associated with cardiovascular death in the general population and the patients with chronic kidney disease [13, 14, 15]. In this study, the 3-year cumulative patient mortality and cardiovascular mortality of the low ALB group were significantly higher than that of the high ALB group. Compared with the high ALB group, the low ALB group had an increased risk of mortality (AHR 3.043, 95% CI 1.085–8.536, p = 0.034) and cardiovascular mortality (AHR 11.587, 95% CI 1.466–91.574, p = 0.020) in CAPD patients after adjustment for sex, age, eGFR, primary renal disease, DM, and cardiovascular disease. The risk of cardiovascular mortality with low serum ALB level was greater than that of patient mortality, which suggested that low serum ALB may increase the risk of patient death by increasing the risk of cardiovascular death.
However, the reasons why low serum ALB increased the risk of cardiovascular mortality in patients on PD, may be as follows: (1) serum ALB is merely a reactivity index, the clinical state of severe inflammation [16], blood volume expansion [17], malnutrition [15], and liver function injury [18] would lead to low serum ALB and a poor prognosis of patients. (2) Serum ALB itself has an effect on the prognosis of PD patients: the colloid osmotic pressure can be reduced in the case of hypoalbuminemia, which can aggravate pulmonary congestion [19], while the pulmonary congestion is significantly associated with adverse outcomes in patients with cardiovascular disease [20]. Serum ALB is widely involved in the process of antioxidant and damage repair [21, 22]. When the serum ALB decreases, these protective effects can be greatly reduced, which increases the risk of cardiovascular death. In this study, in addition to serum ALB, advanced age also increased the risk of all-cause mortality in patients on PD. This may be related to a variety of complications associated with age, especially cardiovascular disease.
Several studies indicated that serum ALB was a significant risk factor associated with technique failure [4, 5, 6]. The findings of our study, similar to most studies on technique failure, showed that serum ALB was an independent risk factor for technique failure in CAPD patients. In addition, males had an increased risk for technique failure in our findings. This is similar to the results of Kitterer et al. [23]. But many other studies have shown that gender had nothing to do with the technique failure of PD [24, 25, 26, 27]. The relationship between technique failure and gender still needs further research.
This research was a single-center observational retrospective cohort study, which discussed the relationship between the serum ALB level at start of PD and the long-term outcomes for Anhui Han patients on CAPD, but the sample was small, follow-up duration was short, the proportion of diabetic nephropathy and elderly patients was low, and there was a certain degree of error according to a simplified MDRD formula to calculate the patient's residual renal function. The results could not exclude the influence of the changes of serum ALB level during 3 years, study time and survivor migration, and other confounding factors (such as social deprivation, dietary factors, medications history, alcohol consumption, and other risk behaviors during PD). And whether our research findings can be generalized to other groups remains uncertain. Therefore, the results of this study still need to be further confirmed.
Conclusion
In Anhui Han patients on CAPD, the levels of serum ALB at start of PD are inversely correlated with patient mortality, cardiovascular mortality, and technique failure, and the long-term outcomes of patients with hypoalbuminemia at start of PD are poor. To improve the long-term outcomes of Anhui Han CAPD patients, patients with hypoalbuminemia at start of PD should be closely monitored.
Statement of Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Anhui Provincial Hospital. Because of the retrospective nature of the study, patient consent for inclusion was waived.
Disclosure Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest to disclose.
Funding Sources
This work was not supported by any grant.
Acknowledgement
The authors would like to express sincere gratitude to their colleagues who contributed to the research while their names did not appear on the paper.
References
- 1.Mehrotra R, Duong U, Jiwakanon S, Kovesdy CP, Moran J, Kopple JD, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis. 2011;58:418–428. doi: 10.1053/j.ajkd.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol. 1996;7:728–736. doi: 10.1681/ASN.V75728. [DOI] [PubMed] [Google Scholar]
- 3.Kim SB, Yang WS, Park JS. Role of hypoalbuminemia in the genesis of cardiovascular disease in dialysis patients. Perit Dial Int. 1999;19:S144–S149. [PubMed] [Google Scholar]
- 4.Cueto-Manzano AM, Quintana-Pina E, Correa-Rotter R. Long-term CAPD survival and analysis of mortality risk factors: 12-year experience of a single Mexican center. Perit Dial Int. 2001;21:148–153. [PubMed] [Google Scholar]
- 5.Khoshhali M, Kazemi I, Hosseini SM, Seirafian S. Relationship between trajectories of serum albumin levels and technique failure according to diabetic status in peritoneal dialysis patients: a joint modeling approach. Kidney Res Clin Pract. 2017;36:182–191. doi: 10.23876/j.krcp.2017.36.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isla RA, Mapiye D, Swanepoel CR, Rozumyk N, Hubahib JE, Okpechi IG. Continuous ambulatory peritoneal dialysis in Limpopo province, South Africa: predictors of patient and technique survival. Perit Dial Int. 2013;34:518–525. doi: 10.3747/pdi.2013.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu PF, Tsai CC, Wu CL, Yang TY, Liou HH, Chen HL, et al. Trajectories of serum albumin predict survival of peritoneal dialysis patients: a 15-year follow-up study. Medicine. 2016;95:e3202. doi: 10.1097/MD.0000000000003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Cao T, Li Z, Wen Q, Lin J, Zhang X, et al. Clinical outcomes of peritoneal dialysis patients transferred from hemodialysis: a matched case-control study. Perit Dial Int. 2013;33:259–266. doi: 10.3747/pdi.2011.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanowsky-Escatell FG, Pazarín-Villaseñor L, Andrade-Sierra J, Zambrano-Velarde MA, Preciado-Figueroa FM, Santana-Arciniega CJ, et al. Association of serum albumin and subjective global assessment on incident peritoneal dialysis patients. Nutr Hosp. 2015;32:2887–2892. doi: 10.3305/nh.2015.32.6.9729. [DOI] [PubMed] [Google Scholar]
- 10.Lee KH, Cho JH, Kwon O, Kim SU, Kim RH, Cho YW, et al. Low prealbumin levels are independently associated with higher mortality in patients on peritoneal dialysis. Kidney Res Clin Pract. 2016;35:169–175. doi: 10.1016/j.krcp.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Liu H, Gong X, Liu F, Peng Y, Cheng M, et al. Risk factors for mortality in Chinese patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2015;35:199–205. doi: 10.3747/pdi.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blake PG, Flowerdew G, Blake RM, Oreopoulos DG. Serum albumin in patients on continuous ambulatory peritoneal dialysis - predictors and correlations with outcomes. J Am Soc Nephrol. 1993;3:1501–1507. doi: 10.1681/ASN.V381501. [DOI] [PubMed] [Google Scholar]
- 13.Richard JL, Warnet JM, Ducimetiere P. Decreased levels of the albumin-alpha-1-globulin fraction of serum proteins, risk factor for ischemic cardiopathy (in French) Rev Epidemiol Sante Publique. 1985;33:347–351. [PubMed] [Google Scholar]
- 14.Kuller LH, Eichner JE, Orchard TJ, Grandits GA, McCallum L, Tracy RP. The relation between serum albumin levels and risk of coronary heart disease in the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1991;134:1266–1277. doi: 10.1093/oxfordjournals.aje.a116030. [DOI] [PubMed] [Google Scholar]
- 15.Zamora E, Lupón J, Vila J, Urrutia A, de Antonio M, Sanz H, et al. Estimated glomerular filtration rate and prognosis in heart failure: value of the Modification of Diet in Renal Disease Study-4, chronic kidney disease, epidemiology collaboration, and Cockroft-Gault formulas. J Am Coll Cardiol. 2012;59:1709–1715. doi: 10.1016/j.jacc.2011.11.066. [DOI] [PubMed] [Google Scholar]
- 16.Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail. 2011;13:1161–1171. doi: 10.1093/eurjhf/hfr122. [DOI] [PubMed] [Google Scholar]
- 17.Adlbrecht C, Kommata S, Hülsmann M, Szekeres T, Bieglmayer C, Strunk G, et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body's red cell volume. Eur Heart J. 2008;29:2343–2350. doi: 10.1093/eurheartj/ehn359. [DOI] [PubMed] [Google Scholar]
- 18.Herzer K, Kneiseler G, Bechmann LP, Post F, Schlattjan M, Sowa JP, et al. Onset of heart failure determines the hepatic cell death pattern. Ann Hepatol. 2011;10:174–179. [PubMed] [Google Scholar]
- 19.Eising GP, Niemeyer M, Günther T, Tassani P, Pfauder M, Schad H, et al. Does a hyperoncotic cardiopulmonary bypass prime affect extravascular lung water and cardiopulmonary function in patients undergoing coronary artery bypass surgery? Eur J Cardiothorac Surg. 2001;20:282–289. doi: 10.1016/s1010-7940(01)00804-1. [DOI] [PubMed] [Google Scholar]
- 20.Gheorghiade M, Shin DD, Thomas TO, Brandimarte F, Fonarow GC, Abraham WT. Congestion is an important diagnostic and therapeutic target in heart failure. Rev Cardiovasc Med. 2006;7((suppl 1)):12–24. [PubMed] [Google Scholar]
- 21.Zoellner H, Höfler M, Beckmann R, Hufnagl P, Vanyek E, Bielek E, et al. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci. 1996;109:2571–2580. doi: 10.1242/jcs.109.10.2571. [DOI] [PubMed] [Google Scholar]
- 22.Era S, Kuwata K, Imai H, Nakamura K, Hayashi T, Sogami M. Age-related change in redox state of human serum albumin. Biochim Biophys Acta. 1995;1247:12–16. doi: 10.1016/0167-4838(94)00166-e. [DOI] [PubMed] [Google Scholar]
- 23.Kitterer D, Segerer S, Braun N, Alscher MD, Latus J. Gender-specific differences in peritoneal dialysis. Kidney Blood Press Res. 2017;42:276–283. doi: 10.1159/000477449. [DOI] [PubMed] [Google Scholar]
- 24.Mujais S, Story K. Peritoneal dialysis in the us: evaluation of outcomes in contemporary cohorts. Kidney Int. 2006;suppl:S21–S26. doi: 10.1038/sj.ki.5001912. [DOI] [PubMed] [Google Scholar]
- 25.Chidambaram M, Bargman JM, Quinn RR, Austin PC, Hux JE, Laupacis A. Patient and physician predictors of peritoneal dialysis technique failure: a population based, retrospective cohort study. Perit Dial Int. 2011;31:565–573. doi: 10.3747/pdi.2010.00096. [DOI] [PubMed] [Google Scholar]
- 26.Han SH, Lee JE, Kim DK, Moon SJ, Kim HW, Chang JH, et al. Long-term clinical outcomes of peritoneal dialysis patients: single center experience from Korea. Perit Dial Int. 2008;28:S21–S26. [PubMed] [Google Scholar]
- 27.Guo A, Mujais S. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int. 2003;suppl:S3–S12. doi: 10.1046/j.1523-1755.2003.08801.x. [DOI] [PubMed] [Google Scholar]