Abstract
Background
Acute kidney injury (AKI) is a common complication following cardiac surgery and percutaneous coronary interventions, with an estimated incidence rate around 30%, depicted by long-term intensive care unit stay and culminating renal dysfunction over time, triggering either perpetual renal damage evolving to chronic kidney disease/end-stage renal disease transitions or high vulnerability for sudden death after surgery. The classical diagnosis of AKI is based on a sharp rise in serum creatinine that takes at least 48 h to be visible and is associated with multiple nonrenal factors.
Objective
We aimed to evaluate the predictive performance of both neutrophil gelatinase-associated lipocalin (NGAL) and Klotho for AKI in patients who underwent cardiothoracic surgery using cardiopulmonary bypass (CPB).
Results
Out of the 182 patients included in the study, 65 had AKI and 117 had non-AKI according to the Kidney Disease: Improving Global Outcomes criteria relying on serum creatinine levels. Baseline serum NGAL was 103.5 ± 41.69 μg/L in the AKI group compared to 79.12 ± 48.02 μg/L in the non-AKI group (p < 0.01) and then manifested a peak-fall-rise pattern until 48 h of CPB, with a more remarkable change in the AKI than in the non-AKI group. ROC curve analysis for all measured biomarkers after 2 h of CPB showed that serum NGAL (0.819, > 75% cutoff, 83.5% accuracy) came after serum creatinine (0.864, > 140% cutoff, 85% accuracy), and troponin I was poorer than both (0.606, > 5.5% cutoff, 60% accuracy). Furthermore, multivariate analysis showed that preoperative serum NGAL, preoperative eGFR ≤60 mL/min/1.73 m2, and arterial hypertension were possible risk factors for AKI with adverse outcomes.
Conclusions
Our study suggests the role of preoperative serum NGAL as a prognostic tool for renal consequences after cardiac surgery. Besides, postoperative serum NGAL is a sensitive marker for AKI, but is less specific than serum creatinine. Troponin I is considered to be a risk confirmatory tool and may help in the prediction of AKI. However, its diagnostic utility is restricted due to age-dependent cutoff values and poor standardization and harmonization because of interassay variations.
Keywords: Serum neutrophil gelatinase-associated lipocalin, Cardiothoracic surgery, Troponin I, Acute kidney injury, Cardiopulmonary bypass
Introduction
Acute kidney injury (AKI) is a critical time-based problem with a pathophysiological impact on renal function ranging from slight temporary to severer permanent insult. Moreover, open heart surgery is a potential reason for prerenal AKI with a proportion of 40%, which badly affects 10% of hospital inpatients, leading to delayed discharge and a highly rate of short- or long-term deaths in the intensive care unit (ICU) [1, 2, 3]. In a related context, AKI was shown to be a firm transition state between normal kidney function and the worse chronic kidney disease/end-stage renal disease stage that absolutely raises the burden of research for alternatives to the poor prognosis routine renal troponins. Many papers have specified the type of AKI that comes after cardiac surgery, such as cardiovascular surgery-associated AKI (CSA-AKI), and have confirmed it to be the second reason for renal function impairment postoperatively due to related surgical procedures [4] that leads to systemic inflammation, ischemic reperfusion, oxidative stress, neurohormonal activation, intermittent hemodilution, as well as renal and tubular insult [5, 6].
The best way to screen for AKI is in the earlier period, in order to have a sufficient time for therapeutic intervention relying on novel biomarkers besides serum creatinine that exhibited slow changes despite AKI progression [7]. Revelation and testing of these biomarkers is not an easy process and should be less influenced by nonrenal factors, high sensitivity and/or specificity, changes synchronically regarding AKI staging and severity, and high discriminative power, depicted with a convergent kinetic behavior in both animals and humans [8].
A standard definition of AKI is still a challenge and is based on recommended international criteria from professionals and expert societies and is generally described as abrupt retrogradation of kidney function resulting in retention of uremic waste products and extracellular fluid volume disturbances [9]. However, the Kidney Disease: Improving Global Outcomes (KDIGO) criteria surpassed the clinical misapproach of baseline creatinine and kinetics associated with interindividual variations via independent case-by-case assessment rather than the whole assessment [10, 11, 12, 13]. Thus, the KDIGO criteria were selected to classify our patients in the study. Besides, serum levels of neutrophil gelatinase-associated lipocalin (NGAL) and Klotho were evaluated in the preoperative (2 h and 1 day) and the postoperative (2 days) period of cardiac surgery using cardiopulmonary bypass (CPB).
NGAL is a secretory glycoprotein that belongs to the lipocalin superfamily, with effective bacteriostatic properties in the innate immunity-related neutrophils, besides iron metabolism regulator functions in other epithelial cells [14]. In response to ischemic renal tubular injury, NGAL is filtered by the glomerulus, reabsorbed via megalin-dependent endocytosis in proximal tubules, and concomitantly rises in urine as well as blood [15]. Previous reports postulated that serum and urine NGAL levels exhibited better sensitivity and/or specificity as an early marker of AKI after cardiac surgery [16, 17]. However, the possible diagnostic utility of NGAL towards acute kidney stress, the pre-injury stage, is still under investigation and is required for understanding the evolution of AKI.
Cardiac troponins isoforms (cTnT, cTnI) are considered to be diagnostic markers for myocardial injury with higher sensitivity and specificity than creatine kinase-MB (CK-MB) [18, 19]. However, the present link between cardiac troponins and the probabilities for chronic kidney disease and end-stage renal disease in cardiovascular disease patients as well as their roles in AKI are still scarce and not well elucidated [20]. A recent study by Omar et al. [21] showed a significant rise in cTnT and overcoming CK-MB levels in AKI patients after cardiac surgery, thus postulating their ability to discriminate high risks for AKI among patients with myocardial injury, ischemia, or even myocardial infarction.
Materials and Methods
Study Population
Our pilot study encompassed 182 age- and sex-matched patients who underwent invasive cardiothoracic surgery using CPB and were transferred to the ICU for postsurgical observation and follow-up. Patients were then classified according to the KDIGO criteria into an AKI and a non-AKI group. The inclusion criteria were age ≤80 years, controlled diabetic patients, and cardiovascular disease patients. The exclusion criteria were patients with diabetic nephropathy, cardiorenal syndrome, hepatorenal syndrome, history of renal disease (either hemodialysis or renal transplants), malignancy, endocrine disorders, autoimmune diseases, and pregnancy.
Methods
Blood samples were collected from all patients five times: before CPB, at the time of CPB (0 h), and 2, 12, and 24 h after CPB. Thereafter, serum aliquots were separated immediately and stored at −20°C to be analyzed as a single serial batch on the Roche Hitachi 912 Chemistry Analyzer for serum creatinine using the BioAssay QuantiChromTM Assay Kit and the Beckman Coulter UniCel DxI 800 Immunoassay Analyzer for serum NGAL, Troponin I Ultra, CK, and C-reactive protein (CRP) using specialized ELISA kits from Elabscience Ltd., Wuhan, China.
Statistics
Data were fed to the computer and analyzed using IBM SPSS software package version 22. Quantitative data were described using range, mean, and standard deviation. The significance of the obtained results was judged at the 5% level. The Student t test was used to compare normally quantitative variables between the two groups. ANOVA was used for comparison between more than two groups. Pearson correlation was used to find the association between each two parameters. ROC curve was used to determine the sensitivity, specificity, and cutoff value of each marker to predict the disease. Multivariate analysis was done to determine the most significant risk factors that affect the outcomes.
Results
All 182 patients studied were distributed according to the KDIGO classification criteria into an AKI group (65 patients) and a non-AKI group (117 patients) according to serum creatinine levels (Fig. 1). The demographic and baseline clinical data of the two studied groups are presented in Table 1. The basic demographic data (age and sex) were matched in the AKI and non-AKI group (p > 0.05).
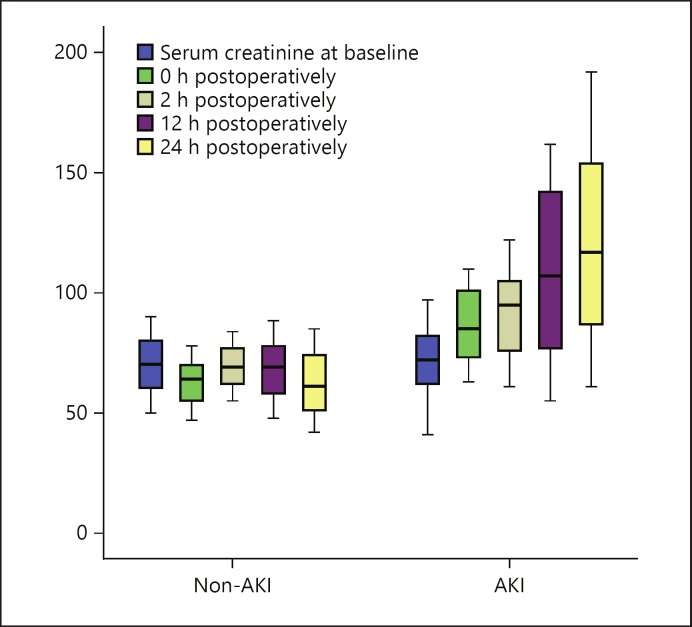
Fig. 1.
Serum creatinine in the AKI and the non-AKI group at different follow-up times. AKI, acute kidney injury.
Table 1.
Demographic and baseline clinical data of the studied groups
| Parameter | Non-AKI group (n = 117) | AKI group (n = 65) | p value |
|---|---|---|---|
| Age, years | 64.15±7.44 | 64.02±7.49 | 0.910 |
| Sex | |||
| Male | 61 (52.1%) | 36 (55.4%) | 0.674 |
| Female | 56 (47.9%) | 29 (44.6%) | |
| eGFR ≤60 mL/min/1.73 m2 | 0 (0.0%) | 42 (64.6%) | 0.0001* |
| Arterial hypertension | 8 (6.8%) | 46 (70.8%) | 0.001* |
| Diabetes mellitus | 38 (32.5%) | 19 (29.2%) | 0.731 |
| Inotropes | 80 (68.4%) | 48 (73.8%) | 0.373 |
| Mechanical ventilation | 23 (19.7%) | 71 (51%) | 0.014* |
| Left ventricular ejection fraction, % | 60.9±9.73 | 58.8±8.94 | 0.224 |
| Body mass index | 29.63±4.02 | 29.44±4.22 | 0.765 |
| Transfusion | |||
| Red blood cells | 2.17±2.90 | 2.46±3.25 | 0.536 |
| Plasma | 1.48±2.43 | 1.92±3.13 | 0.289 |
| Thrombocytes | 0.15±0.42 | 0.15±0.40 | 0.894 |
| Euroscore | 2.6±2.55 | 2.42±2.43 | 0.649 |
| Preoperative NYHA score | |||
| I | 15 (12.8%) | 15 (23.1%) | 0.189 |
| II | 60 (51.3%) | 31 (47.7%) | |
| III | 42 (35.9%) | 19 (29.2%) | |
| Surgery type | |||
| CABG | 14 (12.0%) | 10 (15.4%) | 0.206 |
| CABG+AVR | 26 (22.2%) | 20 (30.8%) | |
| AVR | 63 (53.8%) | 26 (40.0%) | |
| Bentall | 3 (2.6%) | 4 (6.2%) | |
| MVR | 7 (6.0%) | 1 (1.5% | |
| PMV | 4 (3.4%) | 4 (6.2%) | |
| Surgical approach | |||
| Median sternotomy | 96 (82.1%) | 54 (83.1%) | 0.558 |
| Mini-sternotomy | 15 (12.8%) | 5 (7.7%) | |
| Mini-thoracotomy | 4 (3.4%) | 4 (6.2%) | |
| Endoscopic | 2 (1.7%) | 2 (3.1%) | |
| Number of cardiopulmonary bypasses | 0.52±0.95 | 0.71±1.01 | 0.105 |
| Cardiopulmonary bypass time, min | 99.71±33.3 | 100.3±35.17 | 0.911 |
| Aortic cross-clamping time | 74.86±26.98 | 80.62±30.03 | 0.190 |
| Intensive care unit stay, days | 7.53±9.70 | 7.71±9.07 | 0.904 |
Figures are presented as mean ± standard deviation or n (%). AKI, acute kidney injury; AVR, aortic valve replacement; CABG, coronary artery bypass graft; MVR, mitral valve replacement; NYHA, New York Heart Association; PMV, percutaneous mitral valvuloplasty.
Significant at p ≤ 0.05.
Regarding eGFR ≤60 mL/min/1.73 m2, no patients were observed in the non-AKI group, whereas only 42 patients (64.6%) in the AKI group were observed, with an apparent significant difference (p < 0.01). Also, there was a significant increase in the number of patients with arterial hypertension in the AKI group compared to the non-AKI group (p < 0.01). The other clinical data listed in Table 1 showed no significant difference between the studied groups.
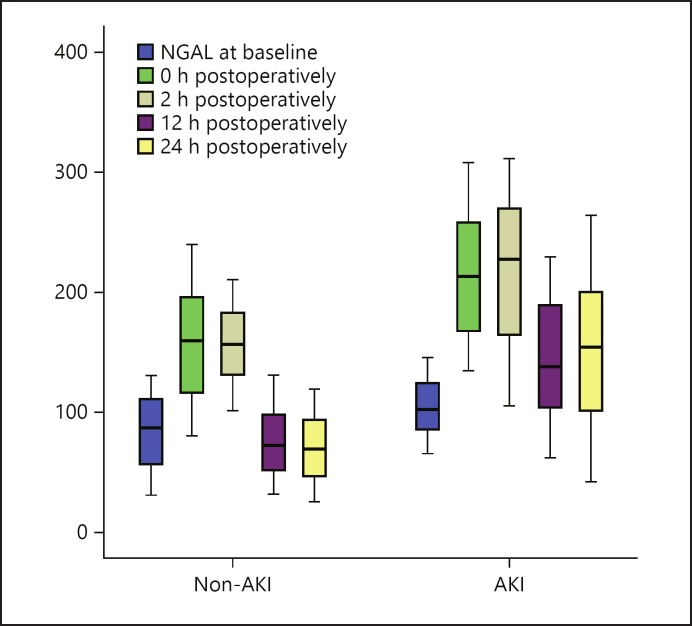
The preoperative baseline serum NGAL levels were superior in the AKI (104.22 ± 23.2 μg/L) compared to the non-AKI group (82.22 ± 30.5 μg/L) (p < 0.01) and persisted in the same manner until the end of time fractions. Subsequently, an increase was observed in the preoperative period for the AKI group (213.52 ± 53.6 μg/L) versus the non-AKI group (156.25 ± 45.9 μg/L) (p < 0.01). A stepwise superfast in the postoperative periods started from 2 h until it reached a maximum peak after 24 h of CPB (154.14 ± 63.5 μg/L in the AKI group and 69.96 ± 28.5 μg/L in the non-AKI group, p < 0.05). Impressively, using the Kruskal-Wallis test to show the extent of divergence between periods revealed a strong significance in the AKI group (p < 0.001) and the non-AKI group (p < 0.01). Table 2 and Figure 2 presented a compendium of serum NGAL concentrations during the studied periods.
Table 2.
Pre- and postoperative values of measured traditional and novel parameters in the AKI and the non-AKI group
| Measured biomarker | Studied groups/measurement times |
p values at definite times |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| non-AKI group (n = 117) |
AKI group (n = 65) |
||||||||||||||
| pre | post |
pre | post |
pre | post |
||||||||||
| 0 h | 2 h | 12 h | 24 h | 0 h | 2 h | 12 h | 24 h | 0 h | 2 h | 12 h | 24 h | ||||
| SCr, µmol/L | 69.93±11.6 | 63.02±9.2 | 69.39±8.5 | 68.37±11.7 | 62.56±12.5 | 70.88±13.8 | 86.32±14.3 | 92.98±17.6 | 109.15±33.3 | 122.29±38.4 | 0.622 | 0.001* | 0.001 * | 0.001 * | 0.001* |
| SNGAL, µg/L | 82.22±30.5 | 156.25±45.9 | 156.32±30.3 | 75.21±28.9 | 69.96±28.5 | 104.22±23.2 | 213.52±53.6 | 215.32±62.3 | 143.58±50.1 | 154.14±63.5 | 0.001* | 0.001* | 0.001 * | 0.001 * | 0.001* |
| SCK, U/L | 5.81±1.7 | 29.82±9.6 | 50.77±14.8 | 85.92±28.5 | 110.61±46.7 | 3.42±1.2 | 6.60±2.4 | 10.34±5.2 | 1.53±0.3 | 8.29±3.7 | 0.524 | 0.547 | 0.001 * | 0.001 * | 0.001* |
| ScTnI, ng/L | 5.29±2.5 | 5.22±2.6 | 5.16±2.6 | 3.47±1.3 | 2.69±1.1 | 5.38±2.3 | 6.80±3.1 | 6.08±2.1 | 3.97±1.4 | 2.98±0.9 | 0.809 | 0.001* | 0.014* | 0.012* | 0.060 |
| SCRP, µg/L | 5.81±1.71 | 29.82±9.61 | 50.77±14.82 | 85.92±28.5 | 110.61±46.7 | 5.93±1.88 | 29.57±9.17 | 50.10±13.92 | 85.71±28.24 | 102.90±49.4 | 0.668 | 0.865 | 0.764 | 0.963 | 0.297 |
| eGFR, mL/min/1.73 m2 | 93.95±15.4 | 98.37±16.90 | 85.36±14.05 | 84.53±14.0 | 84.55±13.6 | 91.44±16.91 | 77.52±14.61 | 75.08±14.91 | 61.85±18.22 | 55.56±14.64 | 0.311 | 0.001* | 0.001 * | 0.001 * | 0.001* |
AKI, acute kidney injury; SCK, serum creatine kinase; SCr, serum creatinine; SCRP, serum C-reactive protein; ScTnI, serum cTnI; SNGAL, serum neutrophil gelatinase-associated lipocalin.
p values were calculated to compare between non-AKI and AKI at the same time and considered to be significant at p ≤ 0.05 using the Student t test.
Fig. 2.
Serum NGAL in the AKI and the non-AKI group at different follow-up times. AKI, acute kidney injury; NGAL, neutrophil gelatinase-associated lipocalin.
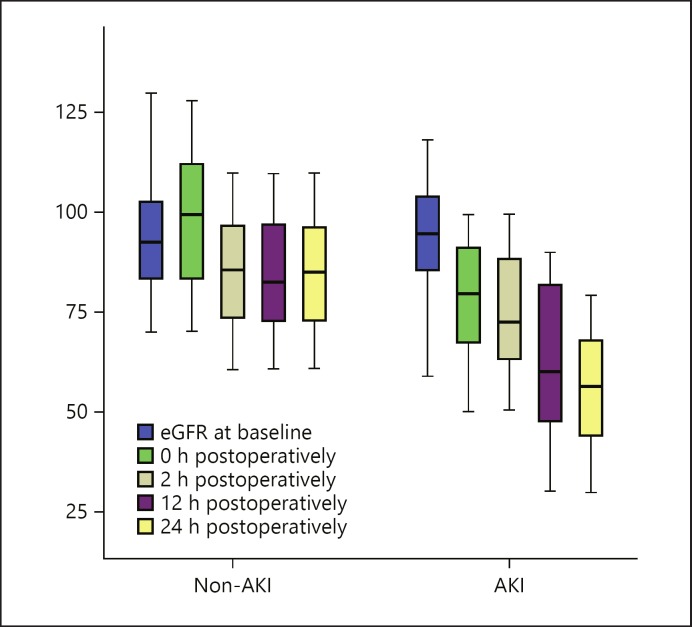
Table 2 also shows the serum creatinine level, eGFR, and serum cTnI in the two groups at different follow-up periods. Preoperative serum creatinine, eGFR, and serum cTnI were matched in the two groups without significant differences. Serum creatinine and serum cTnI increased significantly in the AKI group immediately after operation, and this increase was noticed over the whole follow-up period. On the other hand, eGFR decreased significantly after operation, and the decrease was observed over the whole follow-up period (Fig. 3). Serum CRP showed no significant difference between the two groups over the follow-up period, while serum CK exhibited only a significant difference (p = 0.001) 2–24 h postoperatively in the postoperative periods from preoperative as well as immediately after operation between the AKI and the non-AKI group.
Fig. 3.
eGFR in the AKI and the non-AKI group at different follow-up times. AKI, acute kidney injury.
Evidently, significant positive correlations were found between serum levels of NGAL and creatinine in the postoperative periods (2, 24, and 48 h) only. However, a direct significant relationship was observed postoperatively between 2-h serum CRP and 2-day serum creatinine (r = 0.290 and p ≤ 0.05). Additionally, serum NGAL at 0 h showed positive correlations with preoperative troponin and 2-day postoperative troponin (r = 0.306, p ≤ 0.05 and r = 0.282, p ≤ 0.05, respectively).
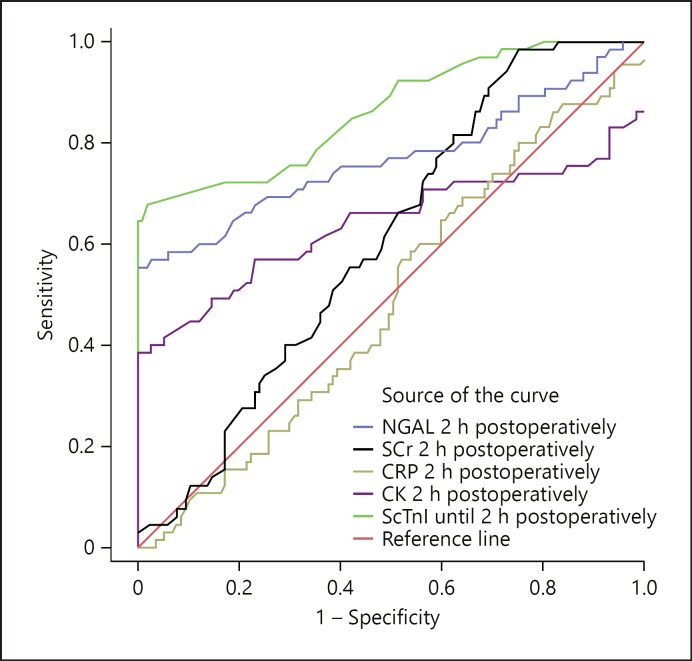
The predictive performance analysis using ROC curve for the measured biomarkers 2 h after CPB is plotted in Figure 4 regarding AUCs, showing the superiority of the classical serum creatinine compared to the remaining biomarkers (0.864; NGAL, CK, troponin I, and CRP: 0.819, 0.640, 0.606, and 0.487, respectively) (Table 3). Further, multivariate analysis of different risk factors showed that the probable risk factors with adverse outcomes were preoperative serum NGAL and preoperative eGFR ≤60 mL/min/1.73 m2 and arterial hypertension (Table 4).
Fig. 4.
ROC curve to determine the cutoffs, sensitivity, specific ity, and cutoff values of each marker. CK, creatine kinase; CRP, C-reactive protein; NGAL, neutrophil gelatinase-associated lipocalin; SCr, serum creatinine; ScTnI, serum cTnI.
Table 3.
The area under the ROC curve data with sensitivity, specificity, and cutoff values of each marker
| Test variable | AUC | Cutoff value | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|
| Cr | 0.864 | >140.0 | 88.0 | 82.0 | 85.0 |
| NGAL | 0.819 | >75.0 | 85.0 | 82.0 | 83.5 |
| CK | 0.640 | >50.0 | 68.0 | 62.0 | 64.6 |
| cTnI | 0.606 | >5.5 | 56.0 | 62.0 | 60.0 |
| CRP | 0.487 | >25.0 | 45.0 | 50.0 | 48.0 |
CK, creatine kinase; Cr, creatinine; CRP, C-reactive protein; NGAL, neutrophil gelatinase-associated lipocalin.
Table 4.
Logistic multivariate regression analysis of different risk factors with short-term outcomes
| Model | Unstandardized coefficients |
Standardized coefficients |
t | p value | |
|---|---|---|---|---|---|
| B | SE | beta | |||
| 1 (Constant) | −0.138 | 0.125 | −1.106 | 0.270 | |
| Baseline NGAL | 0.003 | 0.001 | 0.175 | 4.352 | 0.0001* |
| Baseline serum creatinine | 0.000 | 0.002 | −0.010 | −0.258 | 0.796 |
| Arterial hypertension | 0.453 | 0.045 | 0.432 | 10.050 | 0.0001* |
| eGFR ≤60 mL/min/1.73 m2 | 0.588 | 0.050 | 0.517 | 11.830 | 0.0001* |
Dependent variable: acute kidney injury. NGAL, neutrophil gelatinase-associated lipocalin; SE, standard error.
Significant at p ≤ 0.05.
Discussion
In our study, KDIGO serum creatinine concentrations showed significant disparity among the studied time intervals. Starting with elevated baseline in the AKI compared to the non-AKI group, lasted longer by the same way in both preoperative as well as postoperative timings too. The rise in serum creatinine levels in cardiac patients prior to surgery might explain persistent hypertension and subclinical inflammation derived from atherosclerotic endothelial dysfunction accompanied by cardiovascular diseases that initially upsets renal function [22]. While the deleterious influences of some anesthetics [23], catheterization used in critically ill patients [24], and the circulatory pump machine probably result in fluid overload [25], low blood viscosity [26], hemodilution anemia [27], microembolism [28], tissue hypoperfusion [29], hypothermia [30], and hypotension due to the low cardiac output lead to exaggerated elevations of intra- and postoperative levels [31]. In a related context, CRP levels increased massively after surgery in the AKI versus the non-AKI group, indicating the presence of localized inflammation as one of the heart-lung machine adverse effects [32]. However, the other conventional eGFR parameters decreased markedly compared with serum creatinine and CRP in the AKI compared to the non-AKI group, predicting delayed postoperative renal complications.
Despite being indicative of renal function, classical biomarkers, such as serum creatinine, are badly affected by factors such as age, sex, race, medications, diet, muscle mass, hemodilution, and extracorporeal circulation, which presents an inevitable pitfall to the diagnostic propensity of the test over time [33]. As eGFR calculation is always subsidiary to serum creatinine measurement, it is firmly biased too. To prognosticate AKI at earlier stages, new markers of sufficient sensitivity and specificity than traditional troponins are needed to predict clinical outcomes and potentiate therapeutic interventions.
Assessment of NGAL at serum levels is justified as almost all previous reports on urine and plasma NGAL proved that it could be a surrogate marker for AKI [34, 35]. In addition, studies on serum NGAL are scarce, and its diagnostic utility has not been shown well regarding the CSA-AKI situation. The current study showed that preoperative serum NGAL levels in the non-AKI group were overridden significantly by those in the AKI group, which might be due to the endothelial cell injury accompanying the preexisting coronary artery disease mediated by activated neutrophil recruitment or myocardial contribution beyond congestive heart failure [36, 37].
By the same pattern, serum NGAL concentrations substantially increased during surgery in the AKI compared to the non-AKI group, thus pointing out the impact of the heart-lung machine that upsets cardiac output due to the lack of normalized steady pulsatile flow, lower hematocrit, trigger hypoxemia, launch cell-mediated and humoral immune cascade involving complement activation, and release of inflammatory mediators leading to ischemic renal tubular injury [38, 39]. This observation was consistent with the assumptions of Shemin and Dworkin [40] and Legrand et al. [41] of NGAL being a convenient alert against cumulative structural changes of renal tubular epithelia in patients suffering from consolidated nephrotoxic consequences emanating from CPB or radiocontrast agents. In the postoperative period, a continual increment of serum NGAL levels from 2 to 24 h eventually manifested with a plateau within 48 h compared with a higher starting baseline in the AKI group (228.58 μg/L) than in the other group (178.08 μg/L). Compatibly, Kadry et al. [42] disclosed a profound increase in serum NGAL in the AKI group after 48 h (202 ng/mL). Furthermore, ROC curve analyses elucidated that serum NGAL failed to overcome the superiority of serum creatinine even 2 h earlier in the postoperative period, which supports the findings of Nickolas et al. [43], but contradicts the findings of Mishra et al. [44] The cutoff value of serum NGAL after 2 h was 78 μg/L, with 76.9% sensitivity, 54% specificity, and an AUC of 0.761. In contrast, different cutoff values obtained by Krawczeski et al. [45] in young children and by El-Farghali et al. [46] in neonates were 100 and 117.5 ng/mL, with 82% sensitivity, 89% specificity, and an AUC of 0.95, respectively. These salient interstudy variations suggest the presence of comorbidities affecting the performance of serum NGAL over time, including age, sepsis, diabetes, population size, sex, body fat, and liver function [47, 48]. Impressively, high preoperative concentrations of serum NGAL may predict ongoing renal tubular insult after cardiothoracic surgery, predominantly encompassing the uppermost value by the end of the time course.
Preoperative serum levels of troponin I in both groups were matched without any significant difference. At the perioperative (0 h) and postoperative (2 and 12 h) periods, troponin I levels showed significant increases in the AKI group compared to the non-AKI group that might have been influenced by cardiac surgical consequences, including trauma, sepsis, acute pericarditis, and acute heart failure [49, 50].
Inferior levels 1 day after surgery (p < 0.06), near the statistical significance shown by Croal et al. [51], might predict ongoing peri-/postoperative problems. In a related context, ROC analysis showed low sensitivity, specificity, and accuracy for troponin I. This is in agreement with Omar et al. [21], who suggested the possibility of troponin I being an inaccurate marker of perioperative myocardial infarction in patients who develop AKI after cardiac surgery.
Conclusion
We conclude that preoperative serum NGAL may act as a prognostic predictor for renal tubular injury after cardiothoracic surgery, and that its sensitivity and specificity may be increased by designing a smart combination of preoperative eGFR and serum NGAL. However, postoperative NGAL was found to be a more sensitive marker for AKI than creatinine, but creatinine was found to be more specific than NGAL. Furthermore, we suggest the presence of multiple factors with direct or indirect impact on the diagnostic performance for AKI that will be worth investigating on a large scale. Troponin I was a promising prognostic biomarker for CSA-AKI due to early increase at the immediate (0 h) period with NGAL. However, age-dependent cutoffs, lack of standardization and harmonization because of the interassay variations, and influences of surgical events remains obstacles to the diagnostic utility of troponin I.
Statement of Ethics
Inclusion of the study population after obtaining written consent was in accordance with the declaration of Helsinki code of ethics and the National Egyptian Committee of Biomedical Ethics.
Disclosure Statement
All authors declare that they have no conflicts of interest.
References
- 1.Case J, Khan S, Khalid R, Khan A. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract. 2013;2013:479730. doi: 10.1155/2013/479730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 4.Lema G, Meneses G, Urzua J, Jalil R, Canessa R, Moran S, Irarrazaval MJ, Zalaquett R, Orellana P. Effects of extracorporeal circulation on renal function in coronary surgical patients. Anesth Analg. 1995;81:446–451. doi: 10.1097/00000539-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrman DY, Kellum JA. Epidemiology and pathophysiology of cardiac surgery-associated acute kidney injury. Curr Opin Anaesthesiol. 2017;30:60–65. doi: 10.1097/ACO.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Auriemma S, Fabbri A, D'Onofrio A, Katz N, McCullough PA, Ricci Z, Shaw A, Ronco C. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI) Int J Artif Organs. 2008;31:166–178. doi: 10.1177/039139880803100210. [DOI] [PubMed] [Google Scholar]
- 7.Edelstein CL. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis. 2008;15:222–234. doi: 10.1053/j.ackd.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen MR, Murray PT, Fitzgibbon MC. Establishment of a reference interval for urinary neutrophil gelatinase-associated lipocalin. Ann Clin Biochem. 2012;49:190–193. doi: 10.1258/acb.2011.011105. [DOI] [PubMed] [Google Scholar]
- 9.Waikar SS, Bonventre JV, Acute kidney injury . Harrison's Principles of Internal Medicine. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. ed 19. New York: McGraw-Hill; 2015. [Google Scholar]
- 10.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz DN, Ricci Z, Ronco C. Clinical review: Clinical review: RIFLE and AKIN - time for reappraisal. Crit Care. 2009;13:211. doi: 10.1186/cc7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease. Improving Global Outcomes (KDIGO) Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–141. [Google Scholar]
- 14.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 16.Cruz DN, Ronco C, Katz N. Neutrophil gelatinase-associated lipocalin: a promising biomarker for detecting cardiac surgery-associated acute kidney injury. J Thorac Cardiovasc Surg. 2010;139:1101–1106. doi: 10.1016/j.jtcvs.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Bhayana V, Gougoulias T, Cohoe S, Henderson AR. Discordance between results for serum troponin T and troponin I in renal disease. Clin Chem. 1995;41:312–317. [PubMed] [Google Scholar]
- 19.Croitoru M, Taegtmeyer H. Spurious rises in troponin T in end-stage renal disease. Lancet. 1995;346:974. doi: 10.1016/s0140-6736(95)91598-2. [DOI] [PubMed] [Google Scholar]
- 20.Collinson PO, Hadcocks L, Foo Y, Rosalki SB, Stubbs PJ, Morgan SH, O'Donnell J. Cardiac troponins in patients with renal dysfunction. Ann Clin Biochem. 1998;35:380–386. doi: 10.1177/000456329803500306. [DOI] [PubMed] [Google Scholar]
- 21.Omar AS, Mahmoud K, Hanoura S, Osman H, Sivadasan P, Sudarsanan S, Shouman Y, Singh R, AlKhulaifi A. Acute kidney injury induces high-sensitivity troponin measurement changes after cardiac surgery. BMC Anesthesiol. 2017;17:15. doi: 10.1186/s12871-017-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosa OF, Skitek M, Kalisnik JM, Jerin A. Evaluation of serum cysteine-rich protein 61 and cystatin C levels for assessment of acute kidney injury after cardiac surgery. Ren Fail. 2016;38:699–705. doi: 10.3109/0886022X.2016.1157747. [DOI] [PubMed] [Google Scholar]
- 23.Yoo YC, Shim JK, Song Y, Yang SY, Kwak YL. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014;86:414–422. doi: 10.1038/ki.2013.532. [DOI] [PubMed] [Google Scholar]
- 24.Hennessy SA, LaPar DJ, Stukenborg GJ, Stone ML, Mlynarek RA, Kern JA, Ailawadi G, Kron IL. Cardiac catheterization within 24 h of valve surgery is significantly associated with acute renal failure. J Thorac Cardiovasc Surg. 2010;140:1011–1017. doi: 10.1016/j.jtcvs.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haase-Fielitz A, Haase M, Bellomo R, Calzavacca P, Spura A, Baraki H, Kutschka I, Albert C. Perioperative hemodynamic instability and fluid overload are associated with increasing acute kidney injury severity and worse outcome after cardiac surgery. Blood Purif. 2017;43:298–308. doi: 10.1159/000455061. [DOI] [PubMed] [Google Scholar]
- 26.Messmer K. Hemodilution. Surg Clin North Am. 1975;55:659–678. doi: 10.1016/s0039-6109(16)40641-9. [DOI] [PubMed] [Google Scholar]
- 27.Habib RH, Zacharias A, Schwann TA. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcomes. Crit Care Med. 2005;33:1749–1756. doi: 10.1097/01.ccm.0000171531.06133.b0. [DOI] [PubMed] [Google Scholar]
- 28.Doty JR, Wilentz RE, Salazar JD, Hruban RH, Cameron DE. Atheroembolism in cardiac surgery. Ann Thorac Surg. 2003;75:1221–1226. doi: 10.1016/s0003-4975(02)04712-4. [DOI] [PubMed] [Google Scholar]
- 29.Ranucci M. Perioperative renal failure: hypoperfusion during cardiopulmonary bypass? Semin Cardiothorac Vasc Anesth. 2007;11:265–268. doi: 10.1177/1089253207311141. [DOI] [PubMed] [Google Scholar]
- 30.Boodhwani M, Rubens FD, Wozny D, Nathan HJ. Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting. Ann Thorac Surg. 2009;87:489–495. doi: 10.1016/j.athoracsur.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 31.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adýbelli Z, Giuliani A, Danesi TH, Kim JC, Nayak A, Neri M, Virzi GM, Brocca A, Scalzotto E, Salvador L, Ronco C. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013;3:178–199. doi: 10.1159/000353134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aouifi A, Piriou V, Blanc P, Bouvier H, Bastien O, Chiari P, Rousson R, Evans R, Lehot JJ. Effect of cardiopulmonary bypass on serum procalcitonin and C-reactive protein concentrations. Br J Anaesth. 1999;83:602–607. doi: 10.1093/bja/83.4.602. [DOI] [PubMed] [Google Scholar]
- 33.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 34.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, NGAL Meta-analysis Investigator Group Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Schley G, Köberle C, Manuilova E, Rutz S, Forster C, Weyand M, Formentini I, Kientsch-Engel R, Eckardt KU, Willam C. Comparison of plasma and urine biomarker performance in acute kidney injury. PLoS One. 2015;10:e0145042. doi: 10.1371/journal.pone.0145042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zografos T, Haliassos A, Korovesis S, Giazitzoglou E, Voridis E, Katritsis D. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am J Cardiol. 2009;104:917–920. doi: 10.1016/j.amjcard.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Yndestad A, Landrø L, Ueland T, Dahl CP, Flo TH, Vinge LE, Espevik T, Frøland SS, Husberg C, Christensen G, Dickstein K, Kjekshus J, Øie E, Gullestad L, Aukrust P. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229–1236. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 38.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 39.Royster R, Thomas SJ, Davis RF, Termination of cardiopulmonary bypass . Cardiopulmonary Bypass: Principles and Practices. In: Gravlee GP, Davis RE, Stammers AH, Ungerleider R, editors. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. pp 614–631. [Google Scholar]
- 40.Shemin D, Dworkin LD. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for early acute kidney injury. Crit Care Clin. 2011;27:379–389. doi: 10.1016/j.ccc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Legrand M, Mari A, Mebazaa A. The elusive task of biomarkers of renal injury. Crit Care. 2013;17:132. doi: 10.1186/cc12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadry Y, El Sayed M, Al-Barshomy SM, Omar H. Serum neutrophil gelatinase-associated lipocalin (NGAL) in acute kidney injury and its relation to troponin T in cardiac and renal patients. Int J Adv Res (Indore) 2016;4:499–505. [Google Scholar]
- 43.Nickolas TL, Barasch J, Devarajan P. Biomarkers in acute and chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:127–132. doi: 10.1097/MNH.0b013e3282f4e525. [DOI] [PubMed] [Google Scholar]
- 44.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 45.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–1015. doi: 10.1016/j.jpeds.2010.12.057. e1. [DOI] [PubMed] [Google Scholar]
- 46.El-Farghali OG, El-Raggal NM, Mahmoud NH, Zaina GA. Serum neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury in critically-ill neonates. Pak J Biol Sci. 2012;15:231–237. doi: 10.3923/pjbs.2012.231.237. [DOI] [PubMed] [Google Scholar]
- 47.Helanova K, Spinar J, Parenica J. Diagnostic and prognostic utility of neutrophil gelatinase-associated lipocalin (NGAL) in patients with cardiovascular diseases - review. Kidney Blood Press Res. 2014;39:623–629. doi: 10.1159/000368474. [DOI] [PubMed] [Google Scholar]
- 48.Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 49.Perna ER, Macín SM, Parras JI, Pantich R, Farías EF, Badaracco JR, Jantus E, Medina F, Brizuela M. Cardiac troponin T levels are associated with poor short- and long-term prognosis in patients with acute cardiogenic pulmonary edema. Am Heart J. 2002;143:814–820. doi: 10.1067/mhj.2002.120152. [DOI] [PubMed] [Google Scholar]
- 50.Ammann P, Fehr T, Minder EI, Günter C, Bertel O. Elevation of troponin I in sepsis and septic shock. Intensive Care Med. 2001;27:965–969. doi: 10.1007/s001340100920. [DOI] [PubMed] [Google Scholar]
- 51.Croal BL, Hillis GS, Gibson PH, Fazal MT, El-Shafei H, Gibson G, Jeffrey RR, Buchan KG, West D, Cuthbertson BH. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation. 2006;114:1468–1475. doi: 10.1161/CIRCULATIONAHA.105.602370. [DOI] [PubMed] [Google Scholar]