Summary
Objective
A single brief seizure before learning leads to spatial and contextual memory impairment in rodents without chronic epilepsy. These results suggest that memory can be impacted by seizure activity in the absence of epilepsy pathology. In this study, we investigated the types of memory affected by a seizure and the time course of impairment. We also examined alterations to mammalian target of rapamycin (mTOR) and fragile X mental retardation protein (FMRP) signaling, which modulate elements of the synapse and may underlie impairment.
Methods
We induced a single seizure and investigated hippocampal and nonhippocampal memory using trace fear conditioning, novel object recognition (NOR), and accelerating rotarod to determine the specificity of impairment in mice. We used western blot analysis to examine for changes to cellular signaling and synaptic proteins 1 h, 24 h, and 1 week after a seizure. We also included a histologic examination to determine if cell loss or gross lesions might alternatively explain memory deficits.
Results
Behavioral results indicated that a seizure before learning leads to impairment of trace fear memory that worsens over time. In contrast, nonhippocampal memory was unaffected by a seizure in the NOR and rotarod tasks. Western analysis indicated increased hippocampal phospho‐S6 and total FMRP 1 h following a seizure. Tissue taken 24 h after a seizure indicated increased hippocampal GluA1, suggesting increased α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptor expression. Histologic analysis indicated that neither cell loss nor lesions are present after a single seizure.
Significance
The presence of memory impairment in the absence of damage suggests that memory impairment caused by seizure activity differs from general memory impairment in epilepsy. Instead, memory impairment after a single seizure is associated with alterations to mTOR and FMRP signaling, which leads to a disruption of synaptic proteins involved in consolidation of long‐term memory. These results have implications for understanding memory impairment in epilepsy.
Keywords: Amnesia, Cognitive impairment, Synaptic plasticity, Accelerated long‐term forgetting
Key Points.
A single seizure 1 h before trace fear conditioning leads to hippocampal‐dependent memory impairment that worsens as time progresses
Nonhippocampal memory is unaffected by a seizure before learning in novel object recognition and the accelerating rotarod
One hour after a seizure there is increased phospo‐S6 at ser235/236 and ser 240/244, and an increase in total FMRP, indicating increased translational activity and decreased inhibition of translation, respectively
Histologic analyses indicate that damage/cell loss is unrelated to hippocampal memory impairment after a single seizure
Comorbid memory problems are a common occurrence in individuals with epilepsy. Research using animal models of epilepsy such as pentylenetetrazol kindling, status epilepticus (SE) induced using kainate, or SE‐induced using pilocarpine has confirmed the presence of memory impairment following repeated or prolonged seizures.1, 2, 3 In addition to memory impairments, chronic models of epilepsy also produce seizures of sufficient duration and severity to result in pathologic alterations to the brain including neurodegeneration,4, 5, 6 abnormal neurogenesis,4 oxidative stress,4, 7 inflammation,4, 8, 9, 10 and disruption of the blood–brain barrier.11, 12 Damage and other pathologic changes have been assumed to underlie memory impairment in chronic epilepsy; however, the concurrent presence of multiple alterations to the brain makes defining the direct cause of memory impairment difficult. More recently, studies have shown that a single seizure can produce memory impairment in the absence of chronic pathology. A single seizure prior to learning can impair both hippocampus‐dependent and amygdala‐dependent memory.13, 14, 15, 16 It is still unclear if and which other types of memory are susceptible to impairment after a seizure. The time course of impairment and underlying mechanism are also poorly understood.
A possible mediating mechanism for memory impairment following a seizure involves the mammalian target of rapamycin (mTOR) kinase. The mammalian target of rapamycin complex 1 (mTORC1) is important for controlling long‐lasting synaptic plasticity and for the consolidation of memory.17 Studies have reported that increased mTORC1 activity resulting from deletion of Pten, leads to memory impairment18 and alterations to the translational repressor fragile X mental retardation protein (FMRP).19 One study found that a single seizure induced using pentylenetetrazol (PTZ) can induce hyperactivation of the Phosphoinositide 3‐kinase/Protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.20 Specifically, this study indicated that the increase in hippocampal mTOR occurred by 1 h and persisted for 3–6 h. Therefore, it is plausible that after a single seizure, altered mTOR activation occurs, which could disrupt the synaptic processes governing new memory consolidation.
In the current study, we used trace fear conditioning, novel object recognition, and the accelerating rotarod to examine memory after a seizure to determine the specificity of memory impairments. We used trace fear conditioning to determine whether the acute seizure impacts hippocampal‐dependent memory. Novel object recognition (NOR) was used as a general memory test and is not hippocampal dependent. The accelerating rotarod test was used to determine whether seizures impact motor learning. Memory was examined at multiple time points (24 h, 1 week, and 2 weeks) to determine whether memory recovers or worsens after a seizure. We also examined potential changes to the PI3K/Akt/mTOR signaling pathway and FMRP. We believe an interplay between mTOR and FMRP signaling modulates synaptic proteins in the hippocampus.19, 21, 22, 23 Accordingly, we also determined which synaptic proteins are changed following a brief seizure. We previously reported a transient reduction in locomotor activity 2 h following a flurothyl seizure that recovered by 1 week post‐seizure.16 Due to this potential confound, we assessed changes in activity levels, and anxiety‐like behavior following a seizure. To rule out the contribution of overt damage to memory impairment, we used hematoxylin and eosin (H&E) staining to examine tissue for gross lesions and necrotic cell death.
Materials and methods
Animals
We used 6‐ to 8‐week‐old male C57BL/6J mice that were generated and housed in the Baylor Sciences Building Vivarium at Baylor University. Separate cohorts of mice were used for each behavior test. Mice were group housed in standard mouse cages with access to food and water ad libitum. The home colony was maintained at 22°C on a 12/12‐h light/dark cycle. All procedures performed complied with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and were approved by the Baylor University Institutional Animal Care and Use Committee.
Flurothyl seizures and behavioral testing
Details on the methods for flurothyl seizures have been published previously.16 The latency of first myoclonic jerk, forelimb/hindlimb clonus, and generalized tonic–clonic seizure was live recorded. The latency to first myoclonic jerk was 208.16 ± 4.32 s (mean with the standard error of the mean on both sides of the mean), the latency to forelimb or hindlimb clonus was 247.92 ± 4.69 s, and the latency to generalized seizure (tonic–clonic) was 294.48 ± 5.96 s. None of the mice experienced a seizure during behavior testing or any other period beyond the initial exposure to flurothyl in the inhalation chamber. Control mice were placed in a second inhalation chamber but not exposed to flurothyl.
Trace fear conditioning was used to measure hippocampal‐dependent learning and memory at either 24 h and 1 week, 1 week and 2 weeks, or only 2 weeks after a seizure. Full details for the methods can be found in a previous publication.19 The NOR test was used to examine nonspatial recognition memory either 24 h or 1 week following a brief seizure using previously published methods.24
We assessed mice for deficits in motor learning and memory using the accelerating rotarod. After a 72‐h rest period following a single seizure, we tested subjects in 2 trials per day (1 h rest between trials) over 4 days for a total of 8 trials. Animals were placed on the rotarod (Series 8; IITC Life Science, CA, U.S.A.) which increased rotation speed from 4 to 40 revolutions per minute over 300 s. We live‐scored the latency to fall from the rod for each trial and time of the first complete revolution on the rod.
To assess for seizure‐induced changes to activity level we ran a cohort of mice in the open field following acute flurothyl seizures at 24 h and again at 1 week. The 10 min test was run as described previously.16 To further evaluate activity and anxiety levels we tested a cohort of mice in the elevated plus maze (EPM) 24 h and 1 week following an acute seizure using previously described methods.23
Western blot analysis
We used western blotting to examine potential changes to the PI3K/Akt/mTOR signaling pathway, FMRP, and synaptic proteins that accompanied impairments in trace fear memory after a seizure. Naive mice received flurothyl seizures and were killed 1 h, 24 h, or 1 week later to match the time points of learning and memory tests. Hippocampi, cortex, and cerebellum were rapidly dissected and processed for western blotting as described previously.25 Additional information on the antibodies used can be found in Table S1.
Histology
We evaluated the presence of necrotic cells and gross lesions at the 24‐h and 1‐week time points. Samples were sectioned midsagittally and immersion fixed in 10% neutrally buffered formalin. The tissue samples were dehydrated in graded alcohols, embedded in commercial paraffin mixtures, and sectioned at 5 μm thickness. The sectioned samples were stained for hematoxylin and counterstained for eosin.
Statistical analysis
All data were analyzed using either GraphPad Prism 7 (GraphPad Software Inc., CA, U.S.A.) or IBM SPSS Statistics 23 (IBM, NY, U.S.A.). For the open field, NOR, and rotarod tests, we used a repeated‐measures analysis of variance (ANOVA) design [treatment (control, seizure) x behavior of interest (repeated measures)].To analyze the date for trace fear conditioning we utilized 2‐way mixed‐design ANOVAs using between‐subjects factors [treatment (control, seizure) x interval (Baseline, Tone, Trace, intertrial interval (ITI))], and time as a within‐subjects factor. When we found significant interactions, independent sample t‐tests were used to compare groups during each interval. For western analysis, we used independent sample t‐tests, or a nonparametric Mann‐Whitney U‐test when the homogeneity of variance assumption was violated, to contrast the treatment groups. Statistical significance was defined as p < 0.05.
Results
Trace fear conditioning
Experiment one memory tests
Analysis of freezing levels in the new context during the 24 h cued‐recall test indicated no significant difference between groups, F (1, 18) = 0.50, p = 0.49, but a significant effect of time, F (3, 54) = 21.21, p < 0.001, and significant group x time interaction, F (3, 54) = 3.64, p = 0.02 (Fig. 1B). Follow‐up independent samples t‐tests indicated no significant differences between groups during the baseline, no difference during the tone, no difference during the trace interval, and no difference during the ITI. One week after seizure induction we presented the tone in the new context. There was a significant difference between the groups F (1, 18) = 14.09, p = 0.002, a significant effect of time, F (3, 54) = 15.36, p < 0.001, and significant group x time interaction, F (3, 54) = 2.99, p = 0.04 (Fig. 1C). Post hoc t‐tests for each interval indicated no significant differences between groups during the baseline or trace, p > 0.05, but there were differences in CS and ITI, p > 0.05.
Figure 1.
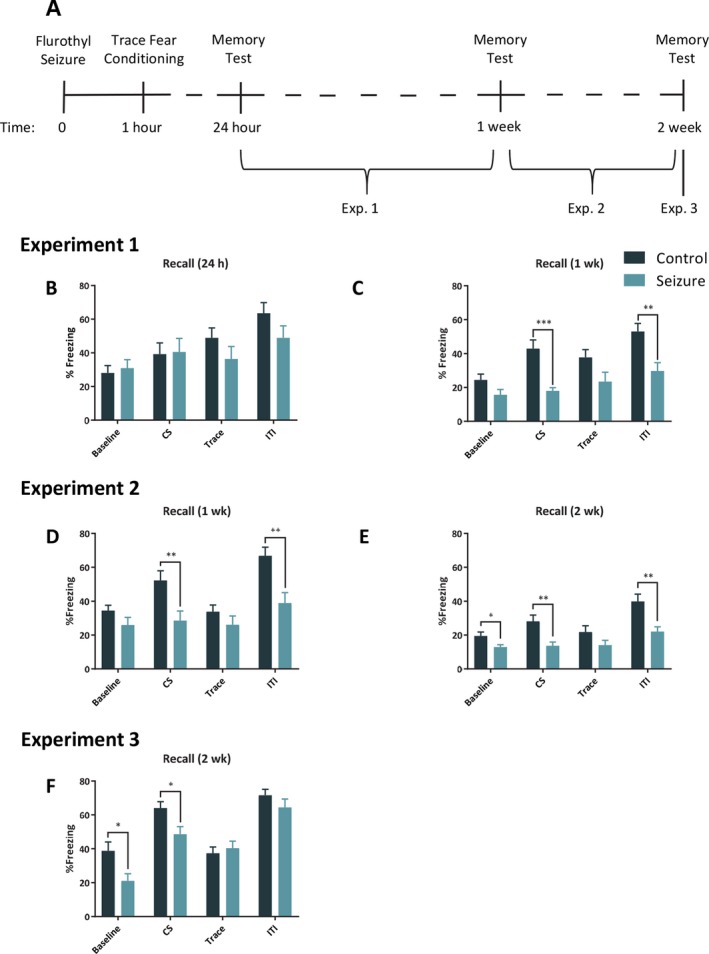
A seizure before learning selectively impairs hippocampal trace memory. A, Timeline of trace fear conditioning experiments. B, The mice that experienced a seizure (n = 10) before training did not differ in freezing levels compared to control (n = 10) mice when the tone was presented again 24 h later in a novel context. C, However, by 1 week after the seizure, mice in the seizure group froze significantly less than those in the control group in the novel context during the tone presentation. Similar to the results in experiment 1 at 1 week there was a significant reduction in freezing in experiment 2 when mice were presented with the tone at one week (D) and 2 weeks (E) after a seizure and learning (control = 10; seizure = 10). F, In our third trace fear experiment in which mice were tested only 2 weeks after a seizure and learning, the seizure (n = 11) group froze significantly less than the control (n = 11) group when presented with the tone in a new context. The bars represent the mean and the error bars indicate standard error of the mean (SEM). * p < .05, ** p < 0.01, *** p < 0.001.
Experiment two memory tests
Examination of the 1‐week memory test in the new context revealed the main effects of group, F (1, 18) = 8.3, p = 0.009, and time, F (3, 54) = 21.5, p < 0.001 (Fig. 1D). There was a group x time interaction, F (3, 54) = 4.97, p = 0.004. Follow‐up t‐tests indicated that control and seizure groups did not differ in freezing during the baseline or during trace interval, p > 0.05, but were significantly different during the CS and ITI, p < 0.05. We tested these animals again in cued recall in the new context the following week. There was a main effect of group, F (1, 18) = 9.28, p = 0.007, time, F (3, 54) = 30.53, p < 0.001, and group x time interaction, F (3, 54) = 5.1, p = 0.004 (Fig. 1E). We followed up the analysis with separate independent sample t‐tests, which indicated a significant difference in freezing between groups during the baseline, CS and ITI, p < 0.05, but no difference during the trace interval.
Experiment three memory test
For the 2‐week test there was an effect of group, F (1, 20) = 5.42, p = 0.03, and an effect of time, F (3, 60) = 43.21, p < 0.001. There was also a significant group x time interaction, F (3, 60) = 3.27, p = 0.03 (Fig. 1 F). Follow‐up independent sample t‐tests for each interval indicated a significant difference in freezing between groups during the baseline, CS, p < 0.5, but no difference during the trace interval and during the ITI.
Novel object recognition
Sample phase
During the sample phase, we found a difference in the percentage of time investigating the left and right objects for the control group, t (38) = 3.11, p = 0.004, but not the seizure group, t (38) = 0.68, p = 0.49 (Fig. 2B). The control group did not differ in the frequency investigating the left and right objects over the 10 min sample phase, t (38) = 0.32, p = 0.75; nor did the seizure group, t (38) = 0.6, p = 0.95.
Figure 2.
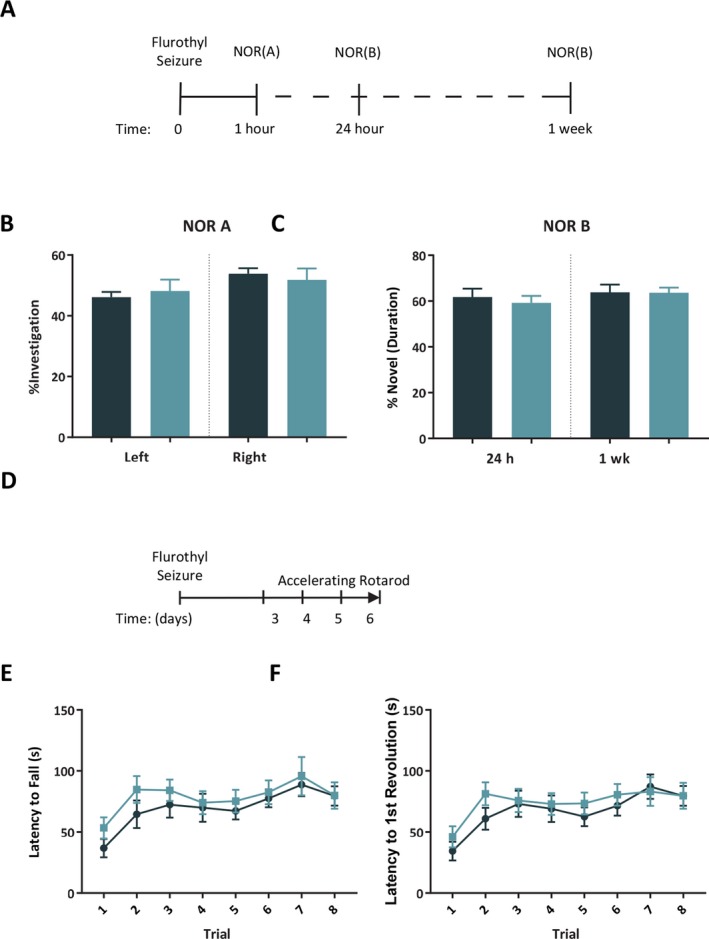
A seizure before learning does not impair nonhippocampal memory in novel object recognition (NOR). A, Timeline of NOR testing. B, The percent of total investigation for the left and right objects during the sample phase of NOR did not differ between control and seizure groups. C, The percentage of novel investigation out of total investigation was not different between control and seizure mice tested at 24 h (control = 10; seizure = 10) or 1 week (control = 10; seizure = 10) after a seizure in the test phase of NOR. D, Timeline of rotarod tests. E‐F, The latency to fall and the latency to first revolution on the rotarod were no different for control (n = 10) and seizure (n = 10) groups over the 4‐day test starting 72 h after a seizure. The bars and symbols represent the mean and the error bars indicate SEM.
Test phase
During the testing phase at 24 h, there was no difference between groups in the percentage of time investigating the novel object, t (18) = 0.53, p = 0.56 (Fig. 2C). During the testing phase at 1 week, we found a similar lack of difference between groups in percentage of time spent investigating the novel object, t (18) = 0.04, p = 0.97.
Accelerating rotarod
Analysis of the time of fall during the 4‐day test using a 2‐way repeated measures ANOVA did not show an effect of group, F (1, 18) = 1.20, p > 0.05, but did indicate a significant effect of trial, F (7, 126) = 4.93, p < 0.001 (Fig. 2E). There was no group x trial interaction, F (7, 126) = 0.3, p = 0.95. The analysis of the time of the first revolution without falling indicated no effect of group, F (1, 18) = 0.78, p > 0.05; however there was a significant effect of trial, F (7, 126) = 5.46, p < 0.001 (Fig. 2F). There was no group x trial interaction, F (7, 126) = 0.45, p = 0.87.
Open field
Activity levels
A repeated‐measures 2‐way ANOVA revealed no significant difference between groups in total distance traveled at 24 h or 1 week after a seizure, F (1, 18) = 0.62, p = 0.44; however there was an effect of time, F (1, 18) = 24.39, p < 0.001, indicating that both groups traveled less at the 1 week time point compared to the 24 h time point (Fig. 3B). There was no treatment x time interaction, F (1, 18) = 4.297, p = 0.053. Additional repeated‐measures 2‐way ANOVA testing of behavior at 24 h and 1 week revealed no difference between treatment groups for horizontal movement, F (1, 18) = 2.62, p = 0.12, of time, F (1, 18) = 0.79, p = 0.39, at 24 h or 1 week, or interaction, F (1, 18) = 0.79, p = 0.14 (Fig. 3C). To further analyze arising differences in locomotion we analyzed ambulatory behavior and found for total ambulatory duration that there was no effect of group F (1, 18) = 4.3, p = 0.053, or time F (1, 18) = 2.18, p = 0.16, but there was a significant interaction between group and time, F (1,18) = 5.23, p = 0.03. Post hoc independent sample t‐tests indicated a significant reduction in duration ambulating for the seizure group compared to controls at 24 h, p < 0.05, but no difference between groups at 1 week (Fig. 3D). Post hoc paired‐sample t‐tests also indicated that the control group showed a reduction in ambulatory time between 24 h and 1 week, p = 0.02, whereas the seizure group showed no change in time ambulating between the 2 testing points p > .05. There was no effect of group for ambulatory episode count F (1, 18) = 1.6, p = 0.22, but there was a significant effect of time, F (1,18) = 8.51, p = .009, and no group x time interaction, F (1, 18) =2.76, p = 0.11 (Fig. 3E).
Figure 3.
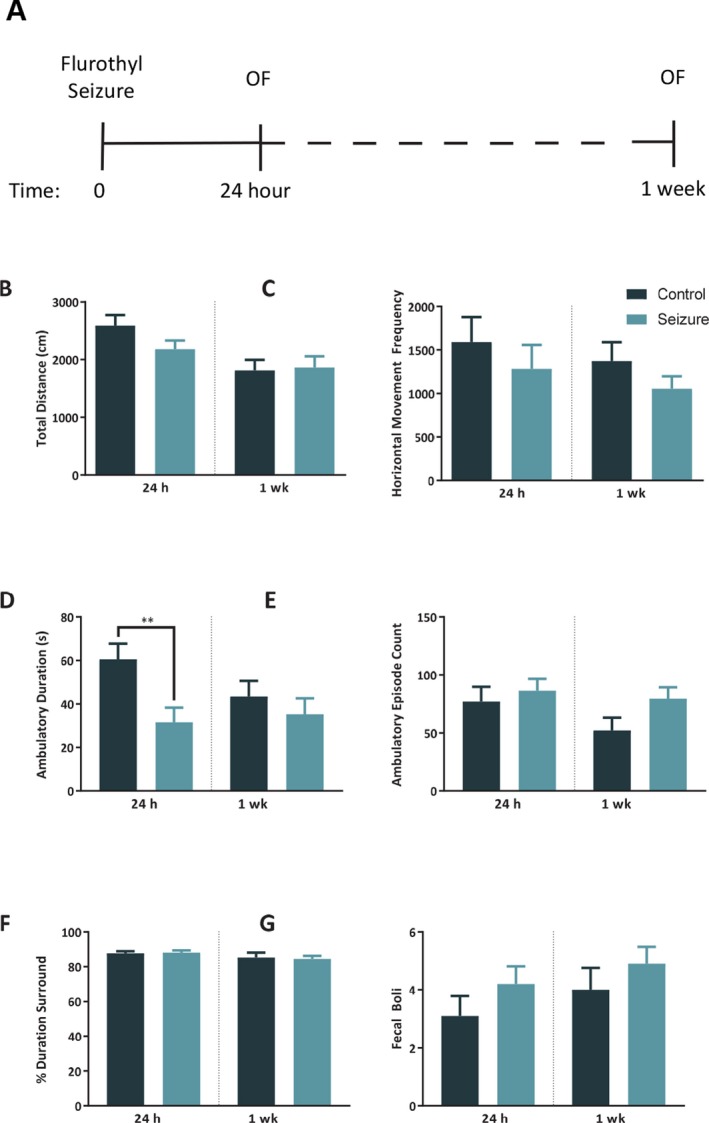
There is a temporary reduction of activity level 24 h after a seizure. A, Timeline for open field tests. There was no difference between control (n = 10) and seizure (n = 10) mice in total distance traveled (B), or horizontal movement frequency (C) at 24 h or 1 week after a seizure. Mice that experienced a seizure (D) spent significantly less time ambulating at 24 h later compared to controls but were no different at 1 week. In comparison, there was no difference between groups in (E) ambulatory episode count at 24 h or 1 week, indicating that the seizure group moved less during each ambulatory episode in comparison to controls. Preliminary measures of anxiety‐like behavior indicated no difference between groups in the percentage of time spent in the center versus surround of the open field (F), and no difference in the quantity of fecal boli (G). The bars represent the mean and the error bars indicate SEM. ** p < 0.01.
Anxiety‐like behavior
We ran a repeated‐measures ANOVA on the percentage of time spent in the perimeter of the open field at 24 h and 1 week and found no difference between groups in the ratio of time spent in the center versus the perimeter of the arena, F (1,18) = 0.01, p = 0.93, time, F (1, 18) = 4.27, p < 0.05, nor a group x time interaction (1, 18) = 0.18, p = 0.67 (Fig. 3F). We similarly analyzed the quantity of fecal boli at the conclusion of each test and found no effect of group F (1, 18) = 1.47, p = 0.24.(Fig. 3G).
Elevated plus maze
Anxiety‐like behavior
A 2‐way repeated‐measures ANOVA indicated no difference between groups at 24 h in the duration of time mice spent in the open arms, closed arms, and the center of the EPM, F (1, 18) = 0.15, p = 0.70; however, there was a significant effect of zone, F (2, 36) = 154.7, p < 0.001. There was not a group x time interaction for duration of time in any zone of the EPM 24 h after a seizure, F (2, 36) = 1.84, p = 0.17 (Fig. 4B). When we tested the same mice again 1 week after a single seizure, there was no effect of group for the duration of time spent in the zones of the maze, F (1, 18) = 0.37, p = 0.55, a significant effect of zone, F (2, 36) = 388.2, p < 0.001, with both groups spending more time in the closed arms, but no group x time interaction, F (2, 36) = 0.80, p = 0.46 (Fig. 4C). When we analyzed the number of fecal boli at 24 h and 1 week, there was no difference between groups in the count of fecal boli, F (1, 18) = 1.02, p = 0.33 (Fig. 4D‐E).
Figure 4.
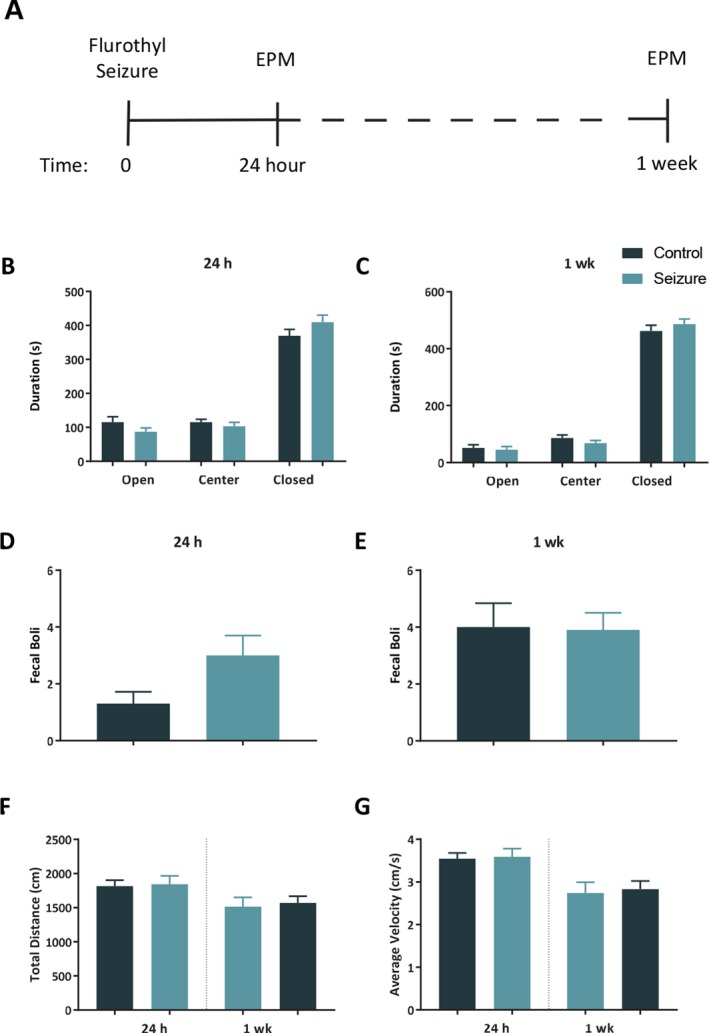
A single seizure does not lead to an increase in anxiety‐like behavior. A, Timeline of elevated plus maze (EPM) tests. There were no differences between control (n = 10) and seizure (n = 10) mice for the duration of time spent in the open arms, closed arms, or center of the EPM at 24 h (B) or 1 week (C) following a seizure. There was not a significant difference between groups in the quantity of fecal boli at 24 h (D), or 1 week (E) after a seizure. There was no difference between groups at 24 h or 1 week in total distance traveled (F) or average movement velocity (G). The bars represent the mean and the error bars indicate SEM.
Activity levels
A 2‐way repeated‐measures ANOVA of total distance traveled at 24 h and 1 week indicated no effect of group, F (1, 18) = 0.09, p = 0.77, and a significant effect of time, F (1, 18) = 12.61, p = 0.002, with both groups moving less at 1 week compared to 24 h. There was not a group x time interaction, F (1, 18) = 0.03, p = 0.87 (Fig. 4F). We also measured average velocity at 24 h and 1 week, and found no effect of group, F (1, 18) = 0.09, p = 0.77, but did find a significant effect of time, F (1, 18) = 23.79, p < 0.001, with both groups moving slower at 1 week in comparison to 24 h. There was no group x time interaction for average velocity, F (1, 18) = 0.02, p = 0.88 (Fig. 4G).
Western blot analysis
To be concise, here we primarily discuss significant hippocampal results, whereas the full results are summarized in Tables S2‐S4 .
One hour
At 1 h following a seizure we detected significant increases in hippocampal phospho‐S6 at Ser235/Ser236, t (16) = 6.39, p < .001, and at Ser240/Ser244, t (16) = 4.68, p < .001; there was no difference in total S6, t (16) = 0.79, p > .05. There was a significant increase in the ratio of phospho‐S6 (Ser235/236)/total S6, t (16) = 4.69, p < .001, and for the ratio of phospho‐S6 (Ser240/244)/total S6, t (16) = 4.47, p < .001 (Fig. 5A‐F), in the hippocampus in the seizure group compared to controls. We also detected an increase of hippocampal total FMRP in the seizure group compared to controls, t (16) = 2.26, p < .05, and a nonsignificant difference in phosphorylated FMRP, t (16) = 1.91, p = .07. There was also a nonsignificant increase in the ratio of phospo‐FMRP/total FMRP in the hippocampus in the seizure group, U = 20, p = .077 (Fig. 5G‐I).
Figure 5.
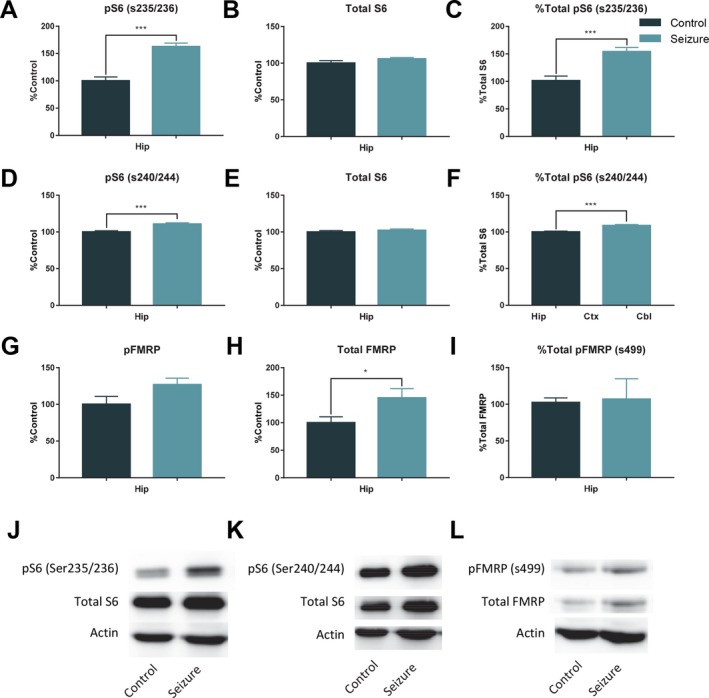
Results of 1 h western blots for pS6, S6, pFMRP, and FMRP. In samples taken 1 h (control = 9; seizure = 9) after a single seizure there was a significant increase in hippocampal pS6 (s235/236) (A), no difference in levels of hippocampal total S6 (B), and a significant increase in the ratio of pS6 (s235/236)/total S6 (C). There was also a significant increase in pS6 (s240/244) (D), but no difference between groups in total S6 (E), and a significant increase in the ratio of pS6 (s240/244) to total S6 (F). G, There was a trending, but nonsignificant increase in pFRMP. However, there was a significant increase in total FMRP 1 h after a seizure (H), and the ratio of pFMRP to total FMRP was no different between groups (I). J‐L, Representative western blots. The bars represent the mean and the error bars indicate SEM. * p < 0.05, *** p < 0.001.
24 Hours
Samples taken at 24 h showed only a significant increase in hippocampal GluA1, t (14) = 2.46, p < .028, in the seizure group when compared to control mice (Fig. 6A,C). There was also a nonsignificant increase in hippocampal PSD‐95, U = 14, p = 0.07 (Fig. 6B,D).
Figure 6.
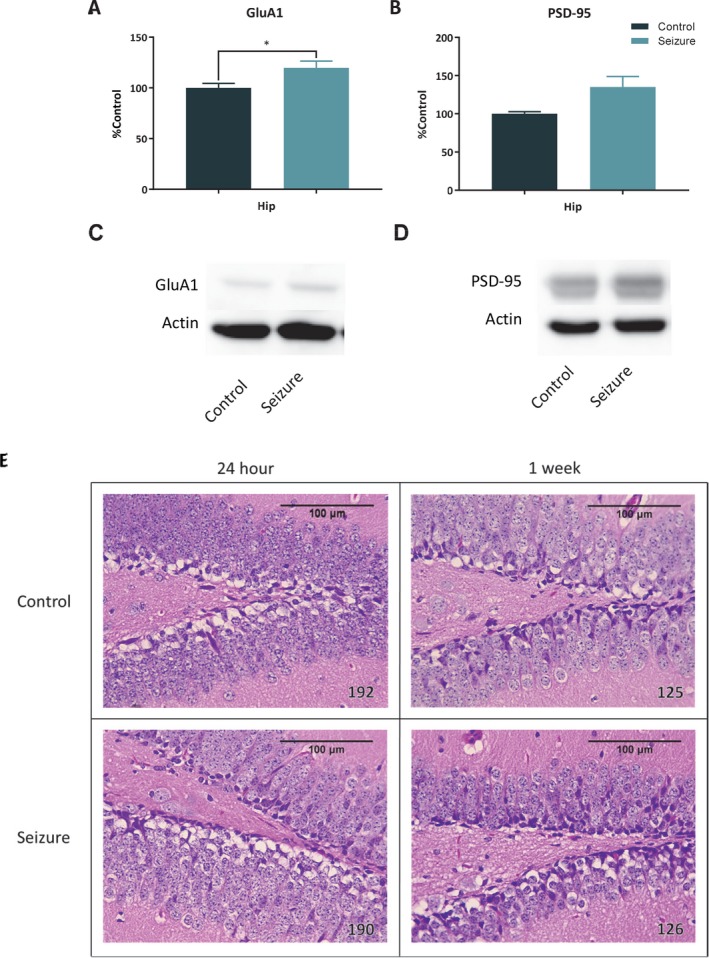
Results of 24 h western blots for GluA1 and PSD‐95 and H&E stained tissue sections. Samples taken from mice 24 h (control = 8; seizure = 8) following a single seizure showed a significant increase in hippocampal GluA1 (A), and a trending, but nonsignificant increase in PSD‐95 (B) (p = 0.065). C‐D, Representative western blots. E, When examined, no necrotic lesions were found in the hippocampus at 24 h or 1 week following a brief flurothyl seizure. The bars represent the mean and the error bars indicate SEM. * p < 0.05.
One week
There were no changes in hippocampal proteins 1 week after a seizure.
Histology
No lesions were detected in brains collected at 24 h or 1 week after seizure induction in comparison to control animals in the hippocampus, cortex, or cerebellum. Photomicrographs of each region were taken (Fig. 6E). See Figure S1 for cortex and cerebellum images.
Discussion
The progressive deterioration of hippocampal‐dependent trace memory that we found complements studies that have found impairments in contextual memory, spatial memory, and cued recall of amygdala‐dependent memory in delay fear conditioning following an acute seizure.13, 14, 15, 16 In addition, our follow‐up trace fear conditioning experiments expand on previous studies, which have only examined memory as far out as 40 h after a seizure15 and indicate that memory impairment persists up to 2 weeks.
Similar to previous results indicating a transient reduction of locomotor activity,16 our results from the open field test indicate that after a seizure there is a reduction in overall locomotion in C57BL/6 mice for at least 24 h that returns to normal by 1 week. The temporary reduction in locomotor activity following a seizure could have masked differences in freezing behavior during the cued‐recall test at 24 h. Despite this concern, there was no difference between groups in baseline freezing during the trace fear tests we ran 24 h or 1 week after a seizure, suggesting that the results were unaffected by altered locomotor activity. In contrast, in the follow‐up fear conditioning experiments, there was lower baseline freezing in the seizure group compared to controls 2 weeks following a seizure. The differences in baseline freezing in both 2‐week tests may indicate contextual memory impairment after a seizure. Balogh and colleagues (2002) examined the time course of fear memory retention and found that C57BL/6J mice display a time‐dependent incubation of contextual fear that generalizes to a novel context 14 days after fear conditioning training.26 A similar generalization of contextual fear likely occurred in our control mice, but not in mice that experienced a seizure.
In comparison to hippocampal trace fear memory, we found that nonhippocampal recognition memory was unaffected in the NOR task. Studies have reported memory impairments in the NOR task after SE induced by intrahippocampal injection of pilocarpine27; however, the greater duration and/or severity of seizures produced by the pilocarpine model likely led to damage and the impairments reported. Another study has reported impairment to recognition memory after PTZ kindling.28 Full PTZ kindling in that study required between 7 and 10 injections of PTZ, injected every 48 h, before the criteria of clonic–tonic seizure was achieved on 3 consecutive injection days. Considering these results, we conclude that without damage, NOR memory likely remains intact after seizures.
We included the accelerating rotarod test to examine whether a single seizure impacts cerebellar learning and memory. We began testing on the rotarod 72 h following a seizure because we observed deficits in locomotor activity in the open field test at 24 h. We did not find any evidence that motor learning and memory was impacted in the rotarod test. However, future studies may include other motor tests such as spontaneous activity in the cylinder test and the adhesive removal test.29 The spontaneous activity in the cylinder test measures limb‐use asymmetry, coordination, rearing, and grooming behavior, whereas the adhesive removal test examines forelimb movement coordination. Using these measures of motor behavior will help determine if more subtle alterations to cerebellar behavior occur following a seizure.
After we established that memory problems after a seizure are limited primarily to memory requiring the hippocampus, we investigated potential mechanisms underlying the impairments. Previous studies have shown that inhibiting the mTOR kinase using rapamycin prior to learning blocks new memories from being formed.30, 31 Similarly, hyperactivation of mTOR caused by the genetic deletion of Pten can also lead to memory impairment.18 Thus, both increased and decreased mTOR activity can have detrimental effects on memory. In addition, when mTOR is hyperactivated, there are also changes to synaptic proteins involved in synaptic plasticity such as FMRP and mGluR5, as well as disruption of numerous scaffolding proteins including PSD‐95 and Shank.23 To test for a role of altered mTOR signaling or synaptic proteins in the memory impairments we observed in trace fear conditioning, we performed western blot analysis on tissue taken at 1 h, 24 h, and 1 week following an acute seizure. One hour after a seizure we found increased hippocampal phospho‐S6 at both Ser235/236 and Ser240/244, confirming hyperactivation of mTOR after a brief seizure. We did not detect increases in phospho‐S6 at either 24 h or 1 week following a seizure, indicating that the increase at 1 h was transient. One hour following a seizure we also detected an increase in hippocampal total FMRP (Ser499), but only a trending increase in phospho‐FMRP. We did not detect any further changes 1 h after a seizure. However, in samples we took at 24 h there was a significant increase in hippocampal GluA1, indicating increased expression of glutamatergic α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptors (AMPARs), and a trending increase in PSD‐95. Our western blot results indicate that multiple translational mechanisms are temporarily disrupted in the hippocampus after a seizure and that disruption may alter synaptic mechanisms underlying hippocampal memory formation and maintenance.
One consideration of the findings of our study is that the relationship between disruptions in mTOR and FMRP and memory deficits are correlational. We did not attempt to rescue memory by using rapamycin, an mTOR inhibitor, after their seizure. Future studies could use mTOR inhibitors at different time points after an acute seizure to determine at which time point inhibition of mTOR is protective against memory impairment. There is one study by Carter et al, 2017, that has provided evidence that inhibition of the upstream regulator of mTOR, PI3K, reduces long‐term memory deficits. Inhibiting PI3K 10 min after a seizure by wortmannin leads to a reduction in downstream phospo‐Akt, phospho‐S6 (ser240/244), and a partial rescue of long‐term auditory memory, but no effect on long‐term contextual memory.15 The lack of full rescue in Carter et al, 2017, may indicate a role for FMRP, which we found to be altered, in seizure‐induced memory impairment. One additional caveat of our study is the inclusion of male mice only. Future studies could use male and female mice to determine if there are sex‐specific effects of an acute seizure on learning and memory. The National Institutes of Health (NIH) has called for the inclusion of both male and female animals, and although we previously reported no effect of sex on seizure‐induced memory impairment, future studies should also include female mice to be in line with this new NIH standard.32
The progressive nature of the impairment in our mice bears a striking resemblance to another phenomenon first described in patients with temporal lobe epilepsy with transient epileptic amnesia, called accelerated long‐term forgetting (ALTF). In ALTF, new memories are encoded and can be recalled initially after standard delays used in neuropsychological assessments (i.e., 30 min), but then over hours, days, or weeks, the memory rapidly deteriorates.33, 34 The forgetting in ALTF appears to differ from general memory impairment in epilepsy, which is detected at shorter delay intervals and is correlated with the extent and location of epilepsy pathology, hippocampal volume, and overall a longer history of seizures.35, 36, 37 Although the mechanism leading to ALTF is unknown, future studies could examine how seizures could lead to ALTF by examining the impact of a single seizure over several time points.
Disclosure
The authors have no conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Hematoxylin and eosin stained brain regions. When examined, no necrotic lesions were found at 24 h or 1 week following a brief flurothyl seizure in cortex (A), or cerebellum (B).
Figure S2. Results of 1 week western blots for p70S6K and GFAP. A, There was a significant increase in cortex p70S6K 1 week following a brief flurothyl seizure. B, There was a significant decrease in cerebellar GFAP 1 week after a brief flurothyl seizure. C‐D, Representative western blots. The bars represent the mean and the error bars indicate SEM. * p < 0.05
Appendix S1. Supplemental Materials and Methods.
Acknowledgments
We would like to extend our gratitude to Bernard S. Jortner, VMD, from the Laboratory for Neurotoxicity Studies at the Virginia‐Maryland Regional College of Veterinary Medicine, Virginia Tech for his work preparing the H&E‐stained samples.
Biography
Andrew J. Holley is a graduate student in the Baylor University Experimental Psychology program.

REFERENCES
- 1. Chauviere L, Rafrafi N, Thinus‐Blanc C, et al. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci 2009;29:5402–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mortazavi F, Ericson M, Story D, et al. Spatial learning deficits and emotional impairments in pentylenetetrazole‐kindled rats. Epilepsy Behav 2005;7:629–638. [DOI] [PubMed] [Google Scholar]
- 3. Pearson JN, Schulz KM, Patel M. Specific alterations in the performance of learning and memory tasks in models of chemoconvulsant‐induced status epilepticus. Epilepsy Res 2014;108:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mishra V, Shuai B, Kodali M, et al. Resveratrol treatment after status epilepticus restrains neurodegeneration and abnormal neurogenesis with suppression of oxidative stress and inflammation. Sci Rep 2015;5:17807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valle‐Dorado MG, Santana‐Gomez CE, Orozco‐Suarez SA, et al. The mast cell stabilizer sodium cromoglycate reduces histamine release and status epilepticus‐induced neuronal damage in the rat hippocampus. Neuropharmacology 2015;92:49–55. [DOI] [PubMed] [Google Scholar]
- 6. Muller CJ, Groticke I, Bankstahl M, et al. Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine‐induced status epilepticus in C57BL/6 mice. Exp Neurol 2009;219:284–297. [DOI] [PubMed] [Google Scholar]
- 7. Imran I, Hillert MH, Klein J. Early metabolic responses to lithium/pilocarpine‐induced status epilepticus in rat brain. J Neurochem 2015;135:1007–1018. [DOI] [PubMed] [Google Scholar]
- 8. Zhu S, Tai C, Petkau TL, et al. Progranulin promotes activation of microglia/macrophage after pilocarpine‐induced status epilepticus. Brain Res 2013;1530:54–65. [DOI] [PubMed] [Google Scholar]
- 9. Fabene PF, Bramanti P, Constantin G. The emerging role for chemokines in epilepsy. J Neuroimmunol 2010;224:22–27. [DOI] [PubMed] [Google Scholar]
- 10. Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 2005;46:1724–1743. [DOI] [PubMed] [Google Scholar]
- 11. Leroy C, Roch C, Koning E, et al. In the lithium‐pilocarpine model of epilepsy, brain lesions are not linked to changes in blood‐brain barrier permeability: an autoradiographic study in adult and developing rats. Exp Neurol 2003;182:361–372. [DOI] [PubMed] [Google Scholar]
- 12. Librizzi L, Noe F, Vezzani A, et al. Seizure‐induced brain‐borne inflammation sustains seizure recurrence and blood‐brain barrier damage. Ann Neurol 2012;72:82–90. [DOI] [PubMed] [Google Scholar]
- 13. Mao RR, Tian M, Yang YX, et al. Effects of pentylenetetrazol‐induced brief convulsive seizures on spatial memory and fear memory. Epilepsy Behav 2009;15:441–444. [DOI] [PubMed] [Google Scholar]
- 14. Mares J, Pometlova M, Deykun K, et al. An isolated epileptic seizure elicits learning impairment which could be prevented by melatonin. Epilepsy Behav 2012;23:199–204. [DOI] [PubMed] [Google Scholar]
- 15. Carter AN, Born HA, Levine AT, et al. Wortmannin Attenuates Seizure‐Induced Hyperactive PI3K/Akt/mTOR Signaling, Impaired Memory, and Spine Dysmorphology in Rats. eNeuro 2017;4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holley AJ, Lugo JN. Effects of an acute seizure on associative learning and memory. Epilepsy Behav 2016;54:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santini E, Huynh TN, Klann E. Mechanisms of translation control underlying long‐lasting synaptic plasticity and the consolidation of long‐term memory. Prog Mol Biol Transl Sci 2014;122:131–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sperow M, Berry RB, Bayazitov IT, et al. Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J Physiol 2012;590:777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lugo JN, Smith GD, Morrison JB, et al. Deletion of PTEN produces deficits in conditioned fear and increases fragile X mental retardation protein. Learn Mem 2013;20:670–673. [DOI] [PubMed] [Google Scholar]
- 20. Zhang B, Wong M. Pentylenetetrazole‐induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia 2012;53:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoeffer CA, Sanchez E, Hagerman RJ, et al. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav 2012;11:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhattacharya A, Kaphzan H, Alvarez‐Dieppa AC, et al. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron 2012;76:325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lugo JN, Smith GD, Arbuckle EP, et al. Deletion of PTEN produces autism‐like behavioral deficits and alterations in synaptic proteins. Front Mol Neurosci 2014;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bevins RA, Besheer J. Object recognition in rats and mice: a one‐trial non‐matching‐to‐sample learning task to study ‘recognition memory’. Nat Protoc 2006;1:1306–1311. [DOI] [PubMed] [Google Scholar]
- 25. Lugo JN, Barnwell LF, Ren Y, et al. Altered phosphorylation and localization of the A‐type channel, Kv4.2 in status epilepticus. J Neurochem 2008;106:1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balogh SA, Radcliffe RA, Logue SF, et al. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behav Neurosci 2002;116:947–957. [DOI] [PubMed] [Google Scholar]
- 27. Lima IVA, Campos AC, Bellozi PMQ, et al. Postictal alterations induced by intrahippocampal injection of pilocarpine in C57BL/6 mice. Epilepsy Behav 2016;64:83–89. [DOI] [PubMed] [Google Scholar]
- 28. Ahmadi M, Dufour JP, Seifritz E, et al. The PTZ kindling mouse model of epilepsy exhibits exploratory drive deficits and aberrant activity amongst VTA dopamine neurons in both familiar and novel space. Behav Brain Res 2017;330:1–7. [DOI] [PubMed] [Google Scholar]
- 29. Fleming SM, Ekhator OR, Ghisays V. Assessment of sensorimotor function in mouse models of Parkinson's disease. J Vis Exp 2013;76:e50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory‐enhancing effect of glucose involves activation of the tuberous sclerosis complex‐Mammalian target of rapamycin pathway. J Neurosci 2006;26:8048–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long‐term fear memory in amygdala neurons. J Neurosci 2006;26:12977–12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holley AJ, Lugo JN. Effects of an acute seizure on associative learning and memory. Epilepsy Behav 2015;54:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fitzgerald Z, Mohamed A, Ricci M, et al. Accelerated long‐term forgetting: a newly identified memory impairment in epilepsy. J Clin Neurosci 2013;20:1486–1491. [DOI] [PubMed] [Google Scholar]
- 34. Audrain S, McAndrews MP. Cognitive and functional correlates of accelerated long‐term forgetting in temporal lobe epilepsy. Cortex 2018;03.022:1–14. [DOI] [PubMed] [Google Scholar]
- 35. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long‐term forgetting and remote memory impairment. Brain 2008;131:2243–2263. [DOI] [PubMed] [Google Scholar]
- 36. Butler CR, Bhaduri A, Acosta‐Cabronero J, et al. Transient epileptic amnesia: regional brain atrophy and its relationship to memory deficits. Brain 2009;132:357–368. [DOI] [PubMed] [Google Scholar]
- 37. Butler C, van Erp W, Bhaduri A, et al. Magnetic resonance volumetry reveals focal brain atrophy in transient epileptic amnesia. Epilepsy Behav 2013;28:363–369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Hematoxylin and eosin stained brain regions. When examined, no necrotic lesions were found at 24 h or 1 week following a brief flurothyl seizure in cortex (A), or cerebellum (B).
Figure S2. Results of 1 week western blots for p70S6K and GFAP. A, There was a significant increase in cortex p70S6K 1 week following a brief flurothyl seizure. B, There was a significant decrease in cerebellar GFAP 1 week after a brief flurothyl seizure. C‐D, Representative western blots. The bars represent the mean and the error bars indicate SEM. * p < 0.05
Appendix S1. Supplemental Materials and Methods.


