Clinical Challenge
A 70-year-old woman nonsmoker, with a history of asthma (no prior pulmonary function tests, not treated with chronic bronchodilators) and gastroesophageal reflux disease presented with abdominal pain, anorexia, and fever. Abdominal imaging revealed a liver mass. Treatment with empiric antibiotics was initiated; all culture data were negative. A diagnostic ultrasound-guided liver biopsy was performed for further evaluation of the hepatic mass. The procedure was uncomplicated. However, within minutes after the procedure, the patient developed acute respiratory distress and fever to 103°F. Her vital signs were notable for tachycardia, with a heart rate of 125 beats/min, blood pressure of 150/90 mm Hg, and tachypnea with a respiratory rate of 28 breaths/min. Her oxygen saturation was measured at 82% with 2 L/min supplemental oxygen by nasal cannula. With increase in the Fio2, oxygen saturation improved to 92%, but respiratory distress persisted. She was extremely anxious and was using accessory muscles of ventilation. Auscultation of the chest revealed diffuse bilateral inspiratory and expiratory wheezes.
An urgent chest radiograph was obtained and demonstrated enlarged lung volumes and a flattened diaphragm; these findings were new compared with prior exam. Imaging showed no evidence of pneumothorax, pulmonary edema, or new infiltrates. An electrocardiogram demonstrated sinus tachycardia. A respiratory code was called for urgent endotracheal intubation.
Questions
1. Under what conditions does dynamic hyperinflation develop?
2. How does dynamic hyperinflation cause dyspnea?
3. How can a clinician ameliorate the deleterious effects of dynamic hyperinflation?
Clinical Reasoning
On the basis of the patient’s acute respiratory decompensation, audible wheezing, and hyperinflation on chest imaging, we inferred that bronchospasm with airflow limitation led to acute dynamic hyperinflation. We posit this was the principal cause of this patient’s worsening respiratory distress; other possible contributing factors are discussed here. There was no evidence of new infiltrates, pneumothorax, or pulmonary edema, the likely alternative explanations for this sudden onset of respiratory distress; hypoxemia had resolved with supplemental oxygen, and PaCO2 was not measured.
We hypothesized that the patient had an acute allergic bronchospastic episode and developed tachypnea in response to breathing discomfort (dyspnea). As she became tachypneic, the time allowed for exhalation decreased; thus, end-expiratory volume increased and tidal volume decreased in the face of expiratory flow limitation. The resulting inspiratory threshold load and decreased sarcomere length of inspiratory muscles led to an increase in the sense of effort and work of breathing. The shift to higher respiratory frequency and lower tidal volume caused increased dead space ventilation and decreased alveolar ventilation. The increased ventilatory drive combined with constrained tidal volume as inspiratory capacity diminished intensified the sensation of air hunger. The increase in work of breathing caused further discomfort, as did the chest tightness associated with bronchospasm, and contributed to her ongoing deterioration with worsening oxygenation and distress.
Clinical Solution
While awaiting initiation of bronchodilators and urgent intubation, morphine 2.5 mg intravenous was administered for dyspnea. This was done in the presence of the code team while preparing for intubation. Over several minutes, the patient appeared more comfortable and less anxious, and her respiratory rate decreased. Endotracheal intubation was averted. Inhaled bronchodilators were administered with continued clinical improvement. The patient was admitted to the intensive care unit and transferred to the medical floor 12 hours later with a presumptive diagnosis of allergic bronchospasm.
The treatment team felt that by reducing the patient’s dyspnea and tachypnea with morphine, there was more time for exhalation, which allowed for a decrease in dynamic hyperinflation, improvement in respiratory muscle mechanics, and consequent increase in tidal volume and diminished dyspnea. Bronchodilators were then administered to reduce the bronchospasm, which was believed to be the acute cause of the air flow limitation.
The Science behind the Solution
Dyspnea often provokes tachypnea. Dynamic hyperinflation occurs because breaths are initiated before complete exhalation of the previous breath, resulting in increased end-expiratory volume, and thus reduction of inspiratory capacity; a vicious circle arises as tidal volume falls and dyspnea further increases. Acute hyperinflation may be unrecognized as the cause of acute dyspnea in patients with acute and chronic obstructive lung disease. Several physiologic conditions occur in concert to produce this phenomenon.
The Equal Pressure Point and Expiratory Flow Limitation
During expiration, gas flows down a pressure gradient from the upstream end, the alveolus, to the downstream end, the airway opening. Positive pressure in the alveolus is generated by elastic recoil of the lung itself, plus pressure exerted by the chest wall via pleural pressure (Equation 1). Because of resistance, the pressure falls along the airways as the gas loses energy to friction; the pressure loss is described by Ohm’s law (Equation 2): the higher the flow, the greater the pressure drop:
| (1) |
where Palv = alveolar pressure; Pel = elastic recoil pressure of the lung; and Ppl = pleural pressure.
| (2) |
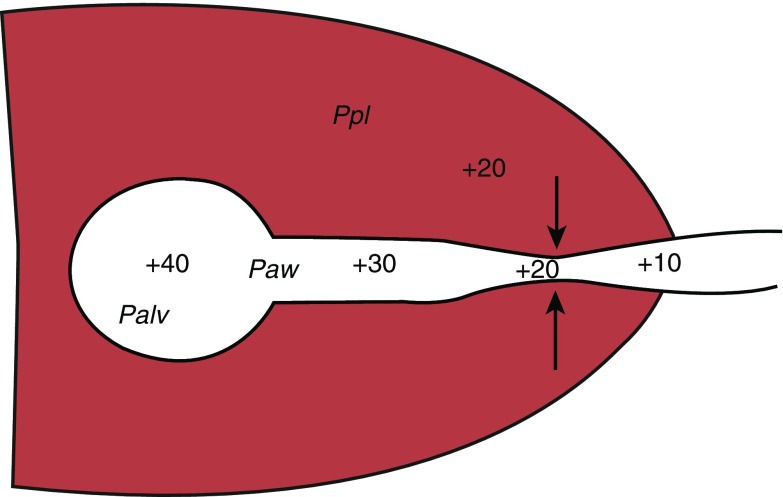
At higher flows, pressure falls more quickly (Equation 2), and at some point along the airway, the pressure inside falls to the level of the pressure outside the airway; that is, the transmural pressure is zero (Figure 1). Downstream of this “equal pressure point,” the airway wall is narrowed by external pressure (pleural pressure), and resistance rises. This occurs during forced exhalation, when pleural pressure is positive (above atmospheric). Increased expiratory effort does not increase expiratory flow under these conditions because both the alveolar driving pressure and the pleural pressure collapsing the airways increase in tandem; that is, expiratory flow cannot increase above a certain limit, regardless of increased expiratory muscle effort, which is termed flow limitation. When equal pressure point conditions exist, the elastic recoil of the lung is the driving force for flow. At lower lung volume, equal pressure point is associated with lower flow because the elastic recoil force of the lung is determined by lung volume.
Figure 1.
Diagram showing equal pressure point. In this example, the pressure in the alveolus is 40 cm H2O (Palv), and the pressure in the airway (Paw) is 30 near the alveolus, and then decreases to 20 and 10 more distally. The pleural pressure (Ppl) is 20. Note that when the pleural pressure and the airway pressure match, the transmural pressure is 0 and the airway collapses. This point, the equal pressure point, is demarcated by the arrows.
Acute Dynamic Hyperinflation and Increased Inspiratory Threshold Load
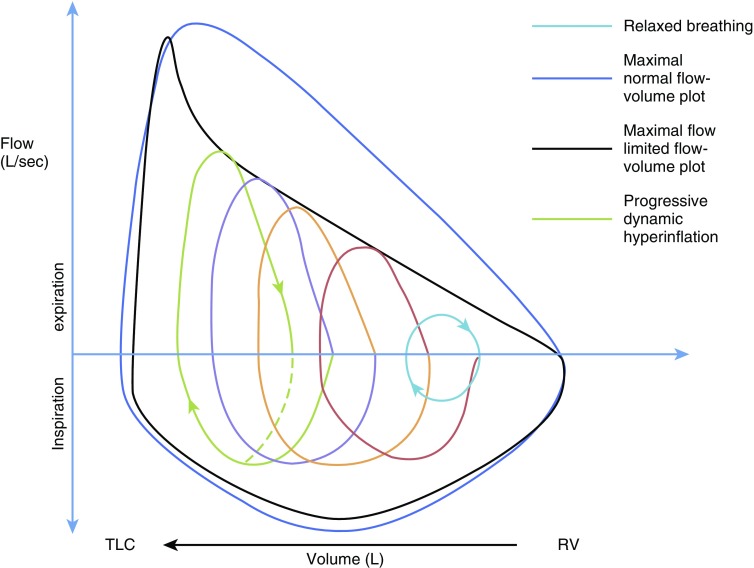
During bronchoconstriction, airway resistance increases, the pressure drop along the airways is greater, and the flow limitation is reached at higher volumes than normally occurs. If expiratory time (TE) is short, there may be insufficient time for the inspired volume to escape, and the next breath will begin at higher lung volume (Figure 2); this process continues until a new equilibrium is reached at a higher lung volume, where higher expiratory flow is available (as a result of greater elastic recoil of the lung, which increases driving pressure, and increased radius of the airways, which reduces resistance). This condition (breathing at higher lung volumes in the setting of increased airway resistance) is termed dynamic hyperinflation, or “air trapping.”
Figure 2.
Dynamic lung hyperinflation. Progression of increase in end-expiratory lung volume on successive breaths in patient with flow limitation with tachypnea and increased ventilation. Red, orange, and purple lines represent individual breaths. RV = residual volume; TLC = total lung capacity.
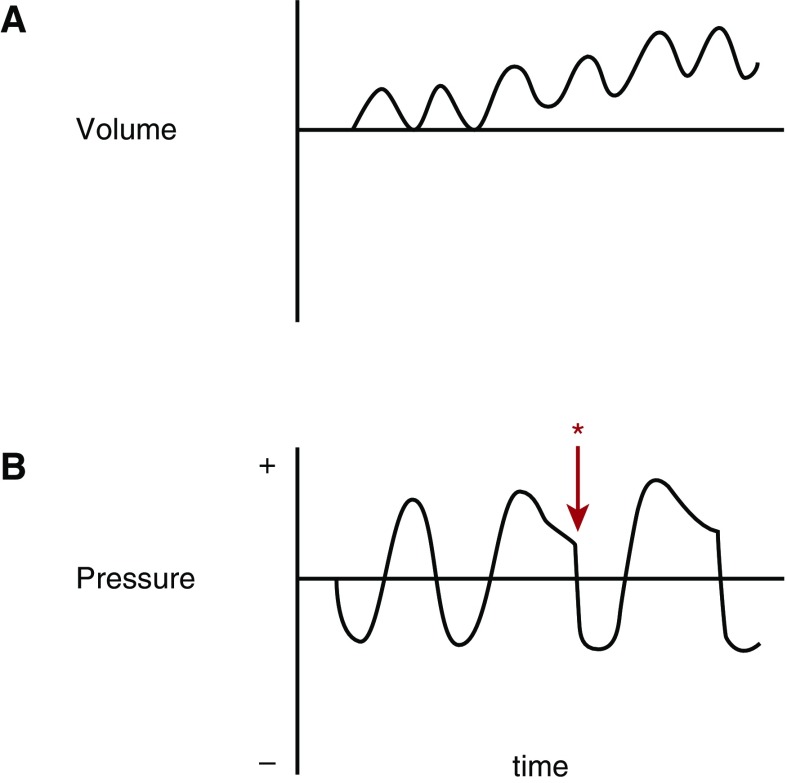
Neurologically, the patient begins inhalation before relaxation volume has been reached; pressure in the airways is positive at end exhalation (intrinsic positive end-expiratory pressure [PEEP]). To generate inspiratory flow, the inspiratory muscles must first overcome intrinsic PEEP; this is termed an inspiratory threshold load and is also conceptualized by the extra work that must be done by inspiratory muscles operating at a shorter, less optimal length for generating tension (Figure 3).
Figure 3.
Inspiratory threshold load. (A) An idealized volume-time curve of a patient who develops increased airway resistance after the first breath, with resulting dynamic hyperinflation. (B) Airway pressure–time curve for the same patient with onset of expiratory resistance after the first breath, with resulting dynamic hyperinflation. With an increase in duty cycle, the brain signals to the inspiratory muscles to contract (*) before the return of lungs to relaxation volume and airway pressure. For air to move from the mouth to the alveoli, the patient must produce airway pressure that is negative relative to atmospheric pressure. The patient must overcome the positive pressure in the airway at the end of exhalation (intrinsic positive end-expiratory pressure) to generate the next breath. The added work associated with this condition has been termed an inspiratory threshold load.
With dynamic hyperinflation and reduced tidal volume, breathing becomes more rapid and shallow, which increases the proportion of ventilation devoted to dead space, hampering gas exchange (1, 2). In addition, at these high lung volumes, the compliance of the respiratory system is reduced, further increasing inspiratory effort. In this situation, one must extend the time allowed for expiratory flow.
Dyspnea
Dyspnea is defined as a “subjective experience of breathing discomfort that consists of qualitatively distinct sensations varying in intensity (3).” This patient was probably experiencing three dyspneic sensations: tightness from bronchoconstriction, excessive effort from the aforementioned mechanical derangements, and air hunger arising from tidal volumes that were too small for the prevailing respiratory drive. Dyspnea often gives rise to behavioral tachypnea, which aggravates the dynamic hyperinflation. Air hunger (a feeling of needing more air and, in the extreme, a sense of suffocation) is the most distressing of these sensations. As efferent neural messages are sent from the motor cortex to the inspiratory muscles, a corollary discharge, or a copy of efferent message, is sent to the sensory cortex and is believed to account for the sense of “effort.” As the respiratory system responds (generating pressure, flow, and volume changes), afferent information from airway, muscle, and joint receptors return to the brain. If the efferent and afferent signals are balanced, there is little air hunger, but if tidal volume is too low for the the respiratory motor drive, air hunger arises (4).
Alleviating air hunger to mitigate behavioral tachypnea may improve a patient’s clinical condition while the underlying condition, in this case bronchospasm, is also being treated. Anxiety associated with dyspnea is a phenomenon well-defined by patients, as has been shown in laboratory and clinical multidimensional dyspnea profile instruments (5, 6). Anxiety commonly accompanies respiratory distress and can contribute to tachypnea. Anxiety has been specifically targeted to alleviate respiratory distress and dyspnea in patients with chronic pulmonary problems in the rehabilitation setting (6, 7).
Role of Morphine in This Case
Opiates have been shown to reduce sensations of breathlessness in patients with a variety of advanced lung diseases, including chronic obstructive pulmonary disease and lung cancer, although none of the studies examined the specific type of respiratory discomfort (3, 8–10). Opiates have also been shown to reduce laboratory-induced air hunger, but not laboratory-induced sense of breathing work. Laboratory experiments suggest morphine reduces dyspnea through two mechanisms: direct action on central synapses to alter perception and decrease anxiety, and action on medullary respiratory centers reducing respiratory drive and associated corollary discharge, thus alleviating the mismatch of motor drive and pulmonary afferent feedback (11).
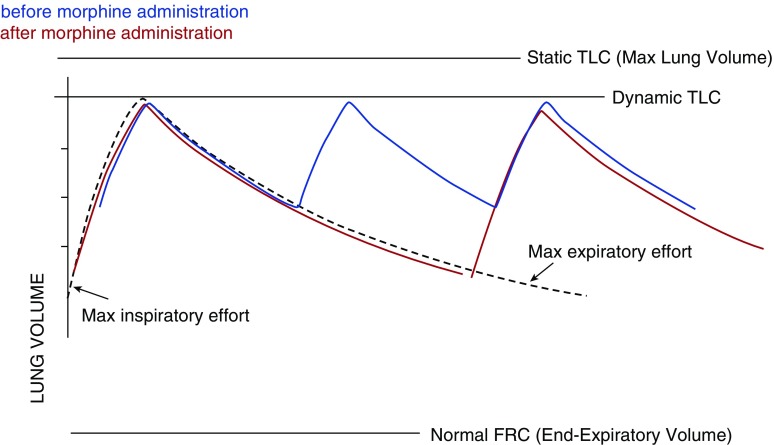
In our patient, we hypothesize that morphine administration slowed her breathing and lengthened her expiratory time, reducing dynamic hyperinflation as well as directly reducing the sensation of air hunger, making respiratory failure less likely (Figure 4). Addressing the underlying etiology of airflow limitation was critical, but we believe that relieving dynamic hyperinflation and air hunger improved symptoms while the primary problem was being addressed. We suggest that low-dose morphine administration could be considered as an adjunctive therapeutic intervention in the setting of increased expiratory resistance to aid in the reversal of respiratory distress in patients who have underlying physiology that can be rapidly reversed (e.g., acute bronchospasm, acute pulmonary edema), have an elevated drive to breath, have a Pco2 less than or equal to 40 mm Hg. This should be done by trained staff in a controlled environment, such as an intensive care unit. The use of morphine in these patients may be used as a bridge or adjunctive measure in patients in whom definitive therapy may be delayed. In normal subjects, morphine used in doses of approximately 5 mg intravenously has been shown to reduce dyspnea without significant depression in ventilation (8).
Figure 4.
As morphine reduces respiratory rate and prolongs time in expiration, end expiratory lung volume decreases and inspiratory capacity improves. The resulting increase in tidal volume improves gas exchange by decreasing the proportion of dead space ventilation, and at the same time it reduces dyspnea. (Blue lines) Before morphine administration. (Red lines) After morphine administration. FRC = functional residual volume; TLC = total lung capacity.
In conclusion, dyspnea may be the consequence of and the driving force for dynamic hyperinflation in patients with airflow limitation. Morphine may be an important therapeutic option to forestall intubation in patients with acute respiratory distress in whom the underlying cause is readily reversible.
Answers
1. Under what conditions does dynamic hyperinflation develop?
Acute dynamic hyperinflation develops in patients with expiratory flow limitation when expiratory time is decreased due to tachypnea, such as during distress or exercise, and end-expiratory volume rises above relaxation volume.
2. How does dynamic hyperinflation cause dyspnea?
Acute dynamic hyperinflation causes dyspnea in three ways: (1) signals from the motor cortex to increase ventilation (efferent signals) are not balanced by an increase in tidal volume (afferent signals sensed by airway, muscle, joint receptors) due to mechanical limitations from the increase in end-expiratory lung volume; this leads to a sensation of air hunger, (2) the work of breathing is increased due to the reduced compliance of the lung when breathing at higher lung volume, and (3) the sense of effort is increased due to the mechanical disadvantage associated with shortened inspiratory muscles when the end expiratory lung volume is elevated.
3. How can a clinician ameliorate the deleterious effects of dynamic hyperinflation?
Acute dynamic hyperinflation is managed by treating acute increases in airway resistance, thereby alleviating expiratory flow limitation, and by increasing expiratory time; both of these will reduce the end-expiratory lung volume. Opiates can decrease respiratory drive and the perception of dyspnea, slowing breathing and allowing for an increase in expiratory time while treatment to decrease airways resistance is underway.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hyatt RE, Wilson TA. Forced Expiration. In: Crystal R, editor. The Lung. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 1393–1401.
- 2.Schwartzstein RM, Parker MJ. Respiratory physiology: a clinical approach. Baltimore, MD: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 3.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, et al. American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartzstein RM, Manning HL, Weiss JW, Weinberger SE. Dyspnea: a sensory experience. Lung. 1990;168:185–199. doi: 10.1007/BF02719692. [DOI] [PubMed] [Google Scholar]
- 5.Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax. 2010;65:21–26. doi: 10.1136/thx.2009.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banzett RB, O’Donnell CR, Guilfoyle TE, Parshall MB, Schwartzstein RM, Meek PM, Gracely RH, Lansing RW. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J. 2015;45:1681–1691. doi: 10.1183/09031936.00038914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrieri-Kohlman V, Gormley JM, Douglas MK, Paul SM, Stulbarg MS. Exercise training decreases dyspnea and the distress and anxiety associated with it. Monitoring alone may be as effective as coaching. Chest. 1996;110:1526–1535. doi: 10.1378/chest.110.6.1526. [DOI] [PubMed] [Google Scholar]
- 8.Banzett RB, Adams L, O’Donnell CR, Gilman SA, Lansing RW, Schwartzstein RM. Using laboratory models to test treatment: morphine reduces dyspnea and hypercapnic ventilatory response. Am J Respir Crit Care Med. 2011;184:920–927. doi: 10.1164/rccm.201101-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pattinson KTS, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG. Opioids depress cortical centers responsible for the volitional control of respiration. J Neurosci. 2009;29:8177–8186. doi: 10.1523/JNEUROSCI.1375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings A-L, Davies AN, Higgins JPT, Gibbs JSR, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57:939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahler DA. Opioids for refractory dyspnea. Expert Rev Respir Med. 2013;7:123–134, quiz 135. doi: 10.1586/ers.13.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.