Abstract
Beginning in 1950, people living on the banks of the Techa River received chronic low-dose rate internal and external radiation exposures as a result of releases from the Mayak nuclear weapons plutonium production facility in the Southern Urals region of the Russian Federation. The Techa River cohort includes about 30,000 people who resided in riverside villages some time between 1950 and 1960. Cumulative red bone marrow doses range up to 2 Gy with a mean of 0.3 Gy. Between 1953 and 2005, 93 cases of leukemia, including 23 cases of chronic lymphatic leukemia (CLL), were ascertained among cohort members. A significant linear dose-response relationship was seen for leukemias other than CLL (p< 0.001), but not for CLL. The estimated excess relative risk per Gy was 4.9 (95% CI: 1.6; 14.3) for leukemias other than CLL and less than 0 (95% upper bound 1.4) for CLL.
Keywords: Techa River Cohort, leukemia, excess relative risk, radiation exposures
Introduction
This paper is one of a series of publications describing the Techa River cohort (TRC) which was established in 1967 by the Urals Center for Radiation Medicine (URCRM) to study the long-term health effects of chronic environmental radiation exposure to the riverside villages (Akleyev and Lyubchansky 1994; Kossenko et al. 1997, 2005; Degteva et al. 2000; Krestinina et al. 2007). The population living near the Techa River was exposed to low-dose rate radiation from radioactive releases from the Mayak nuclear weapons plutonium production facility into the Techa River between 1950 and 1956 (Akleyev and Lyubchansky 1994). The TRC is comprised of an unselected general population of men and women of all ages from two different ethnic groups who lived in similar conditions and have received comparable levels of health care. Cohort members were exposed to protracted external and internal radiation including the bone seeking radionuclide 90Sr through contaminated food and drinking water. Because 90Sr is incorporated into the bone marrow, quantifying the risk of leukemia is a prime interest for this study cohort.
Radiation is a well-established leukemia risk factor as indicated by acute or protracted external exposures among atomic bomb survivors (Preston et al. 1994; 2004) Chernobyl cleanup workers (Kesminiene et al.2008; Romanenko et al. 2008), endometrial cancer patients (Curtis et al. 1994), and Mayak workers (Shilnikova et al. 2003), among others. The TRC is one of the few populations that can provide quantitative estimates of the risk of leukemia following relatively low dose, low-dose rate environmental radiation exposures. Analyses of mortality in the TRC have revealed a dose-response relationship for leukemias other than CLL (non-CLL) (Krestinina et al. 2005). The analyses described in this paper differ from previous analyses in that they make use of data on incidence of non-CLL and CLL leukemias and involve extended follow-up. Because of the unique characteristics of the population, the nature of the exposures, and the length of follow-up, risk estimates from the TRC can play an important role in the development of radiation protection standards for the general public.
Material and methods
Cohort definition, catchment area, follow-up period
The Techa River, which is part of the Ob river system, originates near the Mayak complex and flows about 240 km until it merges with the Iset River. In the 1950's there were 41 villages along the river. The TRC includes 29,756 persons born before 1.1.1950 who lived in any of the Techa riverside villages during the period from 1950 to 1960. More than 80% of the cohort members lived near the river between 1950 and 1953, the period of highest radiation exposure. The cohort includes individuals of all ages, is about 58% female and is comprised of two major ethnic groups, with 80% being of Slav origin and 20% of Tartar or Bashkir origin. About 40% of the cohort members were less than 20 years old when first exposed.
Follow-up begins in 1953 (there are no reported leukemia cases in the cohort prior to 1953 but as discussed below ascertainment is unlikely to be complete from 1950 through 1952) and cohort members are considered to be at risk only for periods of time when they are known to have lived in Chelyabinsk or Kurgan Oblasts. Cohort members are at risk from the first date after 1952 that they lived in either Oblast. Cohort members are considered lost to follow-up when their vital status becomes unknown or when they have emigrated from the study catchment area. Table 1 summarizes vital status and follow-up data for TRC members through 31.12.2005. At this time, 21% of the cohort members were alive and living in the catchment area. Among the 56% of cohort members identified as deceased, the cause of death was established for 90%. Vital status at the end of follow-up was unknown for only 7.5% of cohort members remaining in the study catchment area, but follow-up was censored for about 15% of the cohort when they emigrated from the catchment area (referred to as distal migrants).
Table 1. Vital status for TRC members as of 31.12.2005.
| Vital Status | N | % |
|---|---|---|
| Alive | 6,363 | 21.4 |
| Deceased | 16,619 | 55.9 |
| Cause of death known | 15,025 | 90.4 |
| Cause of death unknown | 1,594 | 9.6 |
| Vital status unknown | 2,219 | 7.5 |
| Distal migrants | 4,555 | 15.3 |
|
| ||
| Total | 29, 756 | 100.0 |
Sources of information
Leukemia cases were ascertained from four main sources: URCRM medical records, regional oncology clinics, regional health centers and death certificates. Since 1950, leukemias have been ascertained from death certificates stored at the government (ZAGS) offices of Chelyabinsk and Kurgan oblasts (19% of all cases). In 1955 Dispensary No 1 of the Institute of Biophysics (subsequently Branch 4 of the Biophysics Institute and currently the URCRM) was given responsibility for the systematic observation and treatment of radiation-exposed individuals with hematological disorders. The medical records for leukemia patients diagnosed between 1953 and 1955 were obtained by URCRM from the Chelyabinsk region hospital. The URCRM was a key source of information on leukemia cases in the first two decades of follow-up, providing data on 39% of all leukemia cases. Since 1956, leukemia notification forms from the Chelyabinsk and Kurgan oblast oncology dispensaries have been linked to the TRC cohort roster and about 37% of all cases were identified from this source. Since the dispensaries are the major cancer treatment facilities in the study catchment area, they have become the primary source for newly diagnosed cases. The remaining cases (about 6% of the total) were ascertained from the records of health centers in Chelyabinsk and Kurgan oblasts. With these data sources, the ascertainment of leukemia cases is largely complete since 1953 for cohort members residing in Chelyabinsk Oblast or Kurgan Oblast. Description of information sources in greater detail provides (Kossenko et al. 2005).
Dosimetry
The population of the Techa riverside villages was exposed to external and internal radiation. External radiation exposure from gamma-emitting radionuclides (l37Cs, 93Zr, 95Nb, l03Ru, l06Ru, and others) was a consequence of contamination of the river water and floodplains. Internal exposure resulted from consumption of water, milk and other food products containing radionuclides (primarily 89Sr, 90Sr, 137Cs). Internal exposure was primarily a result of 90Sr incorporation into the bone structure, for which the bone marrow was the main target-organ.
Red bone marrow doses were estimated using the TRDS-2000 dosimetry system (Degteva et al. 2000; Kossenko et al. 2005; Krestinina et al. 2005; 2007). The system provides individualized internal and external organ dose estimates and information on the uncertainty of these estimates. Estimates of annual total doses were derived from annual village mean dose estimates that allow for the nature of the releases (source term), distance from the release point, relationship of the typical residence to the river flood plain, source of drinking water, and other factors. These were then individualized to account for gender, age, residence history and other factors. The estimated maximum cumulative red bone marrow dose was 2 Gy, and the mean dose was 0.3 Gy. Total cumulative red bone marrow dose estimates were between 0.01 and 0.5 Gy for two-thirds of the cohort members while 19% had estimates in excess of 0.5 Gy. The current analyses were based on two-year lagged cumulative red bone marrow dose.
Data organization and statistical methods
Analyses of background rates and excess risks were carried out using Poisson regression methods (Clayton and Hills 1993; Preston 1993) in a highly stratified table of cases and person years for non-CLL and CLL to provide the most stable site specific estimates feasible with this cohort at this time. Cohort members were considered to be at risk from the earliest of January 1, 1953 or the date at which they first moved into one of the affected villages until their earliest of the date of cancer diagnosis or death. Person-years were accumulated only during periods in which a person was known to be living in Chelyabinsk or Kurgan Oblasts. The radiation effect was described using excess relative risk models (Preston 1993). Similar models have been used to describe radiation effects on cancer risks in the Life Span Study of atomic bomb survivors (Preston 1994; 2004; 1993), in the Mayak worker cohort (Shilnikova et al. 2003) and in the ETRC (Krestinina et al. 2005). Age, birth cohort, gender, ethnicity, and oblast of initial exposure were evaluated as potential risk factors. Linear, linear-quadratic and pure-quadratic dose-response models were considered. Gender, age at entry, attained age, and ethnicity were considered as potential dose-effect modifiers. The Epicure software (Preston et al. 1993) was used for constructing person-year tables and estimating risks.
Results
More than 830,000 person years have accumulated for cohort members residing in Chelyabinsk and Kurgan Oblast over the 53-year follow-up period. During this time 93 leukemia cases were identified, including 75 for which an incidence date could be determined and 18 which were identified solely from death certificates.
Table 2 provides summary information on the distribution of cohort members, person years, and leukemia cases by gender, ethnicity, age-at-exposure, and time since exposure. The crude rates in this table, which do not make allowance for possible dose effects, suggest that rates increase with time or age and are slightly higher for men than for women. Cohort members identified as Tartar or Bashkir ethnicity appear to have higher rates than those identified as Slavs. No leukemia cases were reported among cohort members prior to age 10 and only three cases (one acute and one chronic myeloid leukemia, and one acute leukemia of unspecified cell type) were identified among those aged 10-20 years (data not shown in table).
Table 2. Selected characteristics of leukemia cases and TRC cohort membersa:1953-2005.
| Category | People | Person Years | Leukemia Cases | Rate (per 10,000 PY) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Any | %DCOb | Non-CLLc | Any | Non-CLL | |||
| Gender | |||||||
| Male | 12,565 | 336,660 | 42 | 26% | 30 | 1.25 | 0.89 |
| Female | 17,191 | 495,821 | 51 | 14% | 40 | 1.03 | 0.81 |
|
| |||||||
| Ethnicity | |||||||
| Tartar & Bashkir | 5,950 | 200,594 | 31 | 6% | 26 | 1.55 | 1.30 |
| Slav | 23,806 | 631,887 | 62 | 26% | 44 | 0.98 | 0.70 |
|
| |||||||
| Age at exposure | |||||||
| <20 | 11,769 | 397,045 | 40 | 20% | 34 | 1.01 | 0.86 |
| 20-40 | 9,640 | 296,550 | 31 | 16% | 20 | 1.05 | 0.67 |
| >=40 | 8,347 | 138,886 | 22 | 23% | 10 | 1.58 | 1.15 |
|
| |||||||
| Years since first exposure (average age) | |||||||
| 0 – 5 (32)d | e | 68,684 | 5 | 0% | 5 | 0.73 | 0.73 |
| - 10 (35) | 119,439 | 6 | 0% | 5 | 0.50 | 0.42 | |
| - 20 (41) | 206,818 | 23 | 39% | 18 | 1.11 | 0.87 | |
| - 30 (48) | 171,488 | 18 | 22% | 15 | 1.05 | 0.87 | |
| - 40 (55) | 133,216 | 12 | 17% | 5 | 0.90 | 0.38 | |
| 40+ (63) | 132,836 | 29 | 10% | 22 | 2.18 | 1.66 | |
|
| |||||||
| Total | 29,756 | 832,482 | 93 | 19% | 70 | 1.12 | 0.84 |
Catchment area residents only
% Death certificate only
Leukemias other than chronic lymphocytic leukemia (CLL)
Average attained age for this time-since exposure category
Number of people not shown since this is a time-dependent factor
More than half of the cases (48 cases) were classified as chronic leukemias, 45% (42 cases) as acute or subacute leukemias, while the type of the leukemia could not be determined for 3% (3 cases) of the cases (Table 3). Five-year absolute survival rates were low, but this partly due to the cases diagnosed many years ago when treatment for leukemia was not very sophisticated.
Table 3. Distribution of leukemias by histologic type: Techa River Cohort 1953-2005.
| Type | Incident cases | DCO | Total | Mean age at diagnosis | Five-year absolute Survivala | ||
|---|---|---|---|---|---|---|---|
| Acute leukemias | |||||||
| Myeloid | 8 | 0 | 8 | 48 | 12% | ||
| Other specified typesb | 3 | 0 | 3 | 63 | --c | ||
| Unspecified cell types | 19 | 12 | 31 | 52 | 6% | ||
|
| |||||||
| Chronic leukeimias | |||||||
| Lymphocytic | 22 | 1 | 23 | 62 | 56% | ||
| Myeloid | 22 | 3 | 25 | 57 | 37% | ||
|
| |||||||
| Other or unspecified leukemias | |||||||
| Myeloid | 1 | 2 | 3 | 56 | --c | ||
|
| |||||||
| Total | 75 | 18 | 93 | 55 | 30% | ||
Based on non-DCO cases
Includes: one acute monocytic leukemia and two acute erythremic myelosis
Not computed due to small number of cases
Baseline risks
Log baseline rates for non-CLL leukemias were well-described using gender-dependent quadratic functions of log attained age. After allowing for a linear dose response there was no significant effect of ethnicity (P > 0.5) nor was there any evidence for a log-linear birth cohort effect (P > 0.5). Non-CLL baseline rates for men rose fairly rapid with age increasing from about 0.1 cases per 10,000 person years at age 30 (95% CI 0.04; 0.3) to 0.9 (95% CI 0.4; 1.8) cases at age 70. At these ages, the corresponding baseline rate estimates for women were 0.3 (95% CI 0.1; 0.6) and 0.4 (95% CI 0.2; 0.7), respectively. This gender difference in the temporal pattern of the non-CLL baseline rates was statistically significant (P = 0.01).
The log baseline CLL rates could be described by a quadratic function of log attained age with male rates being 2.1 times the female rates (95% CI 0.9; 4.9), P = 0.08). The fitted rates were essentially 0 up to age 35 and peaked around age 70 when the fitted rates were 1.1 for men (95% CI 0.5; 2.0) and 0.5 for women (95% CI 0.2; 0.9) and decreased slightly at older ages. There was no evidence of ethnic differences (P > 0.5) or a log-linear birth cohort effect (P=0.3) on the CLL baseline rates. CLL cases make up more than half of the cases diagnosed among cohort members in their 50's and 60's.
Radiation risk estimates
There was a statistically significant linear dependence on dose for non-CLL leukemias (P < 0.001). The ERR per Gy estimate was 4.9 with a 95% confidence interval of 1.6 to 14. There was no indication of non-linearity in the non-CLL leukemia dose response (P > 0.5) and the estimated curvature was close to 0 (-0.12 with a 95% CI < -0.5; 17) (Figure 1). Tests for gender or ethnic differences in the dose response provided no indication of effects, P > 0.5. Allowing the dose response to depend on attained age, age at exposure, or time since first exposure did not improve the fit of the model (P > 0.5) and the estimates of the changes were small. In contrast, we found no indication of a dose response for CLL (P > 0.5) and the ERR per Gy estimate was negative (< -0.2 per Gy) with an upper 95% confidence bound of 1.4.
Fig.1. Non-CLL Leukemia dose response function : linear model -solid line, linear-quadratic model- dot-dash line, nonparametric model- points with 95% confidence interval.
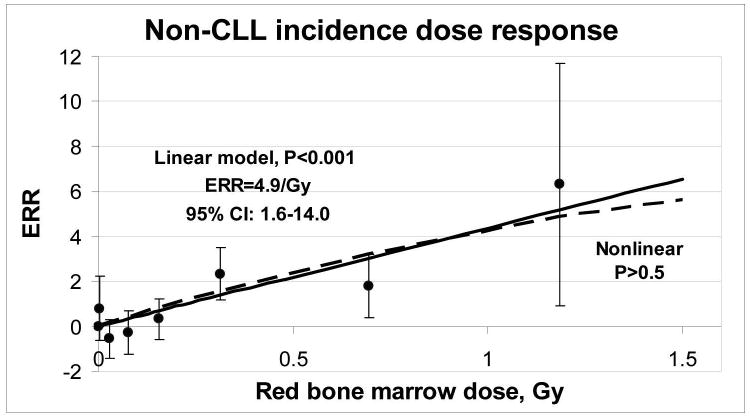
Table 4 presents the number of person-years and cases in categories of cumulative 2-year –lagged red bone marrow dose. The table also includes estimates of the number of radiation-associated non-CLL cases. It was estimated that 59% (95% CI 32%; 80%) of the non-CLL leukemia cases were associated with the radiation-exposure. The proportion of radiation-associated cases was higher for those exposed to more than 0.5 Gy.
Table 4. Distribution of observed and expected leukemia cases by red bone marrow dose category.
| Dose category, Gy | PYK | Non-CLLa | CLL oasesc | |
|---|---|---|---|---|
|
| ||||
| Cases | Excessb | |||
| <0.01 | 103,499 | 6 | 0 | 5 |
| - 0.1 | 137,364 | 3 | 1 | 3 |
| -0.2 | 171,109 | 8 | 4 | 5 |
| -0.5 | 254,262 | 31 | 14 | 5 |
| -1 | 140,848 | 15 | 17 | 5 |
| >=1 | 25,399 | 7 | 5 | 0 |
|
| ||||
| Total | 832,482 | 70 | 41 | 23 |
Leukemias other than chronic lymphocytic leukemia (CLL)
Excess case estimate from a linear dose response model with no effect modification
Excess case estimates not shown since there was no evidence of a dose response (P > 0.5)
Discussion
For over 50 years, the TRC of about 30,000 people with environmental low-dose low-dose-rate radiation exposure has been followed to evaluate radiation-related health effects. Our current results demonstrate a statistically significant linear dependence (p<0.001) of the excess relative risk of non-CLL leukemia incidence on cumulative dose to red bone marrow with almost 60% of the non-CLL cases in the cohort attributable to radiation exposure. The ERR per Gy of 4.9 for non-CLL in this analysis was lower, albeit still statistically compatible with the estimate (6.5; 95% CI 1.8; 24) from an early mortality analyses (Krestinina et al. 2005). Since we are using the same dose estimates and there is little evidence that the ERR is changing with time or age in this cohort, the difference in the ERR estimates is due primarily to the extended follow-up period and the addition of many incident cases. The present analysis includes about 50% more cases than were used in the mortality analyses. The suggestion that the reduction in risk is related to the additional cases is supported by the similar risk estimate (ERR per Gy 4.6; 95% CI: 1.7; 12.3) found in a case-control study of 83 incident leukemia cases conducted in the TRC before TRDS2000 was available (Ostroumova et al. 2007).
Recently two studies of leukemia risk in Chernobyl clean-up workers, who also received low-dose protracted radiation exposure, were published (Kesminiene 2008; Romanenko 2008). The non-CLL ERRs per Gy were 2.73 (95% CI < 0; 13.5) for Ukrainian workers (Romanenko 2008) and 5.0 (90% CI -0.04; 57) for workers from Belarus, Russia and the Baltic countries. The risks in the three studies appear to be statistically compatible. It is of note, that the current TRC non-CLL risk estimate is also consistent with an ERR per Gy of 4 for non-CLL mortality in the atomic bomb survivors who received an acute dose of radiation (Preston et al. 1994).
For more than a decade, we have been focusing on improving the completeness of follow-up and quality of cause of death information. Thus, the proportion of individuals with unknown vital status at the end of the follow-up was reduced to 7.5% and the proportion of deceased cohort members with unknown cause of death was reduced to 10%. These changes led to an increase in statistical power and narrower confidence intervals around the point estimates of risk. The current risk estimates will be updated when a revised dosimetry system becomes available.
Acknowledgments
This work (epidemiological and dosimetry investigations) has been supported by the U.S. Department of Energy, Office of Health Studies and by the U.S. National Institutes of Health, National Cancer Institute. The Federal Department of the Russian Ministry of Health has also provided support for all aspects of this study. Many people have made important contributions to this study. We acknowledge the contributions of Marina Degteva, Nikolai Startsev, Catherine Zhidkova and doctors of URCRM clinical Department.
References
- Akleyev AV, Lyubchansky ER. Environmental and medical effects of nuclear weapon production in the Southern Urals. Sci Total Environ. 1994;42:1–8. doi: 10.1016/0048-9697(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Kossenko MM, Degteva MO, Vyushkova OV, Preston DL, Mabuchi K, Kozheurov VP. Issues in the comparison of risk estimates for the population in the Techa River region and atomic bomb survivors. Radiat Res. 1997;148:54–63. [PubMed] [Google Scholar]
- Degteva MO, Kozheurov VP, Tolstykh EI, et al. The Techa River dosimetry system: methods for the reconstruction of internal dose. Health Phys. 2000;79:24–35. doi: 10.1097/00004032-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Degteva MO, Vorobiova MI, Kozheurov VP, Tolstykh EI, Anspaugh LR, Napier BA. Dose reconstruction system for the exposed population living along the Techa River. Health Phys. 2000;78:542–554. doi: 10.1097/00004032-200005000-00012. [DOI] [PubMed] [Google Scholar]
- Kossenko MM, Thomas TL, Akleyev AV, Krestinina LYu, Startsev NV, Zhidkova CM, Vyushkova OV, Hoffman DA, Preston DL, Davis F, Ron E. The Techa River Cohort: Study Design and Follow-up Methods. Radiat Res. 2005;164:591–601. doi: 10.1667/rr3451.1. [DOI] [PubMed] [Google Scholar]
- Krestinina LY, Preston DL, Ostroumova EV, Degteva MO, Ron E, Vyushkova OV, Startsev NV, Kossenko MM, Akleyev AV. Protracted radiation exposure and cancer mortality in the Techa River Cohort. Radiat Res. 2005;164:602–611. doi: 10.1667/rr3452.1. [DOI] [PubMed] [Google Scholar]
- Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramato A, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat Res. 1994;2(Suppl.):S68–97. [PubMed] [Google Scholar]
- Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res. 2004;162:377–389. doi: 10.1667/rr3232. [DOI] [PubMed] [Google Scholar]
- Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitis J, Stengrevics, et al. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res. 2008;170:721–735. doi: 10.1667/RR1231.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanenko AY, Finch Sc, Hatch M, Lubin JN, Bebeshko VG, Bazyka DA, et al. The Ukrainian-American study of leukemia and related disorders among Chernobyl cleanup workers from Ukraine: III. Radiation risks. Radiat Res. 2008;170:711–720. doi: 10.1667/RR1404.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis RE, Boice JD, Jr, Stovall M, Bernstein L, Holowaty E, Karjalainen S, et al. Relationship of leukemia risk to radiation dose following cancer of the uterine corpus. J Natl Cancer Inst. 1994;86:1315–1324. doi: 10.1093/jnci/86.17.1315. [DOI] [PubMed] [Google Scholar]
- Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, et al. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res. 2003;159:787–798. doi: 10.1667/0033-7587(2003)159[0787:cmrawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Krestinina LY, Davis F, Ostroumova E, Epifanova S, Degteva M, Preston D, et al. Solid cancer incidence and low-dose-rate radiation exposures in the Techa River cohort: 1956 2002. Int J Epidemiol. 2007;36:1038–1046. doi: 10.1093/ije/dym121. [DOI] [PubMed] [Google Scholar]
- Clayton D, Hills M. Statistical Methods in Epidemiology. Oxford University Press; New York: 1993. [Google Scholar]
- Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure Users Guide. Hirosoft International Corporation; Seattle, Washington: 1993. [Google Scholar]
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- Ostroumova EV, Gagnière B, Laurier D, Gudkova NV, Krestinina LY, Verger P, Hubert P, Bard D, Akleyev AV, Tirmarche M, Kossenko MM. Risk analysis of leukaemia incidence among people living along the Techa River: a nested case-control study. J Radiol Prot. 2006;26:17–32. doi: 10.1088/0952-4746/26/1/001. [DOI] [PubMed] [Google Scholar]


