Abstract
Endogenous estrogen plays an integral role in the etiology of breast and endometrial cancer, and conceivably ovarian cancer. However, the underlying mechanisms and the importance of patterns of estrogen metabolism and specific estrogen metabolites have not been adequately explored. Long-standing hypotheses, derived from laboratory experiments, have not been tested in epidemiologic research because of the lack of robust, rapid, accurate measurement techniques appropriate for large-scale studies. We have developed a stable isotope dilution liquid chromatography-tandem mass spectrometry (LC–MS2) method that can measure concurrently all 15 estrogens and estrogen metabolites (EM) in urine and serum with high sensitivity (level of detection = 2.5-3.0 fmol EM/mL serum), specificity, accuracy, and precision [laboratory coefficients of variation (CV’s) ≤5% for nearly all EM]. The assay requires only extraction, a single chemical derivatization, and less than 0.5 mL of serum or urine. By incorporating enzymatic hydrolysis, the assay measures total (glucuronidated + sulfated + unconjugated) EM. If the hydrolysis step is omitted, the assay measures unconjugated EM. Interindividual differences in urinary EM concentrations (pg/mL creatinine), which reflect total EM production, were consistently large, with a range of 10-100-fold for nearly all EM in premenopausal and postmenopausal women and men. Correlational analyses indicated that urinary estrone and estradiol, the most commonly measured EM, do not accurately represent levels of total urinary EM or of the other EM. In serum, all 15 EM were detected as conjugates, but only 5 were detected in unconjugated form. When we compared our assay methods with indirect radioimmunoassays for estrone, estradiol, and estriol and enzyme-linked immunosorbent assays for 2-hydroxyestrone and 16α-hydroxyestrone, ranking of individuals agreed well for premenopausal women [Spearman r (rs) = 0.8-0.9], but only moderately for postmenopausal women (rs = 0.4-0.8). Our absolute readings were consistently lower, especially at the low concentrations characteristic of postmenopausal women, possibly because of improved specificity. We are currently applying our EM measurement techniques in several epidemiologic studies of premenopausal and postmenopausal breast cancer.
Keywords: Estradiol, Estrogen metabolism, Estrone, Liquid chromatography-tandem mass, spectrometry
1. Endogenous estrogen and breast, endometrial, and ovarian cancer
In the last decade, the evidence that endogenous estrogen levels are causally related to breast cancer has strengthened substantially. In 2002, a pooled analysis of the worldwide data from prospective studies, which included 663 women who developed breast cancer and 1765 women who did not, demonstrated that risk of postmenopausal breast cancer increased significantly (p for trend <0.001) with increasing circulating concentrations of estrone sulfate, estrone, and estradiol [1]. For each estrogen, risk doubled between extreme quintiles. Urinary concentrations of estrogens have also been positively associated with subsequent risk of postmenopausal breast cancer, with trends in risk reaching statistical significance [2,3]. However, prospective studies have not yet conclusively shown an association between circulating or urinary estrogens and risk of premenopausal breast cancer, quite possibly because of the complexity of controlling for variation in estrogen levels during the menstrual cycle [3,4]. Fewer data exist for endometrial cancer. In the largest prospective study to date, including 247 incident cases of endometrial cancer and 481 controls from the European Prospective Investigation into Cancer and Nutrition (EPIC), risk of endometrial cancer increased significantly with serum concentrations of estrone and estradiol in postmenopausal women (p for trend <0.01), but was not clearly related in premenopausal women [5]. Among postmenopausal women, associations with endometrial cancer seemed stronger than that for breast cancer, with relative risks reaching 2.7 when extreme tertiles of estrone were compared. Only a few small studies of ovarian cancer have been published, and associations with endogenous estrogen have been inconsistent [6]. For all three cancers, the mechanisms of estrogen-mediated carcinogenesis have yet to be defined, and may differ among the three sites.
2. Estrogen metabolism and cancer
Although experimental, clinical, and epidemiologic research have implicated endogenous estrogens in the etiology of breast and endometrial cancer, and possibly ovarian cancer, the role of individual patterns of estrogen metabolism has been largely unexplored in epidemiologic work [7]. Metabolism of estrogens occurs in the liver and kidneys, and in target tissues, and includes oxidative metabolism (hydroxylation) and conjugative metabolism (methylation, sulfation, and/or glucuronidation) [8]. Oxidation of the parent estrogens, estrone and estradiol, occurs at either the 2-, 4- or 16-position of the carbon skeleton to yield 2-hydroxylated, 4-hydroxylated, or 16-hydroxylated estrogens, respectively (Fig. 1) [9]. At least 15 human cytochrome P450 isoforms, phase I enzymes that vary in their distribution across target tissues, their catalytic activity, and their specificity, are capable of catalyzing these hydroxylations [10]. Catechol estrogens, with adjacent hydroxyl groups at the 2- and 3-positions or the 3- and 4-positions, can be methylated (Fig. 1), which is generally considered an excretory pathway [11]. 16α-hydroxyestrone can be further metabolized by reduction and oxidation at the 17- and 16-positions (Fig. 1). Conjugation with sulfate or glucuronide moieties is known to modulate the bioavailability of estrogens and estrogen metabolites (which we refer to jointly as EM). Sulfation of estrogens may extend the half-life in circulation while glucuronidation is an important excretory pathway for estrogens. Estrogen metabolism yields products that are potentially both estrogenic and genotoxic. Specific estrogen metabolism pathways, such as formation of the 16-hydroxylated estrogens with their strong hormonal and mitogenic activity, are postulated to increase breast cancer risk [12]. Alternatively, specific estrogen metabolites, such as the reactive catechol estrogens, may function as carcinogens by reacting with DNA to form stable or depurinating adducts [7] although it has also been postulated that 2-pathway catechol estrogens may actually be protective since their formation precludes 16-hydroxylation [13]. The 4-pathway catechol estrogens, though substantially less abundant, are potent inducers of DNA damage in animal and in vitro models and have been hypothesized to increase breast cancer risk [14]. Methylation of catechol estrogens weakens binding to the estrogen receptor, thus reducing estrogenicity, and prevents reactive quinone formation; both effects should reduce cancer risk [11]. Estrogen metabolism patterns may also determine how bioavailable estrogen is in target tissue and how efficiently it is removed from circulation.
Fig. 1.
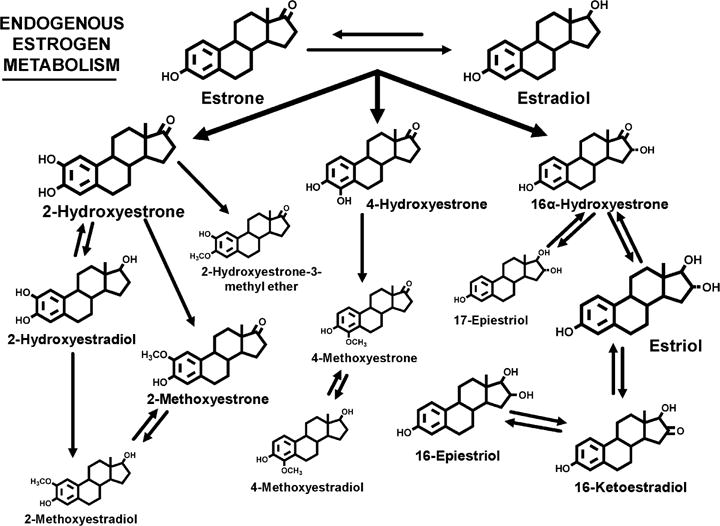
The estrogen metabolites formed by hydroxylation of the parent estrogens, estrone and estradiol, at the 2-, 4-, or 16-positions of the carbon ring. The relative size of the chemical structures indicates the relative concentration in urine in premenopausal women, postmenopausal women, and men [18].
3. Liquid chromatography-tandem mass spectrometry (LC-MS2) assay for estrogens and estrogen metabolites (EM)
Nine years ago, we decided to attempt a “high risk/high reward” project. The laborious “gold standard” mass spectrometry methods for measuring endogenous steroid hormones were being abandoned, and the limitations of commercial radioimmunoassay (RIA), enzyme immunoassay (EIA), and enzyme-linked immunoassay (ELISA) kits were complicating epidemiologic research on hormonal carcinogenesis. Variability over time, between kits, and among labs frustrated individual and pooled analyses. Each hormone was assayed independently, and required 0.2-1.0mL and substantial costs per sample. In addition, long-standing hypotheses about the importance of individual steroid hormone metabolites and patterns of metabolism, based on experimental research, were not being evaluated in epidemiologic studies. Robust, relatively rapid analytic methods capable of characterizing estrogen metabolism in the large number of biologic samples collected in epidemiologic research were required. In a multidisciplinary effort, Drs. Larry Keefer, Tim Veenstra, Xia Xu, and Regina Ziegler have collaborated to develop an accurate, precise, and sensitive high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry method for measuring concurrently the endogenous EM in human serum and urine [15,16]. The procedure is relatively simple and rapid; it requires hydrolysis, extraction, and a single chemical derivatization, and only 0.5 mL of serum or urine (Fig. 2). Enzymatic hydrolysis with Helix pomatia extract removes sulfate and glucuronide residues from the EM. Omitting the hydrolysis step enables us to measure the quantities of unconjugated EM. The single derivatization adds a bulky, charged dansyl (1-dimethylamino-naphthalene-5-sulfonyl) group to the phenolic hydroxyl at the 3-position on each EM. This reactive hydroxyl, characteristic of all estrogens, enables our technique to measure not only the parent estrogens but also all the estrogen metabolites. The dansylation is critical since mass spectrometry separates and detects compounds on the basis of charge and molecular weight, and does not perform efficiently with neutral, fat-soluble compounds, such as steroids. Electrospray ionization is utilized to gently convert complex biological solutions into gas phase ions and link the liquid chromatography to the mass spectrometer. In order to identify unique, well-resolved peaks for each EM, many of which are chemically very similar, we incorporate tandem mass spectrometry, in which a second fragmentation and separation is applied to the ions generated by the initial fragmentation. Finally, a defining characteristic of our approach is reliance on stable isotope dilution. We add stable 2H- or 13C-labeled EM standards at the beginning, before hydrolysis, so that we can quantitatively correct for loss or degradation during all steps of the procedure. With our LC/MS2 technique, we can simultaneously measure the absolute quantities of the two parent EM, estrone and estradiol; the two catechol and three methylated catechol EM in the 2-hydroxylation pathway (2-hydroxyestrone, 2-hydroxyestradiol and 2-methoxyestrone, 2-methoxyestradiol, 2-hydroxyestrone-3-methyl ether); the one catechol and two methylated catechol EM in the 4-hydroxylation pathway (4-hydroxyestrone and 4-methoxyestrone, 4-methoxyestradiol); and the five EM in the 16-hydroxylation pathway (16α-hydroxyestrone, estriol, 17-epiestriol, 16-ketoestradiol, 16-epiestriol) (Fig. 1). Mass spectrometry techniques are increasingly viewed as the most promising approach for improving sensitivity, specificity, accuracy, and precision in steroid hormone measurement, and the “gold standard” against which traditional RIA, EIA, and ELISA should be compared [17]. Our LC/MS2 assays for EM in serum and urine offer the advantages of mass spectrometry and, in addition, analyse parent estrogens and a wide variety of their metabolites in a single run.
Fig. 2.
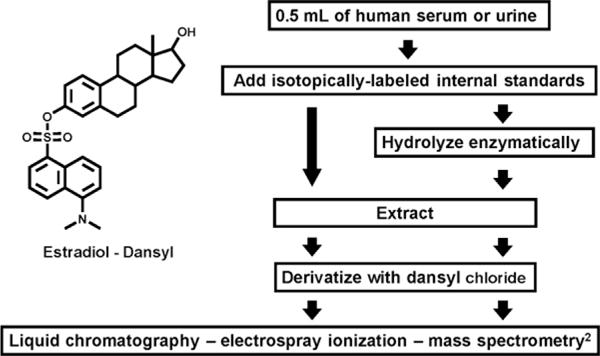
Schematic of liquid chromatography–tandem mass spectrometry procedure for measuring endogenous estrogens and estrogen metabolites (EM) in serum and urine samples. Total (conjugated and unconjugated) EM are measured by including the enzymatic hydrolysis step. Only unconjugated EM are measured if the hydrolysis step is omitted. The chemical structure for dansylated estradiol indicates how the 1-dimethylamino-naphthalene-5-sulfonyl (dansyl) moiety covalently binds to the phenolic hydroxyl at the 3-position, a defining characteristic of all EM.
4. EM in urine
In 2005, we published our LC/MS2 technique for the simultaneous measurement of the absolute quantities of 15 urinary EM, which are presented in Fig. 1 [16]. Because EM are mostly present in urine as glucuronide or sulfate conjugates, we are currently measuring total EM, the sum of the glucuronidated, sulfated, and unconjugated forms of each EM. The conjugated EM within each urine sample are enzymatically hydrolyzed after addition of the isotopically labelled standards. We start with 0.5 mL of urine, and 10% is eventually placed on the column. The lower level of quantitation for each EM is 40 pg/mL urine (~150 fmol/mL). The level of quantitation is the concentration at which we know we have acceptably low coefficients of variation (CV’s) because of sufficiently high signal-to-noise ratios. Our level of detection, which is the “sensitivity” reported in the literature for most steroid hormone assays, is ~4 pg/mL urine (~15 fmol/mL). Accuracy, based on percent recovery of a known amount of unconjugated EM added to charcoal-stripped human urine, is 96-107%. Calibration curves are linear over a 103-fold concentration range. At this point in time, we are relying on five stable isotopically labeled standards for the 15 EM: deuterated estradiol, 2-hydroxyestradiol, 2-methoxyestradiol, estriol, and 16-epiestriol. Using overnight urines from five follicular phase and five luteal phase premenopausal women, five postmenopausal women, and five men, we conducted a formal “proof of performance” of our urinary EM assay by measuring two randomized, blinded aliquots from each subject in each of four batches over four weeks [18]. None of the women were currently using exogenous hormones, such as oral contraceptives or menopausal hormone therapy. Laboratory CV’s, which included the hydrolysis, extraction, and derivatization steps as well as within and between batch variation, were ≤10% for each of the 15 EM, and generally ≤5%. Intraclass correlation coefficients (ICC’s) within each menstrual/sex group, a measure of the variability attributable to interindividual differences, were generally ≥0.98. Thus, within each menstrual/sex group, the range in concentration of each EM was quite large relative to assay variability. The ICC’s may be somewhat imprecise because of the small number of participants in the study but were remarkably consistent across the four menstrual/sex groups. For descriptive analyses, we combined data from an additional 25 subjects with the data from these 20 subjects [18]. Although geometric mean EM concentrations (pg EM/mg creatinine) differed substantially among the four menstrual/sex groups, the rankings of the individual EM were quite similar, with estriol, 2-hydroxyestrone, estrone, estradiol, and 16-ketoestradiol accounting for 60-75% of total urinary EM. The three catechol estrogens comprised 20-25% of total EM, while the five methylated catechol estrogens were 5-10%. What was especially exciting was that within each menstrual/sex group, interindividual differences in urinary EM concentrations, which reflect interindividual differences in EM production and excretion, were consistently large, with a range of 10-100-fold for nearly all EM. This interindividual variation is highlighted in Fig. 3, which shows box plots of urinary concentrations of the three catechol EM, the five methylated catechol EM, and the five 16-pathway EM for each menstrual/sex group. EM concentration is plotted on a logarithmic scale, with a different scale for each boxplot. At this point, we knew that interindividual variability in urinary EM concentrations was substantially larger than laboratory variation. However, we did not know whether variation in urinary EM levels in an individual over secular time would limit our ability to identify associations with risk when we relied upon a single urine collection, as is typical of most epidemiologic studies. Dr. Sue Hankinson at Harvard School of Public Health had collected urine samples in the Nurses’ Health Study II cohort that were appropriate for addressing this question. With Dr. Heather Eliassen, also at Harvard, we examined the reproducibility of urinary EM concentrations in 110 premenopausal women with luteal phase urine samples collected during each of three years [19]. On average, parent EM (estrone and estradiol) were 21% (5th-95th percentiles = 12-34%) of total urinary EM and 2-pathway, 4-pathway, and 16-pathway EM were 36% (12-62%), 4% (1-8%), and 39% (17-67%), respectively; interindividual variation in estrogen metabolism was clearly apparent. Reproducibility within a woman over time was relatively high for the three hydroxylation pathways, with ICC’s ranging from 0.52 (16-pathway EM) to 0.57 (4-pathway EM) to 0.72 (2-pathway EM), which were as high as or higher than the ICC’s for estrone and estradiol (~0.5). ICC’s for the individual catechol EM and 2-pathway methylated catechol EM were comparably high. Because of their low concentrations, ICC’s for the individual methylated catechol EM in the 4-pathway were relatively low (<0.3). Converting absolute EM concentrations (pmol EM/mg creatinine) to relative concentrations (pmol EM expressed as percent of total EM in pmol) noticeably improved the ICC’s. These data indicate that urinary EM levels do vary substantially among individuals when compared to intraindividual variability. It is encouraging that reproducibility in premenopausal women over time for most individual and grouped EM is comparable to or better than that of well-vetted biomarkers, such as circulating cholesterol (ICC = 0.65); blood glucose (ICC = 0.52); and, in postmenopausal women, plasma estradiol (ICC = 0.68), all of which are considered to be reliable predictors of disease in epidemiologic studies [19]. We also evaluated Spearman correlations among the EM [19]. Urinary estrone was only moderately correlated with the individual estrogen metabolites (most rs = 0.3-0.6), while correlations between urinary estradiol and the individual metabolites were still lower (rs = 0.1-0.4). However, individual EM within a pathway were fairly highly correlated. The 2-pathway EM and 4-pathway EM were highly correlated (rs = 0.9), but both pathways were weakly and inversely correlated with the 16-pathway EM (rs = −0.2). These data suggest that urinary concentrations of the parent EM, estrone and estradiol, do not accurately represent the concentrations of individual estrogen metabolites. Potentially important additional information is obtained when the entire estrogen metabolism profile is measured in urine. In preparation for large-scale epidemiologic studies, we have studied the stability of the 15 EM in urine samples, with and without added ascorbic acid (0.1% w/v), during (1) interim storage at 4°C, (2) long-term storage at −80°C, and (3) freeze-thaw cycles [20]. Early morning urine specimens were provided by three premenopausal women. We saw no consistent evidence of >1% loss for any of the EM during interim storage for 24 h, long-term storage for one year, or two additional freeze-thaw cycles in the samples without added ascorbic acid. Given the large interindividual variability in urinary EM concentrations we have observed [18], these changes are unlikely to cause substantial mis-classification in epidemiologic research. To our surprise, ascorbic acid, an antioxidant which has been suggested in the literature as necessary to protect specific EM [21,22], had no clear beneficial effects on individual EM stability in any of these experiments. Therefore, for epidemiologic and clinical studies that will be collecting urine samples in which EM will be measured, we suggest immediately chilling the urine sample to 4 °C on collection, or the individual portions of urine if a 12- or 24 h collection is planned; keeping the urine at 4 °C for no more than 1–2 days before decanting and aliquotting for long-term storage at −70 °C; and adding no preservatives or antioxidants. This stability study validated the urine collection and storage procedures we had already used in several epidemiologic studies in which we wished to measure EM in prospectively stored urine samples and assess associations with subsequent cancer. In epidemiologic studies of endogenous hormones and hormone metabolism, urine samples offer some distinct advantages over blood, including ease of biospecimen collection, potentially higher participation rates, and the integration of exposure over time for hormones with pulsatile, circadian, or menstrual cycle variability.
Fig. 3.
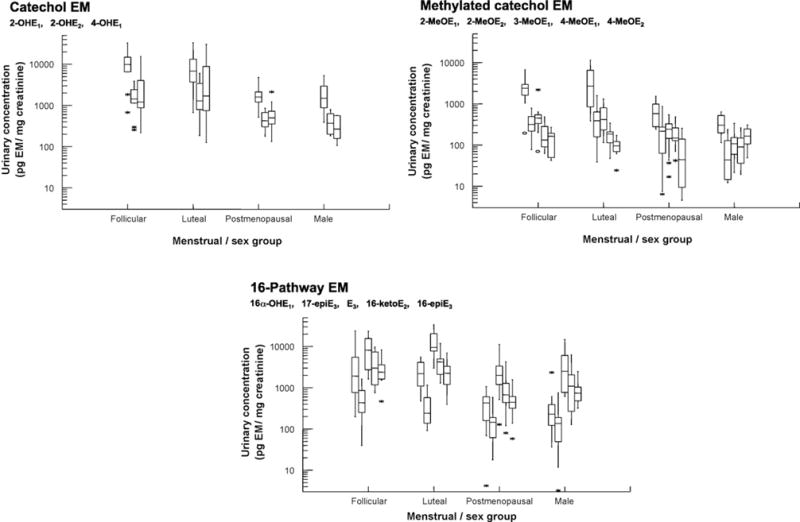
Interindividual variation in urinary concentrations of catechol estrogens and estrogen metabolites (EM), methylated catechol EM, and 16-pathway EM is shown with box plots for 10 premenopausal follicular phase women, 10 premenopausal luteal phase women, 15 postmenopausal women, and 10 men. Urinary EM concentrations are in pg/mg creatinine and presented on a logarithmic scale. The first graph summarizes interindividual variation of the catechol EM, shown in the following order: 2-hydroxyestrone (2-OHE1), 2-hydroxyestradiol (2-OHE2), and 4-hydroxyestrone (4-OHE1). The second graph summarizes interindividual variation of the methylated catechol EM: 2-methoxyestrone (2-MeOE1), 2-methoxyestradiol (2-MeOE2), 2-hydroxyestrone-3-methyl ether(3-MeOE1), 4-methoxyestrone (4-MeOE1), and 4-methoxyestradiol (4-MeOE2).The third graph summarizes interindividual variation of the 16-pathway EM: 16α-hydroxyestrone (16α-OHE1),17-epiestriol (17-epiE3), estriol (E3), 16-ketoestradiol (16-ketoE2), and 16-epiestriol (16-epiE3). The horizontal line within each box is the median of the distribution. The top and bottom of each box are the interquartile range (75 and 25 percentiles, respectively) of the distribution. The vertical lines above and below each box extend to the extreme values that are not outliers (≤1.5 times the interquartile range). Outliers are represented as stars (>1.5 but ≤3 times the interquartile range) and open circles (>3 times the interquartile range).
5. EM in blood
In 2007, we published the details of our LC/MS2 technique for the simultaneous measurement of the absolute quantities of serum EM [15]. In exploratory work, we had found, to our surprise, that all 15 EM we had detected in urine (Fig. 1) were also present in serum in conjugated form, as sulfates or glucuronides, and that five of the EM were present at quantifiable levels in unconjugated, or free, form. Therefore, to accurately capture the concentration of total endogenous estrogen in circulation and the concentrations of all individual EM, we decided that for serum samples, we would do two LC/MS2 analyses: one of total (conjugated + unconjugated) EM and one of unconjugated EM. Unconjugated EM are measured by eliminating the enzymatic hydrolysis step in our method; total EM are measured by including enzymatic hydrolysis; conjugated EM can be calculated as the difference of the two analyses. To enhance accuracy, stable isotopically labeled EM standards are added to 1.0 mL serum samples. Two 0.4 mL aliquots are created from each 1.0 mL sample; only one of the two aliquots is hydroyzed. Both aliquots are then extracted, derivatized, and analysed independently by LC/MS2.
Except for the change described above, where we measure both total and unconjugated EM in each sample, our method for measuring all 15 EM concurrently in serum is similar to our method for urinary EM. A total of 1.0 mL of serum is required to measure both total and unconjugated EM. We currently use newer LC/MS2 systems for the serum analyses than the urine analyses, which has resulted in a 5-fold increase in sensitivity. The lower level of quantitation for each EM is 8 pg/mL serum (26.5-29.6 fmol/mL). The level of detection, which is the “sensitivity” reported in the literature for most steroid hormone assays, is ~0.8 pg/mL serum (<3 fmol/mL). Accuracy, based on recovery of a weighed amount of unconjugated EM added to charcoal-stripped serum, is 91-113%. Calibration curves are linear over a 103-fold concentration range. We are currently relying on six stable isotopically labeled standards for the 15 EM: deuterated 2-hydroxyestradiol, 2-methoxyestradiol, estriol, and 16-epiestriol and C-13 labeled estrone and estradiol. We have not yet completed stability studies of individual EM in serum during interim and long-term storage comparable to the stability studies we performed for urinary EM. For serum collection and storage, we use the protocol adopted by most epidemiologic studies. Blood is kept at room temperature for no more than an hour as it clots; the serum is collected by pipetting or decanting after centrifugation. Once the serum is aliquotted, it is stored at 4 °C for no more than 12 h, and then transferred to −70 °C for long-term storage. No antioxidants or preservatives are used. It is generally accepted that parent EM in serum or plasma are stable for up to 3 days during interim storage at 4 °C, and for years during long-term storage at −70°C. [23].
It is the extremely high sensitivity of our LC–MS2 assay—a level of quantitation for each EM of 8 pg/mL serum and a level of detection of ~0.8 pg/mL serum—that enables us to measure circulating estrogens in postmenopausal women. Distinguishing serum estradiol levels in the low postmenopausal range (<30 pg/mL; <110 fmol/mL) is an important prognostic tool for common chronic diseases of older women, specifically breast cancer, osteoporosis, cardiovascular disease, and possibly cognitive dysfunction [24]. The ability to measure serum estradiol with high sensitivity and specificity is particularly important in monitoring postmenopausal women with hormone-dependent breast cancers who are receiving aromatase inhibitors. Suppression of estrogen production may be influenced by non-compliance, hidden drug-drug interactions, and genetically altered pharmacokinetics and can promote severe bone loss [25]. Both indirect RIA methods, which include extraction and/or chromatography, and direct RIA methods are not accurate or sensitive enough to monitor serum estradiol at these low levels [25]. Bioassays that rely on recombinant yeast methods and HeLa cells may be more sensitive than RIA but lack specificity and convenience [25]. Gas chromatography/tandem mass spectrometry provides the needed specificity, sensitivity, and accuracy, but does not match the tight CV’s of our LC–MS2 method [25]. However, before any mass spectrometry method can be used in a clinical environment to measure estradiol or other EM, detailed reference protocols, calibration and validation requirements, and normative values need to be developed [26]. We have completed a formal “proof of performance” for our serum EM assay in eight postmenopausal women not on hormone therapy. Two samples from each of the eight women were analysed during each of four weeks. Laboratory CV’s were ≤5% for all total and unconjugated EM, except for the two EM at the lowest concentrations: total 4-methoxyestradiol (CV = 6%) and total 17-epiestriol (CV = 7%). The descriptive data from this “proof of performance” were intriguing, and are summarized in Fig. 4. This bar graph shows for all 15 EM the mean serum concentrations, in fmol/mL, of conjugated, unconjugated, and total EM for the eight postmenopausal women. Concentrations are shown on a logarithmic scale, and the standard error of the mean for total EM is included. In general, only circulating levels of estrone sulfate, estrone, and estradiol are measured in epidemiologic and clinical studies. However, these three EM are just a fraction of the physiologic complexity. In all these women, all 15 EM we had previously characterized in urine were also present in serum. The molar concentration in serum of all 15 EM combined was generally more than three times that of estrone sulfate, a biologically inactive estrogen which is thought to be the estrogen reservoir and can be converted to estrone and estradiol in breast and other target tissues [27]. For each of the 15 EM, the molar concentration of the conjugated form was substantially higher than the molar concentration of the unconjugated form; in fact, we detected only five unconjugated EM in circulation: estrone, estradiol, estriol, 2-methoxyestrone, and 2-methoxyestradiol. We could not detect any of the potentially mutagenic and genotoxic catechol estrogens in circulation. Estradiol itself, considered the biologically active form of estrogen and the predominant activator of estrogen receptor-mediated cellular processes, was more abundant conjugated than unconjugated in most of the women. Conjugated estradiol is not currently measured by the indirect or direct RIA assays for estradiol so its influence is generally not evaluted. Yet conjugated estradiol concentrations may be biologically relevant since breast and other tissues contain sulfatases and glucuronidases that can generate biologically active estradiol from conjugated estradiols in circulation. Evidence is increasing that sulfation/desulfation of EM represents a cyclic system important in the regulation of biologically active estrogen in target tissue, while glucuronidation is the major pathway for estrogen excretion in urine and bile [8,28]. We are currently expanding this “proof of performance” for our serum EM assay to include premenopausal women and men. While the laboratory CV’s will be interesting, the descriptive data for total, conjugated, and unconjugated EM in circulation may well be unique. In trying to measure patterns of endogenous estrogen metabolism, we have focused on 15 specific EM in conjugated and unconjugated form, primarily the EM reported in early studies of urinary EM. It is these EM for which we routinely include purified standards in all our LC–MS2 runs. In the future, we will have the opportunity to modify our method and utilize it to identify additional EM present in human urine and serum, including those due to rare gene variants, environmental and lifestyle exposures, and disease/treatment. We have not yet used the structure identification properties of mass spectrometry to identify provocative peaks, nor have we obtained a library of standards for additional EM that might be present.
Fig. 4.
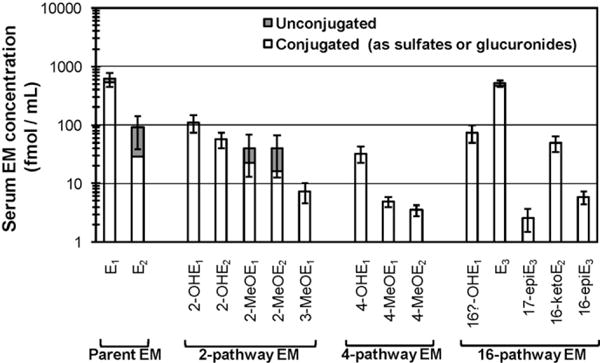
Mean serum concentrations of unconjugated and conjugated forms of 15 estrogens and estrogen metabolites (EM) in eight postmenopausal women. Serum concentrations, in fmol/mL, are plotted on a logarithmic scale. Conjugated forms of each EM are represented by the white section of the bar graph; unconjugated forms are represented by the dark section of the bar graph. Total concentration of each EM is represented by the entire bar graph. The standard error of the mean for total EM concentration is shown by the thatch marks. Parent EM include estrone (E1) and estradiol (E2). 2-pathway EM include 2-hydroxyestrone (2-OHE1), 2-hydroxyestradiol (2-OHE2), 2-methoxyestrone (2-MeOE1), 2-methoxyestradiol (2-MeOE2), and 2-hydroxyestrone-3-methyl ether (3-MeOE1). 4-pathway EM include 4-hydroxyestrone (4-OHE1), 4-methoxyestrone (4-MeOE1), and 4-methoxyestradiol (4-MeOE2). 16-pathway EM include 16α-hydroxyestrone (16α-OHE1), estriol (E3), 17-epiestriol (17-epiE3), 16-ketoestradiol (16-ketoE2), and 16-epiestriol (16-epiE3).
6. Comparing EM measurement by RIA or ELISA and by LC/MS2
Absolute and relative EM concentrations are important for clinical decisions, as well as epidemiologic and experimental research on hormonal carcinogenesis. RIA, EIA, and ELISA are routinely used for measuring EM in blood and urine because of their efficiency, simplicity, and low cost. We wanted to compare these widely accepted, commercially available methods with our new LC/MS2 technique. In a population-based case-control study of breast cancer in Asian–American women aged 20–55 years [29], we had measured five EM in 12 h overnight urines collected from 362 premenopausal and 168 postmenopausal controls. We had chosen state-of-the-art methods and experienced laboratories widely used by epidemiologists and clinicians. Estrone, estradiol, and estriol were assayed at Nichols Institute (San Juan Capistrano, CA) with an indirect method involving enzymatic hydrolysis, extraction, chromatography, and RIA [30]. 2-Hydroxyestrone and 16α-hydroxyestrone were assayed at Strang Cancer Research Laboratory (New York, NY) with a method involving enzymatic hydrolysis and ELISA [31,32]. Recently we re-assayed the same urines with our LC/MS2 method and compared the absolute and relative results with those obtained earlier by RIA and ELISA [33]. For the premenopausal women, ranking subjects by RIA-based measures of urinary estrone, estradiol, and estriol agreed quite well with those obtained using LC/MS2 (rs >0.9), while ranking subjects by ELISA-based measures of urinary 2-hyroxyestrone and 16α-hydroxyestrone agreed reasonably well with LC/MS2 (rs = 0.8-0.9) (Table 1). However, for the postmenopausal women, agreement was noticeably reduced for all five EM (rs = 0.4-0.8). Geometric mean concentrations (pmol/mg creatinine) of estrone, estradiol, and estriol were 1.4-1.9 times higher by RIA than LC/MS2 in premenopausal women, and 1.4-2.7 higher in postmenopausal women (all p <0.0001) (Table 1). Geometric mean concentrations of 2-hydroxyestrone and 16α-hydroxyestrone were 2.0-3.7 times higher by ELISA than LC/MS2 in premenopausal women, and 2.7-11.8 times higher in postmenopausal women (all p <0.0001). These data suggested the RIA and ELISA assays had limited specificity and accuracy, and were detecting additional EM or other steroids. Based on the blinded quality control samples that we had inserted for all the assays, laboratory CV’s for estrone, estradiol, and estriol were <13% for RIA in premenopausal women, ≤18% for RIA in postmenopausal women, and ≤5% for LC/MS2 in both premenopausal and postmenopausal women [33]. Laboratory CV’s for 2-hydroxyestrone and 16α-hydroxyestrone were ≤14% for ELISA in both premenopausal and postmenopausal women, ≤5% for LC/MS2 in premenopausal women, and ≤9% for LC/MS2 in postmenopausal women. Thus, our results for reproducibility, as well as accuracy, indicated that the widely used RIA and ELISA measures for EM might be problematic, particularly at the low concentrations characteristic of postmenopausal women. Although this comparison of state-of-the-art commercial assays with LC/MS2 was performed with urine samples, it is plausible that in serum, a more complicated matrix, the commercial assays would perform even less well, relative to LC/MS2.
Table 1.
Comparison of urinary estrogen/estrogen metabolite (EM) measurement by RIA/ELISA and LC/MS2: Spearman correlations and absolute concentrationsa.
| EM | Premenopausal luteal phase women N = 264 | Premenopausal non-luteal phase women N=98 | Postmenopausal women N =168 |
|---|---|---|---|
| Spearman correlations | |||
| RIA and LC/MS2 | |||
| Estrone | 0.94 | 0.96 | 0.79 |
| Estradiol | 0.91 | 0.95 | 0.63 |
| Estriol | 0.94 | 0.94 | 0.73 |
| ELISA and LC/MS2 | |||
| 2-Hydroxyestrone | 0.81 | 0.89 | 0.37 |
| 16α-Hydroxyestrone | 0.86 | 0.89 | 0.62 |
| Geometric mean concentrations pmol/mg creatinine | ||||||
|---|---|---|---|---|---|---|
| RIA/ELISA | LC/MS2 | RIA/ELISA | LC/MS2 | RIA/ELISA | LC/MS2 | |
| RIA and LC/MS2 | ||||||
| Estrone | 41.9 | 23.4 | 27.9 | 14.6 | 6.9 | 2.6 |
| Estradiol | 17.6 | 10.9 | 12.0 | 7.7 | 2.1 | 1.5 |
| Estriol | 77.2 | 55.5 | 50.1 | 31.2 | 12.9 | 5.7 |
| ELISA and LC/MS2 | ||||||
| 2-Hydroxyestrone | 47.8 | 24.6 | 31.0 | 13.8 | 18.6 | 2.9 |
| 16α-Hydroxyestrone | 32.2 | 11.0 | 23.8 | 6.5 | 14.1 | 1.2 |
Subjects are Asian–American women, aged 20–55 years, selected as controls for a population-based case-control study of breast cancer [29]. 12 h overnight urines were collected.
7. Future directions
We continue to optimize our EM LC/MS2 methods and refine them for the demands of large-scale epidemiologic research. We are concentrating on three issues. (1) At present, we are using either five or six stable isotopically labeled standards in our LC/MS2 methods for measuring 15 EM. In our laboratory, as soon as urines or sera are defrosted for assay, we add the stable isotopically labeled EM standards so that we can correct quantitatively for loss and degradation. Ideally, stable isotope dilution requires a distinct isotopically labeled standard for each analyte so that we do not need to extrapolate results from structurally similar, but not structurally identical, compounds. We have now acquired 12 C-13 labelled standards and will be testing and incorporating them into our assays. (2) Our current throughput per week on one LC–MS2 system is only 40 unknowns (which includes ~4 blinded quality control samples) +8 known quality control samples +14 samples for two calibration curves = 62 samples, only 58% of which are really unknowns. We can receive “real-time” information each week on assay performance from the known quality control samples. Nonetheless, this throughput means that it would require 25 weeks on each of two LC–MS2 systems to measure both total and unconjugated EM concentrations in 1000 serum samples. Clearly, throughput needs to be improved. We are currently testing some faster liquid chromatography systems potentially capable of increasing throughput 3-fold. (3) We have established standard operating procedures for our methods and carefully described the optimized techniques in publications. However, to the extent that personnel may need practical “hands-on” experience before they can successfully implement the assays, we need to clarify, and possibly simplify, our procedures. While our LC/MS2 methods for measuring EM in serum and urine are still being improved, they have been validated and are robust and rapid and, therefore, appropriate for epidemiologic work. We can assess total estrogen exposure, concentrations of specific EM, and individual patterns of estrogen metabolism in epidemiologic studies. In a population-based case-control study of breast cancer in Asian–American migrants, we have explored the relationship between urinary EM and Westernization in the controls. Within these controls, Westernization predicts a 6-fold gradient in risk of breast cancer, comparable to the historic international differences in breast cancer incidence between Asia and the U.S. [29]. We have completed two nested case-control studies of EM and breast cancer in large cohorts. The first, in collaboration with Drs. Hankinson and Eliassen, is of premenopausal breast cancer and utilizes prospectively stored urines from the Nurses’ Health Study; the second is of postmenopausal breast cancer and utilizes prospectively stored serum samples from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. We are designing a nested case-control study of endometrial cancer and circulating EM that will pool biospecimens from PLCO and other cohorts. In collaboration with Drs. Kala Visvanathan and James Yager at Johns Hopkins School of Medicine, we are testing methods to measure EM in breast tissue and will examine the relationships among conjugated and unconjugated EM in breast tissue, blood, and urine. Our LC/MS2 methods for measuring concurrently 15 EM in serum and urine provide outstanding accuracy, precision, sensitivity, and specificity. However, the methods are still relatively labor-intensive and time consuming. We hope to apply our methods in important epidemiologic research where the quality of the study design and potential impact of the results justify the use of our methods. We anticipate that the application of our techniques in epidemiologic research will inform further modification of our methods. Perfecting a method should not be an end in itself. Most important, the results from our expanding portfolio of epidemiologic studies that have utilized these methods should help clarify the role of endogenous estrogen exposure and estrogen metabolism in the etiology of cancer.
Acknowledgments
This project has been funded by the Intramural Research Programs of the Center for Cancer Research and Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, and with federal funds from the National Cancer Institute under Contract N01-CO-12400 to SAIC-Frederick, Inc. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services; nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Footnotes
Article from special issue on “Analysis, Quantification and Metabolic Profiling of Steroids”.
References
- 1.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 2.Key TJ, Wang DY, Brown JB, Hermon C, Allen DS, Moore JW, Bulbrook RD, Fentiman IS, Pike MC. A prospective study of urinary oestrogen excretion and breast cancer risk. Br J Cancer. 1996;73(12):1615–1619. doi: 10.1038/bjc.1996.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onland-Moret NC, Kaaks R, van Noord PA, Rinaldi S, Key T, Grobbee DE, Peeters PH. Urinary endogenous sex hormone levels and the risk of postmenopausal breast cancer. Br J Cancer. 2003;88(9):1394–1399. doi: 10.1038/sj.bjc.6600890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, Clavel–Chapelon F, Fournier A, van Gils CH, Gonzalez CA, Gurrea AB, Critselis E, Khaw KT, Krogh V, Lahmann PH, Nagel G, Olsen A, Onland-Moret NC, Overvad K, Palli D, Panico S, Peeters P, Quires JR, Roddam A, Thiebaut A, Tjonneland A, Chirlaque MD, Trichopoulou A, Trichopoulos D, Tumino R, Vineis P, Norat T, Ferrari P, Slimani N, Riboli E. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition(EPIC) J Natl Cancer Inst. 2005;97(10):755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 5.Allen NE, Key TJ, Dossus L, Rinaldi S, Cust A, Lukanova A, Peeters PH, Onland–Moret NC, Lahmann PH, Berrino F, Panico S, Larranaga N, Pera G, Tormo MJ, Sanchez MJ, Ramon Quiros J, Ardanaz E, Tjonneland A, Olsen A, Chang-Claude J, Linseisen J, Schulz M, Boeing H, Lundin E, Palli D, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Bingham S, Khaw KT, Bas Bueno-de-Mesquita H, Trichopoulou A, Trichopoulos D, Naska A, Tumino R, Riboli E, Kaaks R. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2008;15(2):485–497. doi: 10.1677/ERC-07-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol. 2008;630:148–165. [PubMed] [Google Scholar]
- 7.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 8.Raftogianis R, Creveling C, Weinshilboum R, Weisz J. Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000;(27):113–124. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- 9.Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Yue W, Santner SJ, Tekmal R, Demers L, Pauley R, Naftolin F, Mor G, Berstein L. Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr. 2000;(27):95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 10.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome P450 isoforms. Endocrinology. 2003;144(8):3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 11.Zhu BT. Catechol-O-Methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab. 2002;3(3):321–349. doi: 10.2174/1389200023337586. [DOI] [PubMed] [Google Scholar]
- 12.Rogan EG, Cavalieri EL. Estrogen metabolites, conjugates, and DNA adducts: possible biomarkers for risk of breast, prostate, and other human cancers. Adv Clin Chem. 2004;38:135–149. doi: 10.1016/s0065-2423(04)38005-4. [DOI] [PubMed] [Google Scholar]
- 13.Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-hydroxyestrone: the ‘good’ estrogen. J Endocrinol. 1996;150(Suppl):S259–S265. [PubMed] [Google Scholar]
- 14.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766(1):63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79(20):7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG. Measuring 15 endogenous estrogens simultaneously in human urine by high-performance liquid chromatography–mass spectrometry. Anal Chem. 2005;77(20):6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 17.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16(9):1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 18.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG. A liquid chromatography–mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3411–3418. doi: 10.1158/1055-9965.EPI-08-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, Hankinson SE. Reproducibility of 15 urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2860–2868. doi: 10.1158/1055-9965.EPI-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuhrman BJ, Xu X, Falk RT, Hankinson SE, Veenstra TD, Keefer LK, Ziegler RG. Stability of 15 estrogens and estrogen metabolites in urine samples under processing and storage conditions typically used in epidemiologic studies. [PMC free article] [PubMed] [Google Scholar]
- 21.Adlercreutz H, Kiuru P, Rasku S, Wahala K, Fotsis T. An isotope dilution gas chromatographic-mass spectrometric method for the simultaneous assay of estrogens and phytoestrogens in urine. J Steroid Biochem Mol Biol. 2004;92(5):399–411. doi: 10.1016/j.jsbmb.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Fotsis T, Jarvenpaa P, Adlercreutz H. Purification of urine for quantification of the complete estrogen profile. J Steroid Biochem. 12(1980):503–508. doi: 10.1016/0022-4731(80)90314-3. [DOI] [PubMed] [Google Scholar]
- 23.Tworoger SS, Hankinson SE. Collection, processing, and storage of biological samples in epidemiologic studies: sex hormones, carotenoids, inflammatory markers, and proteomics as examples. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1578–1581. doi: 10.1158/1055-9965.EPI-06-0629. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, Chandler W, Settlage J, Beattie MS, Folkerd E, Dowsett M, Grady D, Cummings SR. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91(10):3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 25.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72(8):666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Rauh M. Steroid measurement with LC–MS/MS in pediatric endocrinology. Mol Cell Endocrinol. 2009;301(1–2):272–281. doi: 10.1016/j.mce.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Pasqualini JR, Chetrite GS. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Mol Biol. 2005;93(2–5):221–236. doi: 10.1016/j.jsbmb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19(1):1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian–American women. J Natl Cancer Inst. 1993;85(22):1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 30.Falk RT, Gail MH, Fears TR, Rossi SC, Stanczyk F, Adlercreutz H, Kiura P, Wahala K, Donaldson JL, Vaught JB, Fillmore CM, Hoover RN, Ziegler RG. Reproducibility and validity of radioimmunoassays for urinary hormones and metabolites in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1999;8(6):567–577. [PubMed] [Google Scholar]
- 31.Falk RT, Fears TR, Xu X, Hoover RN, Pike MC, Wu AH, Nomura AM, Kolonel LN, West DW, Sepkovic DW, Bradlow HL, Ziegler RG. Urinary estrogen metabolites and their ratio among Asian–American women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):221–226. [PubMed] [Google Scholar]
- 32.Falk RT, Rossi SC, Fears TR, Sepkovic DW, Migella A, Adlercreutz H, Donaldson J, Bradlow HL, Ziegler RG. A new ELISA kit for measuring urinary 2-hydroxyestrone, 16alpha-hydroxyestrone, and their ratio: reproducibility, validity, and assay performance after freeze-thaw cycling and preservation by boric acid. Cancer Epidemiol Biomarkers Prev. 2000;9(1):81–87. [PubMed] [Google Scholar]
- 33.Faupel-Badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, Hoover RN, Ziegler RG. Comparison of liquid chromatography–tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomarkers Prev. 2010;19(1):292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]


