ABSTRACT
Transcription of the human mitochondrial genome produces a vast amount of non-coding antisense RNAs. These RNA species can form G-quadraplexes (G4), which affect their decay. We found that the mitochondrial degradosome, a complex of RNA helicase SUPV3L1 (best known as SUV3) and the ribonuclease PNPT1 (also known as PNPase), together with G4-melting protein GRSF1, is a key player in restricting antisense mtRNAs.
Keywords: RNA surveillance, mitochondrial RNA, G-quadraplexes, G4, PNPase, SUV3, GRSF1
Author’s view
Ribonucleolytic enzymes display various specificities for substrate sequence, structure or length. Some enzymes such as the bacterial RNase R are capable of degrading structured RNAs on their own, whereas other RNases cooperate with additional proteins to overcome structural obstacles. RNA molecules can adopt a plethora of structures, some involve non-canonical base pairing, such as G-quadraplexes (G4s). Extensive studies of G4s provide an increasing amount of evidence which shows the critical role of G4 structures in various aspects of RNA biology with a link to human diseases. Intriguingly, mitochondrial genomes of vertebrates are not only GC rich but also exhibit extraordinary GC skews, i.e. high guanine content on one strand. Hence transcription of these genomes produces G-rich RNAs that are likely to form G4s1. These RNAs, which are antisense to functional RNAs, are synthetized at high rate but they are difficult to detect under normal conditions since their amount is limited by RNA-degrading machinery (Figure 1). This swift removal of antisense mitochondrial RNAs seems to be physiologically important, since they potentially affect the functions and expression of sense transcripts. Recently, we described a mechanism by which G4-containing antisense RNAs are recognized and degraded in human mitochondria.1
Figure 1.
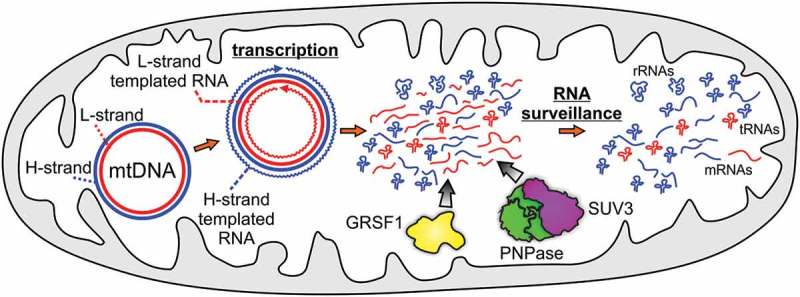
Degradosome shapes the mitochondrial transcriptome. The mitochondrial genome (mtDNA) is a circular double-stranded DNA molecule. Its individual strands differ in their guanine nucleotides content. The G-rich strand is called the H-strand (marked in blue), and the other one is called the L-strand (marked in red). MtDNA encodes 2 rRNAs, 22 tRNAs and 11 mRNAs, of which 8 tRNAs and one mRNA is encoded by the H-strand. Most functional RNAs are produced by H-strand templated transcription whereas the transcription of the L-strand results in the synthesis of many non-coding antisense RNAs. These RNA species are removed by the SUV3-PNPase complex supported by GRSF1, shaping the mitochondrial transcriptome. Please note that for the sake of simplicity not all tRNAs are presented in the figure.
In this study, we found that G-rich RNA sequence binding factor 1 (GRSF1), a member of the quasi-RRM family of RNA binding proteins,2 cooperates with the mitochondrial degradosome, a complex of the Suv3-like RNA helicase SUPV3L1 and the polyribonucleotide nucleotidyltransferase PNPT1 (best known as the helicase SUV3 and the ribonuclease PNPase), to control G4-containing mitochondrial RNAs.1 The human SUV3 was previously identified based on its similarity to its yeast homologue,3 whereas its ribonucleolytic partner was discovered using protein-protein interaction studies4,5 followed by functional experiments.5 Importantly, we revealed that the complex of SUV3 and PNPase is formed in distinct foci, which we named D-foci (since they contain the degradosome).5 This implied that degradosome-dependent RNA transactions are spatially organized. The spatial organization of RNA processing had been described for nuclear and cytosolic compartments but was not attributed to mitochondrial RNA (mtRNA) before.5 After our discovery of D-foci others revealed that GRSF1 protein also functions in a spatially-organized manner.6
The function of SUV3 and PNPase in mtRNA surveillance and decay was established4,5 but it remained unclear how the degradosome recognizes its targets and achieves such a high efficiency of degradation of non-coding mtRNAs. This was intriguing because many non-coding mtRNAs have the potential to adopt G4 structures, which can hamper RNA degradation. Our recent studies showed that the high degradation efficiency of mitochondrial antisense transcripts is achieved, at least partly, by cooperation with the GRSF1 protein.1
A proteomic screen supported by immunofluorescence studies revealed that GRSF1 associates with the degradosome complex,1 which is in agreement with co-immunoprecipitation studies performed by others.7 Using comprehensive RNA sequencing analysis, we identified substrates of the degradosome and GRSF1 and found that non-coding mtRNAs that are prone to form G4s accumulate upon degradosome or GRSF1 dysfunction.1 Among these non-coding mtRNAs we discovered a short mtRNA molecule, which we named tRNA-like, that undergoes post-transcriptional maturation. Biochemical and biophysical analyses revealed that GRSF1/degradosome substrates indeed form G4s, which melt upon GRSF1 binding. This melting facilitates their degradation by the SUV3-PNPase complex both in vitro and in vivo.1
Several lines of evidence support the conclusion that G4 is the cognate target for GRSF1 and the degradosome. First, a group of mtRNAs accumulating upon GRSF1 or degradosome dysfunction includes transcripts which can form G4 but not their complementary counterparts which lack G4 consensus.1 In addition, bioinformatic analysis revealed that a reduction in GRSF1 levels or degradosome activity leads to accumulation of RNAs upstream of G4s, which indicates that under these conditions the degradation of transcripts cannot proceed efficiently across the G4 sequences.1 This is in agreement with the biochemical features of SUV3 and PNPase which function from the 3ʹ to the 5ʹ end of RNA. Second, GRSF1 belongs to quasi-RRM (RNA recognition motif) family for which mechanism of binding and melting of G4 RNA structures was established.2 In agreement with this, our in vitro binding assays1 as well as the results of cross-linking and immunoprecipitation experiments performed by others7 showed that GRSF1 binds RNAs that can adopt G4 structures. Furthermore, studies of McRae et al, who performed a pull-down search for proteins binding G4 RNA, identified GRSF1 as one of the top hits.8 Thus, the ability of GRSF1 to bind G4 RNAs is supported by various data. Third, results of in vitro RNA degradation assays, as well as circular dichroism experiments, indicate that GRSF1 binding affects G4 structure.1 This is in agreement with structural data available for two GRSF1 qRRM domains in a complex with poly Gs. Based on our data we propose a mechanism by which GRSF1 binds RNA and melts/precludes the formation of G4. This enables degradation of destabilized RNA by the degradosome.1 The catalytic activity of SUV3 plays at least two functions: 1) it unwinds classical double-stranded RNA stretches, 2) it displaces GRSF1 from RNA. Otherwise PNPase is unable to degrade dsRNA or single-stranded RNA bound by GRSF11. This seems to be the first documented example of a molecular mechanism involving a G4-melting protein and an RNA decay machinery to control G4 levels.
Notably, the degradosome alone can degrade G4 RNAs, however, its ribonucleolytic activity towards such substrates is strongly enhanced in the presence of GRSF1.1 From the evolutionary point of view the degradosome appeared in mitochondria earlier than GRSF1. Our phylogenetic analysis indicated that GRSF1 was acquired to mitochondria when mitochondrial genomes evolved into molecules encoding many RNAs prone to form G4 structures, namely vertebrates.1 Therefore, it is not surprising that the degradosome has an ability to degrade G4 to some extent as GRSF1-negative organisms are not completely depleted of G4 consensus sequences. GRSF1 and its auxiliary role come to be important when mitochondrial genomes become G4-rich since the activity of the degradosome alone was not sufficient to cope with so many G4 RNAs. Sequence based analysis of quasi-RRM proteins, which are distinguished from classic RRM proteins, including mitochondrial SRA stem-loop interacting RNA binding SLIRP protein, suggests that GRSF1 seems to be the only quasi-RRM protein present in vertebrate mitochondria.1 Thus, the appearance of GRSF1 in mitochondria is an evolutionary adaptation, which enables efficient control of the levels of RNAs that form G4s.1
Our work supports the hypothesis put forward by Guo and Bartel that eukaryotes developed the protein machinery to keep the majority of G4 RNAs in an unfolded state.9 Co-operation of GRSF1 and the degradosome enables efficient elimination of mitochondrial G4 RNAs, which involves G4 melting (GRSF1) and RNA degradation (degradosome).1 Importance of the degradosome-mediated surveillance of mtRNAs is highlighted by the fact that its inactivation can lead to human diseases, including interferonopathies caused by inappropriate activation of antiviral response.10
Funding Statement
This work was supported by the National Science Centre, Poland under Grants UMO-2014/12/W/NZ1/00463 (to R.J.S.), 2013/11/B/NZ1/00089 (to P.P.S.); European Research Council under Starting Grant 309419 PAPs & PUPs (to A.D.).
Acknowledgments
We thank Janina Durys for language editing.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Pietras Z, Wojcik MA, Borowski LS, Szewczyk M, Kulinski TM, Cysewski D, Stepien PP, Dziembowski A, Szczesny RJ.. Dedicated surveillance mechanism controls G-quadruplex forming non-coding RNAs in human mitochondria. Nat Commun. 2018;9:2558. doi: 10.1038/s41467-018-05007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominguez C, Fisette J-F, Chabot B, Allain FH-T.. Structural basis of G-tract recognition and encaging by hnRNP F quasi-RRMs. Nat Struct Mol Biol. 2010;17:853–861. doi: 10.1038/nsmb.1814. [DOI] [PubMed] [Google Scholar]
- 3.Minczuk M, Piwowarski J, Papworth MA, Awiszus K, Schalinski S, Dziembowski A, Dmochowska A, Bartnik E, Tokatlidis K, Stepien PP, et al. Localisation of the human hSuv3p helicase in the mitochondrial matrix and its preferential unwinding of dsDNA. Nucleic Acids Res. 2002;30:5074–5086. doi: 10.1093/nar/gkf647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szczesny RJ, Borowski LS, Brzezniak LK, Dmochowska A, Gewartowski K, Bartnik E, Stepien PP. Human mitochondrial RNA turnover caught in flagranti: involvement of hSuv3p helicase in RNA surveillance. Nucleic Acids Res. 2010;38:279–298. doi: 10.1093/nar/gkp903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borowski LS, Dziembowski A, Hejnowicz MS, Stepien PP, Szczesny RJ. Human mitochondrial RNA decay mediated by PNPase-hSuv3 complex takes place in distinct foci. Nucleic Acids Res. 2013;41:1223–1240. doi: 10.1093/nar/gks1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonicka H, Sasarman F, Nishimura T, Paupe V, Shoubridge EA. The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab. 2013;17:386–398. doi: 10.1016/j.cmet.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Antonicka H, Shoubridge EA. Mitochondrial RNA granules are centers for posttranscriptional RNA processing and ribosome biogenesis. Cell Rep. 2015;10:920–932. doi: 10.1016/j.celrep.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 8.McRae EKS, Booy EP, Moya-Torres A, Ezzati P, Stetefeld J, McKenna SA. Human DDX21 binds and unwinds RNA guanine quadruplexes. Nucleic Acids Res. 2017;45:6656–6668. doi: 10.1093/nar/gkx380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo JU, Bartel DP. RNA G-quadruplexes are globally unfolded in eukaryotic cells and depleted in bacteria. Science. 2016:353. doi: 10.1126/science.aaf5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhir A, Dhir S, Borowski LS, Jimenez L, Teitell M, Rötig A, Crow YJ, Rice GI, Duffy D, Tamby C, et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature. 2018;560:238–242. doi: 10.1038/s41586-018-0363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


