Abstract
The production of ribosomes plays a central role in regulating cell cycle progression and cancer proliferation. A new study by Gaviraghi et al (2018) shows that mRNA decapping coactivator PNRC1 acts as a tumor suppressor by regulating ribosome biogenesis. PNRC1 relocalizes the Dcp1/Dcp2 mRNA decapping complex to the nucleolus and promotes decapping of specific snoRNAs to disrupt the processing of ribosomal RNA. By slowing rRNA processing, and thus ribosome biogenesis, PNRC1 acts as a gatekeeper that restrains oncogenic potential.
Subject Categories: Cancer, RNA Biology
During protein synthesis, ribosomes coordinate peptide bond formation with the decoding of mRNA by tRNA. It has long been appreciated that misregulation of protein synthesis is a hallmark and driving force in many cancers (Robichaud et al, 2018). The rate‐limiting step of protein synthesis for the majority of mRNA transcripts in the cell is thought to be cap‐dependent translation initiation, during which (eukaryotic) initiation factors (eIFs) recruit the ribosome to mRNA using its 5′ terminal m7G cap structure (Merrick & Pavitt, 2018). Overexpression of initiation factors correlates with enhanced translational efficiency of oncogenic mRNAs and transformation of cells (Chu et al, 2016). Targeting eIFs with small molecules can reverse these effects by inhibiting translation initiation. The cell has a number of mechanisms to keep translation initiation in check, including regulation of eIFs by phosphorylation or removal of the 5′ cap structure by decapping enzymes, which is the ultimate form of translational repression leading to digestion of the RNA body by 5′–3′ exonucleases.
Increased rates of ribosome biogenesis are also linked to cancer proliferation and tumorigenesis (Pelletier et al, 2018). How oncogenes hijack factors involved in ribosome biogenesis to promote cellular transformation is poorly understood. In this issue, Gaviraghi et al report that proline‐rich nuclear receptor coactivator 1 (PNRC1) is a tumor suppressor that limits the production of ribosomes by recruiting the Dcp1/Dcp2 decapping complex to the nucleolus to selectively decap the U3 and U8 small nucleolar RNA (snoRNA), thereby inhibiting ribosome biogenesis (Fig 1). This unexpected connection was made by cleverly mining The Cancer Genome Atlas (TCGA) and RNA‐Seq data for genes with copy number alterations and low expression patterns. The authors established a cluster of genes with this property, which included known nucleolar factors linked to cancer. PNRC1 stood out as its function in the nucleolus was uncharacterized. Several observations suggested PNRC1 could act as a tumor suppressor. First, the authors found that PNRC1 was not expressed in patient cancer cells relative to matched controls. Second, in primary cells and in a variety of cell lines, expression of PNRC1 was mutually exclusive with proliferation. Third, ectopic expression of PNRC1 reduced proliferation induced by RAS and MYC, in addition to the ability of these oncogenes to promote focus formation on soft agar when overexpressed.
Figure 1. Model for PNRC1‐mediated snoRNA decapping in the nucleolus to regulate ribosome biogenesis.
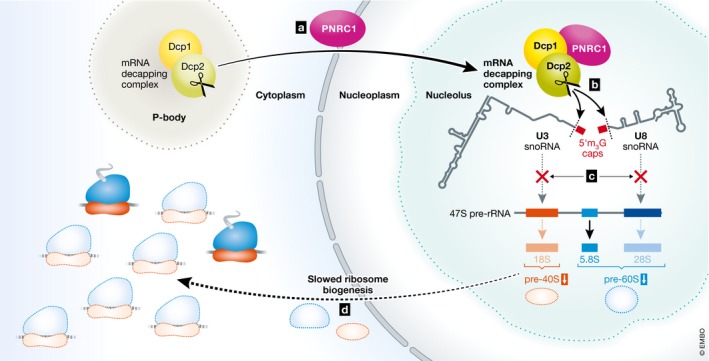
(A) PNRC1 binds to Dcp1/Dcp2 and relocalizes the mRNA decapping complex from cytoplasmic P‐bodies to the nucleolus. (B) PNRC1‐bound decapping complex selectively cleaves the 5′ cap on U3 and U8 snoRNAs. (C) Decapping of U3 and U8 snoRNAs prevents cleavage and processing of the 18S and 28S rRNA isoforms from the 47S pre‐rRNA. (D) Downregulation of 18S and 28S rRNA leads to slowed ribosome biogenesis and tumor suppression.
The nucleolus is a factory for ribosomal RNA (rRNA) transcription and processing. It is here that the single 47S pre‐rRNA is transcribed by RNA Pol I and subsequently cut and modified to generate mature 5.8S, 18S, and 28S rRNA isoforms. The rRNA cleavage and editing are directed by catalytic ribonucleoprotein assemblies guided to specific sites in pre‐rRNA by small nucleolar RNA (snoRNA) through homologous base pairing. The authors show that PNRC1 localizes to sites of rRNA processing in the nucleolus and that expression of PNRC1 reduces the accumulation of mature 18S and 28s rRNA, which are critical components of the large and small ribosomal subunits, respectively. To gain insights into mechanism, the authors performed immunoprecipitation in conjunction with mass spectrometry (IP‐MS) using PNRC1 as bait and found it interacts with the Dcp1/Dcp2 mRNA decapping complex. In cells, the multiprotein eukaryotic decapping complex consists of the catalytic subunit Dcp2, the general activator Dcp1, and a variety of pathway‐specific coactivators that function in bulk mRNA decay, regulated decay, and mRNA quality control pathways (Franks & Lykke‐Andersen, 2008). The coactivator PNRC1, like its paralog PNRC2, contains conserved, short linear motifs that have recently been shown to efficiently bind to and enhance the mRNA decapping activity of Dcp1/Dcp2 in vitro (Mugridge et al, 2018). While mRNA decapping occurs on ribosomes, the decapping complex and its coactivators, such as PNRC2, are often found localized to punctate structures in the cytoplasm known as mRNA processing bodies (P‐bodies). Strikingly, expression of PNRC1 relocalizes Dcp1/Dcp2 to the nucleolus and results in dispersal of P‐bodies, effects that depend on physical association of PNRC1 with Dcp1. But how does PNRC1‐mediated nucleolar localization of the decapping complex result in misregulation of ribosome biogenesis? Both pre‐rRNAs resulting from RNA Pol I and Pol III transcription (e.g., 47S and 5S) and most snoRNAs that are derived from introns of “host transcripts” are uncapped and thus unaffected by decapping activity.
It turns out there are three snoRNAs, U3, U8, and U13, that are transcribed by RNA Pol II and contain a m7G cap structure, which is later trimethylated in the cytoplasm, and reimported into the nucleolus where they can function in rRNA processing (Kiss, 2004). Among these, the U3 snoRNP promotes cleavage of external and internal spacer sequences (ETS1 and ITS1) during 47S pre‐rRNA processing. Since PNRC1 expression increased the ratio of steady‐state levels of 47S pre‐rRNA to 28S product, and this depends on its interaction with Dcp1 and the expression of Dcp2, the authors reasoned that PNRC1 may promote decapping of the U3 snoRNA. Indeed, expression of PNRC1 decreased the amount of U3 snoRNA that could be immunoprecipitated using anti‐cap beads, relative to a mutant of PNRC1 that could not interact with the Dcp1/Dcp2 decapping complex. If PNRC1 promotes decapping of the U3 snoRNA, this should expose a 5′ monophosphate which is susceptible to ligation with an adaptor RNA. Consistent with U3 decapping, the authors observe an increase in ligation efficiency on the U3 snoRNA when PNRC1 is expressed, but not on the capped U1 small nuclear RNA (snRNA), which is involved in splicing. Similar experiments showed PNRC1 promoted decapping of the U8 snoRNA. In sum, PNRC1 makes the Dcp1/Dcp2 decapping complex go nucleolar, directing its specificity toward capped snoRNAs to inhibit ribosome biogenesis and suppress cancer.
This study paves the way for future research looking at the connections between decapping, RNA metabolism, and cancer. For example, loss of the decapping enzyme Nudt16 has recently been linked to C‐MYC activation in leukemia by an unknown mechanism (Anadón et al, 2017). Nudt16 is localized to the nucleolus and the cytoplasm and was shown to decap the U8 snoRNA, like PNRC1, as well as additional mRNA targets (Grudzien‐Nogalska & Kiledjian, 2017). Is Nudt16 a tumor suppressor and does it work to suppress the same or different oncogenes as PNRC1? Genetic interaction studies in mice have the potential to inform on this question. Second, PNRC1‐mediated decapping does not affect steady‐state levels of the U3 or U8 snoRNAs, suggesting that the decapped RNAs are protected from degradation by conserved exoribonucleases such as Xrn2. The authors suggest PNRC1 may misregulate some other aspects of snoRNP biology, such as localization. Future studies will be required to test whether PNRC1 broadly affects capped snoRNA localization or impacts additional targets in cells. Finally, it is noteworthy that the budding yeast decapping coactivator Edc2 contains the same Dcp1‐binding and Dcp2‐activating motifs as PNRC1 and can also be found in the nucleolus (Neef & Thiele, 2009; Mugridge et al, 2018), suggesting that the control of ribosome biogenesis and cell proliferation by Dcp1/Dcp2‐mediated decapping may be a deeply conserved process meriting further exploration.
The EMBO Journal (2018) 37: e100801
See also: https://doi.org/10.15252/embj.201899179 (December 2018)
References
- Anadón C, van Tetering G, Ferreira HJ, Moutinho C, Martínez‐Cardús A, Villanueva A, Soler M, Heyn H, Moran S, de Moura MC, Setien F, Vidal A, Genescà E, Ribera JM, Nomdedeu JF, Guil S, Esteller M (2017) Epigenetic loss of the RNA decapping enzyme NUDT16 mediates C‐MYC activation in T‐cell acute lymphoblastic leukemia. Leukemia 31: 1622–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Cargnello M, Topisirovic I, Pelletier J (2016) Translation initiation factors: reprogramming protein synthesis in cancer. Trends Cell Biol 26: 918–933 [DOI] [PubMed] [Google Scholar]
- Franks TM, Lykke‐Andersen J (2008) The control of mRNA decapping and P‐body formation. Mol Cell 32: 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaviraghi M, Vivori C, Sanchez YP, Invernizzi F, Cattaneo A, Santoliquido BM, Frenquelli M, Segalla S, Bachi A, Doglioni C, Pelechano V, Cittaro D, Tonon G (2018) Tumor suppressor PNRC1 blocks rRNA maturation by recruiting the decapping complex to the nucleolus. EMBO J 37: e99179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzien‐Nogalska E, Kiledjian M (2017) New insights into decapping enzymes and selective mRNA decay. Wiley Interdiscip Rev RNA 8: e1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T (2004) Biogenesis of small nuclear RNPs. J Cell Sci 117: 5949–5951 [DOI] [PubMed] [Google Scholar]
- Merrick WC, Pavitt GD (2018) Protein synthesis initiation in eukaryotic cells. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a033092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugridge JS, Tibble RW, Ziemniak M, Jemielity J, Gross JD (2018) Structure of the activated Edc1‐Dcp1‐Dcp2‐Edc3 mRNA decapping complex with substrate analog poised for catalysis. Nat Commun 9: 1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef DW, Thiele DJ (2009) Enhancer of decapping proteins 1 and 2 are important for translation during heat stress in Saccharomyces cerevisiae . Mol Microbiol 73: 1032–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Thomas G, Volarević S (2018) Ribosome biogenesis in cancer: new players and therapeutic avenues. Nat Rev Cancer 18: 51–63 [DOI] [PubMed] [Google Scholar]
- Robichaud N, Sonenberg N, Ruggero D, Schneider RJ (2018) Translational control in cancer. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a032896 [DOI] [PMC free article] [PubMed] [Google Scholar]


